 Found 15 hits of Enzyme Inhibition Constant Data
Found 15 hits of Enzyme Inhibition Constant Data Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50339081
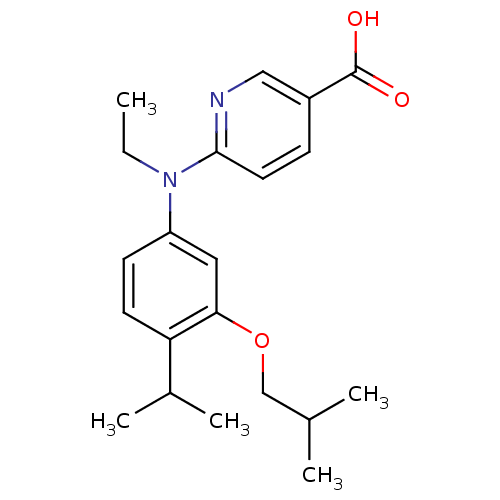
(6-[Ethyl-(3-isobutoxy-4-isopropylphenyl)amino]nico...)Show SMILES CCN(c1ccc(C(C)C)c(OCC(C)C)c1)c1ccc(cn1)C(O)=O Show InChI InChI=1S/C21H28N2O3/c1-6-23(20-10-7-16(12-22-20)21(24)25)17-8-9-18(15(4)5)19(11-17)26-13-14(2)3/h7-12,14-15H,6,13H2,1-5H3,(H,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | n/a | 19 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Agonist activity at RXRalpha transfected in human COS1 cells after 18 hrs by luciferase reporter gene transactivation assay |
ACS Med Chem Lett 1: 521-525 (2010)
Article DOI: 10.1021/ml100184k
BindingDB Entry DOI: 10.7270/Q28S4Q6X |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50339089
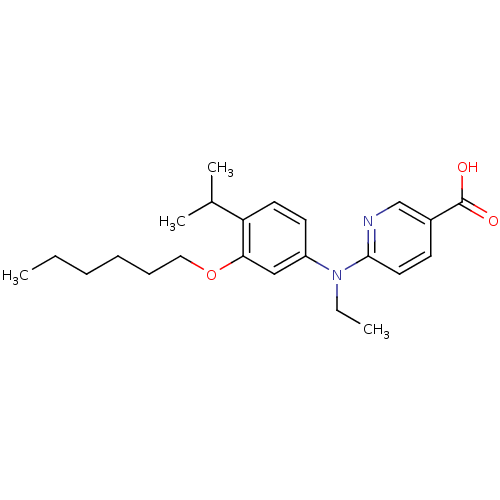
(6-[Ethyl-(3-hexyloxy-4-isopropylphenyl)amino]nicot...)Show SMILES CCCCCCOc1cc(ccc1C(C)C)N(CC)c1ccc(cn1)C(O)=O Show InChI InChI=1S/C23H32N2O3/c1-5-7-8-9-14-28-21-15-19(11-12-20(21)17(3)4)25(6-2)22-13-10-18(16-24-22)23(26)27/h10-13,15-17H,5-9,14H2,1-4H3,(H,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 280 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Agonist activity at RXRalpha transfected in human COS1 cells after 18 hrs by luciferase reporter gene transactivation assay |
ACS Med Chem Lett 1: 521-525 (2010)
Article DOI: 10.1021/ml100184k
BindingDB Entry DOI: 10.7270/Q28S4Q6X |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50339085

(6-{Ethyl-[4-isopropyl-3-(2,2,2-trifluoroethoxy)phe...)Show SMILES CCN(c1ccc(C(C)C)c(OCC(F)(F)F)c1)c1ccc(cn1)C(O)=O Show InChI InChI=1S/C19H21F3N2O3/c1-4-24(17-8-5-13(10-23-17)18(25)26)14-6-7-15(12(2)3)16(9-14)27-11-19(20,21)22/h5-10,12H,4,11H2,1-3H3,(H,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 160 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Agonist activity at RXRalpha transfected in human COS1 cells after 18 hrs by luciferase reporter gene transactivation assay |
