 Found 53 hits of Enzyme Inhibition Constant Data
Found 53 hits of Enzyme Inhibition Constant Data Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50386386
(CHEMBL2046871)Show SMILES CCOc1ccc(Cc2nc3cc(ccc3n2CCC2CCCN2C)[N+]([O-])=O)cc1 Show InChI InChI=1S/C23H28N4O3/c1-3-30-20-9-6-17(7-10-20)15-23-24-21-16-19(27(28)29)8-11-22(21)26(23)14-12-18-5-4-13-25(18)2/h6-11,16,18H,3-5,12-15H2,1-2H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from human mu opioid receptor expressed in HEK-293 cells by scintillation counting |
ACS Med Chem Lett 3: 227-231 (2012)
Article DOI: 10.1021/ml200268w
BindingDB Entry DOI: 10.7270/Q2TX3GF4 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50000092

((-)-(etorphine) | (-)-morphine | (1S,5R,13R,14S)-1...)Show SMILES CN1CC[C@@]23[C@H]4Oc5c2c(C[C@@H]1[C@@H]3C=C[C@@H]4O)ccc5O |r,c:16,TLB:13:12:8.9.10:3.2.1| Show InChI InChI=1S/C17H19NO3/c1-18-7-6-17-10-3-5-13(20)16(17)21-15-12(19)4-2-9(14(15)17)8-11(10)18/h2-5,10-11,13,16,19-20H,6-8H2,1H3/t10-,11+,13-,16-,17-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from human mu opioid receptor expressed in HEK-293 cells by scintillation counting |
ACS Med Chem Lett 3: 227-231 (2012)
Article DOI: 10.1021/ml200268w
BindingDB Entry DOI: 10.7270/Q2TX3GF4 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50386382
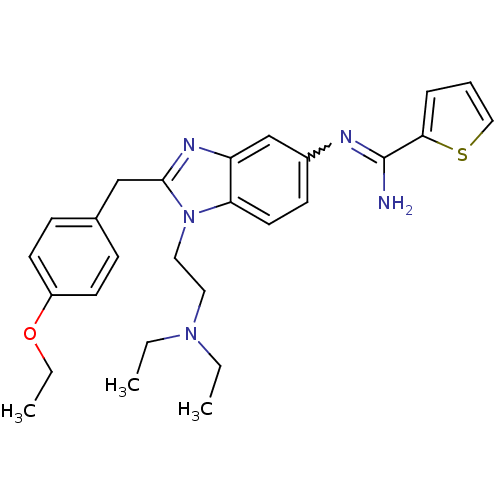
(CHEMBL2046872)Show SMILES CCOc1ccc(Cc2nc3cc(ccc3n2CCN(CC)CC)N=C(N)c2cccs2)cc1 |w:24.25| Show InChI InChI=1S/C27H33N5OS/c1-4-31(5-2)15-16-32-24-14-11-21(29-27(28)25-8-7-17-34-25)19-23(24)30-26(32)18-20-9-12-22(13-10-20)33-6-3/h7-14,17,19H,4-6,15-16,18H2,1-3H3,(H2,28,29) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.41 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from human mu opioid receptor expressed in HEK-293 cells by scintillation counting |
ACS Med Chem Lett 3: 227-231 (2012)
Article DOI: 10.1021/ml200268w
BindingDB Entry DOI: 10.7270/Q2TX3GF4 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50386379
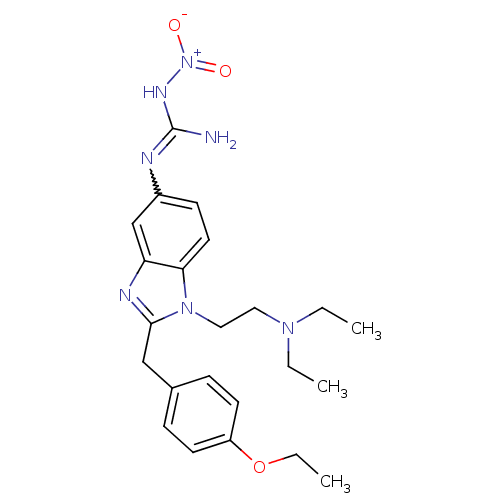
(CHEMBL2048416)Show SMILES CCOc1ccc(Cc2nc3cc(NC(N)=N[N+]([O-])=O)ccc3n2CCN(CC)CC)cc1 |w:16.16| Show InChI InChI=1S/C23H31N7O3/c1-4-28(5-2)13-14-29-21-12-9-18(25-23(24)27-30(31)32)16-20(21)26-22(29)15-17-7-10-19(11-8-17)33-6-3/h7-12,16H,4-6,13-15H2,1-3H3,(H3,24,25,27) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from human mu opioid receptor expressed in HEK-293 cells by scintillation counting |
ACS Med Chem Lett 3: 227-231 (2012)
Article DOI: 10.1021/ml200268w
BindingDB Entry DOI: 10.7270/Q2TX3GF4 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50386380
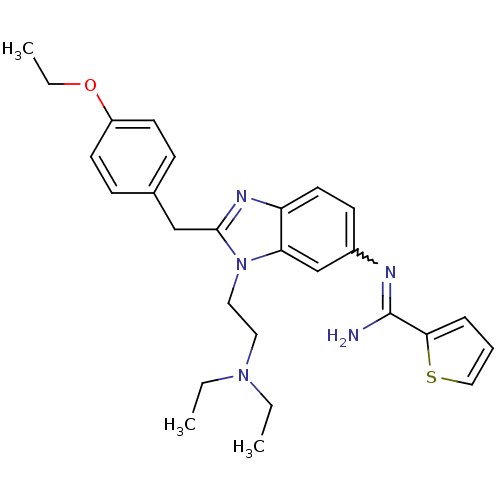
(CHEMBL2048417)Show SMILES CCOc1ccc(Cc2nc3ccc(cc3n2CCN(CC)CC)N=C(N)c2cccs2)cc1 |w:24.25| Show InChI InChI=1S/C27H33N5OS/c1-4-31(5-2)15-16-32-24-19-21(29-27(28)25-8-7-17-34-25)11-14-23(24)30-26(32)18-20-9-12-22(13-10-20)33-6-3/h7-14,17,19H,4-6,15-16,18H2,1-3H3,(H2,28,29) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from human mu opioid receptor expressed in HEK-293 cells by scintillation counting |
ACS Med Chem Lett 3: 227-231 (2012)
Article DOI: 10.1021/ml200268w
BindingDB Entry DOI: 10.7270/Q2TX3GF4 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50386388
(CHEMBL2046877)Show SMILES CCOc1ccc(Cc2nc3cc(ccc3n2CCN(CC)CC)N=C(N)c2ccsc2)cc1 |w:24.25| Show InChI InChI=1S/C27H33N5OS/c1-4-31(5-2)14-15-32-25-12-9-22(29-27(28)21-13-16-34-19-21)18-24(25)30-26(32)17-20-7-10-23(11-8-20)33-6-3/h7-13,16,18-19H,4-6,14-15,17H2,1-3H3,(H2,28,29) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from human mu opioid receptor expressed in HEK-293 cells by scintillation counting |
ACS Med Chem Lett 3: 227-231 (2012)
Article DOI: 10.1021/ml200268w
BindingDB Entry DOI: 10.7270/Q2TX3GF4 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50386385

