 Found 60 hits of Enzyme Inhibition Constant Data
Found 60 hits of Enzyme Inhibition Constant Data Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Muscarinic acetylcholine receptor M3
(RAT) | BDBM50021919
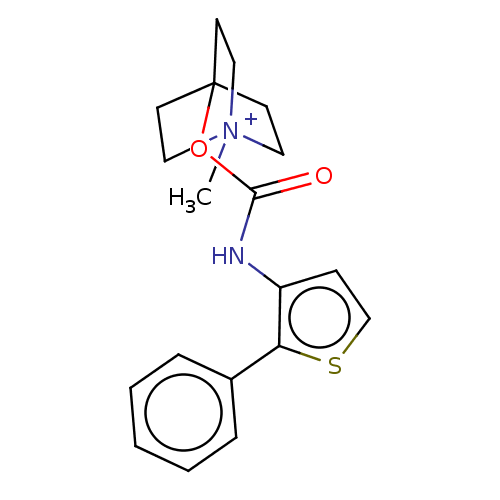
(CHEMBL3298595)Show SMILES [I-].C[N+]12CCC(CC1)(CC2)OC(=O)Nc1ccsc1-c1ccccc1 Show InChI InChI=1S/C19H22N2O2S.HI/c1-21-11-8-19(9-12-21,10-13-21)23-18(22)20-16-7-14-24-17(16)15-5-3-2-4-6-15;/h2-7,14H,8-13H2,1H3;1H | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc
Curated by ChEMBL
| Assay Description
Displacement of [3H]-NMS from Sprague-Dawley rat muscarinic M3 receptor in submandibular gland |
Bioorg Med Chem 22: 3478-87 (2014)
Article DOI: 10.1016/j.bmc.2014.04.031
BindingDB Entry DOI: 10.7270/Q2XS5X00 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(RAT) | BDBM50021919

(CHEMBL3298595)Show SMILES [I-].C[N+]12CCC(CC1)(CC2)OC(=O)Nc1ccsc1-c1ccccc1 Show InChI InChI=1S/C19H22N2O2S.HI/c1-21-11-8-19(9-12-21,10-13-21)23-18(22)20-16-7-14-24-17(16)15-5-3-2-4-6-15;/h2-7,14H,8-13H2,1H3;1H | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc
Curated by ChEMBL
| Assay Description
Displacement of [3H]-NMS from Sprague-Dawley rat muscarinic M1 receptor in cortex membrane |
Bioorg Med Chem 22: 3478-87 (2014)
Article DOI: 10.1016/j.bmc.2014.04.031
BindingDB Entry DOI: 10.7270/Q2XS5X00 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(RAT) | BDBM50021919

(CHEMBL3298595)Show SMILES [I-].C[N+]12CCC(CC1)(CC2)OC(=O)Nc1ccsc1-c1ccccc1 Show InChI InChI=1S/C19H22N2O2S.HI/c1-21-11-8-19(9-12-21,10-13-21)23-18(22)20-16-7-14-24-17(16)15-5-3-2-4-6-15;/h2-7,14H,8-13H2,1H3;1H | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc
Curated by ChEMBL
| Assay Description
Displacement of [3H]-NMS from Sprague-Dawley rat muscarinic M2 receptor in heart |
Bioorg Med Chem 22: 3478-87 (2014)
Article DOI: 10.1016/j.bmc.2014.04.031
BindingDB Entry DOI: 10.7270/Q2XS5X00 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(RAT) | BDBM50021928
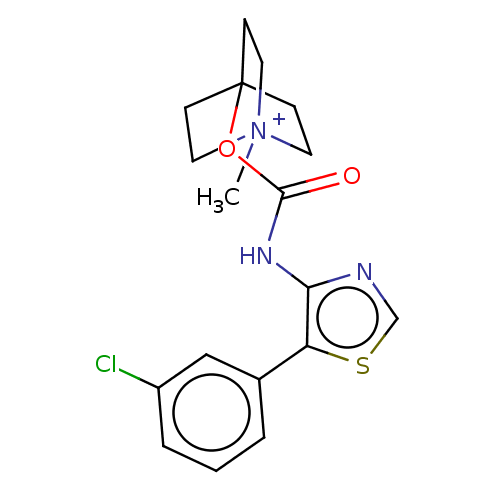
(CHEMBL3298599)Show SMILES [I-].C[N+]12CCC(CC1)(CC2)OC(=O)Nc1ncsc1-c1cccc(Cl)c1 Show InChI InChI=1S/C18H20ClN3O2S.HI/c1-22-8-5-18(6-9-22,7-10-22)24-17(23)21-16-15(25-12-20-16)13-3-2-4-14(19)11-13;/h2-4,11-12H,5-10H2,1H3;1H | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc
Curated by ChEMBL
| Assay Description
Displacement of [3H]-NMS from Sprague-Dawley rat muscarinic M1 receptor in cortex membrane |
Bioorg Med Chem 22: 3478-87 (2014)
Article DOI: 10.1016/j.bmc.2014.04.031
BindingDB Entry DOI: 10.7270/Q2XS5X00 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(RAT) | BDBM50021928

(CHEMBL3298599)Show SMILES [I-].C[N+]12CCC(CC1)(CC2)OC(=O)Nc1ncsc1-c1cccc(Cl)c1 Show InChI InChI=1S/C18H20ClN3O2S.HI/c1-22-8-5-18(6-9-22,7-10-22)24-17(23)21-16-15(25-12-20-16)13-3-2-4-14(19)11-13;/h2-4,11-12H,5-10H2,1H3;1H | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc
Curated by ChEMBL
| Assay Description
Displacement of [3H]-NMS from Sprague-Dawley rat muscarinic M3 receptor in submandibular gland |
Bioorg Med Chem 22: 3478-87 (2014)
Article DOI: 10.1016/j.bmc.2014.04.031
BindingDB Entry DOI: 10.7270/Q2XS5X00 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(RAT) | BDBM50021928

(CHEMBL3298599)Show SMILES [I-].C[N+]12CCC(CC1)(CC2)OC(=O)Nc1ncsc1-c1cccc(Cl)c1 Show InChI InChI=1S/C18H20ClN3O2S.HI/c1-22-8-5-18(6-9-22,7-10-22)24-17(23)21-16-15(25-12-20-16)13-3-2-4-14(19)11-13;/h2-4,11-12H,5-10H2,1H3;1H | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc
Curated by ChEMBL
| Assay Description
Displacement of [3H]-NMS from Sprague-Dawley rat muscarinic M2 receptor in heart |
Bioorg Med Chem 22: 3478-87 (2014)
Article DOI: 10.1016/j.bmc.2014.04.031
BindingDB Entry DOI: 10.7270/Q2XS5X00 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(RAT) | BDBM50021922
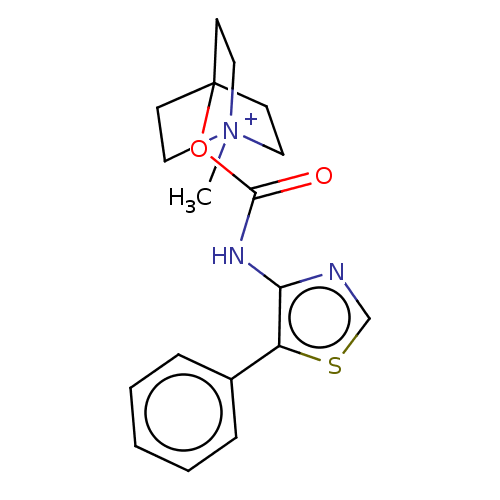
(CHEMBL3298596)Show SMILES [I-].C[N+]12CCC(CC1)(CC2)OC(=O)Nc1ncsc1-c1ccccc1 Show InChI InChI=1S/C18H21N3O2S.HI/c1-21-10-7-18(8-11-21,9-12-21)23-17(22)20-16-15(24-13-19-16)14-5-3-2-4-6-14;/h2-6,13H,7-12H2,1H3;1H | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc
Curated by ChEMBL
| Assay Description
Displacement of [3H]-NMS from Sprague-Dawley rat muscarinic M1 receptor in cortex membrane |
Bioorg Med Chem 22: 3478-87 (2014)
Article DOI: 10.1016/j.bmc.2014.04.031
BindingDB Entry DOI: 10.7270/Q2XS5X00 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(RAT) | BDBM50021904
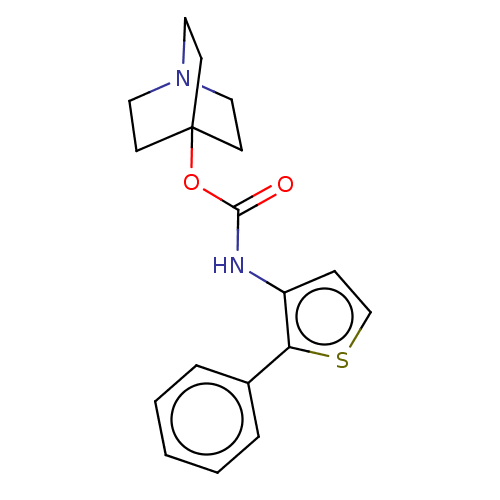
(CHEMBL3298588)Show InChI InChI=1S/C18H20N2O2S.ClH/c21-17(22-18-7-10-20(11-8-18)12-9-18)19-15-6-13-23-16(15)14-4-2-1-3-5-14;/h1-6,13H,7-12H2,(H,19,21);1H | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc
Curated by ChEMBL
| Assay Description
Displacement of [3H]-NMS from Sprague-Dawley rat muscarinic M3 receptor in submandibular gland |
Bioorg Med Chem 22: 3478-87 (2014)
Article DOI: 10.1016/j.bmc.2014.04.031
BindingDB Entry DOI: 10.7270/Q2XS5X00 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(RAT) | BDBM50021922

