 Found 75 hits of Enzyme Inhibition Constant Data
Found 75 hits of Enzyme Inhibition Constant Data Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Prostaglandin G/H synthase 1
(Ovis aries (Sheep)) | BDBM17638

(2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...)Show SMILES COc1ccc2n(C(=O)c3ccc(Cl)cc3)c(C)c(CC(O)=O)c2c1 Show InChI InChI=1S/C19H16ClNO4/c1-11-15(10-18(22)23)16-9-14(25-2)7-8-17(16)21(11)19(24)12-3-5-13(20)6-4-12/h3-9H,10H2,1-2H3,(H,22,23) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Kebangsaan Malaysia
Curated by ChEMBL
| Assay Description
Inhibition of ovine COX1 using arachidonic acid substrate assessed as PGE2 production ELISA method after 2 mins |
Bioorg Med Chem 22: 4151-61 (2014)
Article DOI: 10.1016/j.bmc.2014.05.052
BindingDB Entry DOI: 10.7270/Q2GX4D6F |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Ovis aries (Sheep)) | BDBM50021461

(CHEMBL3289927)Show SMILES CCOC(OCC)c1ccc(\C=C2/CN(C)C\C(=C/c3ccc(cc3)C(OCC)OCC)C2=O)cc1 Show InChI InChI=1S/C30H39NO5/c1-6-33-29(34-7-2)24-14-10-22(11-15-24)18-26-20-31(5)21-27(28(26)32)19-23-12-16-25(17-13-23)30(35-8-3)36-9-4/h10-19,29-30H,6-9,20-21H2,1-5H3/b26-18+,27-19+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 370 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Kebangsaan Malaysia
Curated by ChEMBL
| Assay Description
Inhibition of ovine COX1 using arachidonic acid substrate assessed as PGE2 production ELISA method after 2 mins |
Bioorg Med Chem 22: 4151-61 (2014)
Article DOI: 10.1016/j.bmc.2014.05.052
BindingDB Entry DOI: 10.7270/Q2GX4D6F |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Ovis aries (Sheep)) | BDBM50021468

(CHEMBL3289934)Show SMILES CN(C)c1ccc(\C=C2\CCC\C(=C/c3ccc(cc3[N+]([O-])=O)N(C)C)C2=O)c(c1)[N+]([O-])=O Show InChI InChI=1S/C24H26N4O5/c1-25(2)20-10-8-16(22(14-20)27(30)31)12-18-6-5-7-19(24(18)29)13-17-9-11-21(26(3)4)15-23(17)28(32)33/h8-15H,5-7H2,1-4H3/b18-12-,19-13+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 420 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Kebangsaan Malaysia
Curated by ChEMBL
| Assay Description
Inhibition of ovine COX1 using arachidonic acid substrate assessed as PGE2 production ELISA method after 2 mins |
Bioorg Med Chem 22: 4151-61 (2014)
Article DOI: 10.1016/j.bmc.2014.05.052
BindingDB Entry DOI: 10.7270/Q2GX4D6F |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Ovis aries (Sheep)) | BDBM50021447
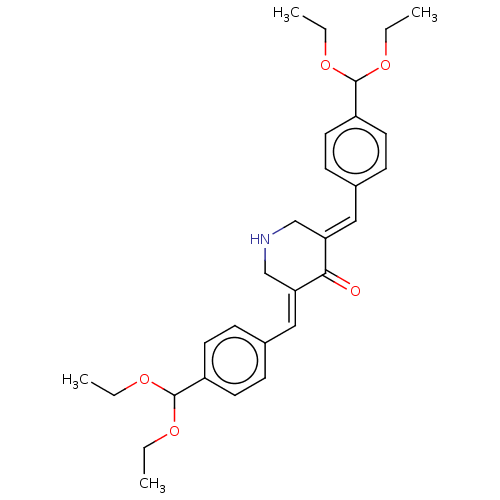
(CHEMBL3289926)Show SMILES CCOC(OCC)c1ccc(\C=C2/CNC\C(=C/c3ccc(cc3)C(OCC)OCC)C2=O)cc1 Show InChI InChI=1S/C29H37NO5/c1-5-32-28(33-6-2)23-13-9-21(10-14-23)17-25-19-30-20-26(27(25)31)18-22-11-15-24(16-12-22)29(34-7-3)35-8-4/h9-18,28-30H,5-8,19-20H2,1-4H3/b25-17+,26-18+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 460 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Kebangsaan Malaysia
Curated by ChEMBL
| Assay Description
Inhibition of ovine COX1 using arachidonic acid substrate assessed as PGE2 production ELISA method after 2 mins |
Bioorg Med Chem 22: 4151-61 (2014)
Article DOI: 10.1016/j.bmc.2014.05.052
BindingDB Entry DOI: 10.7270/Q2GX4D6F |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Ovis aries (Sheep)) | BDBM50045848
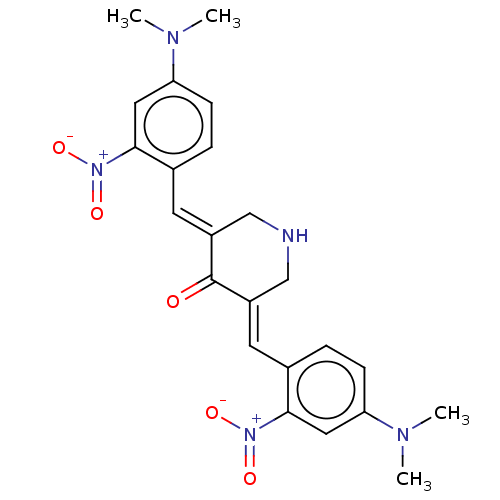
(CHEMBL3309948)Show SMILES CN(C)c1ccc(\C=C2/CNC\C(=C/c3ccc(cc3[N+]([O-])=O)N(C)C)C2=O)c(c1)[N+]([O-])=O Show InChI InChI=1S/C23H25N5O5/c1-25(2)19-7-5-15(21(11-19)27(30)31)9-17-13-24-14-18(23(17)29)10-16-6-8-20(26(3)4)12-22(16)28(32)33/h5-12,24H,13-14H2,1-4H3/b17-9+,18-10+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 670 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Kebangsaan Malaysia
Curated by ChEMBL
| Assay Description
Inhibition of ovine COX1 using arachidonic acid substrate assessed as PGE2 production ELISA method after 2 mins |
Bioorg Med Chem 22: 4151-61 (2014)
Article DOI: 10.1016/j.bmc.2014.05.052
BindingDB Entry DOI: 10.7270/Q2GX4D6F |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Ovis aries (Sheep)) | BDBM50045847

(CHEMBL3309947)Show SMILES CN(C)c1ccc(\C=C2/COC\C(=C/c3ccc(cc3[N+]([O-])=O)N(C)C)C2=O)c(c1)[N+]([O-])=O Show InChI InChI=1S/C23H24N4O6/c1-24(2)19-7-5-15(21(11-19)26(29)30)9-17-13-33-14-18(23(17)28)10-16-6-8-20(25(3)4)12-22(16)27(31)32/h5-12H,13-14H2,1-4H3/b17-9+,18-10+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 720 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Kebangsaan Malaysia
Curated by ChEMBL
| Assay Description
Inhibition of ovine COX1 using arachidonic acid substrate assessed as PGE2 production ELISA method after 2 mins |
Bioorg Med Chem 22: 4151-61 (2014)
Article DOI: 10.1016/j.bmc.2014.05.052
BindingDB Entry DOI: 10.7270/Q2GX4D6F |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Ovis aries (Sheep)) | BDBM50021418
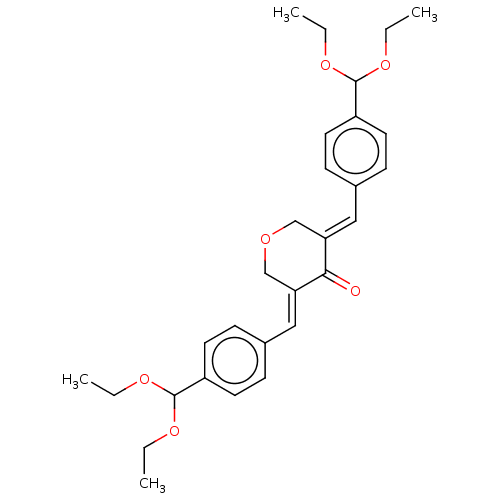
(CHEMBL3289925)Show SMILES CCOC(OCC)c1ccc(\C=C2/COC\C(=C/c3ccc(cc3)C(OCC)OCC)C2=O)cc1 Show InChI InChI=1S/C29H36O6/c1-5-32-28(33-6-2)23-13-9-21(10-14-23)17-25-19-31-20-26(27(25)30)18-22-11-15-24(16-12-22)29(34-7-3)35-8-4/h9-18,28-29H,5-8,19-20H2,1-4H3/b25-17+,26-18+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 990 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Kebangsaan Malaysia
Curated by ChEMBL
| Assay Description
Inhibition of ovine COX1 using arachidonic acid substrate assessed as PGE2 production ELISA method after 2 mins |
Bioorg Med Chem 22: 4151-61 (2014)
Article DOI: 10.1016/j.bmc.2014.05.052
BindingDB Entry DOI: 10.7270/Q2GX4D6F |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Ovis aries (Sheep)) | BDBM50021467

