 Found 71 hits of Enzyme Inhibition Constant Data
Found 71 hits of Enzyme Inhibition Constant Data Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Delta-type opioid receptor
(MOUSE) | BDBM50010652

(CHEMBL3264742)Show SMILES [H][C@]12O[C@]34CC[C@@]5(OCOc6c(O)ccc7C[C@@]3([H])N(CC3CC3)CC[C@]4(c67)[C@]15[H])N(Cc1ccccc1)C2=O |r,TLB:20:19:3:27.15.16,THB:7:6:26:1.2| Show InChI InChI=1S/C30H32N2O5/c33-21-9-8-20-14-22-29-10-11-30-26(25(37-29)27(34)32(30)16-18-4-2-1-3-5-18)28(29,23(20)24(21)35-17-36-30)12-13-31(22)15-19-6-7-19/h1-5,8-9,19,22,25-26,33H,6-7,10-17H2/t22-,25+,26+,28+,29-,30+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.313 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DPDPE from delta opioid receptor in mouse whole brain cerebellum membrane |
ACS Med Chem Lett 5: 368-72 (2014)
Article DOI: 10.1021/ml400491k
BindingDB Entry DOI: 10.7270/Q2GF0W23 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(MOUSE) | BDBM50010658
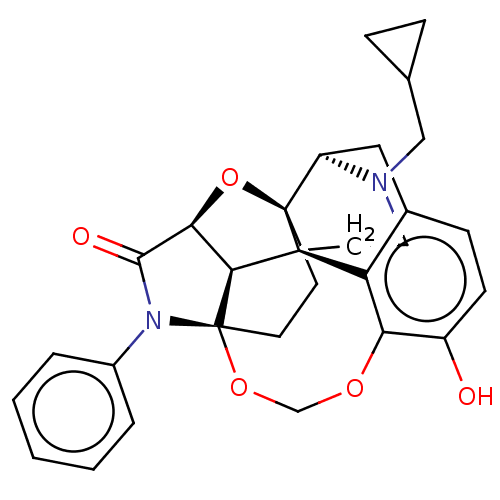
(CHEMBL3264747)Show SMILES [H][C@]12O[C@]34CC[C@@]5(OCOc6c(O)ccc7C[C@@]3([H])N(CC3CC3)CC[C@]4(c67)[C@]15[H])N(C2=O)c1ccccc1 |r,TLB:20:19:3:27.15.16,THB:7:6:26:1.2| Show InChI InChI=1S/C29H30N2O5/c32-20-9-8-18-14-21-28-10-11-29-25(24(36-28)26(33)31(29)19-4-2-1-3-5-19)27(28,22(18)23(20)34-16-35-29)12-13-30(21)15-17-6-7-17/h1-5,8-9,17,21,24-25,32H,6-7,10-16H2/t21-,24+,25+,27+,28-,29+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.534 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DPDPE from delta opioid receptor in mouse whole brain cerebellum membrane |
ACS Med Chem Lett 5: 368-72 (2014)
Article DOI: 10.1021/ml400491k
BindingDB Entry DOI: 10.7270/Q2GF0W23 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(MOUSE) | BDBM50039029

((+)-4-((alpha R)-((2S,5R)-4-allyl-2,5-dimethylpipe...)Show SMILES CCN(CC)C(=O)c1ccc(cc1)[C@@H](N1C[C@@H](C)N(CC=C)C[C@@H]1C)c1cccc(OC)c1 |r| Show InChI InChI=1S/C28H39N3O2/c1-7-17-30-19-22(5)31(20-21(30)4)27(25-11-10-12-26(18-25)33-6)23-13-15-24(16-14-23)28(32)29(8-2)9-3/h7,10-16,18,21-22,27H,1,8-9,17,19-20H2,2-6H3/t21-,22+,27-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DPDPE from delta opioid receptor in mouse whole brain cerebellum membrane |
ACS Med Chem Lett 5: 368-72 (2014)
Article DOI: 10.1021/ml400491k
BindingDB Entry DOI: 10.7270/Q2GF0W23 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(MOUSE) | BDBM50010659
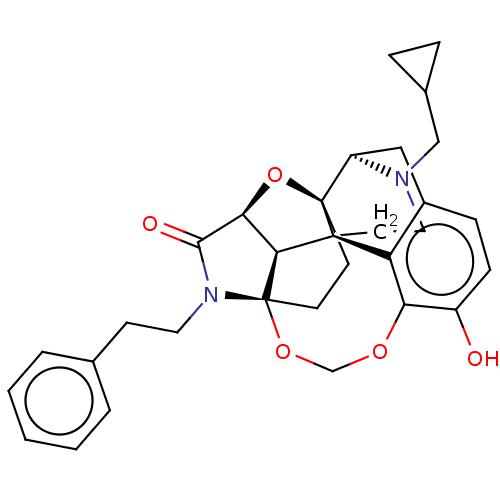
(CHEMBL3264748)Show SMILES [H][C@]12O[C@]34CC[C@@]5(OCOc6c(O)ccc7C[C@@]3([H])N(CC3CC3)CC[C@]4(c67)[C@]15[H])N(CCc1ccccc1)C2=O |r,TLB:20:19:3:27.15.16,THB:7:6:26:1.2| Show InChI InChI=1S/C31H34N2O5/c34-22-9-8-21-16-23-30-11-12-31-27(26(38-30)28(35)33(31)14-10-19-4-2-1-3-5-19)29(30,24(21)25(22)36-18-37-31)13-15-32(23)17-20-6-7-20/h1-5,8-9,20,23,26-27,34H,6-7,10-18H2/t23-,26+,27+,29+,30-,31+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DPDPE from delta opioid receptor in mouse whole brain cerebellum membrane |
ACS Med Chem Lett 5: 368-72 (2014)
Article DOI: 10.1021/ml400491k
BindingDB Entry DOI: 10.7270/Q2GF0W23 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(MOUSE) | BDBM50010656

(CHEMBL3264745)Show SMILES [H][C@]12O[C@]34CC[C@@]5(OCOc6c(O)ccc7C[C@@]3([H])N(CC=C)CC[C@]4(c67)[C@]15[H])N(Cc1ccccc1)C2=O |r,TLB:20:19:3:26.15.16,THB:7:6:25:1.2| Show InChI InChI=1S/C29H30N2O5/c1-2-13-30-14-12-27-22-19-8-9-20(32)23(22)34-17-35-29-11-10-28(27,21(30)15-19)36-24(25(27)29)26(33)31(29)16-18-6-4-3-5-7-18/h2-9,21,24-25,32H,1,10-17H2/t21-,24+,25+,27+,28-,29+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DPDPE from delta opioid receptor in mouse whole brain cerebellum membrane |
ACS Med Chem Lett 5: 368-72 (2014)
Article DOI: 10.1021/ml400491k
BindingDB Entry DOI: 10.7270/Q2GF0W23 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Cavia porcellus (domestic guinea pig)) | BDBM50010658

(CHEMBL3264747)Show SMILES [H][C@]12O[C@]34CC[C@@]5(OCOc6c(O)ccc7C[C@@]3([H])N(CC3CC3)CC[C@]4(c67)[C@]15[H])N(C2=O)c1ccccc1 |r,TLB:20:19:3:27.15.16,THB:7:6:26:1.2| Show InChI InChI=1S/C29H30N2O5/c32-20-9-8-18-14-21-28-10-11-29-25(24(36-28)26(33)31(29)19-4-2-1-3-5-19)27(28,22(18)23(20)34-16-35-29)12-13-30(21)15-17-6-7-17/h1-5,8-9,17,21,24-25,32H,6-7,10-16H2/t21-,24+,25+,27+,28-,29+/m1/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University
Curated by ChEMBL
| Assay Description
Displacement of [3H]U69593 from kappa opioid receptor in guinea pig cerebellum membrane |
ACS Med Chem Lett 5: 368-72 (2014)
Article DOI: 10.1021/ml400491k
BindingDB Entry DOI: 10.7270/Q2GF0W23 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(MOUSE) | BDBM50010659

(CHEMBL3264748)Show SMILES [H][C@]12O[C@]34CC[C@@]5(OCOc6c(O)ccc7C[C@@]3([H])N(CC3CC3)CC[C@]4(c67)[C@]15[H])N(CCc1ccccc1)C2=O |r,TLB:20:19:3:27.15.16,THB:7:6:26:1.2| Show InChI InChI=1S/C31H34N2O5/c34-22-9-8-21-16-23-30-11-12-31-27(26(38-30)28(35)33(31)14-10-19-4-2-1-3-5-19)29(30,24(21)25(22)36-18-37-31)13-15-32(23)17-20-6-7-20/h1-5,8-9,20,23,26-27,34H,6-7,10-18H2/t23-,26+,27+,29+,30-,31+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from mu opioid receptor in mouse whole brain cerebellum membrane |
ACS Med Chem Lett 5: 368-72 (2014)
Article DOI: 10.1021/ml400491k
BindingDB Entry DOI: 10.7270/Q2GF0W23 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Cavia porcellus (domestic guinea pig)) | BDBM50010659

(CHEMBL3264748)Show SMILES [H][C@]12O[C@]34CC[C@@]5(OCOc6c(O)ccc7C[C@@]3([H])N(CC3CC3)CC[C@]4(c67)[C@]15[H])N(CCc1ccccc1)C2=O |r,TLB:20:19:3:27.15.16,THB:7:6:26:1.2| Show InChI InChI=1S/C31H34N2O5/c34-22-9-8-21-16-23-30-11-12-31-27(26(38-30)28(35)33(31)14-10-19-4-2-1-3-5-19)29(30,24(21)25(22)36-18-37-31)13-15-32(23)17-20-6-7-20/h1-5,8-9,20,23,26-27,34H,6-7,10-18H2/t23-,26+,27+,29+,30-,31+/m1/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University
Curated by ChEMBL
| Assay Description
Displacement of [3H]U69593 from kappa opioid receptor in guinea pig cerebellum membrane |
ACS Med Chem Lett 5: 368-72 (2014)
Article DOI: 10.1021/ml400491k
BindingDB Entry DOI: 10.7270/Q2GF0W23 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(MOUSE) | BDBM50010653