(CHEMBL2046876)Show SMILES CCOc1ccc(Cc2nc3cc(ccc3n2CCN(CC)CC)N=C(N)c2ccco2)cc1 |w:24.25| Show InChI InChI=1S/C27H33N5O2/c1-4-31(5-2)15-16-32-24-14-11-21(29-27(28)25-8-7-17-34-25)19-23(24)30-26(32)18-20-9-12-22(13-10-20)33-6-3/h7-14,17,19H,4-6,15-16,18H2,1-3H3,(H2,28,29) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from human mu opioid receptor expressed in HEK-293 cells by scintillation counting |
ACS Med Chem Lett 3: 227-231 (2012)
Article DOI: 10.1021/ml200268w
BindingDB Entry DOI: 10.7270/Q2TX3GF4 |
More data for this
Ligand-Target Pair | |
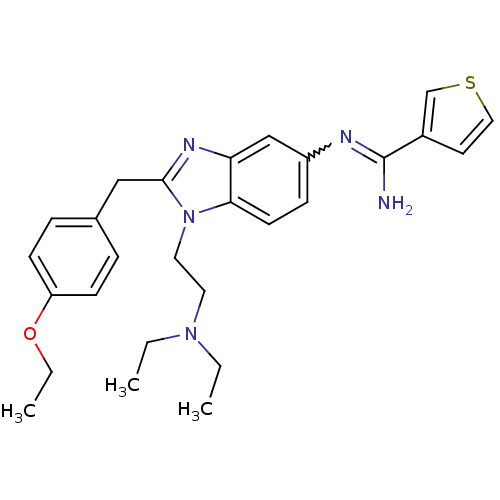
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50386377
(CHEMBL2046878)Show SMILES CCOc1ccc(Cc2nc3cc(ccc3n2CCN(CC)CC)N=C(N)c2ccoc2)cc1 |w:24.25| Show InChI InChI=1S/C27H33N5O2/c1-4-31(5-2)14-15-32-25-12-9-22(29-27(28)21-13-16-33-19-21)18-24(25)30-26(32)17-20-7-10-23(11-8-20)34-6-3/h7-13,16,18-19H,4-6,14-15,17H2,1-3H3,(H2,28,29) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 42 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from human mu opioid receptor expressed in HEK-293 cells by scintillation counting |
ACS Med Chem Lett 3: 227-231 (2012)
Article DOI: 10.1021/ml200268w
BindingDB Entry DOI: 10.7270/Q2TX3GF4 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50386378
(CHEMBL2048415)Show SMILES CCOc1ccc(Cc2nc3cc(ccc3n2CCN(CC)CC)N=C(C)N)cc1 |w:24.25| Show InChI InChI=1S/C24H33N5O/c1-5-28(6-2)14-15-29-23-13-10-20(26-18(4)25)17-22(23)27-24(29)16-19-8-11-21(12-9-19)30-7-3/h8-13,17H,5-7,14-16H2,1-4H3,(H2,25,26) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 48 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from human mu opioid receptor expressed in HEK-293 cells by scintillation counting |
ACS Med Chem Lett 3: 227-231 (2012)
Article DOI: 10.1021/ml200268w
BindingDB Entry DOI: 10.7270/Q2TX3GF4 |
More data for this
Ligand-Target Pair | |
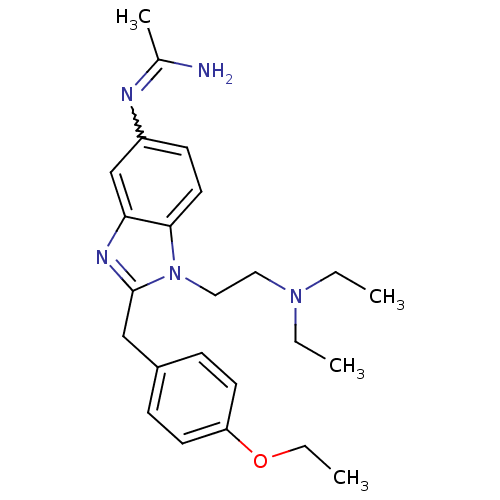
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50386383
(CHEMBL2046873)Show SMILES CCOc1ccc(Cc2nc3cc(ccc3n2CCN(C)C)N=C(N)c2cccs2)cc1 |w:22.23| Show InChI InChI=1S/C25H29N5OS/c1-4-31-20-10-7-18(8-11-20)16-24-28-21-17-19(27-25(26)23-6-5-15-32-23)9-12-22(21)30(24)14-13-29(2)3/h5-12,15,17H,4,13-14,16H2,1-3H3,(H2,26,27) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from human mu opioid receptor expressed in HEK-293 cells by scintillation counting |
ACS Med Chem Lett 3: 227-231 (2012)
Article DOI: 10.1021/ml200268w
BindingDB Entry DOI: 10.7270/Q2TX3GF4 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50386381
(Nucynta | TAPENTADOL HYDROCHLORIDE | Tapentadol)Show InChI InChI=1S/C14H23NO/c1-5-14(11(2)10-15(3)4)12-7-6-8-13(16)9-12/h6-9,11,14,16H,5,10H2,1-4H3/t11-,14+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
PC cid
PC sid
UniChem
Similars
| DrugBank
Article
PubMed
| 160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from human mu opioid receptor expressed in HEK-293 cells by scintillation counting |
ACS Med Chem Lett 3: 227-231 (2012)
Article DOI: 10.1021/ml200268w
BindingDB Entry DOI: 10.7270/Q2TX3GF4 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50386384
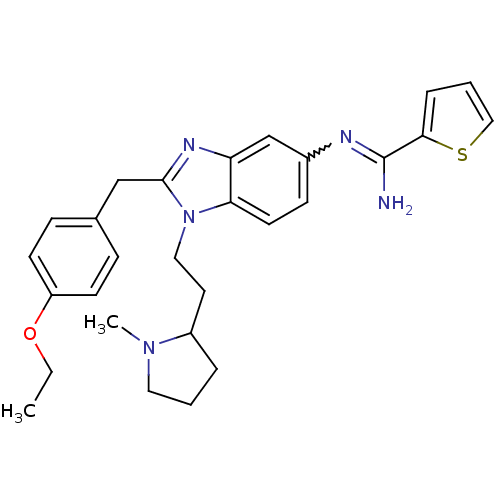
(CHEMBL2046874)Show SMILES CCOc1ccc(Cc2nc3cc(ccc3n2CCC2CCCN2C)N=C(N)c2cccs2)cc1 |w:25.27| Show InChI InChI=1S/C28H33N5OS/c1-3-34-23-11-8-20(9-12-23)18-27-31-24-19-21(30-28(29)26-7-5-17-35-26)10-13-25(24)33(27)16-14-22-6-4-15-32(22)2/h5,7-13,17,19,22H,3-4,6,14-16,18H2,1-2H3,(H2,29,30) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from human mu opioid receptor expressed in HEK-293 cells by scintillation counting |
ACS Med Chem Lett 3: 227-231 (2012)
Article DOI: 10.1021/ml200268w
BindingDB Entry DOI: 10.7270/Q2TX3GF4 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50386387
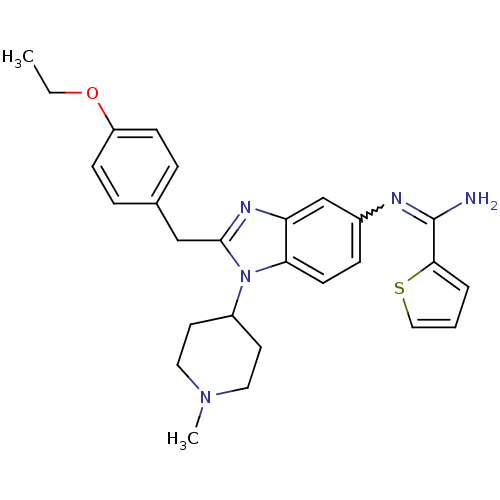
(CHEMBL2046875)Show SMILES CCOc1ccc(Cc2nc3cc(ccc3n2C2CCN(C)CC2)N=C(N)c2cccs2)cc1 |w:24.26| Show InChI InChI=1S/C27H31N5OS/c1-3-33-22-9-6-19(7-10-22)17-26-30-23-18-20(29-27(28)25-5-4-16-34-25)8-11-24(23)32(26)21-12-14-31(2)15-13-21/h4-11,16,18,21H,3,12-15,17H2,1-2H3,(H2,28,29) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from human mu opioid receptor expressed in HEK-293 cells by scintillation counting |
ACS Med Chem Lett 3: 227-231 (2012)
Article DOI: 10.1021/ml200268w
BindingDB Entry DOI: 10.7270/Q2TX3GF4 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50230993