ACS Med Chem Lett 1: 521-525 (2010)
Article DOI: 10.1021/ml100184k
BindingDB Entry DOI: 10.7270/Q28S4Q6X |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50324896
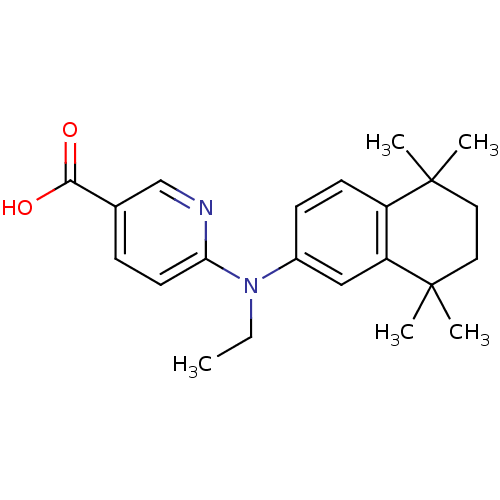
(6-[N-Ethyl(5,5,8,8-tetramethyl-5,6,7,8-tetrahydro-...)Show SMILES CCN(c1ccc2c(c1)C(C)(C)CCC2(C)C)c1ccc(cn1)C(O)=O Show InChI InChI=1S/C22H28N2O2/c1-6-24(19-10-7-15(14-23-19)20(25)26)16-8-9-17-18(13-16)22(4,5)12-11-21(17,2)3/h7-10,13-14H,6,11-12H2,1-5H3,(H,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 5.30 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Agonist activity at RXRalpha transfected in human COS1 cells after 18 hrs by luciferase reporter gene transactivation assay |
ACS Med Chem Lett 1: 521-525 (2010)
Article DOI: 10.1021/ml100184k
BindingDB Entry DOI: 10.7270/Q28S4Q6X |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50339080
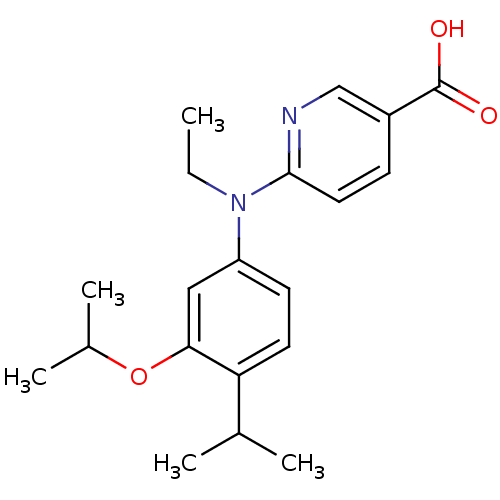
(6-[Ethyl-(3-isopropoxy-4-isopropylphenyl)amino]nic...)Show SMILES CCN(c1ccc(C(C)C)c(OC(C)C)c1)c1ccc(cn1)C(O)=O Show InChI InChI=1S/C20H26N2O3/c1-6-22(19-10-7-15(12-21-19)20(23)24)16-8-9-17(13(2)3)18(11-16)25-14(4)5/h7-14H,6H2,1-5H3,(H,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 66 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Agonist activity at RXRalpha transfected in human COS1 cells after 18 hrs by luciferase reporter gene transactivation assay |
ACS Med Chem Lett 1: 521-525 (2010)
Article DOI: 10.1021/ml100184k
BindingDB Entry DOI: 10.7270/Q28S4Q6X |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50339082

(6-[(3-Cyclopropylmethoxy-4-isopropylphenyl)-ethyla...)Show SMILES CCN(c1ccc(C(C)C)c(OCC2CC2)c1)c1ccc(cn1)C(O)=O Show InChI InChI=1S/C21H26N2O3/c1-4-23(20-10-7-16(12-22-20)21(24)25)17-8-9-18(14(2)3)19(11-17)26-13-15-5-6-15/h7-12,14-15H,4-6,13H2,1-3H3,(H,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 290 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Agonist activity at RXRalpha transfected in human COS1 cells after 18 hrs by luciferase reporter gene transactivation assay |
ACS Med Chem Lett 1: 521-525 (2010)