(CHEMBL3264743)Show SMILES [H][C@]12O[C@]34CC[C@@]5(OCOc6c(O)ccc7C[C@@]3([H])N(C)CC[C@]4(c67)[C@]15[H])N(Cc1ccccc1)C2=O |r,TLB:20:19:3:24.15.16,THB:7:6:23:1.2| Show InChI InChI=1S/C27H28N2O5/c1-28-12-11-25-20-17-7-8-18(30)21(20)32-15-33-27-10-9-26(25,19(28)13-17)34-22(23(25)27)24(31)29(27)14-16-5-3-2-4-6-16/h2-8,19,22-23,30H,9-15H2,1H3/t19-,22+,23+,25+,26-,27+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DPDPE from delta opioid receptor in mouse whole brain cerebellum membrane |
ACS Med Chem Lett 5: 368-72 (2014)
Article DOI: 10.1021/ml400491k
BindingDB Entry DOI: 10.7270/Q2GF0W23 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(MOUSE) | BDBM50010657
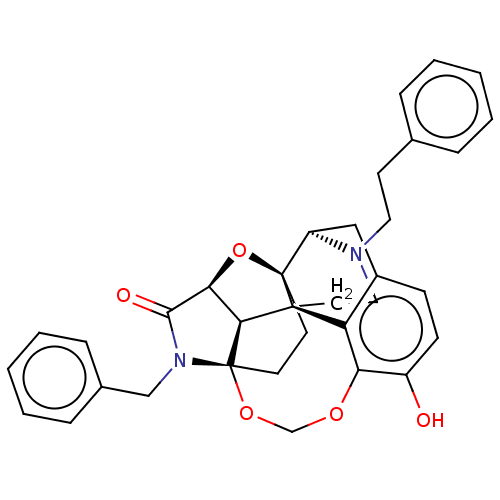
(CHEMBL3264746)Show SMILES [H][C@]12O[C@]34CC[C@@]5(OCOc6c(O)ccc7C[C@@]3([H])N(CCc3ccccc3)CC[C@]4(c67)[C@]15[H])N(Cc1ccccc1)C2=O |r,TLB:20:19:3:31.15.16,THB:7:6:30:1.2| Show InChI InChI=1S/C34H34N2O5/c37-25-12-11-24-19-26-33-14-15-34-30(29(41-33)31(38)36(34)20-23-9-5-2-6-10-23)32(33,27(24)28(25)39-21-40-34)16-18-35(26)17-13-22-7-3-1-4-8-22/h1-12,26,29-30,37H,13-21H2/t26-,29+,30+,32+,33-,34+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DPDPE from delta opioid receptor in mouse whole brain cerebellum membrane |
ACS Med Chem Lett 5: 368-72 (2014)
Article DOI: 10.1021/ml400491k
BindingDB Entry DOI: 10.7270/Q2GF0W23 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(MOUSE) | BDBM50010652

(CHEMBL3264742)Show SMILES [H][C@]12O[C@]34CC[C@@]5(OCOc6c(O)ccc7C[C@@]3([H])N(CC3CC3)CC[C@]4(c67)[C@]15[H])N(Cc1ccccc1)C2=O |r,TLB:20:19:3:27.15.16,THB:7:6:26:1.2| Show InChI InChI=1S/C30H32N2O5/c33-21-9-8-20-14-22-29-10-11-30-26(25(37-29)27(34)32(30)16-18-4-2-1-3-5-18)28(29,23(20)24(21)35-17-36-30)12-13-31(22)15-19-6-7-19/h1-5,8-9,19,22,25-26,33H,6-7,10-17H2/t22-,25+,26+,28+,29-,30+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from mu opioid receptor in mouse whole brain cerebellum membrane |
ACS Med Chem Lett 5: 368-72 (2014)
Article DOI: 10.1021/ml400491k
BindingDB Entry DOI: 10.7270/Q2GF0W23 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(MOUSE) | BDBM50010658

(CHEMBL3264747)Show SMILES [H][C@]12O[C@]34CC[C@@]5(OCOc6c(O)ccc7C[C@@]3([H])N(CC3CC3)CC[C@]4(c67)[C@]15[H])N(C2=O)c1ccccc1 |r,TLB:20:19:3:27.15.16,THB:7:6:26:1.2| Show InChI InChI=1S/C29H30N2O5/c32-20-9-8-18-14-21-28-10-11-29-25(24(36-28)26(33)31(29)19-4-2-1-3-5-19)27(28,22(18)23(20)34-16-35-29)12-13-30(21)15-17-6-7-17/h1-5,8-9,17,21,24-25,32H,6-7,10-16H2/t21-,24+,25+,27+,28-,29+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from mu opioid receptor in mouse whole brain cerebellum membrane |
ACS Med Chem Lett 5: 368-72 (2014)
Article DOI: 10.1021/ml400491k
BindingDB Entry DOI: 10.7270/Q2GF0W23 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Cavia porcellus (domestic guinea pig)) | BDBM50010652

(CHEMBL3264742)Show SMILES [H][C@]12O[C@]34CC[C@@]5(OCOc6c(O)ccc7C[C@@]3([H])N(CC3CC3)CC[C@]4(c67)[C@]15[H])N(Cc1ccccc1)C2=O |r,TLB:20:19:3:27.15.16,THB:7:6:26:1.2| Show InChI InChI=1S/C30H32N2O5/c33-21-9-8-20-14-22-29-10-11-30-26(25(37-29)27(34)32(30)16-18-4-2-1-3-5-18)28(29,23(20)24(21)35-17-36-30)12-13-31(22)15-19-6-7-19/h1-5,8-9,19,22,25-26,33H,6-7,10-17H2/t22-,25+,26+,28+,29-,30+/m1/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 5.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University
Curated by ChEMBL
| Assay Description
Displacement of [3H]U69593 from kappa opioid receptor in guinea pig cerebellum membrane |
ACS Med Chem Lett 5: 368-72 (2014)
Article DOI: 10.1021/ml400491k
BindingDB Entry DOI: 10.7270/Q2GF0W23 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(MOUSE) | BDBM50010654

(CHEMBL3264744)Show SMILES [H][C@]12O[C@]34CC[C@@]5(OCOc6c(O)ccc7C[C@@]3([H])N(CC(C)C)CC[C@]4(c67)[C@]15[H])N(Cc1ccccc1)C2=O |r,TLB:20:19:3:27.15.16,THB:7:6:26:1.2| Show InChI InChI=1S/C30H34N2O5/c1-18(2)15-31-13-12-28-23-20-8-9-21(33)24(23)35-17-36-30-11-10-29(28,22(31)14-20)37-25(26(28)30)27(34)32(30)16-19-6-4-3-5-7-19/h3-9,18,22,25-26,33H,10-17H2,1-2H3/t22-,25+,26+,28+,29-,30+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DPDPE from delta opioid receptor in mouse whole brain cerebellum membrane |
ACS Med Chem Lett 5: 368-72 (2014)
Article DOI: 10.1021/ml400491k
BindingDB Entry DOI: 10.7270/Q2GF0W23 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(MOUSE) | BDBM50010653

(CHEMBL3264743)Show SMILES [H][C@]12O[C@]34CC[C@@]5(OCOc6c(O)ccc7C[C@@]3([H])N(C)CC[C@]4(c67)[C@]15[H])N(Cc1ccccc1)C2=O |r,TLB:20:19:3:24.15.16,THB:7:6:23:1.2| Show InChI InChI=1S/C27H28N2O5/c1-28-12-11-25-20-17-7-8-18(30)21(20)32-15-33-27-10-9-26(25,19(28)13-17)34-22(23(25)27)24(31)29(27)14-16-5-3-2-4-6-16/h2-8,19,22-23,30H,9-15H2,1H3/t19-,22+,23+,25+,26-,27+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from mu opioid receptor in mouse whole brain cerebellum membrane |
ACS Med Chem Lett 5: 368-72 (2014)
Article DOI: 10.1021/ml400491k
BindingDB Entry DOI: 10.7270/Q2GF0W23 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(MOUSE) | BDBM50010657

(CHEMBL3264746)Show SMILES [H][C@]12O[C@]34CC[C@@]5(OCOc6c(O)ccc7C[C@@]3([H])N(CCc3ccccc3)CC[C@]4(c67)[C@]15[H])N(Cc1ccccc1)C2=O |r,TLB:20:19:3:31.15.16,THB:7:6:30:1.2| Show InChI InChI=1S/C34H34N2O5/c37-25-12-11-24-19-26-33-14-15-34-30(29(41-33)31(38)36(34)20-23-9-5-2-6-10-23)32(33,27(24)28(25)39-21-40-34)16-18-35(26)17-13-22-7-3-1-4-8-22/h1-12,26,29-30,37H,13-21H2/t26-,29+,30+,32+,33-,34+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 46 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from mu opioid receptor in mouse whole brain cerebellum membrane |
ACS Med Chem Lett 5: 368-72 (2014)
Article DOI: 10.1021/ml400491k
BindingDB Entry DOI: 10.7270/Q2GF0W23 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(MOUSE) | BDBM50010651
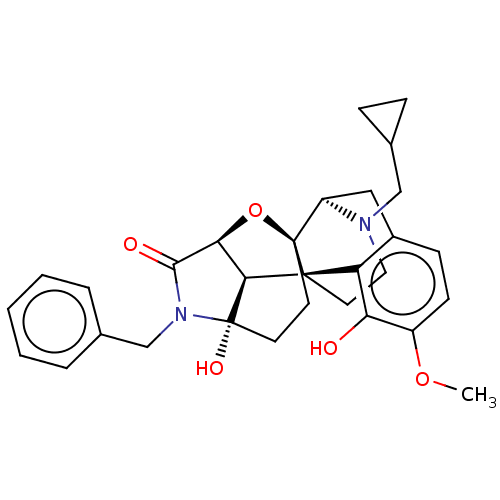
(CHEMBL3264741)Show SMILES [H][C@@]12O[C@@]34CC[C@@](O)(N(Cc5ccccc5)C1=O)[C@]2([H])[C@@]31CCN(CC2CC2)[C@]4([H])Cc2ccc(OC)c(O)c12 |r,TLB:24:23:3:39.31.30,THB:7:6:20:1.2| Show InChI InChI=1S/C30H34N2O5/c1-36-21-10-9-20-15-22-29-11-12-30(35)26(25(37-29)27(34)32(30)17-18-5-3-2-4-6-18)28(29,23(20)24(21)33)13-14-31(22)16-19-7-8-19/h2-6,9-10,19,22,25-26,33,35H,7-8,11-17H2,1H3/t22-,25+,26+,28+,29-,30+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 48 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from mu opioid receptor in mouse whole brain cerebellum membrane |
ACS Med Chem Lett 5: 368-72 (2014)
Article DOI: 10.1021/ml400491k
BindingDB Entry DOI: 10.7270/Q2GF0W23 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Cavia porcellus (domestic guinea pig)) | BDBM50010656