(CHEMBL3298596)Show SMILES [I-].C[N+]12CCC(CC1)(CC2)OC(=O)Nc1ncsc1-c1ccccc1 Show InChI InChI=1S/C18H21N3O2S.HI/c1-21-10-7-18(8-11-21,9-12-21)23-17(22)20-16-15(24-13-19-16)14-5-3-2-4-6-14;/h2-6,13H,7-12H2,1H3;1H | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0640 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc
Curated by ChEMBL
| Assay Description
Displacement of [3H]-NMS from Sprague-Dawley rat muscarinic M3 receptor in submandibular gland |
Bioorg Med Chem 22: 3478-87 (2014)
Article DOI: 10.1016/j.bmc.2014.04.031
BindingDB Entry DOI: 10.7270/Q2XS5X00 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(RAT) | BDBM50021935
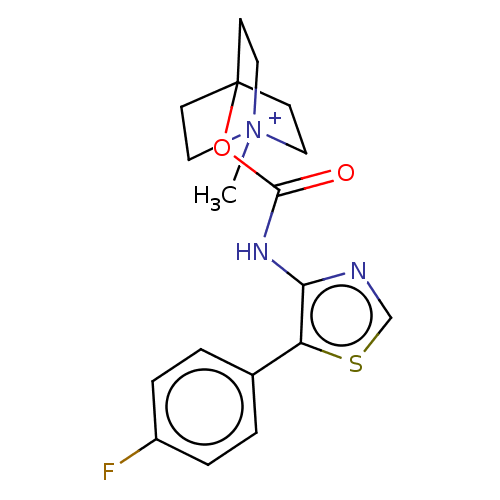
(CHEMBL3298600)Show SMILES [I-].C[N+]12CCC(CC1)(CC2)OC(=O)Nc1ncsc1-c1ccc(F)cc1 Show InChI InChI=1S/C18H20FN3O2S.HI/c1-22-9-6-18(7-10-22,8-11-22)24-17(23)21-16-15(25-12-20-16)13-2-4-14(19)5-3-13;/h2-5,12H,6-11H2,1H3;1H | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0730 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc
Curated by ChEMBL
| Assay Description
Displacement of [3H]-NMS from Sprague-Dawley rat muscarinic M1 receptor in cortex membrane |
Bioorg Med Chem 22: 3478-87 (2014)
Article DOI: 10.1016/j.bmc.2014.04.031
BindingDB Entry DOI: 10.7270/Q2XS5X00 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(RAT) | BDBM50021904

(CHEMBL3298588)Show InChI InChI=1S/C18H20N2O2S.ClH/c21-17(22-18-7-10-20(11-8-18)12-9-18)19-15-6-13-23-16(15)14-4-2-1-3-5-14;/h1-6,13H,7-12H2,(H,19,21);1H | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0950 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc
Curated by ChEMBL
| Assay Description
Displacement of [3H]-NMS from Sprague-Dawley rat muscarinic M1 receptor in cortex membrane |
Bioorg Med Chem 22: 3478-87 (2014)
Article DOI: 10.1016/j.bmc.2014.04.031
BindingDB Entry DOI: 10.7270/Q2XS5X00 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(RAT) | BDBM50021935

(CHEMBL3298600)Show SMILES [I-].C[N+]12CCC(CC1)(CC2)OC(=O)Nc1ncsc1-c1ccc(F)cc1 Show InChI InChI=1S/C18H20FN3O2S.HI/c1-22-9-6-18(7-10-22,8-11-22)24-17(23)21-16-15(25-12-20-16)13-2-4-14(19)5-3-13;/h2-5,12H,6-11H2,1H3;1H | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc
Curated by ChEMBL
| Assay Description
Displacement of [3H]-NMS from Sprague-Dawley rat muscarinic M3 receptor in submandibular gland |
Bioorg Med Chem 22: 3478-87 (2014)
Article DOI: 10.1016/j.bmc.2014.04.031
BindingDB Entry DOI: 10.7270/Q2XS5X00 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(RAT) | BDBM50021922

(CHEMBL3298596)Show SMILES [I-].C[N+]12CCC(CC1)(CC2)OC(=O)Nc1ncsc1-c1ccccc1 Show InChI InChI=1S/C18H21N3O2S.HI/c1-21-10-7-18(8-11-21,9-12-21)23-17(22)20-16-15(24-13-19-16)14-5-3-2-4-6-14;/h2-6,13H,7-12H2,1H3;1H | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc
Curated by ChEMBL
| Assay Description
Displacement of [3H]-NMS from Sprague-Dawley rat muscarinic M2 receptor in heart |
Bioorg Med Chem 22: 3478-87 (2014)
Article DOI: 10.1016/j.bmc.2014.04.031
BindingDB Entry DOI: 10.7270/Q2XS5X00 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(RAT) | BDBM50021893
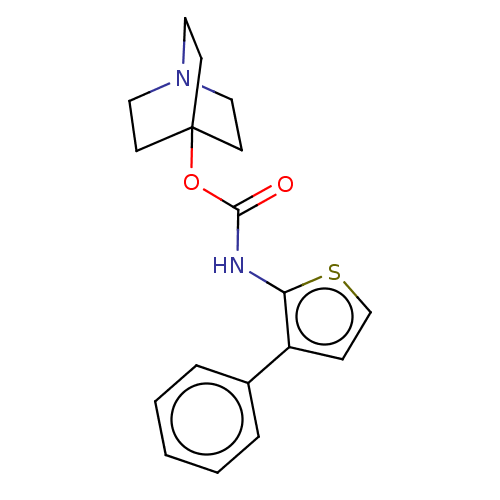
(CHEMBL3298333)Show InChI InChI=1S/C18H20N2O2S/c21-17(22-18-7-10-20(11-8-18)12-9-18)19-16-15(6-13-23-16)14-4-2-1-3-5-14/h1-6,13H,7-12H2,(H,19,21) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc
Curated by ChEMBL
| Assay Description
Displacement of [3H]-NMS from Sprague-Dawley rat muscarinic M3 receptor in submandibular gland |
Bioorg Med Chem 22: 3478-87 (2014)
Article DOI: 10.1016/j.bmc.2014.04.031
BindingDB Entry DOI: 10.7270/Q2XS5X00 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(RAT) | BDBM50021938
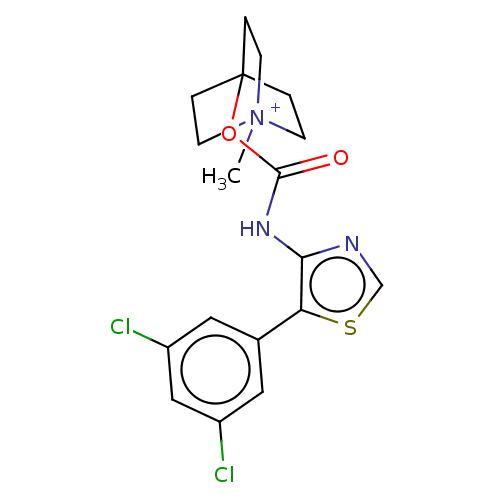
(CHEMBL3298763)Show SMILES [I-].C[N+]12CCC(CC1)(CC2)OC(=O)Nc1ncsc1-c1cc(Cl)cc(Cl)c1 Show InChI InChI=1S/C18H19Cl2N3O2S.HI/c1-23-5-2-18(3-6-23,4-7-23)25-17(24)22-16-15(26-11-21-16)12-8-13(19)10-14(20)9-12;/h8-11H,2-7H2,1H3;1H | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc
Curated by ChEMBL
| Assay Description
Displacement of [3H]-NMS from Sprague-Dawley rat muscarinic M3 receptor in submandibular gland |
Bioorg Med Chem 22: 3478-87 (2014)
Article DOI: 10.1016/j.bmc.2014.04.031
BindingDB Entry DOI: 10.7270/Q2XS5X00 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(RAT) | BDBM50021938