(CHEMBL3289933)Show SMILES CCOC(OCC)c1ccc(\C=C\C(=O)C2CCc3ccccc3C2=O)cc1 Show InChI InChI=1S/C24H26O4/c1-3-27-24(28-4-2)19-12-9-17(10-13-19)11-16-22(25)21-15-14-18-7-5-6-8-20(18)23(21)26/h5-13,16,21,24H,3-4,14-15H2,1-2H3/b16-11+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.12E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Kebangsaan Malaysia
Curated by ChEMBL
| Assay Description
Inhibition of ovine COX1 using arachidonic acid substrate assessed as PGE2 production ELISA method after 2 mins |
Bioorg Med Chem 22: 4151-61 (2014)
Article DOI: 10.1016/j.bmc.2014.05.052
BindingDB Entry DOI: 10.7270/Q2GX4D6F |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Ovis aries (Sheep)) | BDBM50045849
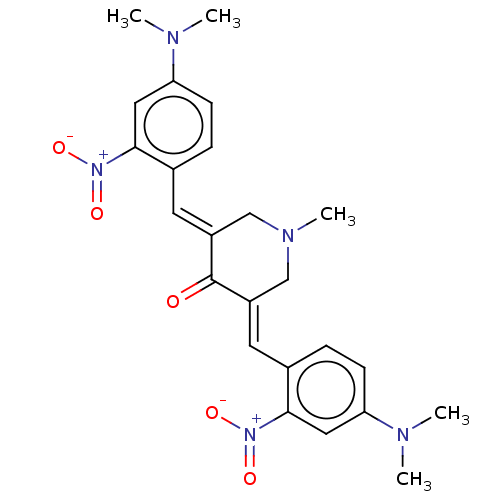
(CHEMBL3309949)Show SMILES CN(C)c1ccc(\C=C2/CN(C)C\C(=C/c3ccc(cc3[N+]([O-])=O)N(C)C)C2=O)c(c1)[N+]([O-])=O Show InChI InChI=1S/C24H27N5O5/c1-25(2)20-8-6-16(22(12-20)28(31)32)10-18-14-27(5)15-19(24(18)30)11-17-7-9-21(26(3)4)13-23(17)29(33)34/h6-13H,14-15H2,1-5H3/b18-10+,19-11+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.22E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Kebangsaan Malaysia
Curated by ChEMBL
| Assay Description
Inhibition of ovine COX1 using arachidonic acid substrate assessed as PGE2 production ELISA method after 2 mins |
Bioorg Med Chem 22: 4151-61 (2014)
Article DOI: 10.1016/j.bmc.2014.05.052
BindingDB Entry DOI: 10.7270/Q2GX4D6F |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Ovis aries (Sheep)) | BDBM50021417
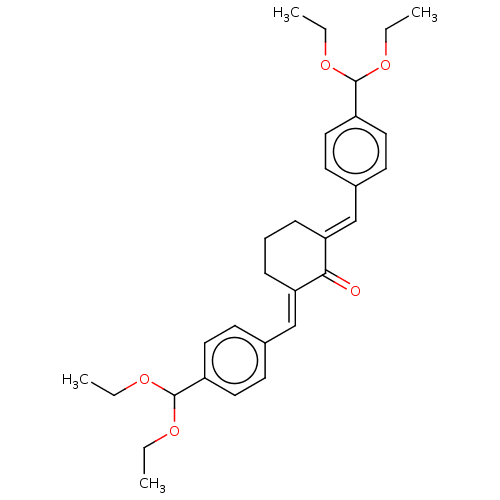
(CHEMBL3289924)Show SMILES CCOC(OCC)c1ccc(\C=C2/CCC\C(=C/c3ccc(cc3)C(OCC)OCC)C2=O)cc1 Show InChI InChI=1S/C30H38O5/c1-5-32-29(33-6-2)24-16-12-22(13-17-24)20-26-10-9-11-27(28(26)31)21-23-14-18-25(19-15-23)30(34-7-3)35-8-4/h12-21,29-30H,5-11H2,1-4H3/b26-20+,27-21+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.77E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Kebangsaan Malaysia
Curated by ChEMBL
| Assay Description
Inhibition of ovine COX1 using arachidonic acid substrate assessed as PGE2 production ELISA method after 2 mins |
Bioorg Med Chem 22: 4151-61 (2014)
Article DOI: 10.1016/j.bmc.2014.05.052
BindingDB Entry DOI: 10.7270/Q2GX4D6F |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Ovis aries (Sheep)) | BDBM50021465
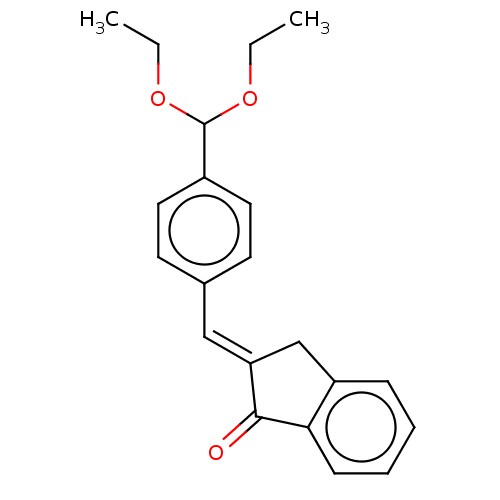
(CHEMBL3289931)Show InChI InChI=1S/C21H22O3/c1-3-23-21(24-4-2)16-11-9-15(10-12-16)13-18-14-17-7-5-6-8-19(17)20(18)22/h5-13,21H,3-4,14H2,1-2H3/b18-13+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.22E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Kebangsaan Malaysia
Curated by ChEMBL
| Assay Description
Inhibition of ovine COX1 using arachidonic acid substrate assessed as PGE2 production ELISA method after 2 mins |
Bioorg Med Chem 22: 4151-61 (2014)
Article DOI: 10.1016/j.bmc.2014.05.052
BindingDB Entry DOI: 10.7270/Q2GX4D6F |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Ovis aries (Sheep)) | BDBM50021464
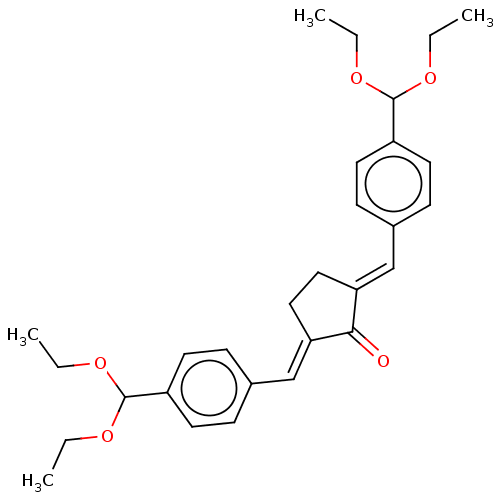
(CHEMBL3289930)Show SMILES CCOC(OCC)c1ccc(\C=C2/CC\C(=C/c3ccc(cc3)C(OCC)OCC)C2=O)cc1 Show InChI InChI=1S/C29H36O5/c1-5-31-28(32-6-2)23-13-9-21(10-14-23)19-25-17-18-26(27(25)30)20-22-11-15-24(16-12-22)29(33-7-3)34-8-4/h9-16,19-20,28-29H,5-8,17-18H2,1-4H3/b25-19+,26-20+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.73E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Kebangsaan Malaysia
Curated by ChEMBL
| Assay Description
Inhibition of ovine COX1 using arachidonic acid substrate assessed as PGE2 production ELISA method after 2 mins |
Bioorg Med Chem 22: 4151-61 (2014)
Article DOI: 10.1016/j.bmc.2014.05.052
BindingDB Entry DOI: 10.7270/Q2GX4D6F |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Ovis aries (Sheep)) | BDBM50021462
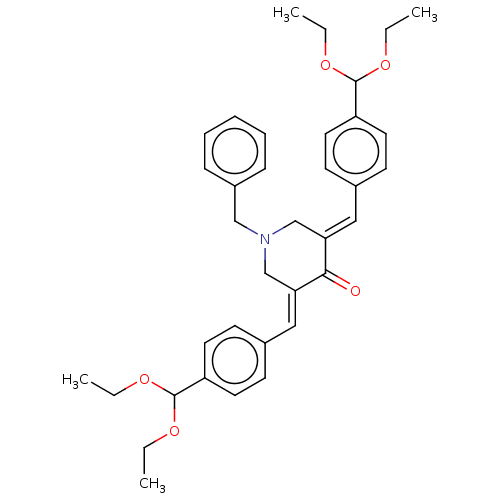
(CHEMBL3289928)Show SMILES CCOC(OCC)c1ccc(\C=C2/CN(Cc3ccccc3)C\C(=C/c3ccc(cc3)C(OCC)OCC)C2=O)cc1 Show InChI InChI=1S/C36H43NO5/c1-5-39-35(40-6-2)30-18-14-27(15-19-30)22-32-25-37(24-29-12-10-9-11-13-29)26-33(34(32)38)23-28-16-20-31(21-17-28)36(41-7-3)42-8-4/h9-23,35-36H,5-8,24-26H2,1-4H3/b32-22+,33-23+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.17E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Kebangsaan Malaysia
Curated by ChEMBL
| Assay Description
Inhibition of ovine COX1 using arachidonic acid substrate assessed as PGE2 production ELISA method after 2 mins |
Bioorg Med Chem 22: 4151-61 (2014)
Article DOI: 10.1016/j.bmc.2014.05.052
BindingDB Entry DOI: 10.7270/Q2GX4D6F |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Ovis aries (Sheep)) | BDBM50021463
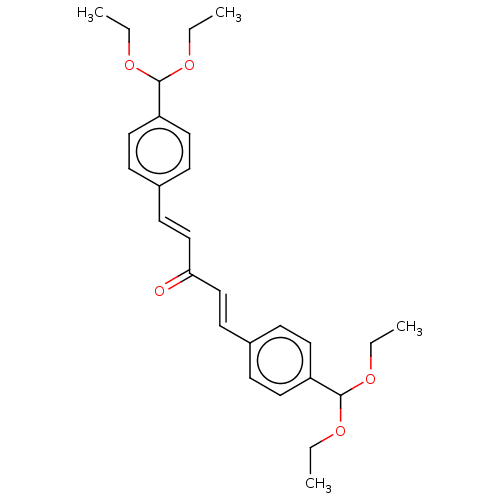
(CHEMBL3289929)Show SMILES CCOC(OCC)c1ccc(\C=C\C(=O)\C=C\c2ccc(cc2)C(OCC)OCC)cc1 Show InChI InChI=1S/C27H34O5/c1-5-29-26(30-6-2)23-15-9-21(10-16-23)13-19-25(28)20-14-22-11-17-24(18-12-22)27(31-7-3)32-8-4/h9-20,26-27H,5-8H2,1-4H3/b19-13+,20-14+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.22E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Kebangsaan Malaysia
Curated by ChEMBL
| Assay Description
Inhibition of ovine COX1 using arachidonic acid substrate assessed as PGE2 production ELISA method after 2 mins |
Bioorg Med Chem 22: 4151-61 (2014)
Article DOI: 10.1016/j.bmc.2014.05.052
BindingDB Entry DOI: 10.7270/Q2GX4D6F |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM17638