(CHEMBL3264745)Show SMILES [H][C@]12O[C@]34CC[C@@]5(OCOc6c(O)ccc7C[C@@]3([H])N(CC=C)CC[C@]4(c67)[C@]15[H])N(Cc1ccccc1)C2=O |r,TLB:20:19:3:26.15.16,THB:7:6:25:1.2| Show InChI InChI=1S/C29H30N2O5/c1-2-13-30-14-12-27-22-19-8-9-20(32)23(22)34-17-35-29-11-10-28(27,21(30)15-19)36-24(25(27)29)26(33)31(29)16-18-6-4-3-5-7-18/h2-9,21,24-25,32H,1,10-17H2/t21-,24+,25+,27+,28-,29+/m1/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 57 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University
Curated by ChEMBL
| Assay Description
Displacement of [3H]U69593 from kappa opioid receptor in guinea pig cerebellum membrane |
ACS Med Chem Lett 5: 368-72 (2014)
Article DOI: 10.1021/ml400491k
BindingDB Entry DOI: 10.7270/Q2GF0W23 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(MOUSE) | BDBM50010656

(CHEMBL3264745)Show SMILES [H][C@]12O[C@]34CC[C@@]5(OCOc6c(O)ccc7C[C@@]3([H])N(CC=C)CC[C@]4(c67)[C@]15[H])N(Cc1ccccc1)C2=O |r,TLB:20:19:3:26.15.16,THB:7:6:25:1.2| Show InChI InChI=1S/C29H30N2O5/c1-2-13-30-14-12-27-22-19-8-9-20(32)23(22)34-17-35-29-11-10-28(27,21(30)15-19)36-24(25(27)29)26(33)31(29)16-18-6-4-3-5-7-18/h2-9,21,24-25,32H,1,10-17H2/t21-,24+,25+,27+,28-,29+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 68 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from mu opioid receptor in mouse whole brain cerebellum membrane |
ACS Med Chem Lett 5: 368-72 (2014)
Article DOI: 10.1021/ml400491k
BindingDB Entry DOI: 10.7270/Q2GF0W23 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Cavia porcellus (domestic guinea pig)) | BDBM50010654

(CHEMBL3264744)Show SMILES [H][C@]12O[C@]34CC[C@@]5(OCOc6c(O)ccc7C[C@@]3([H])N(CC(C)C)CC[C@]4(c67)[C@]15[H])N(Cc1ccccc1)C2=O |r,TLB:20:19:3:27.15.16,THB:7:6:26:1.2| Show InChI InChI=1S/C30H34N2O5/c1-18(2)15-31-13-12-28-23-20-8-9-21(33)24(23)35-17-36-30-11-10-29(28,22(31)14-20)37-25(26(28)30)27(34)32(30)16-19-6-4-3-5-7-19/h3-9,18,22,25-26,33H,10-17H2,1-2H3/t22-,25+,26+,28+,29-,30+/m1/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 119 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University
Curated by ChEMBL
| Assay Description
Displacement of [3H]U69593 from kappa opioid receptor in guinea pig cerebellum membrane |
ACS Med Chem Lett 5: 368-72 (2014)
Article DOI: 10.1021/ml400491k
BindingDB Entry DOI: 10.7270/Q2GF0W23 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(MOUSE) | BDBM50010651

(CHEMBL3264741)Show SMILES [H][C@@]12O[C@@]34CC[C@@](O)(N(Cc5ccccc5)C1=O)[C@]2([H])[C@@]31CCN(CC2CC2)[C@]4([H])Cc2ccc(OC)c(O)c12 |r,TLB:24:23:3:39.31.30,THB:7:6:20:1.2| Show InChI InChI=1S/C30H34N2O5/c1-36-21-10-9-20-15-22-29-11-12-30(35)26(25(37-29)27(34)32(30)17-18-5-3-2-4-6-18)28(29,23(20)24(21)33)13-14-31(22)16-19-7-8-19/h2-6,9-10,19,22,25-26,33,35H,7-8,11-17H2,1H3/t22-,25+,26+,28+,29-,30+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 175 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DPDPE from delta opioid receptor in mouse whole brain cerebellum membrane |
ACS Med Chem Lett 5: 368-72 (2014)
Article DOI: 10.1021/ml400491k
BindingDB Entry DOI: 10.7270/Q2GF0W23 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(MOUSE) | BDBM50010654

(CHEMBL3264744)Show SMILES [H][C@]12O[C@]34CC[C@@]5(OCOc6c(O)ccc7C[C@@]3([H])N(CC(C)C)CC[C@]4(c67)[C@]15[H])N(Cc1ccccc1)C2=O |r,TLB:20:19:3:27.15.16,THB:7:6:26:1.2| Show InChI InChI=1S/C30H34N2O5/c1-18(2)15-31-13-12-28-23-20-8-9-21(33)24(23)35-17-36-30-11-10-29(28,22(31)14-20)37-25(26(28)30)27(34)32(30)16-19-6-4-3-5-7-19/h3-9,18,22,25-26,33H,10-17H2,1-2H3/t22-,25+,26+,28+,29-,30+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 186 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from mu opioid receptor in mouse whole brain cerebellum membrane |
ACS Med Chem Lett 5: 368-72 (2014)
Article DOI: 10.1021/ml400491k
BindingDB Entry DOI: 10.7270/Q2GF0W23 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Cavia porcellus (domestic guinea pig)) | BDBM50010653

(CHEMBL3264743)Show SMILES [H][C@]12O[C@]34CC[C@@]5(OCOc6c(O)ccc7C[C@@]3([H])N(C)CC[C@]4(c67)[C@]15[H])N(Cc1ccccc1)C2=O |r,TLB:20:19:3:24.15.16,THB:7:6:23:1.2| Show InChI InChI=1S/C27H28N2O5/c1-28-12-11-25-20-17-7-8-18(30)21(20)32-15-33-27-10-9-26(25,19(28)13-17)34-22(23(25)27)24(31)29(27)14-16-5-3-2-4-6-16/h2-8,19,22-23,30H,9-15H2,1H3/t19-,22+,23+,25+,26-,27+/m1/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University
Curated by ChEMBL
| Assay Description
Displacement of [3H]U69593 from kappa opioid receptor in guinea pig cerebellum membrane |
ACS Med Chem Lett 5: 368-72 (2014)
Article DOI: 10.1021/ml400491k
BindingDB Entry DOI: 10.7270/Q2GF0W23 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Cavia porcellus (domestic guinea pig)) | BDBM50010651

(CHEMBL3264741)Show SMILES [H][C@@]12O[C@@]34CC[C@@](O)(N(Cc5ccccc5)C1=O)[C@]2([H])[C@@]31CCN(CC2CC2)[C@]4([H])Cc2ccc(OC)c(O)c12 |r,TLB:24:23:3:39.31.30,THB:7:6:20:1.2| Show InChI InChI=1S/C30H34N2O5/c1-36-21-10-9-20-15-22-29-11-12-30(35)26(25(37-29)27(34)32(30)17-18-5-3-2-4-6-18)28(29,23(20)24(21)33)13-14-31(22)16-19-7-8-19/h2-6,9-10,19,22,25-26,33,35H,7-8,11-17H2,1H3/t22-,25+,26+,28+,29-,30+/m1/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 248 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University
Curated by ChEMBL
| Assay Description
Displacement of [3H]U69593 from kappa opioid receptor in guinea pig cerebellum membrane |
ACS Med Chem Lett 5: 368-72 (2014)
Article DOI: 10.1021/ml400491k
BindingDB Entry DOI: 10.7270/Q2GF0W23 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Cavia porcellus (domestic guinea pig)) | BDBM50010657

(CHEMBL3264746)Show SMILES [H][C@]12O[C@]34CC[C@@]5(OCOc6c(O)ccc7C[C@@]3([H])N(CCc3ccccc3)CC[C@]4(c67)[C@]15[H])N(Cc1ccccc1)C2=O |r,TLB:20:19:3:31.15.16,THB:7:6:30:1.2| Show InChI InChI=1S/C34H34N2O5/c37-25-12-11-24-19-26-33-14-15-34-30(29(41-33)31(38)36(34)20-23-9-5-2-6-10-23)32(33,27(24)28(25)39-21-40-34)16-18-35(26)17-13-22-7-3-1-4-8-22/h1-12,26,29-30,37H,13-21H2/t26-,29+,30+,32+,33-,34+/m1/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 588 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University
Curated by ChEMBL
| Assay Description
Displacement of [3H]U69593 from kappa opioid receptor in guinea pig cerebellum membrane |
ACS Med Chem Lett 5: 368-72 (2014)
Article DOI: 10.1021/ml400491k
BindingDB Entry DOI: 10.7270/Q2GF0W23 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(MOUSE) | BDBM50039029