(CHEMBL3298763)Show SMILES [I-].C[N+]12CCC(CC1)(CC2)OC(=O)Nc1ncsc1-c1cc(Cl)cc(Cl)c1 Show InChI InChI=1S/C18H19Cl2N3O2S.HI/c1-23-5-2-18(3-6-23,4-7-23)25-17(24)22-16-15(26-11-21-16)12-8-13(19)10-14(20)9-12;/h8-11H,2-7H2,1H3;1H | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc
Curated by ChEMBL
| Assay Description
Displacement of [3H]-NMS from Sprague-Dawley rat muscarinic M1 receptor in cortex membrane |
Bioorg Med Chem 22: 3478-87 (2014)
Article DOI: 10.1016/j.bmc.2014.04.031
BindingDB Entry DOI: 10.7270/Q2XS5X00 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(RAT) | BDBM50344285
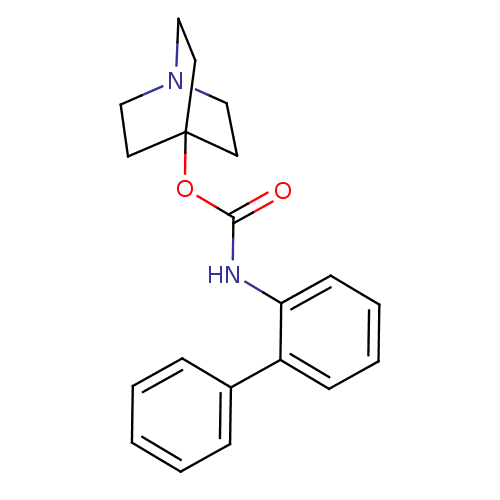
(CHEMBL1779047 | quinuclidin-4-yl biphenyl-2-ylcarb...)Show InChI InChI=1S/C20H22N2O2/c23-19(24-20-10-13-22(14-11-20)15-12-20)21-18-9-5-4-8-17(18)16-6-2-1-3-7-16/h1-9H,10-15H2,(H,21,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc
Curated by ChEMBL
| Assay Description
Displacement of [3H]-NMS from Sprague-Dawley rat muscarinic M3 receptor in submandibular gland |
Bioorg Med Chem 22: 3478-87 (2014)
Article DOI: 10.1016/j.bmc.2014.04.031
BindingDB Entry DOI: 10.7270/Q2XS5X00 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(RAT) | BDBM50296345

((1R,2R,4S,5R,7S)-7-(2-hydroxy-2,2-di-thiophen-2-yl...)Show SMILES C[N+]1(C)[C@@H]2C[C@H](C[C@@H]1[C@@H]1O[C@H]21)OC(=O)C(O)(c1cccs1)c1cccs1 |TLB:11:5:1:8.10| Show InChI InChI=1S/C19H22NO4S2/c1-20(2)12-9-11(10-13(20)17-16(12)24-17)23-18(21)19(22,14-5-3-7-25-14)15-6-4-8-26-15/h3-8,11-13,16-17,22H,9-10H2,1-2H3/q+1/t11-,12-,13-,16-,17+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc
Curated by ChEMBL
| Assay Description
Displacement of [3H]-NMS from Sprague-Dawley rat muscarinic M2 receptor in heart |
Bioorg Med Chem 22: 3478-87 (2014)
Article DOI: 10.1016/j.bmc.2014.04.031
BindingDB Entry DOI: 10.7270/Q2XS5X00 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(RAT) | BDBM50296345

((1R,2R,4S,5R,7S)-7-(2-hydroxy-2,2-di-thiophen-2-yl...)Show SMILES C[N+]1(C)[C@@H]2C[C@H](C[C@@H]1[C@@H]1O[C@H]21)OC(=O)C(O)(c1cccs1)c1cccs1 |TLB:11:5:1:8.10| Show InChI InChI=1S/C19H22NO4S2/c1-20(2)12-9-11(10-13(20)17-16(12)24-17)23-18(21)19(22,14-5-3-7-25-14)15-6-4-8-26-15/h3-8,11-13,16-17,22H,9-10H2,1-2H3/q+1/t11-,12-,13-,16-,17+/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc
Curated by ChEMBL
| Assay Description
Displacement of [3H]-NMS from Sprague-Dawley rat muscarinic M1 receptor in cortex membrane |
Bioorg Med Chem 22: 3478-87 (2014)
Article DOI: 10.1016/j.bmc.2014.04.031
BindingDB Entry DOI: 10.7270/Q2XS5X00 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(RAT) | BDBM50021892
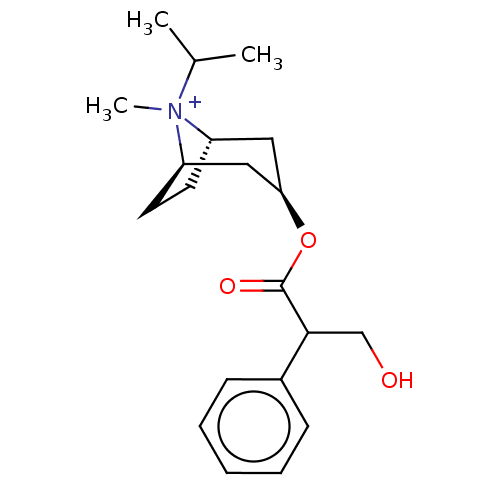
(Ipratropium Bromide)Show SMILES [Br-].[H][C@]12CC[C@]([H])(C[C@@H](C1)OC(=O)C(CO)c1ccccc1)[N+]2(C)C(C)C |TLB:10:8:22:4.3,23:22:8.7.9:4.3,THB:24:22:8.7.9:4.3| Show InChI InChI=1S/C20H30NO3.BrH/c1-14(2)21(3)16-9-10-17(21)12-18(11-16)24-20(23)19(13-22)15-7-5-4-6-8-15;/h4-8,14,16-19,22H,9-13H2,1-3H3;1H/q+1;/p-1/t16-,17+,18+,19?,21?; | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc
Curated by ChEMBL
| Assay Description
Displacement of [3H]-NMS from Sprague-Dawley rat muscarinic M3 receptor in submandibular gland |
Bioorg Med Chem 22: 3478-87 (2014)
Article DOI: 10.1016/j.bmc.2014.04.031
BindingDB Entry DOI: 10.7270/Q2XS5X00 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(RAT) | BDBM50021905
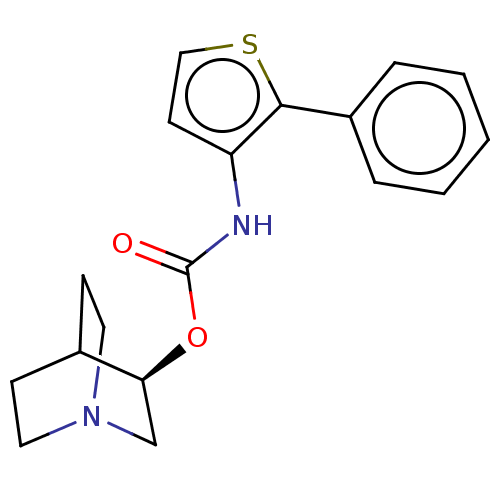
(CHEMBL3298589)Show SMILES Cl.O=C(Nc1ccsc1-c1ccccc1)O[C@H]1CN2CCC1CC2 |r,wD:16.16,(41.69,-11.32,;44.26,-6.71,;42.92,-7.48,;41.59,-6.7,;41.6,-5.16,;42.84,-4.27,;42.37,-2.81,;40.83,-2.8,;40.35,-4.27,;38.89,-4.74,;37.74,-3.7,;36.28,-4.18,;35.96,-5.69,;37.11,-6.72,;38.57,-6.24,;42.92,-9.02,;44.25,-9.79,;44.25,-11.32,;45.58,-12.1,;46.92,-11.33,;46.92,-9.78,;45.61,-9.01,;44.85,-10.35,;46.34,-10.75,)| Show InChI InChI=1S/C18H20N2O2S.ClH/c21-18(22-16-12-20-9-6-13(16)7-10-20)19-15-8-11-23-17(15)14-4-2-1-3-5-14;/h1-5,8,11,13,16H,6-7,9-10,12H2,(H,19,21);1H/t16-;/m0./s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc
Curated by ChEMBL
| Assay Description
Displacement of [3H]-NMS from Sprague-Dawley rat muscarinic M1 receptor in cortex membrane |
Bioorg Med Chem 22: 3478-87 (2014)
Article DOI: 10.1016/j.bmc.2014.04.031
BindingDB Entry DOI: 10.7270/Q2XS5X00 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(RAT) | BDBM50021905