(2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...)Show SMILES COc1ccc2n(C(=O)c3ccc(Cl)cc3)c(C)c(CC(O)=O)c2c1 Show InChI InChI=1S/C19H16ClNO4/c1-11-15(10-18(22)23)16-9-14(25-2)7-8-17(16)21(11)19(24)12-3-5-13(20)6-4-12/h3-9H,10H2,1-2H3,(H,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 3.24E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Kebangsaan Malaysia
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant COX2 using arachidonic acid substrate assessed as PGE2 production ELISA method after 2 mins |
Bioorg Med Chem 22: 4151-61 (2014)
Article DOI: 10.1016/j.bmc.2014.05.052
BindingDB Entry DOI: 10.7270/Q2GX4D6F |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin G/H synthase 1
(Ovis aries (Sheep)) | BDBM50021473
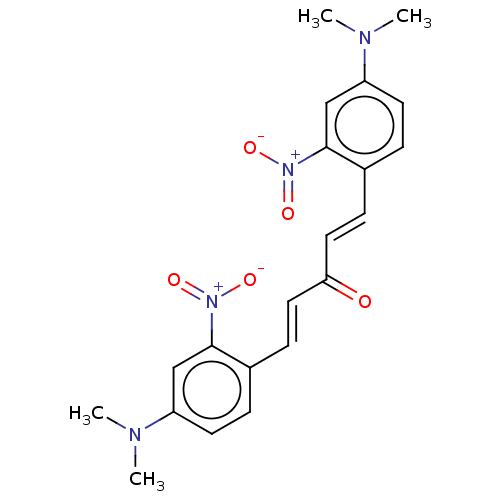
(CHEMBL3289939)Show SMILES CN(C)c1ccc(\C=C\C(=O)\C=C\c2ccc(cc2[N+]([O-])=O)N(C)C)c(c1)[N+]([O-])=O Show InChI InChI=1S/C21H22N4O5/c1-22(2)17-9-5-15(20(13-17)24(27)28)7-11-19(26)12-8-16-6-10-18(23(3)4)14-21(16)25(29)30/h5-14H,1-4H3/b11-7+,12-8+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.96E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Kebangsaan Malaysia
Curated by ChEMBL
| Assay Description
Inhibition of ovine COX1 using arachidonic acid substrate assessed as PGE2 production ELISA method after 2 mins |
Bioorg Med Chem 22: 4151-61 (2014)
Article DOI: 10.1016/j.bmc.2014.05.052
BindingDB Entry DOI: 10.7270/Q2GX4D6F |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50045848

(CHEMBL3309948)Show SMILES CN(C)c1ccc(\C=C2/CNC\C(=C/c3ccc(cc3[N+]([O-])=O)N(C)C)C2=O)c(c1)[N+]([O-])=O Show InChI InChI=1S/C23H25N5O5/c1-25(2)19-7-5-15(21(11-19)27(30)31)9-17-13-24-14-18(23(17)29)10-16-6-8-20(26(3)4)12-22(16)28(32)33/h5-12,24H,13-14H2,1-4H3/b17-9+,18-10+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7.88E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Kebangsaan Malaysia
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant COX2 using arachidonic acid substrate assessed as PGE2 production ELISA method after 2 mins |
Bioorg Med Chem 22: 4151-61 (2014)
Article DOI: 10.1016/j.bmc.2014.05.052
BindingDB Entry DOI: 10.7270/Q2GX4D6F |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Ovis aries (Sheep)) | BDBM50021466
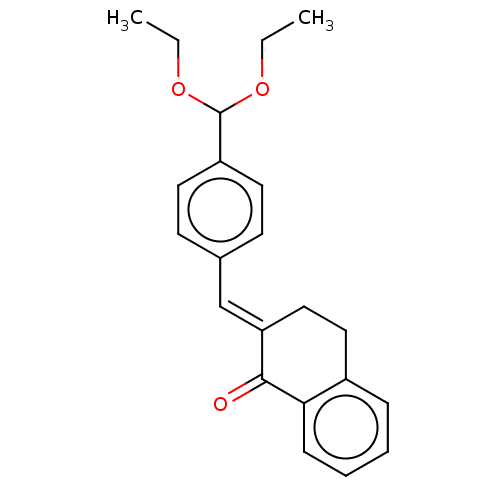
(CHEMBL3289932)Show InChI InChI=1S/C22H24O3/c1-3-24-22(25-4-2)18-11-9-16(10-12-18)15-19-14-13-17-7-5-6-8-20(17)21(19)23/h5-12,15,22H,3-4,13-14H2,1-2H3/b19-15+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.06E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Kebangsaan Malaysia
Curated by ChEMBL
| Assay Description
Inhibition of ovine COX1 using arachidonic acid substrate assessed as PGE2 production ELISA method after 2 mins |
Bioorg Med Chem 22: 4151-61 (2014)
Article DOI: 10.1016/j.bmc.2014.05.052
BindingDB Entry DOI: 10.7270/Q2GX4D6F |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50045849

(CHEMBL3309949)Show SMILES CN(C)c1ccc(\C=C2/CN(C)C\C(=C/c3ccc(cc3[N+]([O-])=O)N(C)C)C2=O)c(c1)[N+]([O-])=O Show InChI InChI=1S/C24H27N5O5/c1-25(2)20-8-6-16(22(12-20)28(31)32)10-18-14-27(5)15-19(24(18)30)11-17-7-9-21(26(3)4)13-23(17)29(33)34/h6-13H,14-15H2,1-5H3/b18-10+,19-11+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 9.12E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Kebangsaan Malaysia
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant COX2 using arachidonic acid substrate assessed as PGE2 production ELISA method after 2 mins |
Bioorg Med Chem 22: 4151-61 (2014)
Article DOI: 10.1016/j.bmc.2014.05.052
BindingDB Entry DOI: 10.7270/Q2GX4D6F |
More data for this
Ligand-Target Pair | |
Lipoxygenase
(Glycine max) | BDBM32020
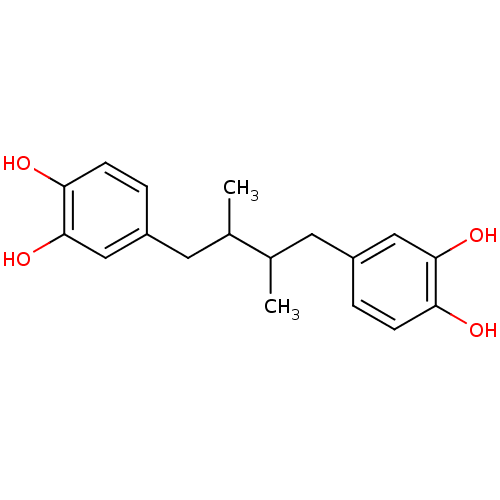
(4-[4-(3,4-dihydroxyphenyl)-2,3-dimethyl-butyl]pyro...)Show InChI InChI=1S/C18H22O4/c1-11(7-13-3-5-15(19)17(21)9-13)12(2)8-14-4-6-16(20)18(22)10-14/h3-6,9-12,19-22H,7-8H2,1-2H3 | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 9.23E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Kebangsaan Malaysia
Curated by ChEMBL
| Assay Description
Inhibition of soybean lipoxygenase after 5 mins by colorimetric assay |
Bioorg Med Chem 22: 4151-61 (2014)
Article DOI: 10.1016/j.bmc.2014.05.052
BindingDB Entry DOI: 10.7270/Q2GX4D6F |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Ovis aries (Sheep)) | BDBM50021475
(CHEMBL3289941)Show SMILES CN(C)c1ccc(\C=C2/Cc3ccccc3C2=O)c(c1)[N+]([O-])=O Show InChI InChI=1S/C18H16N2O3/c1-19(2)15-8-7-13(17(11-15)20(22)23)10-14-9-12-5-3-4-6-16(12)18(14)21/h3-8,10-11H,9H2,1-2H3/b14-10+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.91E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Kebangsaan Malaysia
Curated by ChEMBL
| Assay Description
Inhibition of ovine COX1 using arachidonic acid substrate assessed as PGE2 production ELISA method after 2 mins |
Bioorg Med Chem 22: 4151-61 (2014)
Article DOI: 10.1016/j.bmc.2014.05.052
BindingDB Entry DOI: 10.7270/Q2GX4D6F |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50021418

(CHEMBL3289925)Show SMILES CCOC(OCC)c1ccc(\C=C2/COC\C(=C/c3ccc(cc3)C(OCC)OCC)C2=O)cc1 Show InChI InChI=1S/C29H36O6/c1-5-32-28(33-6-2)23-13-9-21(10-14-23)17-25-19-31-20-26(27(25)30)18-22-11-15-24(16-12-22)29(34-7-3)35-8-4/h9-18,28-29H,5-8,19-20H2,1-4H3/b25-17+,26-18+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.12E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Kebangsaan Malaysia
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant COX2 using arachidonic acid substrate assessed as PGE2 production ELISA method after 2 mins |
Bioorg Med Chem 22: 4151-61 (2014)
Article DOI: 10.1016/j.bmc.2014.05.052
BindingDB Entry DOI: 10.7270/Q2GX4D6F |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50021467