((+)-4-((alpha R)-((2S,5R)-4-allyl-2,5-dimethylpipe...)Show SMILES CCN(CC)C(=O)c1ccc(cc1)[C@@H](N1C[C@@H](C)N(CC=C)C[C@@H]1C)c1cccc(OC)c1 |r| Show InChI InChI=1S/C28H39N3O2/c1-7-17-30-19-22(5)31(20-21(30)4)27(25-11-10-12-26(18-25)33-6)23-13-15-24(16-14-23)28(32)29(8-2)9-3/h7,10-16,18,21-22,27H,1,8-9,17,19-20H2,2-6H3/t21-,22+,27-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 695 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from mu opioid receptor in mouse whole brain cerebellum membrane |
ACS Med Chem Lett 5: 368-72 (2014)
Article DOI: 10.1021/ml400491k
BindingDB Entry DOI: 10.7270/Q2GF0W23 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Cavia porcellus (domestic guinea pig)) | BDBM50039029

((+)-4-((alpha R)-((2S,5R)-4-allyl-2,5-dimethylpipe...)Show SMILES CCN(CC)C(=O)c1ccc(cc1)[C@@H](N1C[C@@H](C)N(CC=C)C[C@@H]1C)c1cccc(OC)c1 |r| Show InChI InChI=1S/C28H39N3O2/c1-7-17-30-19-22(5)31(20-21(30)4)27(25-11-10-12-26(18-25)33-6)23-13-15-24(16-14-23)28(32)29(8-2)9-3/h7,10-16,18,21-22,27H,1,8-9,17,19-20H2,2-6H3/t21-,22+,27-/m1/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University
Curated by ChEMBL
| Assay Description
Displacement of [3H]U69593 from kappa opioid receptor in guinea pig cerebellum membrane |
ACS Med Chem Lett 5: 368-72 (2014)
Article DOI: 10.1021/ml400491k
BindingDB Entry DOI: 10.7270/Q2GF0W23 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50010652

(CHEMBL3264742)Show SMILES [H][C@]12O[C@]34CC[C@@]5(OCOc6c(O)ccc7C[C@@]3([H])N(CC3CC3)CC[C@]4(c67)[C@]15[H])N(Cc1ccccc1)C2=O |r,TLB:20:19:3:27.15.16,THB:7:6:26:1.2| Show InChI InChI=1S/C30H32N2O5/c33-21-9-8-20-14-22-29-10-11-30-26(25(37-29)27(34)32(30)16-18-4-2-1-3-5-18)28(29,23(20)24(21)35-17-36-30)12-13-31(22)15-19-6-7-19/h1-5,8-9,19,22,25-26,33H,6-7,10-17H2/t22-,25+,26+,28+,29-,30+/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a |
Kitasato University
Curated by ChEMBL
| Assay Description
Agonist activity at human mu opioid receptor expressed in HEK293 cells by CellKey method |
ACS Med Chem Lett 5: 368-72 (2014)
Article DOI: 10.1021/ml400491k
BindingDB Entry DOI: 10.7270/Q2GF0W23 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50010654

(CHEMBL3264744)Show SMILES [H][C@]12O[C@]34CC[C@@]5(OCOc6c(O)ccc7C[C@@]3([H])N(CC(C)C)CC[C@]4(c67)[C@]15[H])N(Cc1ccccc1)C2=O |r,TLB:20:19:3:27.15.16,THB:7:6:26:1.2| Show InChI InChI=1S/C30H34N2O5/c1-18(2)15-31-13-12-28-23-20-8-9-21(33)24(23)35-17-36-30-11-10-29(28,22(31)14-20)37-25(26(28)30)27(34)32(30)16-19-6-4-3-5-7-19/h3-9,18,22,25-26,33H,10-17H2,1-2H3/t22-,25+,26+,28+,29-,30+/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 400 | n/a | n/a | n/a | n/a |
Kitasato University
Curated by ChEMBL
| Assay Description
Agonist activity at human mu opioid receptor expressed in HEK293 cells by CellKey method |
ACS Med Chem Lett 5: 368-72 (2014)
Article DOI: 10.1021/ml400491k
BindingDB Entry DOI: 10.7270/Q2GF0W23 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50010657

(CHEMBL3264746)Show SMILES [H][C@]12O[C@]34CC[C@@]5(OCOc6c(O)ccc7C[C@@]3([H])N(CCc3ccccc3)CC[C@]4(c67)[C@]15[H])N(Cc1ccccc1)C2=O |r,TLB:20:19:3:31.15.16,THB:7:6:30:1.2| Show InChI InChI=1S/C34H34N2O5/c37-25-12-11-24-19-26-33-14-15-34-30(29(41-33)31(38)36(34)20-23-9-5-2-6-10-23)32(33,27(24)28(25)39-21-40-34)16-18-35(26)17-13-22-7-3-1-4-8-22/h1-12,26,29-30,37H,13-21H2/t26-,29+,30+,32+,33-,34+/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 639 | n/a | n/a | n/a | n/a |
Kitasato University
Curated by ChEMBL
| Assay Description
Agonist activity at human mu opioid receptor expressed in HEK293 cells by CellKey method |
ACS Med Chem Lett 5: 368-72 (2014)
Article DOI: 10.1021/ml400491k
BindingDB Entry DOI: 10.7270/Q2GF0W23 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50010658

(CHEMBL3264747)Show SMILES [H][C@]12O[C@]34CC[C@@]5(OCOc6c(O)ccc7C[C@@]3([H])N(CC3CC3)CC[C@]4(c67)[C@]15[H])N(C2=O)c1ccccc1 |r,TLB:20:19:3:27.15.16,THB:7:6:26:1.2| Show InChI InChI=1S/C29H30N2O5/c32-20-9-8-18-14-21-28-10-11-29-25(24(36-28)26(33)31(29)19-4-2-1-3-5-19)27(28,22(18)23(20)34-16-35-29)12-13-30(21)15-17-6-7-17/h1-5,8-9,17,21,24-25,32H,6-7,10-16H2/t21-,24+,25+,27+,28-,29+/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a |
Kitasato University
Curated by ChEMBL
| Assay Description
Agonist activity at human mu opioid receptor expressed in HEK293 cells by CellKey method |
ACS Med Chem Lett 5: 368-72 (2014)
Article DOI: 10.1021/ml400491k
BindingDB Entry DOI: 10.7270/Q2GF0W23 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50010656

(CHEMBL3264745)Show SMILES [H][C@]12O[C@]34CC[C@@]5(OCOc6c(O)ccc7C[C@@]3([H])N(CC=C)CC[C@]4(c67)[C@]15[H])N(Cc1ccccc1)C2=O |r,TLB:20:19:3:26.15.16,THB:7:6:25:1.2| Show InChI InChI=1S/C29H30N2O5/c1-2-13-30-14-12-27-22-19-8-9-20(32)23(22)34-17-35-29-11-10-28(27,21(30)15-19)36-24(25(27)29)26(33)31(29)16-18-6-4-3-5-7-18/h2-9,21,24-25,32H,1,10-17H2/t21-,24+,25+,27+,28-,29+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 2 | n/a | n/a | n/a | n/a |
Kitasato University
Curated by ChEMBL
| Assay Description
Agonist activity at human delta opioid receptor expressed in HEK293 cells by CellKey method |
ACS Med Chem Lett 5: 368-72 (2014)
Article DOI: 10.1021/ml400491k
BindingDB Entry DOI: 10.7270/Q2GF0W23 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50010656

(CHEMBL3264745)Show SMILES [H][C@]12O[C@]34CC[C@@]5(OCOc6c(O)ccc7C[C@@]3([H])N(CC=C)CC[C@]4(c67)[C@]15[H])N(Cc1ccccc1)C2=O |r,TLB:20:19:3:26.15.16,THB:7:6:25:1.2| Show InChI InChI=1S/C29H30N2O5/c1-2-13-30-14-12-27-22-19-8-9-20(32)23(22)34-17-35-29-11-10-28(27,21(30)15-19)36-24(25(27)29)26(33)31(29)16-18-6-4-3-5-7-18/h2-9,21,24-25,32H,1,10-17H2/t21-,24+,25+,27+,28-,29+/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 2.70 | n/a | n/a | n/a | n/a |
Kitasato University
Curated by ChEMBL
| Assay Description
Agonist activity at human mu opioid receptor expressed in CHO cells assessed as [35S]-GTP[gammaS] binding |
ACS Med Chem Lett 5: 368-72 (2014)
Article DOI: 10.1021/ml400491k
BindingDB Entry DOI: 10.7270/Q2GF0W23 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50010656

(CHEMBL3264745)Show SMILES [H][C@]12O[C@]34CC[C@@]5(OCOc6c(O)ccc7C[C@@]3([H])N(CC=C)CC[C@]4(c67)[C@]15[H])N(Cc1ccccc1)C2=O |r,TLB:20:19:3:26.15.16,THB:7:6:25:1.2| Show InChI InChI=1S/C29H30N2O5/c1-2-13-30-14-12-27-22-19-8-9-20(32)23(22)34-17-35-29-11-10-28(27,21(30)15-19)36-24(25(27)29)26(33)31(29)16-18-6-4-3-5-7-18/h2-9,21,24-25,32H,1,10-17H2/t21-,24+,25+,27+,28-,29+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 231 | n/a | n/a | n/a | n/a |
Kitasato University
Curated by ChEMBL
| Assay Description
Agonist activity at human kappa opioid receptor expressed in CHO cells assessed as [35S]-GTP[gammaS] binding |
ACS Med Chem Lett 5: 368-72 (2014)
Article DOI: 10.1021/ml400491k
BindingDB Entry DOI: 10.7270/Q2GF0W23 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50039029

((+)-4-((alpha R)-((2S,5R)-4-allyl-2,5-dimethylpipe...)Show SMILES CCN(CC)C(=O)c1ccc(cc1)[C@@H](N1C[C@@H](C)N(CC=C)C[C@@H]1C)c1cccc(OC)c1 |r| Show InChI InChI=1S/C28H39N3O2/c1-7-17-30-19-22(5)31(20-21(30)4)27(25-11-10-12-26(18-25)33-6)23-13-15-24(16-14-23)28(32)29(8-2)9-3/h7,10-16,18,21-22,27H,1,8-9,17,19-20H2,2-6H3/t21-,22+,27-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.140 | n/a | n/a | n/a | n/a |
Kitasato University
Curated by ChEMBL
| Assay Description
Agonist activity at human mu opioid receptor expressed in HEK293 cells by CellKey method |
ACS Med Chem Lett 5: 368-72 (2014)
Article DOI: 10.1021/ml400491k
BindingDB Entry DOI: 10.7270/Q2GF0W23 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50039029