Article DOI: 10.1021/ml100184k
BindingDB Entry DOI: 10.7270/Q28S4Q6X |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50339084

(6-{Ethyl-[4-isopropyl-3-(3-methyl-but-2-enyloxy)ph...)Show SMILES [#6]-[#6]-[#7](-c1ccc(-[#6](-[#6])-[#6])c(-[#8]-[#6]\[#6]=[#6](\[#6])-[#6])c1)-c1ccc(cn1)-[#6](-[#8])=O Show InChI InChI=1S/C22H28N2O3/c1-6-24(21-10-7-17(14-23-21)22(25)26)18-8-9-19(16(4)5)20(13-18)27-12-11-15(2)3/h7-11,13-14,16H,6,12H2,1-5H3,(H,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 160 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Agonist activity at RXRalpha transfected in human COS1 cells after 18 hrs by luciferase reporter gene transactivation assay |
ACS Med Chem Lett 1: 521-525 (2010)
Article DOI: 10.1021/ml100184k
BindingDB Entry DOI: 10.7270/Q28S4Q6X |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50339087
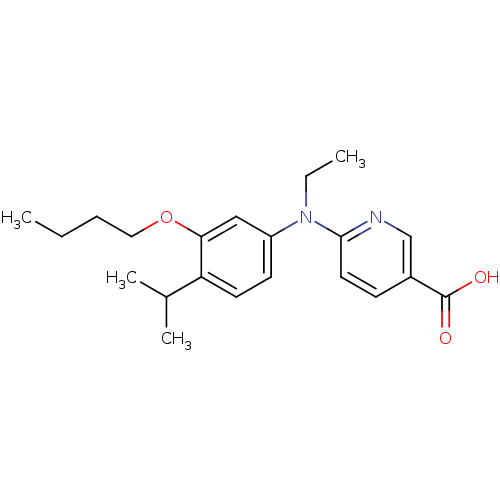
(6-[(3-Butoxy-4-isopropylphenyl)-ethylamino]nicotin...)Show InChI InChI=1S/C21H28N2O3/c1-5-7-12-26-19-13-17(9-10-18(19)15(3)4)23(6-2)20-11-8-16(14-22-20)21(24)25/h8-11,13-15H,5-7,12H2,1-4H3,(H,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 180 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Agonist activity at RXRalpha transfected in human COS1 cells after 18 hrs by luciferase reporter gene transactivation assay |
ACS Med Chem Lett 1: 521-525 (2010)
Article DOI: 10.1021/ml100184k
BindingDB Entry DOI: 10.7270/Q28S4Q6X |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50339088

(6-[Ethyl-(4-isopropyl-3-pentyloxyphenyl)amino]nico...)Show InChI InChI=1S/C22H30N2O3/c1-5-7-8-13-27-20-14-18(10-11-19(20)16(3)4)24(6-2)21-12-9-17(15-23-21)22(25)26/h9-12,14-16H,5-8,13H2,1-4H3,(H,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 160 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Agonist activity at RXRalpha transfected in human COS1 cells after 18 hrs by luciferase reporter gene transactivation assay |
ACS Med Chem Lett 1: 521-525 (2010)
Article DOI: 10.1021/ml100184k
BindingDB Entry DOI: 10.7270/Q28S4Q6X |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50339090