((2S)-2-amino-5-[(N-methylcarbamimidoyl)amino]penta...)Show InChI InChI=1S/C7H16N4O2/c1-10-7(9)11-4-2-3-5(8)6(12)13/h5H,2-4,8H2,1H3,(H,12,13)(H3,9,10,11)/t5-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant iNOS assessed as conversion of [3H]L-arginine to [3H]L-citrulline preincubated for 15 mins measured after 45 mins by ... |
ACS Med Chem Lett 3: 227-231 (2012)
Article DOI: 10.1021/ml200268w
BindingDB Entry DOI: 10.7270/Q2TX3GF4 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50386382

(CHEMBL2046872)Show SMILES CCOc1ccc(Cc2nc3cc(ccc3n2CCN(CC)CC)N=C(N)c2cccs2)cc1 |w:24.25| Show InChI InChI=1S/C27H33N5OS/c1-4-31(5-2)15-16-32-24-14-11-21(29-27(28)25-8-7-17-34-25)19-23(24)30-26(32)18-20-9-12-22(13-10-20)33-6-3/h7-14,17,19H,4-6,15-16,18H2,1-3H3,(H2,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 440 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant nNOS assessed as conversion of [3H]L-arginine to [3H]L-citrulline preincubated for 15 mins measured after 45 mins by ... |
ACS Med Chem Lett 3: 227-231 (2012)
Article DOI: 10.1021/ml200268w
BindingDB Entry DOI: 10.7270/Q2TX3GF4 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, endothelial
(Homo sapiens (Human)) | BDBM50230993

((2S)-2-amino-5-[(N-methylcarbamimidoyl)amino]penta...)Show InChI InChI=1S/C7H16N4O2/c1-10-7(9)11-4-2-3-5(8)6(12)13/h5H,2-4,8H2,1H3,(H,12,13)(H3,9,10,11)/t5-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 650 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant eNOS assessed as conversion of [3H]L-arginine to [3H]L-citrulline preincubated for 15 mins measured after 45 mins by ... |
ACS Med Chem Lett 3: 227-231 (2012)
Article DOI: 10.1021/ml200268w
BindingDB Entry DOI: 10.7270/Q2TX3GF4 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50386383

(CHEMBL2046873)Show SMILES CCOc1ccc(Cc2nc3cc(ccc3n2CCN(C)C)N=C(N)c2cccs2)cc1 |w:22.23| Show InChI InChI=1S/C25H29N5OS/c1-4-31-20-10-7-18(8-11-20)16-24-28-21-17-19(27-25(26)23-6-5-15-32-23)9-12-22(21)30(24)14-13-29(2)3/h5-12,15,17H,4,13-14,16H2,1-3H3,(H2,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 740 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant nNOS assessed as conversion of [3H]L-arginine to [3H]L-citrulline preincubated for 15 mins measured after 45 mins by ... |
ACS Med Chem Lett 3: 227-231 (2012)
Article DOI: 10.1021/ml200268w
BindingDB Entry DOI: 10.7270/Q2TX3GF4 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50230993

((2S)-2-amino-5-[(N-methylcarbamimidoyl)amino]penta...)Show InChI InChI=1S/C7H16N4O2/c1-10-7(9)11-4-2-3-5(8)6(12)13/h5H,2-4,8H2,1H3,(H,12,13)(H3,9,10,11)/t5-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 950 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant nNOS assessed as conversion of [3H]L-arginine to [3H]L-citrulline preincubated for 15 mins measured after 45 mins by ... |
ACS Med Chem Lett 3: 227-231 (2012)
Article DOI: 10.1021/ml200268w
BindingDB Entry DOI: 10.7270/Q2TX3GF4 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50386384

(CHEMBL2046874)Show SMILES CCOc1ccc(Cc2nc3cc(ccc3n2CCC2CCCN2C)N=C(N)c2cccs2)cc1 |w:25.27| Show InChI InChI=1S/C28H33N5OS/c1-3-34-23-11-8-20(9-12-23)18-27-31-24-19-21(30-28(29)26-7-5-17-35-26)10-13-25(24)33(27)16-14-22-6-4-15-32(22)2/h5,7-13,17,19,22H,3-4,6,14-16,18H2,1-2H3,(H2,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.77E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant nNOS assessed as conversion of [3H]L-arginine to [3H]L-citrulline preincubated for 15 mins measured after 45 mins by ... |
ACS Med Chem Lett 3: 227-231 (2012)
Article DOI: 10.1021/ml200268w
BindingDB Entry DOI: 10.7270/Q2TX3GF4 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50386388