((+)-4-((alpha R)-((2S,5R)-4-allyl-2,5-dimethylpipe...)Show SMILES CCN(CC)C(=O)c1ccc(cc1)[C@@H](N1C[C@@H](C)N(CC=C)C[C@@H]1C)c1cccc(OC)c1 |r| Show InChI InChI=1S/C28H39N3O2/c1-7-17-30-19-22(5)31(20-21(30)4)27(25-11-10-12-26(18-25)33-6)23-13-15-24(16-14-23)28(32)29(8-2)9-3/h7,10-16,18,21-22,27H,1,8-9,17,19-20H2,2-6H3/t21-,22+,27-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 5.26E+3 | n/a | n/a | n/a | n/a |
Kitasato University
Curated by ChEMBL
| Assay Description
Agonist activity at human kappa opioid receptor expressed in HEK293 cells by CellKey method |
ACS Med Chem Lett 5: 368-72 (2014)
Article DOI: 10.1021/ml400491k
BindingDB Entry DOI: 10.7270/Q2GF0W23 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50010652

(CHEMBL3264742)Show SMILES [H][C@]12O[C@]34CC[C@@]5(OCOc6c(O)ccc7C[C@@]3([H])N(CC3CC3)CC[C@]4(c67)[C@]15[H])N(Cc1ccccc1)C2=O |r,TLB:20:19:3:27.15.16,THB:7:6:26:1.2| Show InChI InChI=1S/C30H32N2O5/c33-21-9-8-20-14-22-29-10-11-30-26(25(37-29)27(34)32(30)16-18-4-2-1-3-5-18)28(29,23(20)24(21)35-17-36-30)12-13-31(22)15-19-6-7-19/h1-5,8-9,19,22,25-26,33H,6-7,10-17H2/t22-,25+,26+,28+,29-,30+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 40 | n/a | n/a | n/a | n/a |
Kitasato University
Curated by ChEMBL
| Assay Description
Agonist activity at human kappa opioid receptor expressed in HEK293 cells by CellKey method |
ACS Med Chem Lett 5: 368-72 (2014)
Article DOI: 10.1021/ml400491k
BindingDB Entry DOI: 10.7270/Q2GF0W23 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50010657

(CHEMBL3264746)Show SMILES [H][C@]12O[C@]34CC[C@@]5(OCOc6c(O)ccc7C[C@@]3([H])N(CCc3ccccc3)CC[C@]4(c67)[C@]15[H])N(Cc1ccccc1)C2=O |r,TLB:20:19:3:31.15.16,THB:7:6:30:1.2| Show InChI InChI=1S/C34H34N2O5/c37-25-12-11-24-19-26-33-14-15-34-30(29(41-33)31(38)36(34)20-23-9-5-2-6-10-23)32(33,27(24)28(25)39-21-40-34)16-18-35(26)17-13-22-7-3-1-4-8-22/h1-12,26,29-30,37H,13-21H2/t26-,29+,30+,32+,33-,34+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.25E+4 | n/a | n/a | n/a | n/a |
Kitasato University
Curated by ChEMBL
| Assay Description
Agonist activity at human kappa opioid receptor expressed in HEK293 cells by CellKey method |
ACS Med Chem Lett 5: 368-72 (2014)
Article DOI: 10.1021/ml400491k
BindingDB Entry DOI: 10.7270/Q2GF0W23 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50039029

((+)-4-((alpha R)-((2S,5R)-4-allyl-2,5-dimethylpipe...)Show SMILES CCN(CC)C(=O)c1ccc(cc1)[C@@H](N1C[C@@H](C)N(CC=C)C[C@@H]1C)c1cccc(OC)c1 |r| Show InChI InChI=1S/C28H39N3O2/c1-7-17-30-19-22(5)31(20-21(30)4)27(25-11-10-12-26(18-25)33-6)23-13-15-24(16-14-23)28(32)29(8-2)9-3/h7,10-16,18,21-22,27H,1,8-9,17,19-20H2,2-6H3/t21-,22+,27-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a |
Kitasato University
Curated by ChEMBL
| Assay Description
Agonist activity at human delta opioid receptor expressed in CHO cells assessed as [35S]-GTP[gammaS] binding |
ACS Med Chem Lett 5: 368-72 (2014)
Article DOI: 10.1021/ml400491k
BindingDB Entry DOI: 10.7270/Q2GF0W23 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50010653

(CHEMBL3264743)Show SMILES [H][C@]12O[C@]34CC[C@@]5(OCOc6c(O)ccc7C[C@@]3([H])N(C)CC[C@]4(c67)[C@]15[H])N(Cc1ccccc1)C2=O |r,TLB:20:19:3:24.15.16,THB:7:6:23:1.2| Show InChI InChI=1S/C27H28N2O5/c1-28-12-11-25-20-17-7-8-18(30)21(20)32-15-33-27-10-9-26(25,19(28)13-17)34-22(23(25)27)24(31)29(27)14-16-5-3-2-4-6-16/h2-8,19,22-23,30H,9-15H2,1H3/t19-,22+,23+,25+,26-,27+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 307 | n/a | n/a | n/a | n/a |
Kitasato University
Curated by ChEMBL
| Assay Description
Agonist activity at human kappa opioid receptor expressed in HEK293 cells by CellKey method |
ACS Med Chem Lett 5: 368-72 (2014)
Article DOI: 10.1021/ml400491k
BindingDB Entry DOI: 10.7270/Q2GF0W23 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50010656

(CHEMBL3264745)Show SMILES [H][C@]12O[C@]34CC[C@@]5(OCOc6c(O)ccc7C[C@@]3([H])N(CC=C)CC[C@]4(c67)[C@]15[H])N(Cc1ccccc1)C2=O |r,TLB:20:19:3:26.15.16,THB:7:6:25:1.2| Show InChI InChI=1S/C29H30N2O5/c1-2-13-30-14-12-27-22-19-8-9-20(32)23(22)34-17-35-29-11-10-28(27,21(30)15-19)36-24(25(27)29)26(33)31(29)16-18-6-4-3-5-7-18/h2-9,21,24-25,32H,1,10-17H2/t21-,24+,25+,27+,28-,29+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 6.5 | n/a | n/a | n/a | n/a |
Kitasato University
Curated by ChEMBL
| Assay Description
Agonist activity at human delta opioid receptor expressed in CHO cells assessed as [35S]-GTP[gammaS] binding |
ACS Med Chem Lett 5: 368-72 (2014)
Article DOI: 10.1021/ml400491k
BindingDB Entry DOI: 10.7270/Q2GF0W23 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50010658

(CHEMBL3264747)Show SMILES [H][C@]12O[C@]34CC[C@@]5(OCOc6c(O)ccc7C[C@@]3([H])N(CC3CC3)CC[C@]4(c67)[C@]15[H])N(C2=O)c1ccccc1 |r,TLB:20:19:3:27.15.16,THB:7:6:26:1.2| Show InChI InChI=1S/C29H30N2O5/c32-20-9-8-18-14-21-28-10-11-29-25(24(36-28)26(33)31(29)19-4-2-1-3-5-19)27(28,22(18)23(20)34-16-35-29)12-13-30(21)15-17-6-7-17/h1-5,8-9,17,21,24-25,32H,6-7,10-16H2/t21-,24+,25+,27+,28-,29+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a |
Kitasato University
Curated by ChEMBL
| Assay Description
Agonist activity at human kappa opioid receptor expressed in HEK293 cells by CellKey method |
ACS Med Chem Lett 5: 368-72 (2014)
Article DOI: 10.1021/ml400491k
BindingDB Entry DOI: 10.7270/Q2GF0W23 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50010653

(CHEMBL3264743)Show SMILES [H][C@]12O[C@]34CC[C@@]5(OCOc6c(O)ccc7C[C@@]3([H])N(C)CC[C@]4(c67)[C@]15[H])N(Cc1ccccc1)C2=O |r,TLB:20:19:3:24.15.16,THB:7:6:23:1.2| Show InChI InChI=1S/C27H28N2O5/c1-28-12-11-25-20-17-7-8-18(30)21(20)32-15-33-27-10-9-26(25,19(28)13-17)34-22(23(25)27)24(31)29(27)14-16-5-3-2-4-6-16/h2-8,19,22-23,30H,9-15H2,1H3/t19-,22+,23+,25+,26-,27+/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 113 | n/a | n/a | n/a | n/a |
Kitasato University
Curated by ChEMBL
| Assay Description
Agonist activity at human mu opioid receptor expressed in CHO cells assessed as [35S]-GTP[gammaS] binding |
ACS Med Chem Lett 5: 368-72 (2014)
Article DOI: 10.1021/ml400491k
BindingDB Entry DOI: 10.7270/Q2GF0W23 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50010659

(CHEMBL3264748)Show SMILES [H][C@]12O[C@]34CC[C@@]5(OCOc6c(O)ccc7C[C@@]3([H])N(CC3CC3)CC[C@]4(c67)[C@]15[H])N(CCc1ccccc1)C2=O |r,TLB:20:19:3:27.15.16,THB:7:6:26:1.2| Show InChI InChI=1S/C31H34N2O5/c34-22-9-8-21-16-23-30-11-12-31-27(26(38-30)28(35)33(31)14-10-19-4-2-1-3-5-19)29(30,24(21)25(22)36-18-37-31)13-15-32(23)17-20-6-7-20/h1-5,8-9,20,23,26-27,34H,6-7,10-18H2/t23-,26+,27+,29+,30-,31+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.410 | n/a | n/a | n/a | n/a |
Kitasato University
Curated by ChEMBL
| Assay Description
Agonist activity at human delta opioid receptor expressed in CHO cells assessed as [35S]-GTP[gammaS] binding |
ACS Med Chem Lett 5: 368-72 (2014)
Article DOI: 10.1021/ml400491k
BindingDB Entry DOI: 10.7270/Q2GF0W23 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50010659