(CHEMBL3298589)Show SMILES Cl.O=C(Nc1ccsc1-c1ccccc1)O[C@H]1CN2CCC1CC2 |r,wD:16.16,(41.69,-11.32,;44.26,-6.71,;42.92,-7.48,;41.59,-6.7,;41.6,-5.16,;42.84,-4.27,;42.37,-2.81,;40.83,-2.8,;40.35,-4.27,;38.89,-4.74,;37.74,-3.7,;36.28,-4.18,;35.96,-5.69,;37.11,-6.72,;38.57,-6.24,;42.92,-9.02,;44.25,-9.79,;44.25,-11.32,;45.58,-12.1,;46.92,-11.33,;46.92,-9.78,;45.61,-9.01,;44.85,-10.35,;46.34,-10.75,)| Show InChI InChI=1S/C18H20N2O2S.ClH/c21-18(22-16-12-20-9-6-13(16)7-10-20)19-15-8-11-23-17(15)14-4-2-1-3-5-14;/h1-5,8,11,13,16H,6-7,9-10,12H2,(H,19,21);1H/t16-;/m0./s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc
Curated by ChEMBL
| Assay Description
Displacement of [3H]-NMS from Sprague-Dawley rat muscarinic M3 receptor in submandibular gland |
Bioorg Med Chem 22: 3478-87 (2014)
Article DOI: 10.1016/j.bmc.2014.04.031
BindingDB Entry DOI: 10.7270/Q2XS5X00 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(RAT) | BDBM50021909
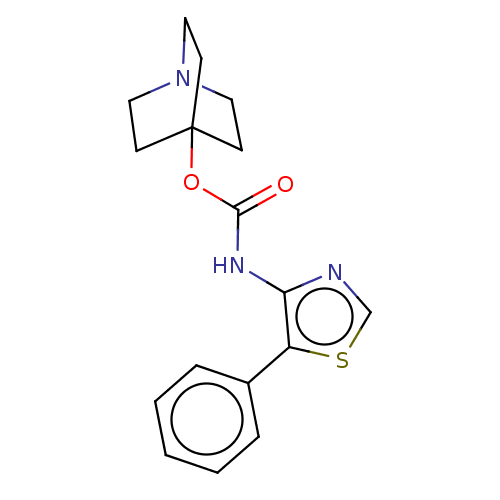
(CHEMBL3298591)Show InChI InChI=1S/C17H19N3O2S/c21-16(22-17-6-9-20(10-7-17)11-8-17)19-15-14(23-12-18-15)13-4-2-1-3-5-13/h1-5,12H,6-11H2,(H,19,21) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc
Curated by ChEMBL
| Assay Description
Displacement of [3H]-NMS from Sprague-Dawley rat muscarinic M3 receptor in submandibular gland |
Bioorg Med Chem 22: 3478-87 (2014)
Article DOI: 10.1016/j.bmc.2014.04.031
BindingDB Entry DOI: 10.7270/Q2XS5X00 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(RAT) | BDBM50021935

(CHEMBL3298600)Show SMILES [I-].C[N+]12CCC(CC1)(CC2)OC(=O)Nc1ncsc1-c1ccc(F)cc1 Show InChI InChI=1S/C18H20FN3O2S.HI/c1-22-9-6-18(7-10-22,8-11-22)24-17(23)21-16-15(25-12-20-16)13-2-4-14(19)5-3-13;/h2-5,12H,6-11H2,1H3;1H | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc
Curated by ChEMBL
| Assay Description
Displacement of [3H]-NMS from Sprague-Dawley rat muscarinic M2 receptor in heart |
Bioorg Med Chem 22: 3478-87 (2014)
Article DOI: 10.1016/j.bmc.2014.04.031
BindingDB Entry DOI: 10.7270/Q2XS5X00 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(RAT) | BDBM50021901
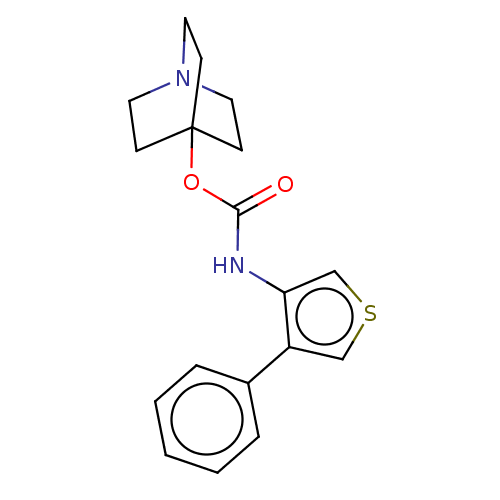
(CHEMBL3298334)Show InChI InChI=1S/C18H20N2O2S.ClH/c21-17(22-18-6-9-20(10-7-18)11-8-18)19-16-13-23-12-15(16)14-4-2-1-3-5-14;/h1-5,12-13H,6-11H2,(H,19,21);1H | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc
Curated by ChEMBL
| Assay Description
Displacement of [3H]-NMS from Sprague-Dawley rat muscarinic M3 receptor in submandibular gland |
Bioorg Med Chem 22: 3478-87 (2014)
Article DOI: 10.1016/j.bmc.2014.04.031
BindingDB Entry DOI: 10.7270/Q2XS5X00 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(RAT) | BDBM50021926
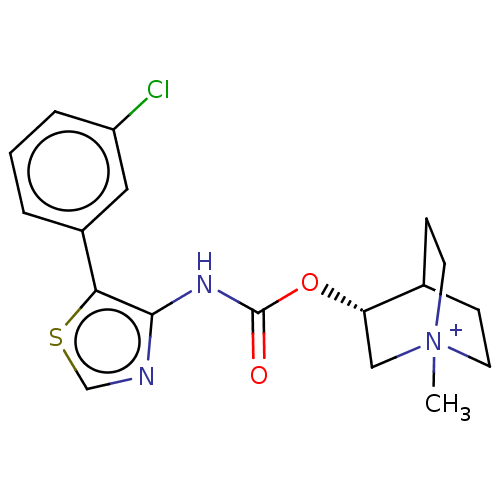
(CHEMBL3298598)Show SMILES C[N+]12CCC(CC1)[C@H](C2)OC(=O)Nc1ncsc1-c1cccc(Cl)c1 |r| Show InChI InChI=1S/C18H20ClN3O2S.HI/c1-22-7-5-12(6-8-22)15(10-22)24-18(23)21-17-16(25-11-20-17)13-3-2-4-14(19)9-13;/h2-4,9,11-12,15H,5-8,10H2,1H3;1H/t12?,15-,22?;/m0./s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc
Curated by ChEMBL
| Assay Description
Displacement of [3H]-NMS from Sprague-Dawley rat muscarinic M3 receptor in submandibular gland |
Bioorg Med Chem 22: 3478-87 (2014)
Article DOI: 10.1016/j.bmc.2014.04.031
BindingDB Entry DOI: 10.7270/Q2XS5X00 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(RAT) | BDBM50021892

(Ipratropium Bromide)Show SMILES [Br-].[H][C@]12CC[C@]([H])(C[C@@H](C1)OC(=O)C(CO)c1ccccc1)[N+]2(C)C(C)C |TLB:10:8:22:4.3,23:22:8.7.9:4.3,THB:24:22:8.7.9:4.3| Show InChI InChI=1S/C20H30NO3.BrH/c1-14(2)21(3)16-9-10-17(21)12-18(11-16)24-20(23)19(13-22)15-7-5-4-6-8-15;/h4-8,14,16-19,22H,9-13H2,1-3H3;1H/q+1;/p-1/t16-,17+,18+,19?,21?; | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc
Curated by ChEMBL
| Assay Description
Displacement of [3H]-NMS from Sprague-Dawley rat muscarinic M1 receptor in cortex membrane |
Bioorg Med Chem 22: 3478-87 (2014)
Article DOI: 10.1016/j.bmc.2014.04.031
BindingDB Entry DOI: 10.7270/Q2XS5X00 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(RAT) | BDBM50296345

((1R,2R,4S,5R,7S)-7-(2-hydroxy-2,2-di-thiophen-2-yl...)Show SMILES C[N+]1(C)[C@@H]2C[C@H](C[C@@H]1[C@@H]1O[C@H]21)OC(=O)C(O)(c1cccs1)c1cccs1 |TLB:11:5:1:8.10| Show InChI InChI=1S/C19H22NO4S2/c1-20(2)12-9-11(10-13(20)17-16(12)24-17)23-18(21)19(22,14-5-3-7-25-14)15-6-4-8-26-15/h3-8,11-13,16-17,22H,9-10H2,1-2H3/q+1/t11-,12-,13-,16-,17+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc
Curated by ChEMBL
| Assay Description
Displacement of [3H]-NMS from Sprague-Dawley rat muscarinic M3 receptor in submandibular gland |
Bioorg Med Chem 22: 3478-87 (2014)
Article DOI: 10.1016/j.bmc.2014.04.031
BindingDB Entry DOI: 10.7270/Q2XS5X00 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(RAT) | BDBM50021926

(CHEMBL3298598)Show SMILES C[N+]12CCC(CC1)[C@H](C2)OC(=O)Nc1ncsc1-c1cccc(Cl)c1 |r| Show InChI InChI=1S/C18H20ClN3O2S.HI/c1-22-7-5-12(6-8-22)15(10-22)24-18(23)21-17-16(25-11-20-17)13-3-2-4-14(19)9-13;/h2-4,9,11-12,15H,5-8,10H2,1H3;1H/t12?,15-,22?;/m0./s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc
Curated by ChEMBL
| Assay Description
Displacement of [3H]-NMS from Sprague-Dawley rat muscarinic M1 receptor in cortex membrane |
Bioorg Med Chem 22: 3478-87 (2014)
Article DOI: 10.1016/j.bmc.2014.04.031
BindingDB Entry DOI: 10.7270/Q2XS5X00 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(RAT) | BDBM50344285

(CHEMBL1779047 | quinuclidin-4-yl biphenyl-2-ylcarb...)Show InChI InChI=1S/C20H22N2O2/c23-19(24-20-10-13-22(14-11-20)15-12-20)21-18-9-5-4-8-17(18)16-6-2-1-3-7-16/h1-9H,10-15H2,(H,21,23) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc
Curated by ChEMBL
| Assay Description
Displacement of [3H]-NMS from Sprague-Dawley rat muscarinic M1 receptor in cortex membrane |
Bioorg Med Chem 22: 3478-87 (2014)
Article DOI: 10.1016/j.bmc.2014.04.031
BindingDB Entry DOI: 10.7270/Q2XS5X00 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(RAT) | BDBM50021893