(CHEMBL2046877)Show SMILES CCOc1ccc(Cc2nc3cc(ccc3n2CCN(CC)CC)N=C(N)c2ccsc2)cc1 |w:24.25| Show InChI InChI=1S/C27H33N5OS/c1-4-31(5-2)14-15-32-25-12-9-22(29-27(28)21-13-16-34-19-21)18-24(25)30-26(32)17-20-7-10-23(11-8-20)33-6-3/h7-13,16,18-19H,4-6,14-15,17H2,1-3H3,(H2,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.76E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant nNOS assessed as conversion of [3H]L-arginine to [3H]L-citrulline preincubated for 15 mins measured after 45 mins by ... |
ACS Med Chem Lett 3: 227-231 (2012)
Article DOI: 10.1021/ml200268w
BindingDB Entry DOI: 10.7270/Q2TX3GF4 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, endothelial
(Homo sapiens (Human)) | BDBM50386384

(CHEMBL2046874)Show SMILES CCOc1ccc(Cc2nc3cc(ccc3n2CCC2CCCN2C)N=C(N)c2cccs2)cc1 |w:25.27| Show InChI InChI=1S/C28H33N5OS/c1-3-34-23-11-8-20(9-12-23)18-27-31-24-19-21(30-28(29)26-7-5-17-35-26)10-13-25(24)33(27)16-14-22-6-4-15-32(22)2/h5,7-13,17,19,22H,3-4,6,14-16,18H2,1-2H3,(H2,29,30) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.04E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant eNOS assessed as conversion of [3H]L-arginine to [3H]L-citrulline preincubated for 15 mins measured after 45 mins by ... |
ACS Med Chem Lett 3: 227-231 (2012)
Article DOI: 10.1021/ml200268w
BindingDB Entry DOI: 10.7270/Q2TX3GF4 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, endothelial
(Homo sapiens (Human)) | BDBM50386382

(CHEMBL2046872)Show SMILES CCOc1ccc(Cc2nc3cc(ccc3n2CCN(CC)CC)N=C(N)c2cccs2)cc1 |w:24.25| Show InChI InChI=1S/C27H33N5OS/c1-4-31(5-2)15-16-32-24-14-11-21(29-27(28)25-8-7-17-34-25)19-23(24)30-26(32)18-20-9-12-22(13-10-20)33-6-3/h7-14,17,19H,4-6,15-16,18H2,1-3H3,(H2,28,29) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.74E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant eNOS assessed as conversion of [3H]L-arginine to [3H]L-citrulline preincubated for 15 mins measured after 45 mins by ... |
ACS Med Chem Lett 3: 227-231 (2012)
Article DOI: 10.1021/ml200268w
BindingDB Entry DOI: 10.7270/Q2TX3GF4 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50386385

(CHEMBL2046876)Show SMILES CCOc1ccc(Cc2nc3cc(ccc3n2CCN(CC)CC)N=C(N)c2ccco2)cc1 |w:24.25| Show InChI InChI=1S/C27H33N5O2/c1-4-31(5-2)15-16-32-24-14-11-21(29-27(28)25-8-7-17-34-25)19-23(24)30-26(32)18-20-9-12-22(13-10-20)33-6-3/h7-14,17,19H,4-6,15-16,18H2,1-3H3,(H2,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.05E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant nNOS assessed as conversion of [3H]L-arginine to [3H]L-citrulline preincubated for 15 mins measured after 45 mins by ... |
ACS Med Chem Lett 3: 227-231 (2012)
Article DOI: 10.1021/ml200268w
BindingDB Entry DOI: 10.7270/Q2TX3GF4 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, endothelial
(Homo sapiens (Human)) | BDBM50386387

(CHEMBL2046875)Show SMILES CCOc1ccc(Cc2nc3cc(ccc3n2C2CCN(C)CC2)N=C(N)c2cccs2)cc1 |w:24.26| Show InChI InChI=1S/C27H31N5OS/c1-3-33-22-9-6-19(7-10-22)17-26-30-23-18-20(29-27(28)25-5-4-16-34-25)8-11-24(23)32(26)21-12-14-31(2)15-13-21/h4-11,16,18,21H,3,12-15,17H2,1-2H3,(H2,28,29) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant eNOS assessed as conversion of [3H]L-arginine to [3H]L-citrulline preincubated for 15 mins measured after 45 mins by ... |
ACS Med Chem Lett 3: 227-231 (2012)
Article DOI: 10.1021/ml200268w
BindingDB Entry DOI: 10.7270/Q2TX3GF4 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50386387

(CHEMBL2046875)Show SMILES CCOc1ccc(Cc2nc3cc(ccc3n2C2CCN(C)CC2)N=C(N)c2cccs2)cc1 |w:24.26| Show InChI InChI=1S/C27H31N5OS/c1-3-33-22-9-6-19(7-10-22)17-26-30-23-18-20(29-27(28)25-5-4-16-34-25)8-11-24(23)32(26)21-12-14-31(2)15-13-21/h4-11,16,18,21H,3,12-15,17H2,1-2H3,(H2,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.28E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant nNOS assessed as conversion of [3H]L-arginine to [3H]L-citrulline preincubated for 15 mins measured after 45 mins by ... |
ACS Med Chem Lett 3: 227-231 (2012)
Article DOI: 10.1021/ml200268w
BindingDB Entry DOI: 10.7270/Q2TX3GF4 |
More data for this
Ligand-Target Pair | |
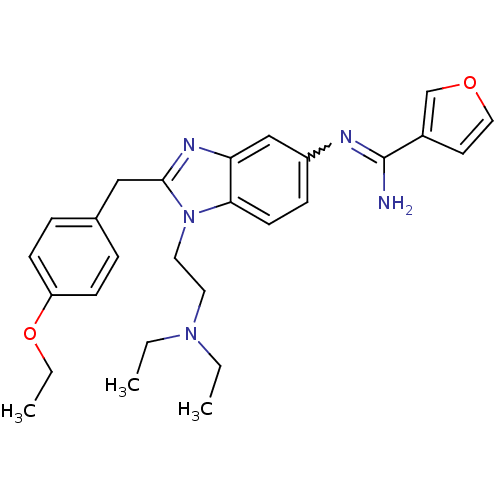
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50386377

(CHEMBL2046878)Show SMILES CCOc1ccc(Cc2nc3cc(ccc3n2CCN(CC)CC)N=C(N)c2ccoc2)cc1 |w:24.25| Show InChI InChI=1S/C27H33N5O2/c1-4-31(5-2)14-15-32-25-12-9-22(29-27(28)21-13-16-33-19-21)18-24(25)30-26(32)17-20-7-10-23(11-8-20)34-6-3/h7-13,16,18-19H,4-6,14-15,17H2,1-3H3,(H2,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.44E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant nNOS assessed as conversion of [3H]L-arginine to [3H]L-citrulline preincubated for 15 mins measured after 45 mins by ... |
ACS Med Chem Lett 3: 227-231 (2012)
Article DOI: 10.1021/ml200268w
BindingDB Entry DOI: 10.7270/Q2TX3GF4 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50386385