(CHEMBL3289933)Show SMILES CCOC(OCC)c1ccc(\C=C\C(=O)C2CCc3ccccc3C2=O)cc1 Show InChI InChI=1S/C24H26O4/c1-3-27-24(28-4-2)19-12-9-17(10-13-19)11-16-22(25)21-15-14-18-7-5-6-8-20(18)23(21)26/h5-13,16,21,24H,3-4,14-15H2,1-2H3/b16-11+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.33E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Kebangsaan Malaysia
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant COX2 using arachidonic acid substrate assessed as PGE2 production ELISA method after 2 mins |
Bioorg Med Chem 22: 4151-61 (2014)
Article DOI: 10.1016/j.bmc.2014.05.052
BindingDB Entry DOI: 10.7270/Q2GX4D6F |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Ovis aries (Sheep)) | BDBM50045850
(CHEMBL3309950)Show SMILES CN(C)c1ccc(\C=C2/CN(Cc3ccccc3)C\C(=C/c3ccc(cc3[N+]([O-])=O)N(C)C)C2=O)c(c1)[N+]([O-])=O Show InChI InChI=1S/C30H31N5O5/c1-31(2)26-12-10-22(28(16-26)34(37)38)14-24-19-33(18-21-8-6-5-7-9-21)20-25(30(24)36)15-23-11-13-27(32(3)4)17-29(23)35(39)40/h5-17H,18-20H2,1-4H3/b24-14+,25-15+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.46E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Kebangsaan Malaysia
Curated by ChEMBL
| Assay Description
Inhibition of ovine COX1 using arachidonic acid substrate assessed as PGE2 production ELISA method after 2 mins |
Bioorg Med Chem 22: 4151-61 (2014)
Article DOI: 10.1016/j.bmc.2014.05.052
BindingDB Entry DOI: 10.7270/Q2GX4D6F |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50021464

(CHEMBL3289930)Show SMILES CCOC(OCC)c1ccc(\C=C2/CC\C(=C/c3ccc(cc3)C(OCC)OCC)C2=O)cc1 Show InChI InChI=1S/C29H36O5/c1-5-31-28(32-6-2)23-13-9-21(10-14-23)19-25-17-18-26(27(25)30)20-22-11-15-24(16-12-22)29(33-7-3)34-8-4/h9-16,19-20,28-29H,5-8,17-18H2,1-4H3/b25-19+,26-20+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.48E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Kebangsaan Malaysia
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant COX2 using arachidonic acid substrate assessed as PGE2 production ELISA method after 2 mins |
Bioorg Med Chem 22: 4151-61 (2014)
Article DOI: 10.1016/j.bmc.2014.05.052
BindingDB Entry DOI: 10.7270/Q2GX4D6F |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50021475

(CHEMBL3289941)Show SMILES CN(C)c1ccc(\C=C2/Cc3ccccc3C2=O)c(c1)[N+]([O-])=O Show InChI InChI=1S/C18H16N2O3/c1-19(2)15-8-7-13(17(11-15)20(22)23)10-14-9-12-5-3-4-6-16(12)18(14)21/h3-8,10-11H,9H2,1-2H3/b14-10+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.49E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Kebangsaan Malaysia
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant COX2 using arachidonic acid substrate assessed as PGE2 production ELISA method after 2 mins |
Bioorg Med Chem 22: 4151-61 (2014)
Article DOI: 10.1016/j.bmc.2014.05.052
BindingDB Entry DOI: 10.7270/Q2GX4D6F |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50021473

(CHEMBL3289939)Show SMILES CN(C)c1ccc(\C=C\C(=O)\C=C\c2ccc(cc2[N+]([O-])=O)N(C)C)c(c1)[N+]([O-])=O Show InChI InChI=1S/C21H22N4O5/c1-22(2)17-9-5-15(20(13-17)24(27)28)7-11-19(26)12-8-16-6-10-18(23(3)4)14-21(16)25(29)30/h5-14H,1-4H3/b11-7+,12-8+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Kebangsaan Malaysia
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant COX2 using arachidonic acid substrate assessed as PGE2 production ELISA method after 2 mins |
Bioorg Med Chem 22: 4151-61 (2014)
Article DOI: 10.1016/j.bmc.2014.05.052
BindingDB Entry DOI: 10.7270/Q2GX4D6F |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50021417

(CHEMBL3289924)Show SMILES CCOC(OCC)c1ccc(\C=C2/CCC\C(=C/c3ccc(cc3)C(OCC)OCC)C2=O)cc1 Show InChI InChI=1S/C30H38O5/c1-5-32-29(33-6-2)24-16-12-22(13-17-24)20-26-10-9-11-27(28(26)31)21-23-14-18-25(19-15-23)30(34-7-3)35-8-4/h12-21,29-30H,5-11H2,1-4H3/b26-20+,27-21+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.74E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Kebangsaan Malaysia
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant COX2 using arachidonic acid substrate assessed as PGE2 production ELISA method after 2 mins |
Bioorg Med Chem 22: 4151-61 (2014)
Article DOI: 10.1016/j.bmc.2014.05.052
BindingDB Entry DOI: 10.7270/Q2GX4D6F |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50045847

(CHEMBL3309947)Show SMILES CN(C)c1ccc(\C=C2/COC\C(=C/c3ccc(cc3[N+]([O-])=O)N(C)C)C2=O)c(c1)[N+]([O-])=O Show InChI InChI=1S/C23H24N4O6/c1-24(2)19-7-5-15(21(11-19)26(29)30)9-17-13-33-14-18(23(17)28)10-16-6-8-20(25(3)4)12-22(16)27(31)32/h5-12H,13-14H2,1-4H3/b17-9+,18-10+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.86E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Kebangsaan Malaysia
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant COX2 using arachidonic acid substrate assessed as PGE2 production ELISA method after 2 mins |
Bioorg Med Chem 22: 4151-61 (2014)
Article DOI: 10.1016/j.bmc.2014.05.052
BindingDB Entry DOI: 10.7270/Q2GX4D6F |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50045850

(CHEMBL3309950)Show SMILES CN(C)c1ccc(\C=C2/CN(Cc3ccccc3)C\C(=C/c3ccc(cc3[N+]([O-])=O)N(C)C)C2=O)c(c1)[N+]([O-])=O Show InChI InChI=1S/C30H31N5O5/c1-31(2)26-12-10-22(28(16-26)34(37)38)14-24-19-33(18-21-8-6-5-7-9-21)20-25(30(24)36)15-23-11-13-27(32(3)4)17-29(23)35(39)40/h5-17H,18-20H2,1-4H3/b24-14+,25-15+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.92E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Kebangsaan Malaysia
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant COX2 using arachidonic acid substrate assessed as PGE2 production ELISA method after 2 mins |
Bioorg Med Chem 22: 4151-61 (2014)
Article DOI: 10.1016/j.bmc.2014.05.052
BindingDB Entry DOI: 10.7270/Q2GX4D6F |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50021465

(CHEMBL3289931)Show InChI InChI=1S/C21H22O3/c1-3-23-21(24-4-2)16-11-9-15(10-12-16)13-18-14-17-7-5-6-8-19(17)20(18)22/h5-13,21H,3-4,14H2,1-2H3/b18-13+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.93E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Kebangsaan Malaysia
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant COX2 using arachidonic acid substrate assessed as PGE2 production ELISA method after 2 mins |
Bioorg Med Chem 22: 4151-61 (2014)
Article DOI: 10.1016/j.bmc.2014.05.052
BindingDB Entry DOI: 10.7270/Q2GX4D6F |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Ovis aries (Sheep)) | BDBM50021476
(CHEMBL3289942)Show SMILES CN(C)c1ccc(\C=C2/CCc3ccccc3C2=O)c(c1)[N+]([O-])=O Show InChI InChI=1S/C19H18N2O3/c1-20(2)16-10-9-14(18(12-16)21(23)24)11-15-8-7-13-5-3-4-6-17(13)19(15)22/h3-6,9-12H,7-8H2,1-2H3/b15-11+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.15E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Kebangsaan Malaysia
Curated by ChEMBL
| Assay Description
Inhibition of ovine COX1 using arachidonic acid substrate assessed as PGE2 production ELISA method after 2 mins |
Bioorg Med Chem 22: 4151-61 (2014)
Article DOI: 10.1016/j.bmc.2014.05.052
BindingDB Entry DOI: 10.7270/Q2GX4D6F |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50021447

(CHEMBL3289926)Show SMILES CCOC(OCC)c1ccc(\C=C2/CNC\C(=C/c3ccc(cc3)C(OCC)OCC)C2=O)cc1 Show InChI InChI=1S/C29H37NO5/c1-5-32-28(33-6-2)23-13-9-21(10-14-23)17-25-19-30-20-26(27(25)31)18-22-11-15-24(16-12-22)29(34-7-3)35-8-4/h9-18,28-30H,5-8,19-20H2,1-4H3/b25-17+,26-18+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.77E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Kebangsaan Malaysia
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant COX2 using arachidonic acid substrate assessed as PGE2 production ELISA method after 2 mins |
Bioorg Med Chem 22: 4151-61 (2014)
Article DOI: 10.1016/j.bmc.2014.05.052
BindingDB Entry DOI: 10.7270/Q2GX4D6F |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50021476

(CHEMBL3289942)Show SMILES CN(C)c1ccc(\C=C2/CCc3ccccc3C2=O)c(c1)[N+]([O-])=O Show InChI InChI=1S/C19H18N2O3/c1-20(2)16-10-9-14(18(12-16)21(23)24)11-15-8-7-13-5-3-4-6-17(13)19(15)22/h3-6,9-12H,7-8H2,1-2H3/b15-11+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Kebangsaan Malaysia
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant COX2 using arachidonic acid substrate assessed as PGE2 production ELISA method after 2 mins |
Bioorg Med Chem 22: 4151-61 (2014)
Article DOI: 10.1016/j.bmc.2014.05.052
BindingDB Entry DOI: 10.7270/Q2GX4D6F |
More data for this
Ligand-Target Pair | |
Lipoxygenase
(Glycine max) | BDBM50021468