(CHEMBL3298333)Show InChI InChI=1S/C18H20N2O2S/c21-17(22-18-7-10-20(11-8-18)12-9-18)19-16-15(6-13-23-16)14-4-2-1-3-5-14/h1-6,13H,7-12H2,(H,19,21) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc
Curated by ChEMBL
| Assay Description
Displacement of [3H]-NMS from Sprague-Dawley rat muscarinic M1 receptor in cortex membrane |
Bioorg Med Chem 22: 3478-87 (2014)
Article DOI: 10.1016/j.bmc.2014.04.031
BindingDB Entry DOI: 10.7270/Q2XS5X00 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(RAT) | BDBM50021924

(CHEMBL3298597)Show SMILES C[N+]12CCC(CC1)[C@H](C2)OC(=O)Nc1ncsc1-c1ccccc1 |r| Show InChI InChI=1S/C18H21N3O2S.HI/c1-21-9-7-13(8-10-21)15(11-21)23-18(22)20-17-16(24-12-19-17)14-5-3-2-4-6-14;/h2-6,12-13,15H,7-11H2,1H3;1H/t13?,15-,21?;/m0./s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc
Curated by ChEMBL
| Assay Description
Displacement of [3H]-NMS from Sprague-Dawley rat muscarinic M3 receptor in submandibular gland |
Bioorg Med Chem 22: 3478-87 (2014)
Article DOI: 10.1016/j.bmc.2014.04.031
BindingDB Entry DOI: 10.7270/Q2XS5X00 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(RAT) | BDBM50021924

(CHEMBL3298597)Show SMILES C[N+]12CCC(CC1)[C@H](C2)OC(=O)Nc1ncsc1-c1ccccc1 |r| Show InChI InChI=1S/C18H21N3O2S.HI/c1-21-9-7-13(8-10-21)15(11-21)23-18(22)20-17-16(24-12-19-17)14-5-3-2-4-6-14;/h2-6,12-13,15H,7-11H2,1H3;1H/t13?,15-,21?;/m0./s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc
Curated by ChEMBL
| Assay Description
Displacement of [3H]-NMS from Sprague-Dawley rat muscarinic M1 receptor in cortex membrane |
Bioorg Med Chem 22: 3478-87 (2014)
Article DOI: 10.1016/j.bmc.2014.04.031
BindingDB Entry DOI: 10.7270/Q2XS5X00 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(RAT) | BDBM50021909

(CHEMBL3298591)Show InChI InChI=1S/C17H19N3O2S/c21-16(22-17-6-9-20(10-7-17)11-8-17)19-15-14(23-12-18-15)13-4-2-1-3-5-13/h1-5,12H,6-11H2,(H,19,21) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc
Curated by ChEMBL
| Assay Description
Displacement of [3H]-NMS from Sprague-Dawley rat muscarinic M1 receptor in cortex membrane |
Bioorg Med Chem 22: 3478-87 (2014)
Article DOI: 10.1016/j.bmc.2014.04.031
BindingDB Entry DOI: 10.7270/Q2XS5X00 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(RAT) | BDBM50021904

(CHEMBL3298588)Show InChI InChI=1S/C18H20N2O2S.ClH/c21-17(22-18-7-10-20(11-8-18)12-9-18)19-15-6-13-23-16(15)14-4-2-1-3-5-14;/h1-6,13H,7-12H2,(H,19,21);1H | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc
Curated by ChEMBL
| Assay Description
Displacement of [3H]-NMS from Sprague-Dawley rat muscarinic M2 receptor in heart |
Bioorg Med Chem 22: 3478-87 (2014)
Article DOI: 10.1016/j.bmc.2014.04.031
BindingDB Entry DOI: 10.7270/Q2XS5X00 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(RAT) | BDBM50021901

(CHEMBL3298334)Show InChI InChI=1S/C18H20N2O2S.ClH/c21-17(22-18-6-9-20(10-7-18)11-8-18)19-16-13-23-12-15(16)14-4-2-1-3-5-14;/h1-5,12-13H,6-11H2,(H,19,21);1H | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc
Curated by ChEMBL
| Assay Description
Displacement of [3H]-NMS from Sprague-Dawley rat muscarinic M1 receptor in cortex membrane |
Bioorg Med Chem 22: 3478-87 (2014)
Article DOI: 10.1016/j.bmc.2014.04.031
BindingDB Entry DOI: 10.7270/Q2XS5X00 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(RAT) | BDBM50021938

(CHEMBL3298763)Show SMILES [I-].C[N+]12CCC(CC1)(CC2)OC(=O)Nc1ncsc1-c1cc(Cl)cc(Cl)c1 Show InChI InChI=1S/C18H19Cl2N3O2S.HI/c1-23-5-2-18(3-6-23,4-7-23)25-17(24)22-16-15(26-11-21-16)12-8-13(19)10-14(20)9-12;/h8-11H,2-7H2,1H3;1H | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.640 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc
Curated by ChEMBL
| Assay Description
Displacement of [3H]-NMS from Sprague-Dawley rat muscarinic M2 receptor in heart |
Bioorg Med Chem 22: 3478-87 (2014)
Article DOI: 10.1016/j.bmc.2014.04.031
BindingDB Entry DOI: 10.7270/Q2XS5X00 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(RAT) | BDBM50021910
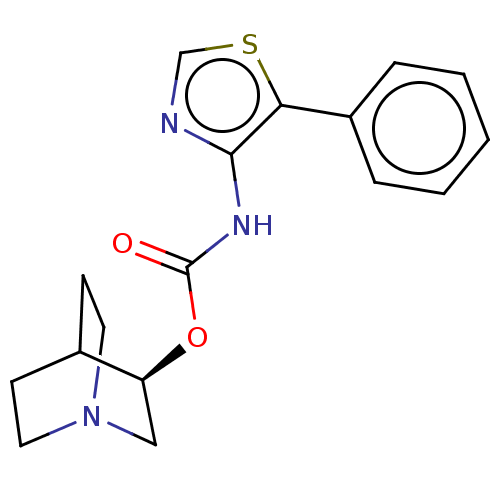
(CHEMBL3298592)Show SMILES Cl.O=C(Nc1ncsc1-c1ccccc1)O[C@H]1CN2CCC1CC2 |r,wD:16.16,(29.91,-10.86,;32.24,-6.16,;30.91,-6.93,;29.57,-6.15,;29.58,-4.61,;30.82,-3.72,;30.35,-2.26,;28.81,-2.25,;28.33,-3.72,;26.87,-4.18,;25.72,-3.15,;24.26,-3.63,;23.94,-5.14,;25.09,-6.17,;26.55,-5.68,;30.9,-8.47,;32.23,-9.24,;32.23,-10.77,;33.57,-11.55,;34.9,-10.78,;34.91,-9.23,;33.59,-8.46,;32.83,-9.8,;34.33,-10.2,)| Show InChI InChI=1S/C17H19N3O2S.ClH/c21-17(22-14-10-20-8-6-12(14)7-9-20)19-16-15(23-11-18-16)13-4-2-1-3-5-13;/h1-5,11-12,14H,6-10H2,(H,19,21);1H/t14-;/m0./s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.75 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc
Curated by ChEMBL
| Assay Description
Displacement of [3H]-NMS from Sprague-Dawley rat muscarinic M1 receptor in cortex membrane |
Bioorg Med Chem 22: 3478-87 (2014)
Article DOI: 10.1016/j.bmc.2014.04.031
BindingDB Entry DOI: 10.7270/Q2XS5X00 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(RAT) | BDBM50021892

(Ipratropium Bromide)Show SMILES [Br-].[H][C@]12CC[C@]([H])(C[C@@H](C1)OC(=O)C(CO)c1ccccc1)[N+]2(C)C(C)C |TLB:10:8:22:4.3,23:22:8.7.9:4.3,THB:24:22:8.7.9:4.3| Show InChI InChI=1S/C20H30NO3.BrH/c1-14(2)21(3)16-9-10-17(21)12-18(11-16)24-20(23)19(13-22)15-7-5-4-6-8-15;/h4-8,14,16-19,22H,9-13H2,1-3H3;1H/q+1;/p-1/t16-,17+,18+,19?,21?; | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.780 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc
Curated by ChEMBL
| Assay Description
Displacement of [3H]-NMS from Sprague-Dawley rat muscarinic M2 receptor in heart |
Bioorg Med Chem 22: 3478-87 (2014)
Article DOI: 10.1016/j.bmc.2014.04.031
BindingDB Entry DOI: 10.7270/Q2XS5X00 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(RAT) | BDBM50021909

(CHEMBL3298591)Show InChI InChI=1S/C17H19N3O2S/c21-16(22-17-6-9-20(10-7-17)11-8-17)19-15-14(23-12-18-15)13-4-2-1-3-5-13/h1-5,12H,6-11H2,(H,19,21) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc
Curated by ChEMBL
| Assay Description
Displacement of [3H]-NMS from Sprague-Dawley rat muscarinic M2 receptor in heart |
Bioorg Med Chem 22: 3478-87 (2014)
Article DOI: 10.1016/j.bmc.2014.04.031
BindingDB Entry DOI: 10.7270/Q2XS5X00 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(RAT) | BDBM50021905