(CHEMBL2046876)Show SMILES CCOc1ccc(Cc2nc3cc(ccc3n2CCN(CC)CC)N=C(N)c2ccco2)cc1 |w:24.25| Show InChI InChI=1S/C27H33N5O2/c1-4-31(5-2)15-16-32-24-14-11-21(29-27(28)25-8-7-17-34-25)19-23(24)30-26(32)18-20-9-12-22(13-10-20)33-6-3/h7-14,17,19H,4-6,15-16,18H2,1-3H3,(H2,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant iNOS assessed as conversion of [3H]L-arginine to [3H]L-citrulline preincubated for 15 mins measured after 45 mins by ... |
ACS Med Chem Lett 3: 227-231 (2012)
Article DOI: 10.1021/ml200268w
BindingDB Entry DOI: 10.7270/Q2TX3GF4 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, endothelial
(Homo sapiens (Human)) | BDBM50386383

(CHEMBL2046873)Show SMILES CCOc1ccc(Cc2nc3cc(ccc3n2CCN(C)C)N=C(N)c2cccs2)cc1 |w:22.23| Show InChI InChI=1S/C25H29N5OS/c1-4-31-20-10-7-18(8-11-20)16-24-28-21-17-19(27-25(26)23-6-5-15-32-23)9-12-22(21)30(24)14-13-29(2)3/h5-12,15,17H,4,13-14,16H2,1-3H3,(H2,26,27) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant eNOS assessed as conversion of [3H]L-arginine to [3H]L-citrulline preincubated for 15 mins measured after 45 mins by ... |
ACS Med Chem Lett 3: 227-231 (2012)
Article DOI: 10.1021/ml200268w
BindingDB Entry DOI: 10.7270/Q2TX3GF4 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, endothelial
(Homo sapiens (Human)) | BDBM50386385

(CHEMBL2046876)Show SMILES CCOc1ccc(Cc2nc3cc(ccc3n2CCN(CC)CC)N=C(N)c2ccco2)cc1 |w:24.25| Show InChI InChI=1S/C27H33N5O2/c1-4-31(5-2)15-16-32-24-14-11-21(29-27(28)25-8-7-17-34-25)19-23(24)30-26(32)18-20-9-12-22(13-10-20)33-6-3/h7-14,17,19H,4-6,15-16,18H2,1-3H3,(H2,28,29) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.87E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant eNOS assessed as conversion of [3H]L-arginine to [3H]L-citrulline preincubated for 15 mins measured after 45 mins by ... |
ACS Med Chem Lett 3: 227-231 (2012)
Article DOI: 10.1021/ml200268w
BindingDB Entry DOI: 10.7270/Q2TX3GF4 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, endothelial
(Homo sapiens (Human)) | BDBM50386388

(CHEMBL2046877)Show SMILES CCOc1ccc(Cc2nc3cc(ccc3n2CCN(CC)CC)N=C(N)c2ccsc2)cc1 |w:24.25| Show InChI InChI=1S/C27H33N5OS/c1-4-31(5-2)14-15-32-25-12-9-22(29-27(28)21-13-16-34-19-21)18-24(25)30-26(32)17-20-7-10-23(11-8-20)33-6-3/h7-13,16,18-19H,4-6,14-15,17H2,1-3H3,(H2,28,29) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.95E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant eNOS assessed as conversion of [3H]L-arginine to [3H]L-citrulline preincubated for 15 mins measured after 45 mins by ... |
ACS Med Chem Lett 3: 227-231 (2012)
Article DOI: 10.1021/ml200268w
BindingDB Entry DOI: 10.7270/Q2TX3GF4 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50386379

(CHEMBL2048416)Show SMILES CCOc1ccc(Cc2nc3cc(NC(N)=N[N+]([O-])=O)ccc3n2CCN(CC)CC)cc1 |w:16.16| Show InChI InChI=1S/C23H31N7O3/c1-4-28(5-2)13-14-29-21-12-9-18(25-23(24)27-30(31)32)16-20(21)26-22(29)15-17-7-10-19(11-8-17)33-6-3/h7-12,16H,4-6,13-15H2,1-3H3,(H3,24,25,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.57E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant nNOS assessed as conversion of [3H]L-arginine to [3H]L-citrulline preincubated for 15 mins measured after 45 mins by ... |
ACS Med Chem Lett 3: 227-231 (2012)
Article DOI: 10.1021/ml200268w
BindingDB Entry DOI: 10.7270/Q2TX3GF4 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, endothelial
(Homo sapiens (Human)) | BDBM50386379

(CHEMBL2048416)Show SMILES CCOc1ccc(Cc2nc3cc(NC(N)=N[N+]([O-])=O)ccc3n2CCN(CC)CC)cc1 |w:16.16| Show InChI InChI=1S/C23H31N7O3/c1-4-28(5-2)13-14-29-21-12-9-18(25-23(24)27-30(31)32)16-20(21)26-22(29)15-17-7-10-19(11-8-17)33-6-3/h7-12,16H,4-6,13-15H2,1-3H3,(H3,24,25,27) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.71E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant eNOS assessed as conversion of [3H]L-arginine to [3H]L-citrulline preincubated for 15 mins measured after 45 mins by ... |
ACS Med Chem Lett 3: 227-231 (2012)
Article DOI: 10.1021/ml200268w
BindingDB Entry DOI: 10.7270/Q2TX3GF4 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50386380

(CHEMBL2048417)Show SMILES CCOc1ccc(Cc2nc3ccc(cc3n2CCN(CC)CC)N=C(N)c2cccs2)cc1 |w:24.25| Show InChI InChI=1S/C27H33N5OS/c1-4-31(5-2)15-16-32-24-19-21(29-27(28)25-8-7-17-34-25)11-14-23(24)30-26(32)18-20-9-12-22(13-10-20)33-6-3/h7-14,17,19H,4-6,15-16,18H2,1-3H3,(H2,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.72E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant nNOS assessed as conversion of [3H]L-arginine to [3H]L-citrulline preincubated for 15 mins measured after 45 mins by ... |
ACS Med Chem Lett 3: 227-231 (2012)
Article DOI: 10.1021/ml200268w
BindingDB Entry DOI: 10.7270/Q2TX3GF4 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, endothelial
(Homo sapiens (Human)) | BDBM50386380

(CHEMBL2048417)Show SMILES CCOc1ccc(Cc2nc3ccc(cc3n2CCN(CC)CC)N=C(N)c2cccs2)cc1 |w:24.25| Show InChI InChI=1S/C27H33N5OS/c1-4-31(5-2)15-16-32-24-19-21(29-27(28)25-8-7-17-34-25)11-14-23(24)30-26(32)18-20-9-12-22(13-10-20)33-6-3/h7-14,17,19H,4-6,15-16,18H2,1-3H3,(H2,28,29) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.71E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant eNOS assessed as conversion of [3H]L-arginine to [3H]L-citrulline preincubated for 15 mins measured after 45 mins by ... |
ACS Med Chem Lett 3: 227-231 (2012)
Article DOI: 10.1021/ml200268w
BindingDB Entry DOI: 10.7270/Q2TX3GF4 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50386382