(CHEMBL3264748)Show SMILES [H][C@]12O[C@]34CC[C@@]5(OCOc6c(O)ccc7C[C@@]3([H])N(CC3CC3)CC[C@]4(c67)[C@]15[H])N(CCc1ccccc1)C2=O |r,TLB:20:19:3:27.15.16,THB:7:6:26:1.2| Show InChI InChI=1S/C31H34N2O5/c34-22-9-8-21-16-23-30-11-12-31-27(26(38-30)28(35)33(31)14-10-19-4-2-1-3-5-19)29(30,24(21)25(22)36-18-37-31)13-15-32(23)17-20-6-7-20/h1-5,8-9,20,23,26-27,34H,6-7,10-18H2/t23-,26+,27+,29+,30-,31+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 3.90 | n/a | n/a | n/a | n/a |
Kitasato University
Curated by ChEMBL
| Assay Description
Agonist activity at human kappa opioid receptor expressed in CHO cells assessed as [35S]-GTP[gammaS] binding |
ACS Med Chem Lett 5: 368-72 (2014)
Article DOI: 10.1021/ml400491k
BindingDB Entry DOI: 10.7270/Q2GF0W23 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50010657

(CHEMBL3264746)Show SMILES [H][C@]12O[C@]34CC[C@@]5(OCOc6c(O)ccc7C[C@@]3([H])N(CCc3ccccc3)CC[C@]4(c67)[C@]15[H])N(Cc1ccccc1)C2=O |r,TLB:20:19:3:31.15.16,THB:7:6:30:1.2| Show InChI InChI=1S/C34H34N2O5/c37-25-12-11-24-19-26-33-14-15-34-30(29(41-33)31(38)36(34)20-23-9-5-2-6-10-23)32(33,27(24)28(25)39-21-40-34)16-18-35(26)17-13-22-7-3-1-4-8-22/h1-12,26,29-30,37H,13-21H2/t26-,29+,30+,32+,33-,34+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 21 | n/a | n/a | n/a | n/a |
Kitasato University
Curated by ChEMBL
| Assay Description
Agonist activity at human delta opioid receptor expressed in HEK293 cells by CellKey method |
ACS Med Chem Lett 5: 368-72 (2014)
Article DOI: 10.1021/ml400491k
BindingDB Entry DOI: 10.7270/Q2GF0W23 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50010653

(CHEMBL3264743)Show SMILES [H][C@]12O[C@]34CC[C@@]5(OCOc6c(O)ccc7C[C@@]3([H])N(C)CC[C@]4(c67)[C@]15[H])N(Cc1ccccc1)C2=O |r,TLB:20:19:3:24.15.16,THB:7:6:23:1.2| Show InChI InChI=1S/C27H28N2O5/c1-28-12-11-25-20-17-7-8-18(30)21(20)32-15-33-27-10-9-26(25,19(28)13-17)34-22(23(25)27)24(31)29(27)14-16-5-3-2-4-6-16/h2-8,19,22-23,30H,9-15H2,1H3/t19-,22+,23+,25+,26-,27+/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.35E+3 | n/a | n/a | n/a | n/a |
Kitasato University
Curated by ChEMBL
| Assay Description
Agonist activity at human mu opioid receptor expressed in HEK293 cells by CellKey method |
ACS Med Chem Lett 5: 368-72 (2014)
Article DOI: 10.1021/ml400491k
BindingDB Entry DOI: 10.7270/Q2GF0W23 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50010652

(CHEMBL3264742)Show SMILES [H][C@]12O[C@]34CC[C@@]5(OCOc6c(O)ccc7C[C@@]3([H])N(CC3CC3)CC[C@]4(c67)[C@]15[H])N(Cc1ccccc1)C2=O |r,TLB:20:19:3:27.15.16,THB:7:6:26:1.2| Show InChI InChI=1S/C30H32N2O5/c33-21-9-8-20-14-22-29-10-11-30-26(25(37-29)27(34)32(30)16-18-4-2-1-3-5-18)28(29,23(20)24(21)35-17-36-30)12-13-31(22)15-19-6-7-19/h1-5,8-9,19,22,25-26,33H,6-7,10-17H2/t22-,25+,26+,28+,29-,30+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a |
Kitasato University
Curated by ChEMBL
| Assay Description
Agonist activity at human delta opioid receptor expressed in HEK293 cells by CellKey method |
ACS Med Chem Lett 5: 368-72 (2014)
Article DOI: 10.1021/ml400491k
BindingDB Entry DOI: 10.7270/Q2GF0W23 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50010659

(CHEMBL3264748)Show SMILES [H][C@]12O[C@]34CC[C@@]5(OCOc6c(O)ccc7C[C@@]3([H])N(CC3CC3)CC[C@]4(c67)[C@]15[H])N(CCc1ccccc1)C2=O |r,TLB:20:19:3:27.15.16,THB:7:6:26:1.2| Show InChI InChI=1S/C31H34N2O5/c34-22-9-8-21-16-23-30-11-12-31-27(26(38-30)28(35)33(31)14-10-19-4-2-1-3-5-19)29(30,24(21)25(22)36-18-37-31)13-15-32(23)17-20-6-7-20/h1-5,8-9,20,23,26-27,34H,6-7,10-18H2/t23-,26+,27+,29+,30-,31+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.620 | n/a | n/a | n/a | n/a |
Kitasato University
Curated by ChEMBL
| Assay Description
Agonist activity at human delta opioid receptor expressed in HEK293 cells by CellKey method |
ACS Med Chem Lett 5: 368-72 (2014)
Article DOI: 10.1021/ml400491k
BindingDB Entry DOI: 10.7270/Q2GF0W23 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50010654

(CHEMBL3264744)Show SMILES [H][C@]12O[C@]34CC[C@@]5(OCOc6c(O)ccc7C[C@@]3([H])N(CC(C)C)CC[C@]4(c67)[C@]15[H])N(Cc1ccccc1)C2=O |r,TLB:20:19:3:27.15.16,THB:7:6:26:1.2| Show InChI InChI=1S/C30H34N2O5/c1-18(2)15-31-13-12-28-23-20-8-9-21(33)24(23)35-17-36-30-11-10-29(28,22(31)14-20)37-25(26(28)30)27(34)32(30)16-19-6-4-3-5-7-19/h3-9,18,22,25-26,33H,10-17H2,1-2H3/t22-,25+,26+,28+,29-,30+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 333 | n/a | n/a | n/a | n/a |
Kitasato University
Curated by ChEMBL
| Assay Description
Agonist activity at human kappa opioid receptor expressed in HEK293 cells by CellKey method |
ACS Med Chem Lett 5: 368-72 (2014)
Article DOI: 10.1021/ml400491k
BindingDB Entry DOI: 10.7270/Q2GF0W23 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50010659

(CHEMBL3264748)Show SMILES [H][C@]12O[C@]34CC[C@@]5(OCOc6c(O)ccc7C[C@@]3([H])N(CC3CC3)CC[C@]4(c67)[C@]15[H])N(CCc1ccccc1)C2=O |r,TLB:20:19:3:27.15.16,THB:7:6:26:1.2| Show InChI InChI=1S/C31H34N2O5/c34-22-9-8-21-16-23-30-11-12-31-27(26(38-30)28(35)33(31)14-10-19-4-2-1-3-5-19)29(30,24(21)25(22)36-18-37-31)13-15-32(23)17-20-6-7-20/h1-5,8-9,20,23,26-27,34H,6-7,10-18H2/t23-,26+,27+,29+,30-,31+/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a |
Kitasato University
Curated by ChEMBL
| Assay Description
Agonist activity at human mu opioid receptor expressed in CHO cells assessed as [35S]-GTP[gammaS] binding |
ACS Med Chem Lett 5: 368-72 (2014)
Article DOI: 10.1021/ml400491k
BindingDB Entry DOI: 10.7270/Q2GF0W23 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50010653

(CHEMBL3264743)Show SMILES [H][C@]12O[C@]34CC[C@@]5(OCOc6c(O)ccc7C[C@@]3([H])N(C)CC[C@]4(c67)[C@]15[H])N(Cc1ccccc1)C2=O |r,TLB:20:19:3:24.15.16,THB:7:6:23:1.2| Show InChI InChI=1S/C27H28N2O5/c1-28-12-11-25-20-17-7-8-18(30)21(20)32-15-33-27-10-9-26(25,19(28)13-17)34-22(23(25)27)24(31)29(27)14-16-5-3-2-4-6-16/h2-8,19,22-23,30H,9-15H2,1H3/t19-,22+,23+,25+,26-,27+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 11 | n/a | n/a | n/a | n/a |
Kitasato University
Curated by ChEMBL
| Assay Description
Agonist activity at human delta opioid receptor expressed in CHO cells assessed as [35S]-GTP[gammaS] binding |
ACS Med Chem Lett 5: 368-72 (2014)
Article DOI: 10.1021/ml400491k
BindingDB Entry DOI: 10.7270/Q2GF0W23 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50010653

(CHEMBL3264743)Show SMILES [H][C@]12O[C@]34CC[C@@]5(OCOc6c(O)ccc7C[C@@]3([H])N(C)CC[C@]4(c67)[C@]15[H])N(Cc1ccccc1)C2=O |r,TLB:20:19:3:24.15.16,THB:7:6:23:1.2| Show InChI InChI=1S/C27H28N2O5/c1-28-12-11-25-20-17-7-8-18(30)21(20)32-15-33-27-10-9-26(25,19(28)13-17)34-22(23(25)27)24(31)29(27)14-16-5-3-2-4-6-16/h2-8,19,22-23,30H,9-15H2,1H3/t19-,22+,23+,25+,26-,27+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 478 | n/a | n/a | n/a | n/a |
Kitasato University
Curated by ChEMBL
| Assay Description
Agonist activity at human kappa opioid receptor expressed in CHO cells assessed as [35S]-GTP[gammaS] binding |
ACS Med Chem Lett 5: 368-72 (2014)
Article DOI: 10.1021/ml400491k
BindingDB Entry DOI: 10.7270/Q2GF0W23 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50010654