(CHEMBL3289934)Show SMILES CN(C)c1ccc(\C=C2\CCC\C(=C/c3ccc(cc3[N+]([O-])=O)N(C)C)C2=O)c(c1)[N+]([O-])=O Show InChI InChI=1S/C24H26N4O5/c1-25(2)20-10-8-16(22(14-20)27(30)31)12-18-6-5-7-19(24(18)29)13-17-9-11-21(26(3)4)15-23(17)28(32)33/h8-15H,5-7H2,1-4H3/b18-12-,19-13+ | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.93E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Kebangsaan Malaysia
Curated by ChEMBL
| Assay Description
Inhibition of soybean lipoxygenase after 5 mins by colorimetric assay |
Bioorg Med Chem 22: 4151-61 (2014)
Article DOI: 10.1016/j.bmc.2014.05.052
BindingDB Entry DOI: 10.7270/Q2GX4D6F |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Ovis aries (Sheep)) | BDBM50045851
(CHEMBL3357954)Show SMILES CN(C)c1ccc(\C=C\C(=O)C2CCc3ccccc3C2=O)c(c1)[N+]([O-])=O Show InChI InChI=1S/C21H20N2O4/c1-22(2)16-10-7-15(19(13-16)23(26)27)9-12-20(24)18-11-8-14-5-3-4-6-17(14)21(18)25/h3-7,9-10,12-13,18H,8,11H2,1-2H3/b12-9+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.93E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Kebangsaan Malaysia
Curated by ChEMBL
| Assay Description
Inhibition of ovine COX1 using arachidonic acid substrate assessed as PGE2 production ELISA method after 2 mins |
Bioorg Med Chem 22: 4151-61 (2014)
Article DOI: 10.1016/j.bmc.2014.05.052
BindingDB Entry DOI: 10.7270/Q2GX4D6F |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Ovis aries (Sheep)) | BDBM50067040
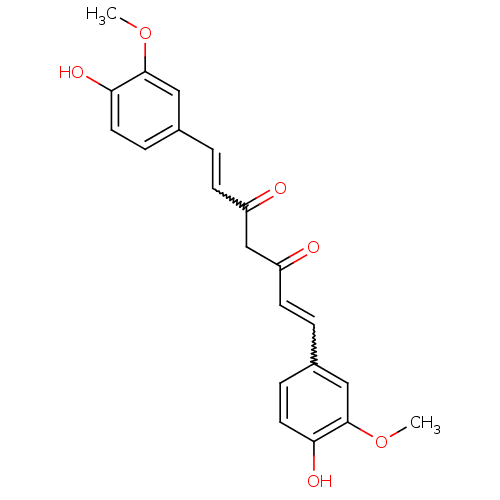
(((E,E)-1,7-bis(4-hydroxy-3-methoxyphenyl)-1,6-hept...)Show SMILES COc1cc(C=CC(=O)CC(=O)C=Cc2ccc(O)c(OC)c2)ccc1O |w:12.11,5.4| Show InChI InChI=1S/C21H20O6/c1-26-20-11-14(5-9-18(20)24)3-7-16(22)13-17(23)8-4-15-6-10-19(25)21(12-15)27-2/h3-12,24-25H,13H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.97E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Kebangsaan Malaysia
Curated by ChEMBL
| Assay Description
Inhibition of ovine COX1 using arachidonic acid substrate assessed as PGE2 production ELISA method after 2 mins |
Bioorg Med Chem 22: 4151-61 (2014)
Article DOI: 10.1016/j.bmc.2014.05.052
BindingDB Entry DOI: 10.7270/Q2GX4D6F |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50021463

(CHEMBL3289929)Show SMILES CCOC(OCC)c1ccc(\C=C\C(=O)\C=C\c2ccc(cc2)C(OCC)OCC)cc1 Show InChI InChI=1S/C27H34O5/c1-5-29-26(30-6-2)23-15-9-21(10-16-23)13-19-25(28)20-14-22-11-17-24(18-12-22)27(31-7-3)32-8-4/h9-20,26-27H,5-8H2,1-4H3/b19-13+,20-14+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.15E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Kebangsaan Malaysia
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant COX2 using arachidonic acid substrate assessed as PGE2 production ELISA method after 2 mins |
Bioorg Med Chem 22: 4151-61 (2014)
Article DOI: 10.1016/j.bmc.2014.05.052
BindingDB Entry DOI: 10.7270/Q2GX4D6F |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Ovis aries (Sheep)) | BDBM50021487
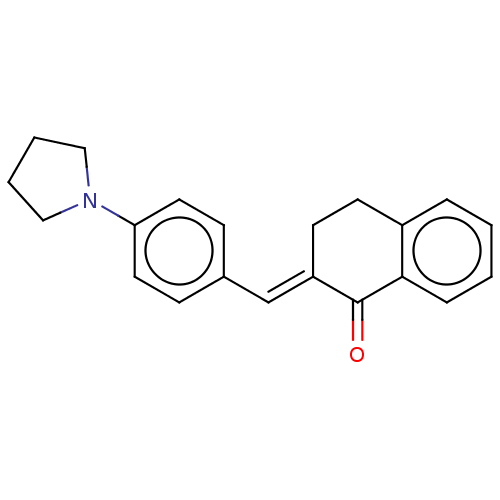
(CHEMBL3290195)Show InChI InChI=1S/C21H21NO/c23-21-18(10-9-17-5-1-2-6-20(17)21)15-16-7-11-19(12-8-16)22-13-3-4-14-22/h1-2,5-8,11-12,15H,3-4,9-10,13-14H2/b18-15+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.35E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Kebangsaan Malaysia
Curated by ChEMBL
| Assay Description
Inhibition of ovine COX1 using arachidonic acid substrate assessed as PGE2 production ELISA method after 2 mins |
Bioorg Med Chem 22: 4151-61 (2014)
Article DOI: 10.1016/j.bmc.2014.05.052
BindingDB Entry DOI: 10.7270/Q2GX4D6F |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Ovis aries (Sheep)) | BDBM50045852

(CHEMBL3309953)Show SMILES O=C1\C(CCC\C1=C/c1ccc(cc1)N1CCCC1)=C\c1ccc(cc1)N1CCCC1 Show InChI InChI=1S/C28H32N2O/c31-28-24(20-22-8-12-26(13-9-22)29-16-1-2-17-29)6-5-7-25(28)21-23-10-14-27(15-11-23)30-18-3-4-19-30/h8-15,20-21H,1-7,16-19H2/b24-20+,25-21+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.35E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Kebangsaan Malaysia
Curated by ChEMBL
| Assay Description
Inhibition of ovine COX1 using arachidonic acid substrate assessed as PGE2 production ELISA method after 2 mins |
Bioorg Med Chem 22: 4151-61 (2014)
Article DOI: 10.1016/j.bmc.2014.05.052
BindingDB Entry DOI: 10.7270/Q2GX4D6F |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50045851

(CHEMBL3357954)Show SMILES CN(C)c1ccc(\C=C\C(=O)C2CCc3ccccc3C2=O)c(c1)[N+]([O-])=O Show InChI InChI=1S/C21H20N2O4/c1-22(2)16-10-7-15(19(13-16)23(26)27)9-12-20(24)18-11-8-14-5-3-4-6-17(14)21(18)25/h3-7,9-10,12-13,18H,8,11H2,1-2H3/b12-9+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.77E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Kebangsaan Malaysia
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant COX2 using arachidonic acid substrate assessed as PGE2 production ELISA method after 2 mins |
Bioorg Med Chem 22: 4151-61 (2014)
Article DOI: 10.1016/j.bmc.2014.05.052
BindingDB Entry DOI: 10.7270/Q2GX4D6F |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Ovis aries (Sheep)) | BDBM50021485
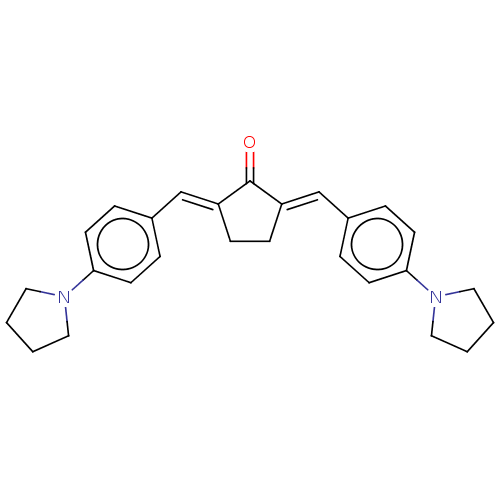
(CHEMBL3290193)Show SMILES O=C1\C(CC\C1=C/c1ccc(cc1)N1CCCC1)=C\c1ccc(cc1)N1CCCC1 Show InChI InChI=1S/C27H30N2O/c30-27-23(19-21-5-11-25(12-6-21)28-15-1-2-16-28)9-10-24(27)20-22-7-13-26(14-8-22)29-17-3-4-18-29/h5-8,11-14,19-20H,1-4,9-10,15-18H2/b23-19+,24-20+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.78E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Kebangsaan Malaysia
Curated by ChEMBL
| Assay Description
Inhibition of ovine COX1 using arachidonic acid substrate assessed as PGE2 production ELISA method after 2 mins |
Bioorg Med Chem 22: 4151-61 (2014)
Article DOI: 10.1016/j.bmc.2014.05.052
BindingDB Entry DOI: 10.7270/Q2GX4D6F |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50021461

(CHEMBL3289927)Show SMILES CCOC(OCC)c1ccc(\C=C2/CN(C)C\C(=C/c3ccc(cc3)C(OCC)OCC)C2=O)cc1 Show InChI InChI=1S/C30H39NO5/c1-6-33-29(34-7-2)24-14-10-22(11-15-24)18-26-20-31(5)21-27(28(26)32)19-23-12-16-25(17-13-23)30(35-8-3)36-9-4/h10-19,29-30H,6-9,20-21H2,1-5H3/b26-18+,27-19+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.82E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Kebangsaan Malaysia
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant COX2 using arachidonic acid substrate assessed as PGE2 production ELISA method after 2 mins |
Bioorg Med Chem 22: 4151-61 (2014)
Article DOI: 10.1016/j.bmc.2014.05.052
BindingDB Entry DOI: 10.7270/Q2GX4D6F |
More data for this
Ligand-Target Pair | |
Lipoxygenase
(Glycine max) | BDBM50045849