(CHEMBL2046872)Show SMILES CCOc1ccc(Cc2nc3cc(ccc3n2CCN(CC)CC)N=C(N)c2cccs2)cc1 |w:24.25| Show InChI InChI=1S/C27H33N5OS/c1-4-31(5-2)15-16-32-24-14-11-21(29-27(28)25-8-7-17-34-25)19-23(24)30-26(32)18-20-9-12-22(13-10-20)33-6-3/h7-14,17,19H,4-6,15-16,18H2,1-3H3,(H2,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.52E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant iNOS assessed as conversion of [3H]L-arginine to [3H]L-citrulline preincubated for 15 mins measured after 45 mins by ... |
ACS Med Chem Lett 3: 227-231 (2012)
Article DOI: 10.1021/ml200268w
BindingDB Entry DOI: 10.7270/Q2TX3GF4 |
More data for this
Ligand-Target Pair | |
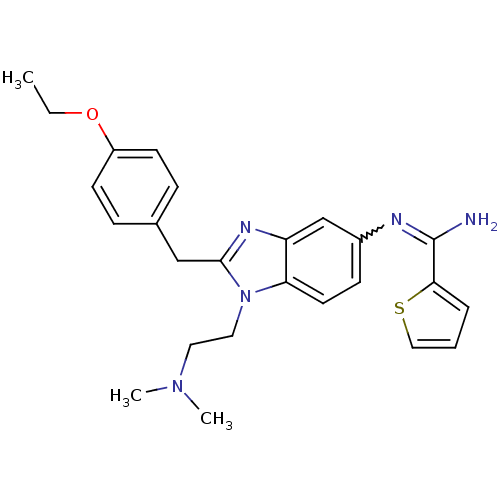
Nitric oxide synthase, endothelial
(Homo sapiens (Human)) | BDBM50386378

(CHEMBL2048415)Show SMILES CCOc1ccc(Cc2nc3cc(ccc3n2CCN(CC)CC)N=C(C)N)cc1 |w:24.25| Show InChI InChI=1S/C24H33N5O/c1-5-28(6-2)14-15-29-23-13-10-20(26-18(4)25)17-22(23)27-24(29)16-19-8-11-21(12-9-19)30-7-3/h8-13,17H,5-7,14-16H2,1-4H3,(H2,25,26) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant eNOS assessed as conversion of [3H]L-arginine to [3H]L-citrulline preincubated for 15 mins measured after 45 mins by ... |
ACS Med Chem Lett 3: 227-231 (2012)
Article DOI: 10.1021/ml200268w
BindingDB Entry DOI: 10.7270/Q2TX3GF4 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50386378

(CHEMBL2048415)Show SMILES CCOc1ccc(Cc2nc3cc(ccc3n2CCN(CC)CC)N=C(C)N)cc1 |w:24.25| Show InChI InChI=1S/C24H33N5O/c1-5-28(6-2)14-15-29-23-13-10-20(26-18(4)25)17-22(23)27-24(29)16-19-8-11-21(12-9-19)30-7-3/h8-13,17H,5-7,14-16H2,1-4H3,(H2,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant nNOS assessed as conversion of [3H]L-arginine to [3H]L-citrulline preincubated for 15 mins measured after 45 mins by ... |
ACS Med Chem Lett 3: 227-231 (2012)
Article DOI: 10.1021/ml200268w
BindingDB Entry DOI: 10.7270/Q2TX3GF4 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50386386

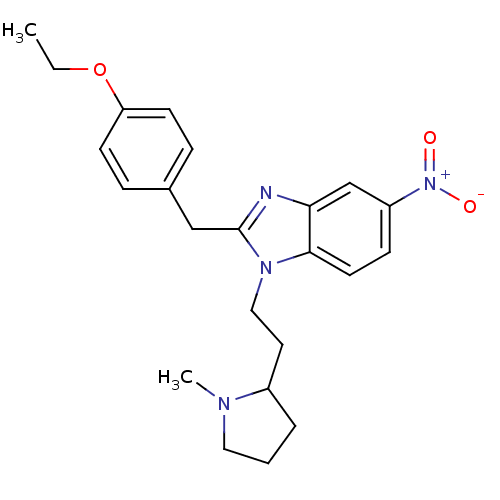
(CHEMBL2046871)Show SMILES CCOc1ccc(Cc2nc3cc(ccc3n2CCC2CCCN2C)[N+]([O-])=O)cc1 Show InChI InChI=1S/C23H28N4O3/c1-3-30-20-9-6-17(7-10-20)15-23-24-21-16-19(27(28)29)8-11-22(21)26(23)14-12-18-5-4-13-25(18)2/h6-11,16,18H,3-5,12-15H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.13E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant nNOS assessed as conversion of [3H]L-arginine to [3H]L-citrulline preincubated for 15 mins measured after 45 mins by ... |
ACS Med Chem Lett 3: 227-231 (2012)
Article DOI: 10.1021/ml200268w
BindingDB Entry DOI: 10.7270/Q2TX3GF4 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, endothelial
(Homo sapiens (Human)) | BDBM50386377

(CHEMBL2046878)Show SMILES CCOc1ccc(Cc2nc3cc(ccc3n2CCN(CC)CC)N=C(N)c2ccoc2)cc1 |w:24.25| Show InChI InChI=1S/C27H33N5O2/c1-4-31(5-2)14-15-32-25-12-9-22(29-27(28)21-13-16-33-19-21)18-24(25)30-26(32)17-20-7-10-23(11-8-20)34-6-3/h7-13,16,18-19H,4-6,14-15,17H2,1-3H3,(H2,28,29) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.17E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant eNOS assessed as conversion of [3H]L-arginine to [3H]L-citrulline preincubated for 15 mins measured after 45 mins by ... |
ACS Med Chem Lett 3: 227-231 (2012)
Article DOI: 10.1021/ml200268w
BindingDB Entry DOI: 10.7270/Q2TX3GF4 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50386388

(CHEMBL2046877)Show SMILES CCOc1ccc(Cc2nc3cc(ccc3n2CCN(CC)CC)N=C(N)c2ccsc2)cc1 |w:24.25| Show InChI InChI=1S/C27H33N5OS/c1-4-31(5-2)14-15-32-25-12-9-22(29-27(28)21-13-16-34-19-21)18-24(25)30-26(32)17-20-7-10-23(11-8-20)33-6-3/h7-13,16,18-19H,4-6,14-15,17H2,1-3H3,(H2,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.18E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant iNOS assessed as conversion of [3H]L-arginine to [3H]L-citrulline preincubated for 15 mins measured after 45 mins by ... |
ACS Med Chem Lett 3: 227-231 (2012)
Article DOI: 10.1021/ml200268w
BindingDB Entry DOI: 10.7270/Q2TX3GF4 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, endothelial
(Homo sapiens (Human)) | BDBM50386386