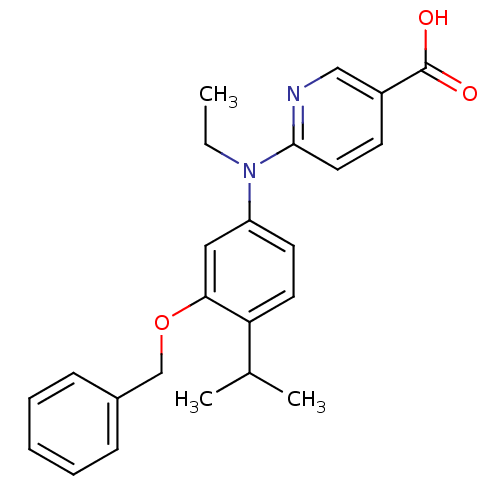
(6-[(3-Benzyloxy-4-isopropylphenyl)-ethylamino]nico...)Show SMILES CCN(c1ccc(C(C)C)c(OCc2ccccc2)c1)c1ccc(cn1)C(O)=O Show InChI InChI=1S/C24H26N2O3/c1-4-26(23-13-10-19(15-25-23)24(27)28)20-11-12-21(17(2)3)22(14-20)29-16-18-8-6-5-7-9-18/h5-15,17H,4,16H2,1-3H3,(H,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 160 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Agonist activity at RXRalpha transfected in human COS1 cells after 18 hrs by luciferase reporter gene transactivation assay |
ACS Med Chem Lett 1: 521-525 (2010)
Article DOI: 10.1021/ml100184k
BindingDB Entry DOI: 10.7270/Q28S4Q6X |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50032675

(4-[1-(3,5,5,8,8-pentamethyl-5,6,7,8-tetrahydronaph...)Show SMILES Cc1cc2c(cc1C(=C)c1ccc(cc1)C(O)=O)C(C)(C)CCC2(C)C Show InChI InChI=1S/C24H28O2/c1-15-13-20-21(24(5,6)12-11-23(20,3)4)14-19(15)16(2)17-7-9-18(10-8-17)22(25)26/h7-10,13-14H,2,11-12H2,1,3-6H3,(H,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | n/a | n/a | 20 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Agonist activity at RXRalpha transfected in human COS1 cells after 18 hrs by luciferase reporter gene transactivation assay |
ACS Med Chem Lett 1: 521-525 (2010)
Article DOI: 10.1021/ml100184k
BindingDB Entry DOI: 10.7270/Q28S4Q6X |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50339092

(6-{Ethyl-[4-isopropyl-3-(3-phenylpropoxy)phenyl]am...)Show SMILES CCN(c1ccc(C(C)C)c(OCCCc2ccccc2)c1)c1ccc(cn1)C(O)=O Show InChI InChI=1S/C26H30N2O3/c1-4-28(25-15-12-21(18-27-25)26(29)30)22-13-14-23(19(2)3)24(17-22)31-16-8-11-20-9-6-5-7-10-20/h5-7,9-10,12-15,17-19H,4,8,11,16H2,1-3H3,(H,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 820 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Agonist activity at RXRalpha transfected in human COS1 cells after 18 hrs by luciferase reporter gene transactivation assay |
ACS Med Chem Lett 1: 521-525 (2010)
Article DOI: 10.1021/ml100184k
BindingDB Entry DOI: 10.7270/Q28S4Q6X |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50339083