(CHEMBL3309949)Show SMILES CN(C)c1ccc(\C=C2/CN(C)C\C(=C/c3ccc(cc3[N+]([O-])=O)N(C)C)C2=O)c(c1)[N+]([O-])=O Show InChI InChI=1S/C24H27N5O5/c1-25(2)20-8-6-16(22(12-20)28(31)32)10-18-14-27(5)15-19(24(18)30)11-17-7-9-21(26(3)4)13-23(17)29(33)34/h6-13H,14-15H2,1-5H3/b18-10+,19-11+ | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.83E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Kebangsaan Malaysia
Curated by ChEMBL
| Assay Description
Inhibition of soybean lipoxygenase after 5 mins by colorimetric assay |
Bioorg Med Chem 22: 4151-61 (2014)
Article DOI: 10.1016/j.bmc.2014.05.052
BindingDB Entry DOI: 10.7270/Q2GX4D6F |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Ovis aries (Sheep)) | BDBM50045853
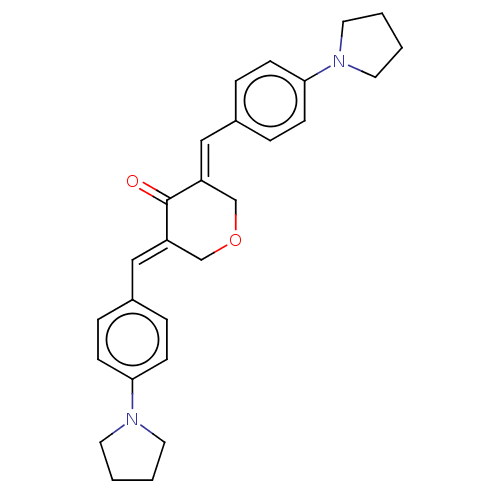
(CHEMBL3309954)Show SMILES O=C1\C(COC\C1=C/c1ccc(cc1)N1CCCC1)=C\c1ccc(cc1)N1CCCC1 Show InChI InChI=1S/C27H30N2O2/c30-27-23(17-21-5-9-25(10-6-21)28-13-1-2-14-28)19-31-20-24(27)18-22-7-11-26(12-8-22)29-15-3-4-16-29/h5-12,17-18H,1-4,13-16,19-20H2/b23-17+,24-18+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.94E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Kebangsaan Malaysia
Curated by ChEMBL
| Assay Description
Inhibition of ovine COX1 using arachidonic acid substrate assessed as PGE2 production ELISA method after 2 mins |
Bioorg Med Chem 22: 4151-61 (2014)
Article DOI: 10.1016/j.bmc.2014.05.052
BindingDB Entry DOI: 10.7270/Q2GX4D6F |
More data for this
Ligand-Target Pair | |
Lipoxygenase
(Glycine max) | BDBM50045848

(CHEMBL3309948)Show SMILES CN(C)c1ccc(\C=C2/CNC\C(=C/c3ccc(cc3[N+]([O-])=O)N(C)C)C2=O)c(c1)[N+]([O-])=O Show InChI InChI=1S/C23H25N5O5/c1-25(2)19-7-5-15(21(11-19)27(30)31)9-17-13-24-14-18(23(17)29)10-16-6-8-20(26(3)4)12-22(16)28(32)33/h5-12,24H,13-14H2,1-4H3/b17-9+,18-10+ | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.95E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Kebangsaan Malaysia
Curated by ChEMBL
| Assay Description
Inhibition of soybean lipoxygenase after 5 mins by colorimetric assay |
Bioorg Med Chem 22: 4151-61 (2014)
Article DOI: 10.1016/j.bmc.2014.05.052
BindingDB Entry DOI: 10.7270/Q2GX4D6F |
More data for this
Ligand-Target Pair | |
Lipoxygenase
(Glycine max) | BDBM50045847

(CHEMBL3309947)Show SMILES CN(C)c1ccc(\C=C2/COC\C(=C/c3ccc(cc3[N+]([O-])=O)N(C)C)C2=O)c(c1)[N+]([O-])=O Show InChI InChI=1S/C23H24N4O6/c1-24(2)19-7-5-15(21(11-19)26(29)30)9-17-13-33-14-18(23(17)28)10-16-6-8-20(25(3)4)12-22(16)27(31)32/h5-12H,13-14H2,1-4H3/b17-9+,18-10+ | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.47E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Kebangsaan Malaysia
Curated by ChEMBL
| Assay Description
Inhibition of soybean lipoxygenase after 5 mins by colorimetric assay |
Bioorg Med Chem 22: 4151-61 (2014)
Article DOI: 10.1016/j.bmc.2014.05.052
BindingDB Entry DOI: 10.7270/Q2GX4D6F |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50021485

(CHEMBL3290193)Show SMILES O=C1\C(CC\C1=C/c1ccc(cc1)N1CCCC1)=C\c1ccc(cc1)N1CCCC1 Show InChI InChI=1S/C27H30N2O/c30-27-23(19-21-5-11-25(12-6-21)28-15-1-2-16-28)9-10-24(27)20-22-7-13-26(14-8-22)29-17-3-4-18-29/h5-8,11-14,19-20H,1-4,9-10,15-18H2/b23-19+,24-20+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.62E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Kebangsaan Malaysia
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant COX2 using arachidonic acid substrate assessed as PGE2 production ELISA method after 2 mins |
Bioorg Med Chem 22: 4151-61 (2014)
Article DOI: 10.1016/j.bmc.2014.05.052
BindingDB Entry DOI: 10.7270/Q2GX4D6F |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Ovis aries (Sheep)) | BDBM50021483
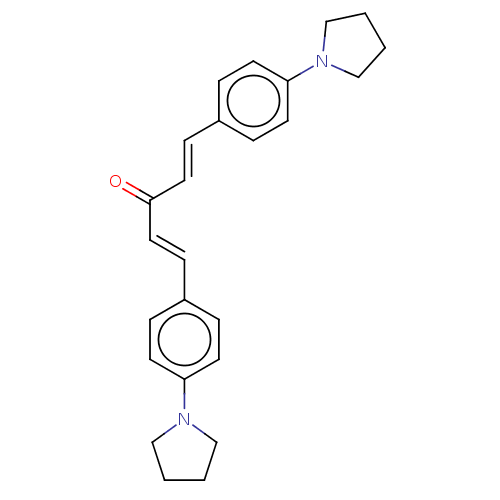
(CHEMBL3290192)Show SMILES O=C(\C=C\c1ccc(cc1)N1CCCC1)/C=C/c1ccc(cc1)N1CCCC1 Show InChI InChI=1S/C25H28N2O/c28-25(15-9-21-5-11-23(12-6-21)26-17-1-2-18-26)16-10-22-7-13-24(14-8-22)27-19-3-4-20-27/h5-16H,1-4,17-20H2/b15-9+,16-10+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.93E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Kebangsaan Malaysia
Curated by ChEMBL
| Assay Description
Inhibition of ovine COX1 using arachidonic acid substrate assessed as PGE2 production ELISA method after 2 mins |
Bioorg Med Chem 22: 4151-61 (2014)
Article DOI: 10.1016/j.bmc.2014.05.052
BindingDB Entry DOI: 10.7270/Q2GX4D6F |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Ovis aries (Sheep)) | BDBM50046029

(CHEMBL3309957)Show SMILES O=C1\C(CN(Cc2ccccc2)C\C1=C/c1ccc(cc1)N1CCCC1)=C\c1ccc(cc1)N1CCCC1 Show InChI InChI=1S/C34H37N3O/c38-34-30(22-27-10-14-32(15-11-27)36-18-4-5-19-36)25-35(24-29-8-2-1-3-9-29)26-31(34)23-28-12-16-33(17-13-28)37-20-6-7-21-37/h1-3,8-17,22-23H,4-7,18-21,24-26H2/b30-22+,31-23+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.13E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Kebangsaan Malaysia
Curated by ChEMBL
| Assay Description
Inhibition of ovine COX1 using arachidonic acid substrate assessed as PGE2 production ELISA method after 2 mins |
Bioorg Med Chem 22: 4151-61 (2014)
Article DOI: 10.1016/j.bmc.2014.05.052
BindingDB Entry DOI: 10.7270/Q2GX4D6F |
More data for this
Ligand-Target Pair | |
Lipoxygenase
(Glycine max) | BDBM50021467

(CHEMBL3289933)Show SMILES CCOC(OCC)c1ccc(\C=C\C(=O)C2CCc3ccccc3C2=O)cc1 Show InChI InChI=1S/C24H26O4/c1-3-27-24(28-4-2)19-12-9-17(10-13-19)11-16-22(25)21-15-14-18-7-5-6-8-20(18)23(21)26/h5-13,16,21,24H,3-4,14-15H2,1-2H3/b16-11+ | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.22E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Kebangsaan Malaysia
Curated by ChEMBL
| Assay Description
Inhibition of soybean lipoxygenase after 5 mins by colorimetric assay |
Bioorg Med Chem 22: 4151-61 (2014)
Article DOI: 10.1016/j.bmc.2014.05.052
BindingDB Entry DOI: 10.7270/Q2GX4D6F |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50045852

(CHEMBL3309953)Show SMILES O=C1\C(CCC\C1=C/c1ccc(cc1)N1CCCC1)=C\c1ccc(cc1)N1CCCC1 Show InChI InChI=1S/C28H32N2O/c31-28-24(20-22-8-12-26(13-9-22)29-16-1-2-17-29)6-5-7-25(28)21-23-10-14-27(15-11-23)30-18-3-4-19-30/h8-15,20-21H,1-7,16-19H2/b24-20+,25-21+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.43E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Kebangsaan Malaysia
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant COX2 using arachidonic acid substrate assessed as PGE2 production ELISA method after 2 mins |
Bioorg Med Chem 22: 4151-61 (2014)
Article DOI: 10.1016/j.bmc.2014.05.052
BindingDB Entry DOI: 10.7270/Q2GX4D6F |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50021486

(CHEMBL3290194)Show InChI InChI=1S/C20H19NO/c22-20-17(14-16-5-1-2-6-19(16)20)13-15-7-9-18(10-8-15)21-11-3-4-12-21/h1-2,5-10,13H,3-4,11-12,14H2/b17-13+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.46E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Kebangsaan Malaysia
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant COX2 using arachidonic acid substrate assessed as PGE2 production ELISA method after 2 mins |
Bioorg Med Chem 22: 4151-61 (2014)
Article DOI: 10.1016/j.bmc.2014.05.052
BindingDB Entry DOI: 10.7270/Q2GX4D6F |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50046029