(CHEMBL2046871)Show SMILES CCOc1ccc(Cc2nc3cc(ccc3n2CCC2CCCN2C)[N+]([O-])=O)cc1 Show InChI InChI=1S/C23H28N4O3/c1-3-30-20-9-6-17(7-10-20)15-23-24-21-16-19(27(28)29)8-11-22(21)26(23)14-12-18-5-4-13-25(18)2/h6-11,16,18H,3-5,12-15H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.17E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant eNOS assessed as conversion of [3H]L-arginine to [3H]L-citrulline preincubated for 15 mins measured after 45 mins by ... |
ACS Med Chem Lett 3: 227-231 (2012)
Article DOI: 10.1021/ml200268w
BindingDB Entry DOI: 10.7270/Q2TX3GF4 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50386380

(CHEMBL2048417)Show SMILES CCOc1ccc(Cc2nc3ccc(cc3n2CCN(CC)CC)N=C(N)c2cccs2)cc1 |w:24.25| Show InChI InChI=1S/C27H33N5OS/c1-4-31(5-2)15-16-32-24-19-21(29-27(28)25-8-7-17-34-25)11-14-23(24)30-26(32)18-20-9-12-22(13-10-20)33-6-3/h7-14,17,19H,4-6,15-16,18H2,1-3H3,(H2,28,29) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 170 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Agonist activity at human mu opioid receptor expressed in CHO cells assessed as inhibition of forskolin-induced cAMP accumulation after 1 hr by HTRF ... |
ACS Med Chem Lett 3: 227-231 (2012)
Article DOI: 10.1021/ml200268w
BindingDB Entry DOI: 10.7270/Q2TX3GF4 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50386382

(CHEMBL2046872)Show SMILES CCOc1ccc(Cc2nc3cc(ccc3n2CCN(CC)CC)N=C(N)c2cccs2)cc1 |w:24.25| Show InChI InChI=1S/C27H33N5OS/c1-4-31(5-2)15-16-32-24-14-11-21(29-27(28)25-8-7-17-34-25)19-23(24)30-26(32)18-20-9-12-22(13-10-20)33-6-3/h7-14,17,19H,4-6,15-16,18H2,1-3H3,(H2,28,29) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 340 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Agonist activity at human mu opioid receptor expressed in CHO cells assessed as inhibition of forskolin-induced cAMP accumulation after 1 hr by HTRF ... |
ACS Med Chem Lett 3: 227-231 (2012)
Article DOI: 10.1021/ml200268w
BindingDB Entry DOI: 10.7270/Q2TX3GF4 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50000092

((-)-(etorphine) | (-)-morphine | (1S,5R,13R,14S)-1...)Show SMILES CN1CC[C@@]23[C@H]4Oc5c2c(C[C@@H]1[C@@H]3C=C[C@@H]4O)ccc5O |r,c:16,TLB:13:12:8.9.10:3.2.1| Show InChI InChI=1S/C17H19NO3/c1-18-7-6-17-10-3-5-13(20)16(17)21-15-12(19)4-2-9(14(15)17)8-11(10)18/h2-5,10-11,13,16,19-20H,6-8H2,1H3/t10-,11+,13-,16-,17-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | n/a | n/a | 3.70 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Agonist activity at human mu opioid receptor expressed in CHO cells assessed as inhibition of forskolin-induced cAMP accumulation after 1 hr by HTRF ... |
ACS Med Chem Lett 3: 227-231 (2012)
Article DOI: 10.1021/ml200268w
BindingDB Entry DOI: 10.7270/Q2TX3GF4 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50386381

(Nucynta | TAPENTADOL HYDROCHLORIDE | Tapentadol)Show InChI InChI=1S/C14H23NO/c1-5-14(11(2)10-15(3)4)12-7-6-8-13(16)9-12/h6-9,11,14,16H,5,10H2,1-4H3/t11-,14+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
PC cid
PC sid
UniChem
Similars
| DrugBank
Article
PubMed
| n/a | n/a | n/a | n/a | 670 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Agonist activity at human mu opioid receptor expressed in CHO cells assessed as inhibition of forskolin-induced cAMP accumulation after 1 hr by HTRF ... |
ACS Med Chem Lett 3: 227-231 (2012)
Article DOI: 10.1021/ml200268w
BindingDB Entry DOI: 10.7270/Q2TX3GF4 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
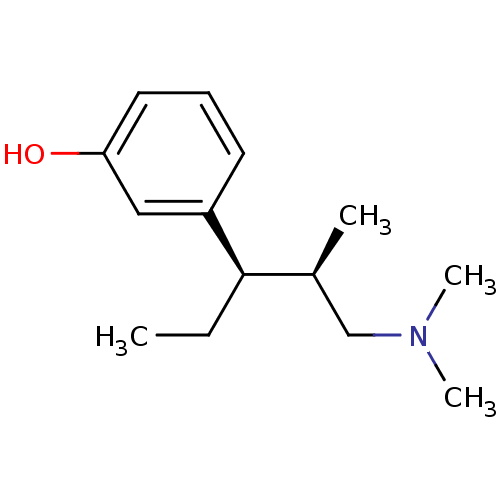
(Homo sapiens (Human)) | BDBM50176263
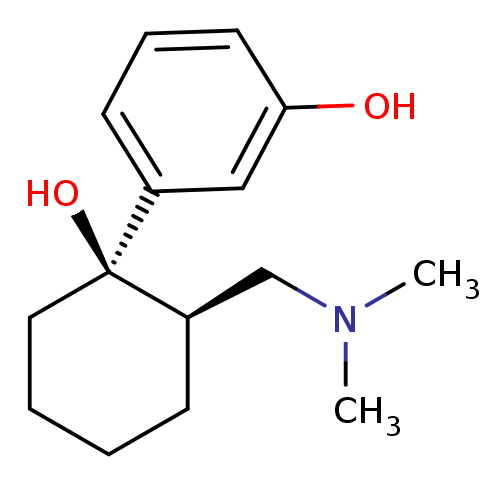
(3-((1R,2R)-2-((dimethylamino)methyl)-1-hydroxycycl...)Show InChI InChI=1S/C15H23NO2/c1-16(2)11-13-6-3-4-9-15(13,18)12-7-5-8-14(17)10-12/h5,7-8,10,13,17-18H,3-4,6,9,11H2,1-2H3/t13-,15+/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 120 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Agonist activity at human mu opioid receptor expressed in CHO cells assessed as inhibition of forskolin-induced cAMP accumulation after 1 hr by HTRF ... |
ACS Med Chem Lett 3: 227-231 (2012)
Article DOI: 10.1021/ml200268w
BindingDB Entry DOI: 10.7270/Q2TX3GF4 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50386383