(CHEMBL3298589)Show SMILES Cl.O=C(Nc1ccsc1-c1ccccc1)O[C@H]1CN2CCC1CC2 |r,wD:16.16,(41.69,-11.32,;44.26,-6.71,;42.92,-7.48,;41.59,-6.7,;41.6,-5.16,;42.84,-4.27,;42.37,-2.81,;40.83,-2.8,;40.35,-4.27,;38.89,-4.74,;37.74,-3.7,;36.28,-4.18,;35.96,-5.69,;37.11,-6.72,;38.57,-6.24,;42.92,-9.02,;44.25,-9.79,;44.25,-11.32,;45.58,-12.1,;46.92,-11.33,;46.92,-9.78,;45.61,-9.01,;44.85,-10.35,;46.34,-10.75,)| Show InChI InChI=1S/C18H20N2O2S.ClH/c21-18(22-16-12-20-9-6-13(16)7-10-20)19-15-8-11-23-17(15)14-4-2-1-3-5-14;/h1-5,8,11,13,16H,6-7,9-10,12H2,(H,19,21);1H/t16-;/m0./s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.820 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc
Curated by ChEMBL
| Assay Description
Displacement of [3H]-NMS from Sprague-Dawley rat muscarinic M2 receptor in heart |
Bioorg Med Chem 22: 3478-87 (2014)
Article DOI: 10.1016/j.bmc.2014.04.031
BindingDB Entry DOI: 10.7270/Q2XS5X00 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(RAT) | BDBM50021910

(CHEMBL3298592)Show SMILES Cl.O=C(Nc1ncsc1-c1ccccc1)O[C@H]1CN2CCC1CC2 |r,wD:16.16,(29.91,-10.86,;32.24,-6.16,;30.91,-6.93,;29.57,-6.15,;29.58,-4.61,;30.82,-3.72,;30.35,-2.26,;28.81,-2.25,;28.33,-3.72,;26.87,-4.18,;25.72,-3.15,;24.26,-3.63,;23.94,-5.14,;25.09,-6.17,;26.55,-5.68,;30.9,-8.47,;32.23,-9.24,;32.23,-10.77,;33.57,-11.55,;34.9,-10.78,;34.91,-9.23,;33.59,-8.46,;32.83,-9.8,;34.33,-10.2,)| Show InChI InChI=1S/C17H19N3O2S.ClH/c21-17(22-14-10-20-8-6-12(14)7-9-20)19-16-15(23-11-18-16)13-4-2-1-3-5-13;/h1-5,11-12,14H,6-10H2,(H,19,21);1H/t14-;/m0./s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.930 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc
Curated by ChEMBL
| Assay Description
Displacement of [3H]-NMS from Sprague-Dawley rat muscarinic M3 receptor in submandibular gland |
Bioorg Med Chem 22: 3478-87 (2014)
Article DOI: 10.1016/j.bmc.2014.04.031
BindingDB Entry DOI: 10.7270/Q2XS5X00 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(RAT) | BDBM50021926

(CHEMBL3298598)Show SMILES C[N+]12CCC(CC1)[C@H](C2)OC(=O)Nc1ncsc1-c1cccc(Cl)c1 |r| Show InChI InChI=1S/C18H20ClN3O2S.HI/c1-22-7-5-12(6-8-22)15(10-22)24-18(23)21-17-16(25-11-20-17)13-3-2-4-14(19)9-13;/h2-4,9,11-12,15H,5-8,10H2,1H3;1H/t12?,15-,22?;/m0./s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc
Curated by ChEMBL
| Assay Description
Displacement of [3H]-NMS from Sprague-Dawley rat muscarinic M2 receptor in heart |
Bioorg Med Chem 22: 3478-87 (2014)
Article DOI: 10.1016/j.bmc.2014.04.031
BindingDB Entry DOI: 10.7270/Q2XS5X00 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(RAT) | BDBM50021924

(CHEMBL3298597)Show SMILES C[N+]12CCC(CC1)[C@H](C2)OC(=O)Nc1ncsc1-c1ccccc1 |r| Show InChI InChI=1S/C18H21N3O2S.HI/c1-21-9-7-13(8-10-21)15(11-21)23-18(22)20-17-16(24-12-19-17)14-5-3-2-4-6-14;/h2-6,12-13,15H,7-11H2,1H3;1H/t13?,15-,21?;/m0./s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc
Curated by ChEMBL
| Assay Description
Displacement of [3H]-NMS from Sprague-Dawley rat muscarinic M2 receptor in heart |
Bioorg Med Chem 22: 3478-87 (2014)
Article DOI: 10.1016/j.bmc.2014.04.031
BindingDB Entry DOI: 10.7270/Q2XS5X00 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(RAT) | BDBM50021893

(CHEMBL3298333)Show InChI InChI=1S/C18H20N2O2S/c21-17(22-18-7-10-20(11-8-18)12-9-18)19-16-15(6-13-23-16)14-4-2-1-3-5-14/h1-6,13H,7-12H2,(H,19,21) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc
Curated by ChEMBL
| Assay Description
Displacement of [3H]-NMS from Sprague-Dawley rat muscarinic M2 receptor in heart |
Bioorg Med Chem 22: 3478-87 (2014)
Article DOI: 10.1016/j.bmc.2014.04.031
BindingDB Entry DOI: 10.7270/Q2XS5X00 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(RAT) | BDBM50021936
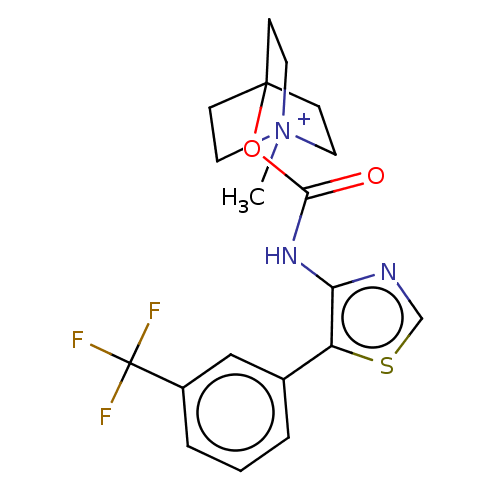
(CHEMBL3298601)Show SMILES [I-].C[N+]12CCC(CC1)(CC2)OC(=O)Nc1ncsc1-c1cccc(c1)C(F)(F)F Show InChI InChI=1S/C19H20F3N3O2S.HI/c1-25-8-5-18(6-9-25,7-10-25)27-17(26)24-16-15(28-12-23-16)13-3-2-4-14(11-13)19(20,21)22;/h2-4,11-12H,5-10H2,1H3;1H | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc
Curated by ChEMBL
| Assay Description
Displacement of [3H]-NMS from Sprague-Dawley rat muscarinic M3 receptor in submandibular gland |
Bioorg Med Chem 22: 3478-87 (2014)
Article DOI: 10.1016/j.bmc.2014.04.031
BindingDB Entry DOI: 10.7270/Q2XS5X00 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(RAT) | BDBM50021910

(CHEMBL3298592)Show SMILES Cl.O=C(Nc1ncsc1-c1ccccc1)O[C@H]1CN2CCC1CC2 |r,wD:16.16,(29.91,-10.86,;32.24,-6.16,;30.91,-6.93,;29.57,-6.15,;29.58,-4.61,;30.82,-3.72,;30.35,-2.26,;28.81,-2.25,;28.33,-3.72,;26.87,-4.18,;25.72,-3.15,;24.26,-3.63,;23.94,-5.14,;25.09,-6.17,;26.55,-5.68,;30.9,-8.47,;32.23,-9.24,;32.23,-10.77,;33.57,-11.55,;34.9,-10.78,;34.91,-9.23,;33.59,-8.46,;32.83,-9.8,;34.33,-10.2,)| Show InChI InChI=1S/C17H19N3O2S.ClH/c21-17(22-14-10-20-8-6-12(14)7-9-20)19-16-15(23-11-18-16)13-4-2-1-3-5-13;/h1-5,11-12,14H,6-10H2,(H,19,21);1H/t14-;/m0./s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc
Curated by ChEMBL
| Assay Description
Displacement of [3H]-NMS from Sprague-Dawley rat muscarinic M2 receptor in heart |
Bioorg Med Chem 22: 3478-87 (2014)
Article DOI: 10.1016/j.bmc.2014.04.031
BindingDB Entry DOI: 10.7270/Q2XS5X00 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(RAT) | BDBM50021936

(CHEMBL3298601)Show SMILES [I-].C[N+]12CCC(CC1)(CC2)OC(=O)Nc1ncsc1-c1cccc(c1)C(F)(F)F Show InChI InChI=1S/C19H20F3N3O2S.HI/c1-25-8-5-18(6-9-25,7-10-25)27-17(26)24-16-15(28-12-23-16)13-3-2-4-14(11-13)19(20,21)22;/h2-4,11-12H,5-10H2,1H3;1H | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc
Curated by ChEMBL
| Assay Description
Displacement of [3H]-NMS from Sprague-Dawley rat muscarinic M1 receptor in cortex membrane |
Bioorg Med Chem 22: 3478-87 (2014)
Article DOI: 10.1016/j.bmc.2014.04.031
BindingDB Entry DOI: 10.7270/Q2XS5X00 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(RAT) | BDBM50021901