(CHEMBL3309957)Show SMILES O=C1\C(CN(Cc2ccccc2)C\C1=C/c1ccc(cc1)N1CCCC1)=C\c1ccc(cc1)N1CCCC1 Show InChI InChI=1S/C34H37N3O/c38-34-30(22-27-10-14-32(15-11-27)36-18-4-5-19-36)25-35(24-29-8-2-1-3-9-29)26-31(34)23-28-12-16-33(17-13-28)37-20-6-7-21-37/h1-3,8-17,22-23H,4-7,18-21,24-26H2/b30-22+,31-23+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.72E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Kebangsaan Malaysia
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant COX2 using arachidonic acid substrate assessed as PGE2 production ELISA method after 2 mins |
Bioorg Med Chem 22: 4151-61 (2014)
Article DOI: 10.1016/j.bmc.2014.05.052
BindingDB Entry DOI: 10.7270/Q2GX4D6F |
More data for this
Ligand-Target Pair | |
Lipoxygenase
(Glycine max) | BDBM50067040

(((E,E)-1,7-bis(4-hydroxy-3-methoxyphenyl)-1,6-hept...)Show SMILES COc1cc(C=CC(=O)CC(=O)C=Cc2ccc(O)c(OC)c2)ccc1O |w:12.11,5.4| Show InChI InChI=1S/C21H20O6/c1-26-20-11-14(5-9-18(20)24)3-7-16(22)13-17(23)8-4-15-6-10-19(25)21(12-15)27-2/h3-12,24-25H,13H2,1-2H3 | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.95E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Kebangsaan Malaysia
Curated by ChEMBL
| Assay Description
Inhibition of soybean lipoxygenase after 5 mins by colorimetric assay |
Bioorg Med Chem 22: 4151-61 (2014)
Article DOI: 10.1016/j.bmc.2014.05.052
BindingDB Entry DOI: 10.7270/Q2GX4D6F |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50021488

(CHEMBL3290196)Show SMILES O=C(\C=C\c1ccc(cc1)N1CCCC1)C1CCc2ccccc2C1=O Show InChI InChI=1S/C23H23NO2/c25-22(21-13-10-18-5-1-2-6-20(18)23(21)26)14-9-17-7-11-19(12-8-17)24-15-3-4-16-24/h1-2,5-9,11-12,14,21H,3-4,10,13,15-16H2/b14-9+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.12E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Kebangsaan Malaysia
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant COX2 using arachidonic acid substrate assessed as PGE2 production ELISA method after 2 mins |
Bioorg Med Chem 22: 4151-61 (2014)
Article DOI: 10.1016/j.bmc.2014.05.052
BindingDB Entry DOI: 10.7270/Q2GX4D6F |
More data for this
Ligand-Target Pair | |
Lipoxygenase
(Glycine max) | BDBM50021461

(CHEMBL3289927)Show SMILES CCOC(OCC)c1ccc(\C=C2/CN(C)C\C(=C/c3ccc(cc3)C(OCC)OCC)C2=O)cc1 Show InChI InChI=1S/C30H39NO5/c1-6-33-29(34-7-2)24-14-10-22(11-15-24)18-26-20-31(5)21-27(28(26)32)19-23-12-16-25(17-13-23)30(35-8-3)36-9-4/h10-19,29-30H,6-9,20-21H2,1-5H3/b26-18+,27-19+ | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.56E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Kebangsaan Malaysia
Curated by ChEMBL
| Assay Description
Inhibition of soybean lipoxygenase after 5 mins by colorimetric assay |
Bioorg Med Chem 22: 4151-61 (2014)
Article DOI: 10.1016/j.bmc.2014.05.052
BindingDB Entry DOI: 10.7270/Q2GX4D6F |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Ovis aries (Sheep)) | BDBM50046028

(CHEMBL3309956)Show SMILES CN1C\C(=C/c2ccc(cc2)N2CCCC2)C(=O)\C(C1)=C\c1ccc(cc1)N1CCCC1 Show InChI InChI=1S/C28H33N3O/c1-29-20-24(18-22-6-10-26(11-7-22)30-14-2-3-15-30)28(32)25(21-29)19-23-8-12-27(13-9-23)31-16-4-5-17-31/h6-13,18-19H,2-5,14-17,20-21H2,1H3/b24-18+,25-19+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.92E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Kebangsaan Malaysia
Curated by ChEMBL
| Assay Description
Inhibition of ovine COX1 using arachidonic acid substrate assessed as PGE2 production ELISA method after 2 mins |
Bioorg Med Chem 22: 4151-61 (2014)
Article DOI: 10.1016/j.bmc.2014.05.052
BindingDB Entry DOI: 10.7270/Q2GX4D6F |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50021483

(CHEMBL3290192)Show SMILES O=C(\C=C\c1ccc(cc1)N1CCCC1)/C=C/c1ccc(cc1)N1CCCC1 Show InChI InChI=1S/C25H28N2O/c28-25(15-9-21-5-11-23(12-6-21)26-17-1-2-18-26)16-10-22-7-13-24(14-8-22)27-19-3-4-20-27/h5-16H,1-4,17-20H2/b15-9+,16-10+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.98E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Kebangsaan Malaysia
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant COX2 using arachidonic acid substrate assessed as PGE2 production ELISA method after 2 mins |
Bioorg Med Chem 22: 4151-61 (2014)
Article DOI: 10.1016/j.bmc.2014.05.052
BindingDB Entry DOI: 10.7270/Q2GX4D6F |
More data for this
Ligand-Target Pair | |
Lipoxygenase
(Glycine max) | BDBM50021417

(CHEMBL3289924)Show SMILES CCOC(OCC)c1ccc(\C=C2/CCC\C(=C/c3ccc(cc3)C(OCC)OCC)C2=O)cc1 Show InChI InChI=1S/C30H38O5/c1-5-32-29(33-6-2)24-16-12-22(13-17-24)20-26-10-9-11-27(28(26)31)21-23-14-18-25(19-15-23)30(34-7-3)35-8-4/h12-21,29-30H,5-11H2,1-4H3/b26-20+,27-21+ | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8.31E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Kebangsaan Malaysia
Curated by ChEMBL
| Assay Description
Inhibition of soybean lipoxygenase after 5 mins by colorimetric assay |
Bioorg Med Chem 22: 4151-61 (2014)
Article DOI: 10.1016/j.bmc.2014.05.052
BindingDB Entry DOI: 10.7270/Q2GX4D6F |
More data for this
Ligand-Target Pair | |
Lipoxygenase
(Glycine max) | BDBM50021447

(CHEMBL3289926)Show SMILES CCOC(OCC)c1ccc(\C=C2/CNC\C(=C/c3ccc(cc3)C(OCC)OCC)C2=O)cc1 Show InChI InChI=1S/C29H37NO5/c1-5-32-28(33-6-2)23-13-9-21(10-14-23)17-25-19-30-20-26(27(25)31)18-22-11-15-24(16-12-22)29(34-7-3)35-8-4/h9-18,28-30H,5-8,19-20H2,1-4H3/b25-17+,26-18+ | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.84E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Kebangsaan Malaysia
Curated by ChEMBL
| Assay Description
Inhibition of soybean lipoxygenase after 5 mins by colorimetric assay |
Bioorg Med Chem 22: 4151-61 (2014)
Article DOI: 10.1016/j.bmc.2014.05.052
BindingDB Entry DOI: 10.7270/Q2GX4D6F |
More data for this
Ligand-Target Pair | |
Lipoxygenase
(Glycine max) | BDBM50021418

(CHEMBL3289925)Show SMILES CCOC(OCC)c1ccc(\C=C2/COC\C(=C/c3ccc(cc3)C(OCC)OCC)C2=O)cc1 Show InChI InChI=1S/C29H36O6/c1-5-32-28(33-6-2)23-13-9-21(10-14-23)17-25-19-31-20-26(27(25)30)18-22-11-15-24(16-12-22)29(34-7-3)35-8-4/h9-18,28-29H,5-8,19-20H2,1-4H3/b25-17+,26-18+ | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Kebangsaan Malaysia
Curated by ChEMBL
| Assay Description
Inhibition of soybean lipoxygenase after 5 mins by colorimetric assay |
Bioorg Med Chem 22: 4151-61 (2014)
Article DOI: 10.1016/j.bmc.2014.05.052
BindingDB Entry DOI: 10.7270/Q2GX4D6F |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50021462

(CHEMBL3289928)Show SMILES CCOC(OCC)c1ccc(\C=C2/CN(Cc3ccccc3)C\C(=C/c3ccc(cc3)C(OCC)OCC)C2=O)cc1 Show InChI InChI=1S/C36H43NO5/c1-5-39-35(40-6-2)30-18-14-27(15-19-30)22-32-25-37(24-29-12-10-9-11-13-29)26-33(34(32)38)23-28-16-20-31(21-17-28)36(41-7-3)42-8-4/h9-23,35-36H,5-8,24-26H2,1-4H3/b32-22+,33-23+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Kebangsaan Malaysia
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant COX2 using arachidonic acid substrate assessed as PGE2 production ELISA method after 2 mins |
Bioorg Med Chem 22: 4151-61 (2014)
Article DOI: 10.1016/j.bmc.2014.05.052
BindingDB Entry DOI: 10.7270/Q2GX4D6F |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50021466

(CHEMBL3289932)Show InChI InChI=1S/C22H24O3/c1-3-24-22(25-4-2)18-11-9-16(10-12-18)15-19-14-13-17-7-5-6-8-20(17)21(19)23/h5-12,15,22H,3-4,13-14H2,1-2H3/b19-15+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Kebangsaan Malaysia
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant COX2 using arachidonic acid substrate assessed as PGE2 production ELISA method after 2 mins |
Bioorg Med Chem 22: 4151-61 (2014)
Article DOI: 10.1016/j.bmc.2014.05.052
BindingDB Entry DOI: 10.7270/Q2GX4D6F |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50067040