(CHEMBL2046873)Show SMILES CCOc1ccc(Cc2nc3cc(ccc3n2CCN(C)C)N=C(N)c2cccs2)cc1 |w:22.23| Show InChI InChI=1S/C25H29N5OS/c1-4-31-20-10-7-18(8-11-20)16-24-28-21-17-19(27-25(26)23-6-5-15-32-23)9-12-22(21)30(24)14-13-29(2)3/h5-12,15,17H,4,13-14,16H2,1-3H3,(H2,26,27) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Agonist activity at human mu opioid receptor expressed in CHO cells assessed as inhibition of forskolin-induced cAMP accumulation after 1 hr by HTRF ... |
ACS Med Chem Lett 3: 227-231 (2012)
Article DOI: 10.1021/ml200268w
BindingDB Entry DOI: 10.7270/Q2TX3GF4 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50386385

(CHEMBL2046876)Show SMILES CCOc1ccc(Cc2nc3cc(ccc3n2CCN(CC)CC)N=C(N)c2ccco2)cc1 |w:24.25| Show InChI InChI=1S/C27H33N5O2/c1-4-31(5-2)15-16-32-24-14-11-21(29-27(28)25-8-7-17-34-25)19-23(24)30-26(32)18-20-9-12-22(13-10-20)33-6-3/h7-14,17,19H,4-6,15-16,18H2,1-3H3,(H2,28,29) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 400 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Agonist activity at human mu opioid receptor expressed in CHO cells assessed as inhibition of forskolin-induced cAMP accumulation after 1 hr by HTRF ... |
ACS Med Chem Lett 3: 227-231 (2012)
Article DOI: 10.1021/ml200268w
BindingDB Entry DOI: 10.7270/Q2TX3GF4 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50386377

(CHEMBL2046878)Show SMILES CCOc1ccc(Cc2nc3cc(ccc3n2CCN(CC)CC)N=C(N)c2ccoc2)cc1 |w:24.25| Show InChI InChI=1S/C27H33N5O2/c1-4-31(5-2)14-15-32-25-12-9-22(29-27(28)21-13-16-33-19-21)18-24(25)30-26(32)17-20-7-10-23(11-8-20)34-6-3/h7-13,16,18-19H,4-6,14-15,17H2,1-3H3,(H2,28,29) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 590 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Agonist activity at human mu opioid receptor expressed in CHO cells assessed as inhibition of forskolin-induced cAMP accumulation after 1 hr by HTRF ... |
ACS Med Chem Lett 3: 227-231 (2012)
Article DOI: 10.1021/ml200268w
BindingDB Entry DOI: 10.7270/Q2TX3GF4 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50386379

(CHEMBL2048416)Show SMILES CCOc1ccc(Cc2nc3cc(NC(N)=N[N+]([O-])=O)ccc3n2CCN(CC)CC)cc1 |w:16.16| Show InChI InChI=1S/C23H31N7O3/c1-4-28(5-2)13-14-29-21-12-9-18(25-23(24)27-30(31)32)16-20(21)26-22(29)15-17-7-10-19(11-8-17)33-6-3/h7-12,16H,4-6,13-15H2,1-3H3,(H3,24,25,27) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 52 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Agonist activity at human mu opioid receptor expressed in CHO cells assessed as inhibition of forskolin-induced cAMP accumulation after 1 hr by HTRF ... |
ACS Med Chem Lett 3: 227-231 (2012)
Article DOI: 10.1021/ml200268w
BindingDB Entry DOI: 10.7270/Q2TX3GF4 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50176259
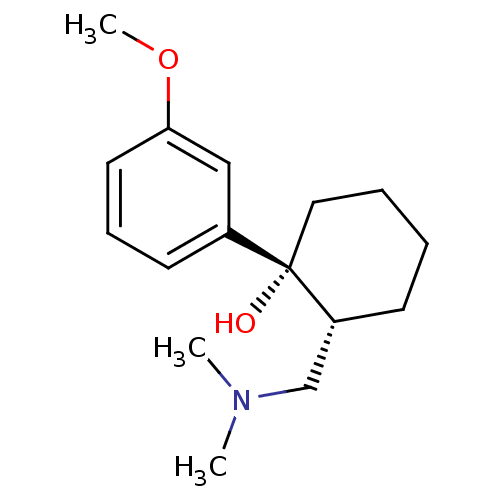
((1R,2R)-2-[(dimethylamino)methyl]-1-(3-methoxyphen...)Show InChI InChI=1S/C16H25NO2/c1-17(2)12-14-7-4-5-10-16(14,18)13-8-6-9-15(11-13)19-3/h6,8-9,11,14,18H,4-5,7,10,12H2,1-3H3/t14-,16+/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Agonist activity at human mu opioid receptor expressed in CHO cells assessed as inhibition of forskolin-induced cAMP accumulation after 1 hr by HTRF ... |
ACS Med Chem Lett 3: 227-231 (2012)
Article DOI: 10.1021/ml200268w
BindingDB Entry DOI: 10.7270/Q2TX3GF4 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50386384

(CHEMBL2046874)Show SMILES CCOc1ccc(Cc2nc3cc(ccc3n2CCC2CCCN2C)N=C(N)c2cccs2)cc1 |w:25.27| Show InChI InChI=1S/C28H33N5OS/c1-3-34-23-11-8-20(9-12-23)18-27-31-24-19-21(30-28(29)26-7-5-17-35-26)10-13-25(24)33(27)16-14-22-6-4-15-32(22)2/h5,7-13,17,19,22H,3-4,6,14-16,18H2,1-2H3,(H2,29,30) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Agonist activity at human mu opioid receptor expressed in CHO cells assessed as inhibition of forskolin-induced cAMP accumulation after 1 hr by HTRF ... |
ACS Med Chem Lett 3: 227-231 (2012)
Article DOI: 10.1021/ml200268w
BindingDB Entry DOI: 10.7270/Q2TX3GF4 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50386378

(CHEMBL2048415)Show SMILES CCOc1ccc(Cc2nc3cc(ccc3n2CCN(CC)CC)N=C(C)N)cc1 |w:24.25| Show InChI InChI=1S/C24H33N5O/c1-5-28(6-2)14-15-29-23-13-10-20(26-18(4)25)17-22(23)27-24(29)16-19-8-11-21(12-9-19)30-7-3/h8-13,17H,5-7,14-16H2,1-4H3,(H2,25,26) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 940 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Agonist activity at human mu opioid receptor expressed in CHO cells assessed as inhibition of forskolin-induced cAMP accumulation after 1 hr by HTRF ... |
ACS Med Chem Lett 3: 227-231 (2012)
Article DOI: 10.1021/ml200268w
BindingDB Entry DOI: 10.7270/Q2TX3GF4 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data