(CHEMBL3264744)Show SMILES [H][C@]12O[C@]34CC[C@@]5(OCOc6c(O)ccc7C[C@@]3([H])N(CC(C)C)CC[C@]4(c67)[C@]15[H])N(Cc1ccccc1)C2=O |r,TLB:20:19:3:27.15.16,THB:7:6:26:1.2| Show InChI InChI=1S/C30H34N2O5/c1-18(2)15-31-13-12-28-23-20-8-9-21(33)24(23)35-17-36-30-11-10-29(28,22(31)14-20)37-25(26(28)30)27(34)32(30)16-19-6-4-3-5-7-19/h3-9,18,22,25-26,33H,10-17H2,1-2H3/t22-,25+,26+,28+,29-,30+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 760 | n/a | n/a | n/a | n/a |
Kitasato University
Curated by ChEMBL
| Assay Description
Agonist activity at human kappa opioid receptor expressed in CHO cells assessed as [35S]-GTP[gammaS] binding |
ACS Med Chem Lett 5: 368-72 (2014)
Article DOI: 10.1021/ml400491k
BindingDB Entry DOI: 10.7270/Q2GF0W23 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50010658

(CHEMBL3264747)Show SMILES [H][C@]12O[C@]34CC[C@@]5(OCOc6c(O)ccc7C[C@@]3([H])N(CC3CC3)CC[C@]4(c67)[C@]15[H])N(C2=O)c1ccccc1 |r,TLB:20:19:3:27.15.16,THB:7:6:26:1.2| Show InChI InChI=1S/C29H30N2O5/c32-20-9-8-18-14-21-28-10-11-29-25(24(36-28)26(33)31(29)19-4-2-1-3-5-19)27(28,22(18)23(20)34-16-35-29)12-13-30(21)15-17-6-7-17/h1-5,8-9,17,21,24-25,32H,6-7,10-16H2/t21-,24+,25+,27+,28-,29+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 6.5 | n/a | n/a | n/a | n/a |
Kitasato University
Curated by ChEMBL
| Assay Description
Agonist activity at human kappa opioid receptor expressed in CHO cells assessed as [35S]-GTP[gammaS] binding |
ACS Med Chem Lett 5: 368-72 (2014)
Article DOI: 10.1021/ml400491k
BindingDB Entry DOI: 10.7270/Q2GF0W23 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50010654

(CHEMBL3264744)Show SMILES [H][C@]12O[C@]34CC[C@@]5(OCOc6c(O)ccc7C[C@@]3([H])N(CC(C)C)CC[C@]4(c67)[C@]15[H])N(Cc1ccccc1)C2=O |r,TLB:20:19:3:27.15.16,THB:7:6:26:1.2| Show InChI InChI=1S/C30H34N2O5/c1-18(2)15-31-13-12-28-23-20-8-9-21(33)24(23)35-17-36-30-11-10-29(28,22(31)14-20)37-25(26(28)30)27(34)32(30)16-19-6-4-3-5-7-19/h3-9,18,22,25-26,33H,10-17H2,1-2H3/t22-,25+,26+,28+,29-,30+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 140 | n/a | n/a | n/a | n/a |
Kitasato University
Curated by ChEMBL
| Assay Description
Agonist activity at human delta opioid receptor expressed in HEK293 cells by CellKey method |
ACS Med Chem Lett 5: 368-72 (2014)
Article DOI: 10.1021/ml400491k
BindingDB Entry DOI: 10.7270/Q2GF0W23 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50010659

(CHEMBL3264748)Show SMILES [H][C@]12O[C@]34CC[C@@]5(OCOc6c(O)ccc7C[C@@]3([H])N(CC3CC3)CC[C@]4(c67)[C@]15[H])N(CCc1ccccc1)C2=O |r,TLB:20:19:3:27.15.16,THB:7:6:26:1.2| Show InChI InChI=1S/C31H34N2O5/c34-22-9-8-21-16-23-30-11-12-31-27(26(38-30)28(35)33(31)14-10-19-4-2-1-3-5-19)29(30,24(21)25(22)36-18-37-31)13-15-32(23)17-20-6-7-20/h1-5,8-9,20,23,26-27,34H,6-7,10-18H2/t23-,26+,27+,29+,30-,31+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a |
Kitasato University
Curated by ChEMBL
| Assay Description
Agonist activity at human kappa opioid receptor expressed in HEK293 cells by CellKey method |
ACS Med Chem Lett 5: 368-72 (2014)
Article DOI: 10.1021/ml400491k
BindingDB Entry DOI: 10.7270/Q2GF0W23 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50010652

(CHEMBL3264742)Show SMILES [H][C@]12O[C@]34CC[C@@]5(OCOc6c(O)ccc7C[C@@]3([H])N(CC3CC3)CC[C@]4(c67)[C@]15[H])N(Cc1ccccc1)C2=O |r,TLB:20:19:3:27.15.16,THB:7:6:26:1.2| Show InChI InChI=1S/C30H32N2O5/c33-21-9-8-20-14-22-29-10-11-30-26(25(37-29)27(34)32(30)16-18-4-2-1-3-5-18)28(29,23(20)24(21)35-17-36-30)12-13-31(22)15-19-6-7-19/h1-5,8-9,19,22,25-26,33H,6-7,10-17H2/t22-,25+,26+,28+,29-,30+/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 2.80 | n/a | n/a | n/a | n/a |
Kitasato University
Curated by ChEMBL
| Assay Description
Agonist activity at human mu opioid receptor expressed in CHO cells assessed as [35S]-GTP[gammaS] binding |
ACS Med Chem Lett 5: 368-72 (2014)
Article DOI: 10.1021/ml400491k
BindingDB Entry DOI: 10.7270/Q2GF0W23 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50010657

(CHEMBL3264746)Show SMILES [H][C@]12O[C@]34CC[C@@]5(OCOc6c(O)ccc7C[C@@]3([H])N(CCc3ccccc3)CC[C@]4(c67)[C@]15[H])N(Cc1ccccc1)C2=O |r,TLB:20:19:3:31.15.16,THB:7:6:30:1.2| Show InChI InChI=1S/C34H34N2O5/c37-25-12-11-24-19-26-33-14-15-34-30(29(41-33)31(38)36(34)20-23-9-5-2-6-10-23)32(33,27(24)28(25)39-21-40-34)16-18-35(26)17-13-22-7-3-1-4-8-22/h1-12,26,29-30,37H,13-21H2/t26-,29+,30+,32+,33-,34+/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a |
Kitasato University
Curated by ChEMBL
| Assay Description
Agonist activity at human mu opioid receptor expressed in CHO cells assessed as [35S]-GTP[gammaS] binding |
ACS Med Chem Lett 5: 368-72 (2014)
Article DOI: 10.1021/ml400491k
BindingDB Entry DOI: 10.7270/Q2GF0W23 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50010658

(CHEMBL3264747)Show SMILES [H][C@]12O[C@]34CC[C@@]5(OCOc6c(O)ccc7C[C@@]3([H])N(CC3CC3)CC[C@]4(c67)[C@]15[H])N(C2=O)c1ccccc1 |r,TLB:20:19:3:27.15.16,THB:7:6:26:1.2| Show InChI InChI=1S/C29H30N2O5/c32-20-9-8-18-14-21-28-10-11-29-25(24(36-28)26(33)31(29)19-4-2-1-3-5-19)27(28,22(18)23(20)34-16-35-29)12-13-30(21)15-17-6-7-17/h1-5,8-9,17,21,24-25,32H,6-7,10-16H2/t21-,24+,25+,27+,28-,29+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.390 | n/a | n/a | n/a | n/a |
Kitasato University
Curated by ChEMBL
| Assay Description
Agonist activity at human delta opioid receptor expressed in HEK293 cells by CellKey method |
ACS Med Chem Lett 5: 368-72 (2014)
Article DOI: 10.1021/ml400491k
BindingDB Entry DOI: 10.7270/Q2GF0W23 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50010652

(CHEMBL3264742)Show SMILES [H][C@]12O[C@]34CC[C@@]5(OCOc6c(O)ccc7C[C@@]3([H])N(CC3CC3)CC[C@]4(c67)[C@]15[H])N(Cc1ccccc1)C2=O |r,TLB:20:19:3:27.15.16,THB:7:6:26:1.2| Show InChI InChI=1S/C30H32N2O5/c33-21-9-8-20-14-22-29-10-11-30-26(25(37-29)27(34)32(30)16-18-4-2-1-3-5-18)28(29,23(20)24(21)35-17-36-30)12-13-31(22)15-19-6-7-19/h1-5,8-9,19,22,25-26,33H,6-7,10-17H2/t22-,25+,26+,28+,29-,30+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 81 | n/a | n/a | n/a | n/a |
Kitasato University
Curated by ChEMBL
| Assay Description
Agonist activity at human kappa opioid receptor expressed in CHO cells assessed as [35S]-GTP[gammaS] binding |
ACS Med Chem Lett 5: 368-72 (2014)
Article DOI: 10.1021/ml400491k
BindingDB Entry DOI: 10.7270/Q2GF0W23 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50010658

(CHEMBL3264747)Show SMILES [H][C@]12O[C@]34CC[C@@]5(OCOc6c(O)ccc7C[C@@]3([H])N(CC3CC3)CC[C@]4(c67)[C@]15[H])N(C2=O)c1ccccc1 |r,TLB:20:19:3:27.15.16,THB:7:6:26:1.2| Show InChI InChI=1S/C29H30N2O5/c32-20-9-8-18-14-21-28-10-11-29-25(24(36-28)26(33)31(29)19-4-2-1-3-5-19)27(28,22(18)23(20)34-16-35-29)12-13-30(21)15-17-6-7-17/h1-5,8-9,17,21,24-25,32H,6-7,10-16H2/t21-,24+,25+,27+,28-,29+/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 9 | n/a | n/a | n/a | n/a |
Kitasato University
Curated by ChEMBL
| Assay Description
Agonist activity at human mu opioid receptor expressed in CHO cells assessed as [35S]-GTP[gammaS] binding |
ACS Med Chem Lett 5: 368-72 (2014)
Article DOI: 10.1021/ml400491k
BindingDB Entry DOI: 10.7270/Q2GF0W23 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50010654