(CHEMBL3298334)Show InChI InChI=1S/C18H20N2O2S.ClH/c21-17(22-18-6-9-20(10-7-18)11-8-18)19-16-13-23-12-15(16)14-4-2-1-3-5-14;/h1-5,12-13H,6-11H2,(H,19,21);1H | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc
Curated by ChEMBL
| Assay Description
Displacement of [3H]-NMS from Sprague-Dawley rat muscarinic M2 receptor in heart |
Bioorg Med Chem 22: 3478-87 (2014)
Article DOI: 10.1016/j.bmc.2014.04.031
BindingDB Entry DOI: 10.7270/Q2XS5X00 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(RAT) | BDBM50344285

(CHEMBL1779047 | quinuclidin-4-yl biphenyl-2-ylcarb...)Show InChI InChI=1S/C20H22N2O2/c23-19(24-20-10-13-22(14-11-20)15-12-20)21-18-9-5-4-8-17(18)16-6-2-1-3-7-16/h1-9H,10-15H2,(H,21,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc
Curated by ChEMBL
| Assay Description
Displacement of [3H]-NMS from Sprague-Dawley rat muscarinic M2 receptor in heart |
Bioorg Med Chem 22: 3478-87 (2014)
Article DOI: 10.1016/j.bmc.2014.04.031
BindingDB Entry DOI: 10.7270/Q2XS5X00 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(RAT) | BDBM50021907
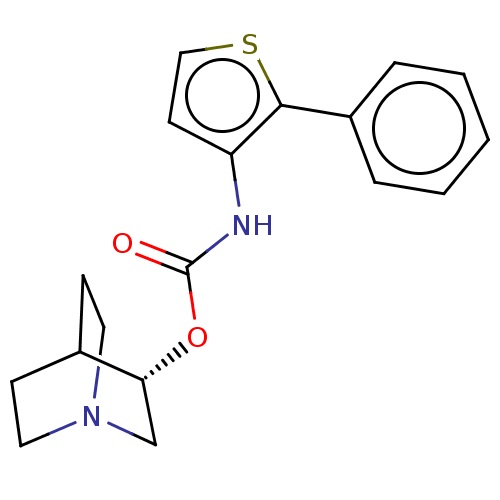
(CHEMBL3298590)Show SMILES Cl.O=C(Nc1ccsc1-c1ccccc1)O[C@@H]1CN2CCC1CC2 |r,wU:16.16,(31.36,-12.32,;32.24,-6.16,;30.91,-6.93,;29.57,-6.15,;29.58,-4.61,;30.82,-3.72,;30.35,-2.26,;28.81,-2.25,;28.33,-3.72,;26.87,-4.18,;25.72,-3.15,;24.26,-3.63,;23.94,-5.14,;25.09,-6.17,;26.55,-5.68,;30.9,-8.47,;32.23,-9.24,;32.23,-10.77,;33.57,-11.55,;34.9,-10.78,;34.91,-9.23,;33.59,-8.46,;32.83,-9.8,;34.33,-10.2,)| Show InChI InChI=1S/C18H20N2O2S.ClH/c21-18(22-16-12-20-9-6-13(16)7-10-20)19-15-8-11-23-17(15)14-4-2-1-3-5-14;/h1-5,8,11,13,16H,6-7,9-10,12H2,(H,19,21);1H/t16-;/m1./s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc
Curated by ChEMBL
| Assay Description
Displacement of [3H]-NMS from Sprague-Dawley rat muscarinic M3 receptor in submandibular gland |
Bioorg Med Chem 22: 3478-87 (2014)
Article DOI: 10.1016/j.bmc.2014.04.031
BindingDB Entry DOI: 10.7270/Q2XS5X00 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(RAT) | BDBM50021907

(CHEMBL3298590)Show SMILES Cl.O=C(Nc1ccsc1-c1ccccc1)O[C@@H]1CN2CCC1CC2 |r,wU:16.16,(31.36,-12.32,;32.24,-6.16,;30.91,-6.93,;29.57,-6.15,;29.58,-4.61,;30.82,-3.72,;30.35,-2.26,;28.81,-2.25,;28.33,-3.72,;26.87,-4.18,;25.72,-3.15,;24.26,-3.63,;23.94,-5.14,;25.09,-6.17,;26.55,-5.68,;30.9,-8.47,;32.23,-9.24,;32.23,-10.77,;33.57,-11.55,;34.9,-10.78,;34.91,-9.23,;33.59,-8.46,;32.83,-9.8,;34.33,-10.2,)| Show InChI InChI=1S/C18H20N2O2S.ClH/c21-18(22-16-12-20-9-6-13(16)7-10-20)19-15-8-11-23-17(15)14-4-2-1-3-5-14;/h1-5,8,11,13,16H,6-7,9-10,12H2,(H,19,21);1H/t16-;/m1./s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc
Curated by ChEMBL
| Assay Description
Displacement of [3H]-NMS from Sprague-Dawley rat muscarinic M1 receptor in cortex membrane |
Bioorg Med Chem 22: 3478-87 (2014)
Article DOI: 10.1016/j.bmc.2014.04.031
BindingDB Entry DOI: 10.7270/Q2XS5X00 |
More data for this
Ligand-Target Pair | |
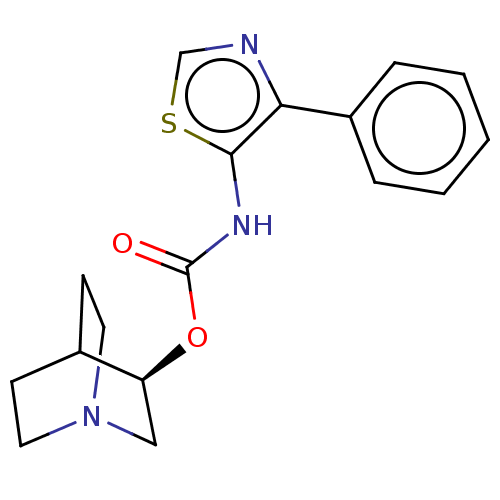
Muscarinic acetylcholine receptor M1
(RAT) | BDBM50021911
(CHEMBL3298593)Show SMILES Cl.O=C(Nc1scnc1-c1ccccc1)O[C@H]1CN2CCC1CC2 |r,wD:16.16,(29.91,-10.86,;32.24,-6.16,;30.91,-6.93,;29.57,-6.15,;29.58,-4.61,;30.82,-3.72,;30.35,-2.26,;28.81,-2.25,;28.33,-3.72,;26.87,-4.18,;25.72,-3.15,;24.26,-3.63,;23.94,-5.14,;25.09,-6.17,;26.55,-5.68,;30.9,-8.47,;32.23,-9.24,;32.23,-10.77,;33.57,-11.55,;34.9,-10.78,;34.91,-9.23,;33.59,-8.46,;32.83,-9.8,;34.33,-10.2,)| Show InChI InChI=1S/C17H19N3O2S.ClH/c21-17(22-14-10-20-8-6-12(14)7-9-20)19-16-15(18-11-23-16)13-4-2-1-3-5-13;/h1-5,11-12,14H,6-10H2,(H,19,21);1H/t14-;/m0./s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc
Curated by ChEMBL
| Assay Description
Displacement of [3H]-NMS from Sprague-Dawley rat muscarinic M1 receptor in cortex membrane |
Bioorg Med Chem 22: 3478-87 (2014)
Article DOI: 10.1016/j.bmc.2014.04.031
BindingDB Entry DOI: 10.7270/Q2XS5X00 |
More data for this
Ligand-Target Pair | |
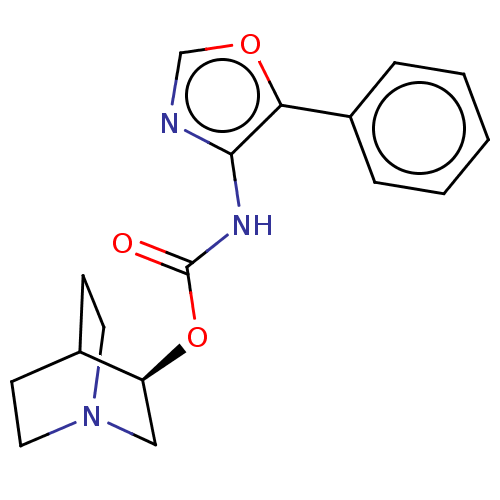
Muscarinic acetylcholine receptor M1
(RAT) | BDBM50021918
(CHEMBL3298594)Show SMILES Cl.O=C(Nc1ncoc1-c1ccccc1)O[C@H]1CN2CCC1CC2 |r,wD:16.16,(29.91,-10.86,;32.24,-6.16,;30.91,-6.93,;29.57,-6.15,;29.58,-4.61,;30.82,-3.72,;30.35,-2.26,;28.81,-2.25,;28.33,-3.72,;26.87,-4.18,;25.72,-3.15,;24.26,-3.63,;23.94,-5.14,;25.09,-6.17,;26.55,-5.68,;30.9,-8.47,;32.23,-9.24,;32.23,-10.77,;33.57,-11.55,;34.9,-10.78,;34.91,-9.23,;33.59,-8.46,;32.83,-9.8,;34.33,-10.2,)| Show InChI InChI=1S/C17H19N3O3.ClH/c21-17(23-14-10-20-8-6-12(14)7-9-20)19-16-15(22-11-18-16)13-4-2-1-3-5-13;/h1-5,11-12,14H,6-10H2,(H,19,21);1H/t14-;/m0./s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc
Curated by ChEMBL
| Assay Description
Displacement of [3H]-NMS from Sprague-Dawley rat muscarinic M1 receptor in cortex membrane |
Bioorg Med Chem 22: 3478-87 (2014)
Article DOI: 10.1016/j.bmc.2014.04.031
BindingDB Entry DOI: 10.7270/Q2XS5X00 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(RAT) | BDBM50021918