(((E,E)-1,7-bis(4-hydroxy-3-methoxyphenyl)-1,6-hept...)Show SMILES COc1cc(C=CC(=O)CC(=O)C=Cc2ccc(O)c(OC)c2)ccc1O |w:12.11,5.4| Show InChI InChI=1S/C21H20O6/c1-26-20-11-14(5-9-18(20)24)3-7-16(22)13-17(23)8-4-15-6-10-19(25)21(12-15)27-2/h3-12,24-25H,13H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Kebangsaan Malaysia
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant COX2 using arachidonic acid substrate assessed as PGE2 production ELISA method after 2 mins |
Bioorg Med Chem 22: 4151-61 (2014)
Article DOI: 10.1016/j.bmc.2014.05.052
BindingDB Entry DOI: 10.7270/Q2GX4D6F |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50046000

(CHEMBL3309955)Show SMILES O=C1\C(CNC\C1=C/c1ccc(cc1)N1CCCC1)=C\c1ccc(cc1)N1CCCC1 Show InChI InChI=1S/C27H31N3O/c31-27-23(17-21-5-9-25(10-6-21)29-13-1-2-14-29)19-28-20-24(27)18-22-7-11-26(12-8-22)30-15-3-4-16-30/h5-12,17-18,28H,1-4,13-16,19-20H2/b23-17+,24-18+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Kebangsaan Malaysia
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant COX2 using arachidonic acid substrate assessed as PGE2 production ELISA method after 2 mins |
Bioorg Med Chem 22: 4151-61 (2014)
Article DOI: 10.1016/j.bmc.2014.05.052
BindingDB Entry DOI: 10.7270/Q2GX4D6F |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50045853

(CHEMBL3309954)Show SMILES O=C1\C(COC\C1=C/c1ccc(cc1)N1CCCC1)=C\c1ccc(cc1)N1CCCC1 Show InChI InChI=1S/C27H30N2O2/c30-27-23(17-21-5-9-25(10-6-21)28-13-1-2-14-28)19-31-20-24(27)18-22-7-11-26(12-8-22)29-15-3-4-16-29/h5-12,17-18H,1-4,13-16,19-20H2/b23-17+,24-18+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Kebangsaan Malaysia
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant COX2 using arachidonic acid substrate assessed as PGE2 production ELISA method after 2 mins |
Bioorg Med Chem 22: 4151-61 (2014)
Article DOI: 10.1016/j.bmc.2014.05.052
BindingDB Entry DOI: 10.7270/Q2GX4D6F |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50021468

(CHEMBL3289934)Show SMILES CN(C)c1ccc(\C=C2\CCC\C(=C/c3ccc(cc3[N+]([O-])=O)N(C)C)C2=O)c(c1)[N+]([O-])=O Show InChI InChI=1S/C24H26N4O5/c1-25(2)20-10-8-16(22(14-20)27(30)31)12-18-6-5-7-19(24(18)29)13-17-9-11-21(26(3)4)15-23(17)28(32)33/h8-15H,5-7H2,1-4H3/b18-12-,19-13+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Kebangsaan Malaysia
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant COX2 using arachidonic acid substrate assessed as PGE2 production ELISA method after 2 mins |
Bioorg Med Chem 22: 4151-61 (2014)
Article DOI: 10.1016/j.bmc.2014.05.052
BindingDB Entry DOI: 10.7270/Q2GX4D6F |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50021474
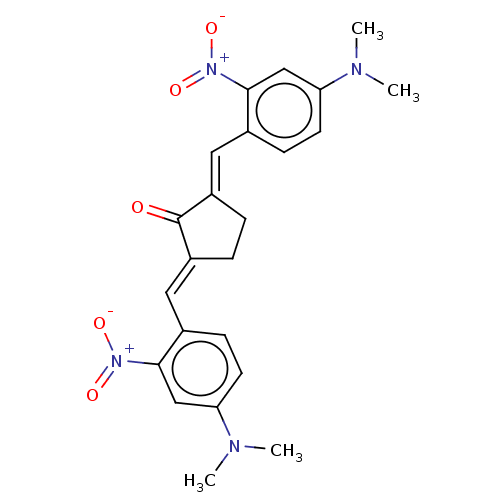
(CHEMBL3289940)Show SMILES CN(C)c1ccc(\C=C2/CC\C(=C/c3ccc(cc3[N+]([O-])=O)N(C)C)C2=O)c(c1)[N+]([O-])=O Show InChI InChI=1S/C23H24N4O5/c1-24(2)19-9-7-15(21(13-19)26(29)30)11-17-5-6-18(23(17)28)12-16-8-10-20(25(3)4)14-22(16)27(31)32/h7-14H,5-6H2,1-4H3/b17-11+,18-12+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Kebangsaan Malaysia
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant COX2 using arachidonic acid substrate assessed as PGE2 production ELISA method after 2 mins |
Bioorg Med Chem 22: 4151-61 (2014)
Article DOI: 10.1016/j.bmc.2014.05.052
BindingDB Entry DOI: 10.7270/Q2GX4D6F |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50021487

(CHEMBL3290195)Show InChI InChI=1S/C21H21NO/c23-21-18(10-9-17-5-1-2-6-20(17)21)15-16-7-11-19(12-8-16)22-13-3-4-14-22/h1-2,5-8,11-12,15H,3-4,9-10,13-14H2/b18-15+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Kebangsaan Malaysia
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant COX2 using arachidonic acid substrate assessed as PGE2 production ELISA method after 2 mins |
Bioorg Med Chem 22: 4151-61 (2014)
Article DOI: 10.1016/j.bmc.2014.05.052
BindingDB Entry DOI: 10.7270/Q2GX4D6F |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50046028

(CHEMBL3309956)Show SMILES CN1C\C(=C/c2ccc(cc2)N2CCCC2)C(=O)\C(C1)=C\c1ccc(cc1)N1CCCC1 Show InChI InChI=1S/C28H33N3O/c1-29-20-24(18-22-6-10-26(11-7-22)30-14-2-3-15-30)28(32)25(21-29)19-23-8-12-27(13-9-23)31-16-4-5-17-31/h6-13,18-19H,2-5,14-17,20-21H2,1H3/b24-18+,25-19+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Kebangsaan Malaysia
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant COX2 using arachidonic acid substrate assessed as PGE2 production ELISA method after 2 mins |
Bioorg Med Chem 22: 4151-61 (2014)
Article DOI: 10.1016/j.bmc.2014.05.052
BindingDB Entry DOI: 10.7270/Q2GX4D6F |
More data for this
Ligand-Target Pair | |
Lipoxygenase
(Glycine max) | BDBM50021473

(CHEMBL3289939)Show SMILES CN(C)c1ccc(\C=C\C(=O)\C=C\c2ccc(cc2[N+]([O-])=O)N(C)C)c(c1)[N+]([O-])=O Show InChI InChI=1S/C21H22N4O5/c1-22(2)17-9-5-15(20(13-17)24(27)28)7-11-19(26)12-8-16-6-10-18(23(3)4)14-21(16)25(29)30/h5-14H,1-4H3/b11-7+,12-8+ | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.03E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Kebangsaan Malaysia
Curated by ChEMBL
| Assay Description
Inhibition of soybean lipoxygenase after 5 mins by colorimetric assay |
Bioorg Med Chem 22: 4151-61 (2014)
Article DOI: 10.1016/j.bmc.2014.05.052
BindingDB Entry DOI: 10.7270/Q2GX4D6F |
More data for this
Ligand-Target Pair | |
Lipoxygenase
(Glycine max) | BDBM50045851

(CHEMBL3357954)Show SMILES CN(C)c1ccc(\C=C\C(=O)C2CCc3ccccc3C2=O)c(c1)[N+]([O-])=O Show InChI InChI=1S/C21H20N2O4/c1-22(2)16-10-7-15(19(13-16)23(26)27)9-12-20(24)18-11-8-14-5-3-4-6-17(14)21(18)25/h3-7,9-10,12-13,18H,8,11H2,1-2H3/b12-9+ | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.57E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Kebangsaan Malaysia
Curated by ChEMBL
| Assay Description
Inhibition of soybean lipoxygenase after 5 mins by colorimetric assay |
Bioorg Med Chem 22: 4151-61 (2014)
Article DOI: 10.1016/j.bmc.2014.05.052
BindingDB Entry DOI: 10.7270/Q2GX4D6F |
More data for this
Ligand-Target Pair | |
Lipoxygenase
(Glycine max) | BDBM50021463

(CHEMBL3289929)Show SMILES CCOC(OCC)c1ccc(\C=C\C(=O)\C=C\c2ccc(cc2)C(OCC)OCC)cc1 Show InChI InChI=1S/C27H34O5/c1-5-29-26(30-6-2)23-15-9-21(10-16-23)13-19-25(28)20-14-22-11-17-24(18-12-22)27(31-7-3)32-8-4/h9-20,26-27H,5-8H2,1-4H3/b19-13+,20-14+ | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.10E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Kebangsaan Malaysia
Curated by ChEMBL
| Assay Description
Inhibition of soybean lipoxygenase after 5 mins by colorimetric assay |
Bioorg Med Chem 22: 4151-61 (2014)
Article DOI: 10.1016/j.bmc.2014.05.052
BindingDB Entry DOI: 10.7270/Q2GX4D6F |
More data for this
Ligand-Target Pair | |
Lipoxygenase
(Glycine max) | BDBM50045850

(CHEMBL3309950)Show SMILES CN(C)c1ccc(\C=C2/CN(Cc3ccccc3)C\C(=C/c3ccc(cc3[N+]([O-])=O)N(C)C)C2=O)c(c1)[N+]([O-])=O Show InChI InChI=1S/C30H31N5O5/c1-31(2)26-12-10-22(28(16-26)34(37)38)14-24-19-33(18-21-8-6-5-7-9-21)20-25(30(24)36)15-23-11-13-27(32(3)4)17-29(23)35(39)40/h5-17H,18-20H2,1-4H3/b24-14+,25-15+ | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.11E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Kebangsaan Malaysia
Curated by ChEMBL
| Assay Description
Inhibition of soybean lipoxygenase after 5 mins by colorimetric assay |
Bioorg Med Chem 22: 4151-61 (2014)
Article DOI: 10.1016/j.bmc.2014.05.052
BindingDB Entry DOI: 10.7270/Q2GX4D6F |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data