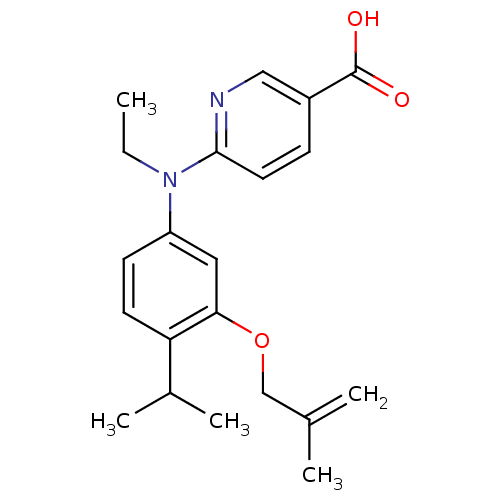
(6-{Ethyl-[4-isopropyl-3-(2-methylallyloxy)phenyl]a...)Show SMILES CCN(c1ccc(C(C)C)c(OCC(C)=C)c1)c1ccc(cn1)C(O)=O Show InChI InChI=1S/C21H26N2O3/c1-6-23(20-10-7-16(12-22-20)21(24)25)17-8-9-18(15(4)5)19(11-17)26-13-14(2)3/h7-12,15H,2,6,13H2,1,3-5H3,(H,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 110 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Agonist activity at RXRalpha transfected in human COS1 cells after 18 hrs by luciferase reporter gene transactivation assay |
ACS Med Chem Lett 1: 521-525 (2010)
Article DOI: 10.1021/ml100184k
BindingDB Entry DOI: 10.7270/Q28S4Q6X |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50339086
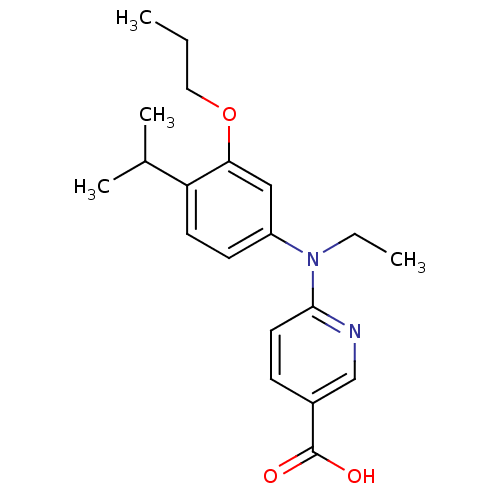
(6-[Ethyl-(4-isopropyl-3-propoxyphenyl)amino]nicoti...)Show InChI InChI=1S/C20H26N2O3/c1-5-11-25-18-12-16(8-9-17(18)14(3)4)22(6-2)19-10-7-15(13-21-19)20(23)24/h7-10,12-14H,5-6,11H2,1-4H3,(H,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 450 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Agonist activity at RXRalpha transfected in human COS1 cells after 18 hrs by luciferase reporter gene transactivation assay |
ACS Med Chem Lett 1: 521-525 (2010)
Article DOI: 10.1021/ml100184k
BindingDB Entry DOI: 10.7270/Q28S4Q6X |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50339091
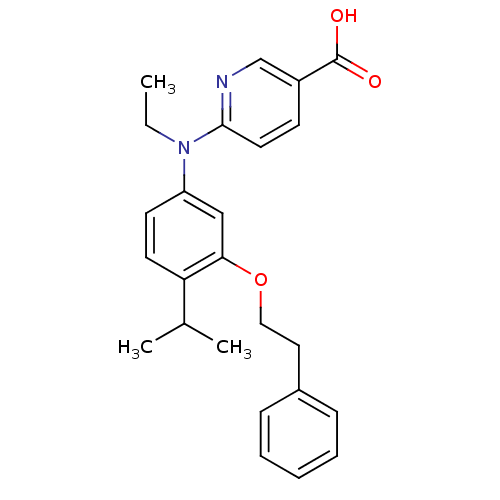
(6-[Ethyl-(4-isopropyl-3-phenethyloxyphenyl)amino]n...)Show SMILES CCN(c1ccc(C(C)C)c(OCCc2ccccc2)c1)c1ccc(cn1)C(O)=O Show InChI InChI=1S/C25H28N2O3/c1-4-27(24-13-10-20(17-26-24)25(28)29)21-11-12-22(18(2)3)23(16-21)30-15-14-19-8-6-5-7-9-19/h5-13,16-18H,4,14-15H2,1-3H3,(H,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 330 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Agonist activity at RXRalpha transfected in human COS1 cells after 18 hrs by luciferase reporter gene transactivation assay |
ACS Med Chem Lett 1: 521-525 (2010)
Article DOI: 10.1021/ml100184k
BindingDB Entry DOI: 10.7270/Q28S4Q6X |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data