(CHEMBL3298594)Show SMILES Cl.O=C(Nc1ncoc1-c1ccccc1)O[C@H]1CN2CCC1CC2 |r,wD:16.16,(29.91,-10.86,;32.24,-6.16,;30.91,-6.93,;29.57,-6.15,;29.58,-4.61,;30.82,-3.72,;30.35,-2.26,;28.81,-2.25,;28.33,-3.72,;26.87,-4.18,;25.72,-3.15,;24.26,-3.63,;23.94,-5.14,;25.09,-6.17,;26.55,-5.68,;30.9,-8.47,;32.23,-9.24,;32.23,-10.77,;33.57,-11.55,;34.9,-10.78,;34.91,-9.23,;33.59,-8.46,;32.83,-9.8,;34.33,-10.2,)| Show InChI InChI=1S/C17H19N3O3.ClH/c21-17(23-14-10-20-8-6-12(14)7-9-20)19-16-15(22-11-18-16)13-4-2-1-3-5-13;/h1-5,11-12,14H,6-10H2,(H,19,21);1H/t14-;/m0./s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc
Curated by ChEMBL
| Assay Description
Displacement of [3H]-NMS from Sprague-Dawley rat muscarinic M3 receptor in submandibular gland |
Bioorg Med Chem 22: 3478-87 (2014)
Article DOI: 10.1016/j.bmc.2014.04.031
BindingDB Entry DOI: 10.7270/Q2XS5X00 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(RAT) | BDBM50021911

(CHEMBL3298593)Show SMILES Cl.O=C(Nc1scnc1-c1ccccc1)O[C@H]1CN2CCC1CC2 |r,wD:16.16,(29.91,-10.86,;32.24,-6.16,;30.91,-6.93,;29.57,-6.15,;29.58,-4.61,;30.82,-3.72,;30.35,-2.26,;28.81,-2.25,;28.33,-3.72,;26.87,-4.18,;25.72,-3.15,;24.26,-3.63,;23.94,-5.14,;25.09,-6.17,;26.55,-5.68,;30.9,-8.47,;32.23,-9.24,;32.23,-10.77,;33.57,-11.55,;34.9,-10.78,;34.91,-9.23,;33.59,-8.46,;32.83,-9.8,;34.33,-10.2,)| Show InChI InChI=1S/C17H19N3O2S.ClH/c21-17(22-14-10-20-8-6-12(14)7-9-20)19-16-15(18-11-23-16)13-4-2-1-3-5-13;/h1-5,11-12,14H,6-10H2,(H,19,21);1H/t14-;/m0./s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc
Curated by ChEMBL
| Assay Description
Displacement of [3H]-NMS from Sprague-Dawley rat muscarinic M3 receptor in submandibular gland |
Bioorg Med Chem 22: 3478-87 (2014)
Article DOI: 10.1016/j.bmc.2014.04.031
BindingDB Entry DOI: 10.7270/Q2XS5X00 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(RAT) | BDBM50021907

(CHEMBL3298590)Show SMILES Cl.O=C(Nc1ccsc1-c1ccccc1)O[C@@H]1CN2CCC1CC2 |r,wU:16.16,(31.36,-12.32,;32.24,-6.16,;30.91,-6.93,;29.57,-6.15,;29.58,-4.61,;30.82,-3.72,;30.35,-2.26,;28.81,-2.25,;28.33,-3.72,;26.87,-4.18,;25.72,-3.15,;24.26,-3.63,;23.94,-5.14,;25.09,-6.17,;26.55,-5.68,;30.9,-8.47,;32.23,-9.24,;32.23,-10.77,;33.57,-11.55,;34.9,-10.78,;34.91,-9.23,;33.59,-8.46,;32.83,-9.8,;34.33,-10.2,)| Show InChI InChI=1S/C18H20N2O2S.ClH/c21-18(22-16-12-20-9-6-13(16)7-10-20)19-15-8-11-23-17(15)14-4-2-1-3-5-14;/h1-5,8,11,13,16H,6-7,9-10,12H2,(H,19,21);1H/t16-;/m1./s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc
Curated by ChEMBL
| Assay Description
Displacement of [3H]-NMS from Sprague-Dawley rat muscarinic M2 receptor in heart |
Bioorg Med Chem 22: 3478-87 (2014)
Article DOI: 10.1016/j.bmc.2014.04.031
BindingDB Entry DOI: 10.7270/Q2XS5X00 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(RAT) | BDBM50021936

(CHEMBL3298601)Show SMILES [I-].C[N+]12CCC(CC1)(CC2)OC(=O)Nc1ncsc1-c1cccc(c1)C(F)(F)F Show InChI InChI=1S/C19H20F3N3O2S.HI/c1-25-8-5-18(6-9-25,7-10-25)27-17(26)24-16-15(28-12-23-16)13-3-2-4-14(11-13)19(20,21)22;/h2-4,11-12H,5-10H2,1H3;1H | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc
Curated by ChEMBL
| Assay Description
Displacement of [3H]-NMS from Sprague-Dawley rat muscarinic M2 receptor in heart |
Bioorg Med Chem 22: 3478-87 (2014)
Article DOI: 10.1016/j.bmc.2014.04.031
BindingDB Entry DOI: 10.7270/Q2XS5X00 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(RAT) | BDBM50021911

(CHEMBL3298593)Show SMILES Cl.O=C(Nc1scnc1-c1ccccc1)O[C@H]1CN2CCC1CC2 |r,wD:16.16,(29.91,-10.86,;32.24,-6.16,;30.91,-6.93,;29.57,-6.15,;29.58,-4.61,;30.82,-3.72,;30.35,-2.26,;28.81,-2.25,;28.33,-3.72,;26.87,-4.18,;25.72,-3.15,;24.26,-3.63,;23.94,-5.14,;25.09,-6.17,;26.55,-5.68,;30.9,-8.47,;32.23,-9.24,;32.23,-10.77,;33.57,-11.55,;34.9,-10.78,;34.91,-9.23,;33.59,-8.46,;32.83,-9.8,;34.33,-10.2,)| Show InChI InChI=1S/C17H19N3O2S.ClH/c21-17(22-14-10-20-8-6-12(14)7-9-20)19-16-15(18-11-23-16)13-4-2-1-3-5-13;/h1-5,11-12,14H,6-10H2,(H,19,21);1H/t14-;/m0./s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 39 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc
Curated by ChEMBL
| Assay Description
Displacement of [3H]-NMS from Sprague-Dawley rat muscarinic M2 receptor in heart |
Bioorg Med Chem 22: 3478-87 (2014)
Article DOI: 10.1016/j.bmc.2014.04.031
BindingDB Entry DOI: 10.7270/Q2XS5X00 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(RAT) | BDBM50021918

(CHEMBL3298594)Show SMILES Cl.O=C(Nc1ncoc1-c1ccccc1)O[C@H]1CN2CCC1CC2 |r,wD:16.16,(29.91,-10.86,;32.24,-6.16,;30.91,-6.93,;29.57,-6.15,;29.58,-4.61,;30.82,-3.72,;30.35,-2.26,;28.81,-2.25,;28.33,-3.72,;26.87,-4.18,;25.72,-3.15,;24.26,-3.63,;23.94,-5.14,;25.09,-6.17,;26.55,-5.68,;30.9,-8.47,;32.23,-9.24,;32.23,-10.77,;33.57,-11.55,;34.9,-10.78,;34.91,-9.23,;33.59,-8.46,;32.83,-9.8,;34.33,-10.2,)| Show InChI InChI=1S/C17H19N3O3.ClH/c21-17(23-14-10-20-8-6-12(14)7-9-20)19-16-15(22-11-18-16)13-4-2-1-3-5-13;/h1-5,11-12,14H,6-10H2,(H,19,21);1H/t14-;/m0./s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 59 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc
Curated by ChEMBL
| Assay Description
Displacement of [3H]-NMS from Sprague-Dawley rat muscarinic M2 receptor in heart |
Bioorg Med Chem 22: 3478-87 (2014)
Article DOI: 10.1016/j.bmc.2014.04.031
BindingDB Entry DOI: 10.7270/Q2XS5X00 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data