

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM21221 ((2S,3S,4R,5R)-5-(2-chloro-6-{[(3-iodophenyl)methyl...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ghent University Curated by ChEMBL | Assay Description Binding affinity to A3AR (unknown origin) | Bioorg Med Chem 22: 4257-68 (2014) Article DOI: 10.1016/j.bmc.2014.05.036 BindingDB Entry DOI: 10.7270/Q24Q7WN1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50118812 ((2S,3S,4R,5R)-3,4-Dihydroxy-5-[6-(3-iodo-benzylami...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ghent University Curated by ChEMBL | Assay Description Binding affinity to A3AR (unknown origin) | Bioorg Med Chem 22: 4257-68 (2014) Article DOI: 10.1016/j.bmc.2014.05.036 BindingDB Entry DOI: 10.7270/Q24Q7WN1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50045595 (CHEMBL3311505) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 978 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ghent University Curated by ChEMBL | Assay Description Displacement of [125I]N6-(4-amino-3-iodobenzyl)adenosine-50-N-methyluronamide from human recombinant A3AR expressed in CHO cells | Bioorg Med Chem 22: 4257-68 (2014) Article DOI: 10.1016/j.bmc.2014.05.036 BindingDB Entry DOI: 10.7270/Q24Q7WN1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50045596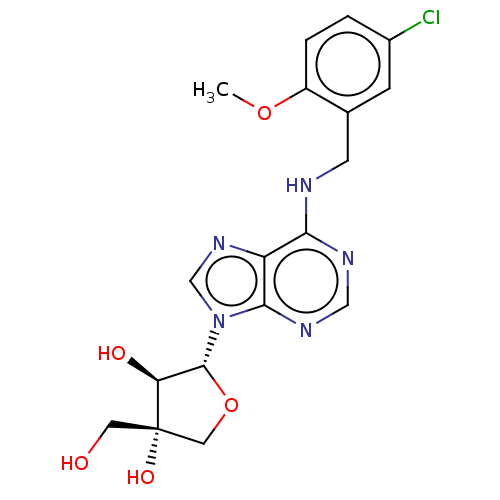 (CHEMBL3311508) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.07E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ghent University Curated by ChEMBL | Assay Description Displacement of [125I]N6-(4-amino-3-iodobenzyl)adenosine-50-N-methyluronamide from human recombinant A3AR expressed in CHO cells | Bioorg Med Chem 22: 4257-68 (2014) Article DOI: 10.1016/j.bmc.2014.05.036 BindingDB Entry DOI: 10.7270/Q24Q7WN1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM21221 ((2S,3S,4R,5R)-5-(2-chloro-6-{[(3-iodophenyl)methyl...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Ghent University Curated by ChEMBL | Assay Description Agonist activity at human recombinant A3AR expressed in CHO cells assessed as inhibition of forskolin-induced stimulation of cAMP production | Bioorg Med Chem 22: 4257-68 (2014) Article DOI: 10.1016/j.bmc.2014.05.036 BindingDB Entry DOI: 10.7270/Q24Q7WN1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50045596 (CHEMBL3311508) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.56E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ghent University Curated by ChEMBL | Assay Description Agonist activity at human recombinant A3AR expressed in CHO cells assessed as inhibition of forskolin-induced stimulation of cAMP production | Bioorg Med Chem 22: 4257-68 (2014) Article DOI: 10.1016/j.bmc.2014.05.036 BindingDB Entry DOI: 10.7270/Q24Q7WN1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||