(CHEMBL3264744)Show SMILES [H][C@]12O[C@]34CC[C@@]5(OCOc6c(O)ccc7C[C@@]3([H])N(CC(C)C)CC[C@]4(c67)[C@]15[H])N(Cc1ccccc1)C2=O |r,TLB:20:19:3:27.15.16,THB:7:6:26:1.2| Show InChI InChI=1S/C30H34N2O5/c1-18(2)15-31-13-12-28-23-20-8-9-21(33)24(23)35-17-36-30-11-10-29(28,22(31)14-20)37-25(26(28)30)27(34)32(30)16-19-6-4-3-5-7-19/h3-9,18,22,25-26,33H,10-17H2,1-2H3/t22-,25+,26+,28+,29-,30+/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 223 | n/a | n/a | n/a | n/a |
Kitasato University
Curated by ChEMBL
| Assay Description
Agonist activity at human mu opioid receptor expressed in CHO cells assessed as [35S]-GTP[gammaS] binding |
ACS Med Chem Lett 5: 368-72 (2014)
Article DOI: 10.1021/ml400491k
BindingDB Entry DOI: 10.7270/Q2GF0W23 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50010653

(CHEMBL3264743)Show SMILES [H][C@]12O[C@]34CC[C@@]5(OCOc6c(O)ccc7C[C@@]3([H])N(C)CC[C@]4(c67)[C@]15[H])N(Cc1ccccc1)C2=O |r,TLB:20:19:3:24.15.16,THB:7:6:23:1.2| Show InChI InChI=1S/C27H28N2O5/c1-28-12-11-25-20-17-7-8-18(30)21(20)32-15-33-27-10-9-26(25,19(28)13-17)34-22(23(25)27)24(31)29(27)14-16-5-3-2-4-6-16/h2-8,19,22-23,30H,9-15H2,1H3/t19-,22+,23+,25+,26-,27+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 141 | n/a | n/a | n/a | n/a |
Kitasato University
Curated by ChEMBL
| Assay Description
Agonist activity at human delta opioid receptor expressed in HEK293 cells by CellKey method |
ACS Med Chem Lett 5: 368-72 (2014)
Article DOI: 10.1021/ml400491k
BindingDB Entry DOI: 10.7270/Q2GF0W23 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50039029

((+)-4-((alpha R)-((2S,5R)-4-allyl-2,5-dimethylpipe...)Show SMILES CCN(CC)C(=O)c1ccc(cc1)[C@@H](N1C[C@@H](C)N(CC=C)C[C@@H]1C)c1cccc(OC)c1 |r| Show InChI InChI=1S/C28H39N3O2/c1-7-17-30-19-22(5)31(20-21(30)4)27(25-11-10-12-26(18-25)33-6)23-13-15-24(16-14-23)28(32)29(8-2)9-3/h7,10-16,18,21-22,27H,1,8-9,17,19-20H2,2-6H3/t21-,22+,27-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a |
Kitasato University
Curated by ChEMBL
| Assay Description
Agonist activity at human delta opioid receptor expressed in HEK293 cells by CellKey method |
ACS Med Chem Lett 5: 368-72 (2014)
Article DOI: 10.1021/ml400491k
BindingDB Entry DOI: 10.7270/Q2GF0W23 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50010659

(CHEMBL3264748)Show SMILES [H][C@]12O[C@]34CC[C@@]5(OCOc6c(O)ccc7C[C@@]3([H])N(CC3CC3)CC[C@]4(c67)[C@]15[H])N(CCc1ccccc1)C2=O |r,TLB:20:19:3:27.15.16,THB:7:6:26:1.2| Show InChI InChI=1S/C31H34N2O5/c34-22-9-8-21-16-23-30-11-12-31-27(26(38-30)28(35)33(31)14-10-19-4-2-1-3-5-19)29(30,24(21)25(22)36-18-37-31)13-15-32(23)17-20-6-7-20/h1-5,8-9,20,23,26-27,34H,6-7,10-18H2/t23-,26+,27+,29+,30-,31+/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 5.20 | n/a | n/a | n/a | n/a |
Kitasato University
Curated by ChEMBL
| Assay Description
Agonist activity at human mu opioid receptor expressed in HEK293 cells by CellKey method |
ACS Med Chem Lett 5: 368-72 (2014)
Article DOI: 10.1021/ml400491k
BindingDB Entry DOI: 10.7270/Q2GF0W23 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50010656

(CHEMBL3264745)Show SMILES [H][C@]12O[C@]34CC[C@@]5(OCOc6c(O)ccc7C[C@@]3([H])N(CC=C)CC[C@]4(c67)[C@]15[H])N(Cc1ccccc1)C2=O |r,TLB:20:19:3:26.15.16,THB:7:6:25:1.2| Show InChI InChI=1S/C29H30N2O5/c1-2-13-30-14-12-27-22-19-8-9-20(32)23(22)34-17-35-29-11-10-28(27,21(30)15-19)36-24(25(27)29)26(33)31(29)16-18-6-4-3-5-7-18/h2-9,21,24-25,32H,1,10-17H2/t21-,24+,25+,27+,28-,29+/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 5.10 | n/a | n/a | n/a | n/a |
Kitasato University
Curated by ChEMBL
| Assay Description
Agonist activity at human mu opioid receptor expressed in HEK293 cells by CellKey method |
ACS Med Chem Lett 5: 368-72 (2014)
Article DOI: 10.1021/ml400491k
BindingDB Entry DOI: 10.7270/Q2GF0W23 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50010658

(CHEMBL3264747)Show SMILES [H][C@]12O[C@]34CC[C@@]5(OCOc6c(O)ccc7C[C@@]3([H])N(CC3CC3)CC[C@]4(c67)[C@]15[H])N(C2=O)c1ccccc1 |r,TLB:20:19:3:27.15.16,THB:7:6:26:1.2| Show InChI InChI=1S/C29H30N2O5/c32-20-9-8-18-14-21-28-10-11-29-25(24(36-28)26(33)31(29)19-4-2-1-3-5-19)27(28,22(18)23(20)34-16-35-29)12-13-30(21)15-17-6-7-17/h1-5,8-9,17,21,24-25,32H,6-7,10-16H2/t21-,24+,25+,27+,28-,29+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.980 | n/a | n/a | n/a | n/a |
Kitasato University
Curated by ChEMBL
| Assay Description
Agonist activity at human delta opioid receptor expressed in CHO cells assessed as [35S]-GTP[gammaS] binding |
ACS Med Chem Lett 5: 368-72 (2014)
Article DOI: 10.1021/ml400491k
BindingDB Entry DOI: 10.7270/Q2GF0W23 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50010657

(CHEMBL3264746)Show SMILES [H][C@]12O[C@]34CC[C@@]5(OCOc6c(O)ccc7C[C@@]3([H])N(CCc3ccccc3)CC[C@]4(c67)[C@]15[H])N(Cc1ccccc1)C2=O |r,TLB:20:19:3:31.15.16,THB:7:6:30:1.2| Show InChI InChI=1S/C34H34N2O5/c37-25-12-11-24-19-26-33-14-15-34-30(29(41-33)31(38)36(34)20-23-9-5-2-6-10-23)32(33,27(24)28(25)39-21-40-34)16-18-35(26)17-13-22-7-3-1-4-8-22/h1-12,26,29-30,37H,13-21H2/t26-,29+,30+,32+,33-,34+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 9.20 | n/a | n/a | n/a | n/a |
Kitasato University
Curated by ChEMBL
| Assay Description
Agonist activity at human delta opioid receptor expressed in CHO cells assessed as [35S]-GTP[gammaS] binding |
ACS Med Chem Lett 5: 368-72 (2014)
Article DOI: 10.1021/ml400491k
BindingDB Entry DOI: 10.7270/Q2GF0W23 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50010652

(CHEMBL3264742)Show SMILES [H][C@]12O[C@]34CC[C@@]5(OCOc6c(O)ccc7C[C@@]3([H])N(CC3CC3)CC[C@]4(c67)[C@]15[H])N(Cc1ccccc1)C2=O |r,TLB:20:19:3:27.15.16,THB:7:6:26:1.2| Show InChI InChI=1S/C30H32N2O5/c33-21-9-8-20-14-22-29-10-11-30-26(25(37-29)27(34)32(30)16-18-4-2-1-3-5-18)28(29,23(20)24(21)35-17-36-30)12-13-31(22)15-19-6-7-19/h1-5,8-9,19,22,25-26,33H,6-7,10-17H2/t22-,25+,26+,28+,29-,30+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a |
Kitasato University
Curated by ChEMBL
| Assay Description
Agonist activity at human delta opioid receptor expressed in CHO cells assessed as [35S]-GTP[gammaS] binding |
ACS Med Chem Lett 5: 368-72 (2014)
Article DOI: 10.1021/ml400491k
BindingDB Entry DOI: 10.7270/Q2GF0W23 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50010654

(CHEMBL3264744)Show SMILES [H][C@]12O[C@]34CC[C@@]5(OCOc6c(O)ccc7C[C@@]3([H])N(CC(C)C)CC[C@]4(c67)[C@]15[H])N(Cc1ccccc1)C2=O |r,TLB:20:19:3:27.15.16,THB:7:6:26:1.2| Show InChI InChI=1S/C30H34N2O5/c1-18(2)15-31-13-12-28-23-20-8-9-21(33)24(23)35-17-36-30-11-10-29(28,22(31)14-20)37-25(26(28)30)27(34)32(30)16-19-6-4-3-5-7-19/h3-9,18,22,25-26,33H,10-17H2,1-2H3/t22-,25+,26+,28+,29-,30+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 16 | n/a | n/a | n/a | n/a |
Kitasato University
Curated by ChEMBL
| Assay Description
Agonist activity at human delta opioid receptor expressed in CHO cells assessed as [35S]-GTP[gammaS] binding |
ACS Med Chem Lett 5: 368-72 (2014)
Article DOI: 10.1021/ml400491k
BindingDB Entry DOI: 10.7270/Q2GF0W23 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data