

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50020730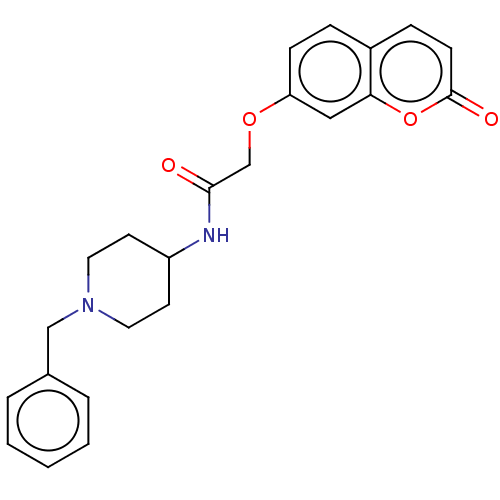 (CHEMBL1469070) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.32E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine as substrate by Lineweavere-Burk plot | Eur J Med Chem 82: 536-44 (2014) Article DOI: 10.1016/j.ejmech.2014.05.056 BindingDB Entry DOI: 10.7270/Q27D2WQV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM8960 ((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate by spectrophotometer analysis | Eur J Med Chem 82: 536-44 (2014) Article DOI: 10.1016/j.ejmech.2014.05.056 BindingDB Entry DOI: 10.7270/Q27D2WQV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50020730 (CHEMBL1469070) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate by spectrophotometer analysis | Eur J Med Chem 82: 536-44 (2014) Article DOI: 10.1016/j.ejmech.2014.05.056 BindingDB Entry DOI: 10.7270/Q27D2WQV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50020733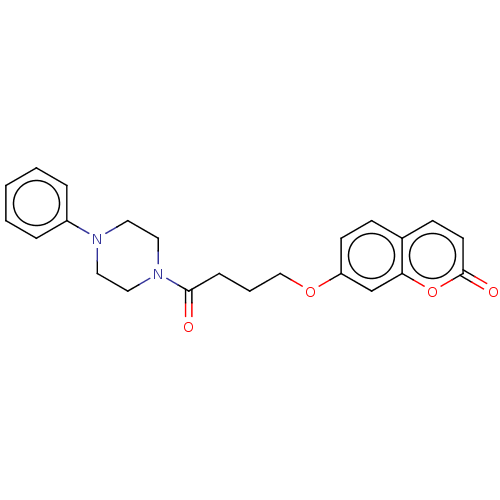 (CHEMBL3286721) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.43E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate by spectrophotometer analysis | Eur J Med Chem 82: 536-44 (2014) Article DOI: 10.1016/j.ejmech.2014.05.056 BindingDB Entry DOI: 10.7270/Q27D2WQV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50020745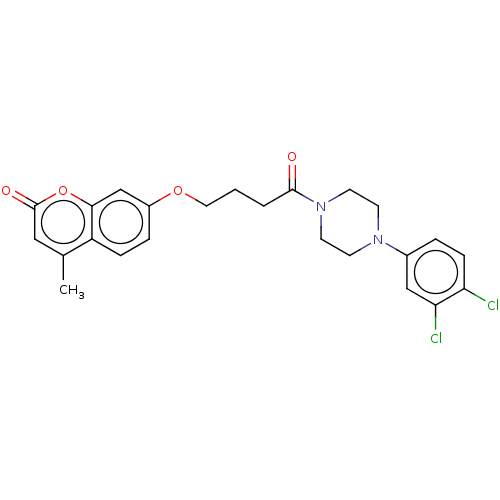 (CHEMBL3286944) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.38E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate by spectrophotometer analysis | Eur J Med Chem 82: 536-44 (2014) Article DOI: 10.1016/j.ejmech.2014.05.056 BindingDB Entry DOI: 10.7270/Q27D2WQV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50020741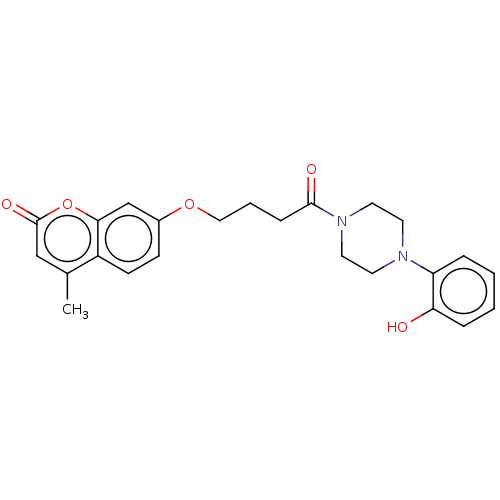 (CHEMBL3286941) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate by spectrophotometer analysis | Eur J Med Chem 82: 536-44 (2014) Article DOI: 10.1016/j.ejmech.2014.05.056 BindingDB Entry DOI: 10.7270/Q27D2WQV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50020735 (CHEMBL3286723) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate by spectrophotometer analysis | Eur J Med Chem 82: 536-44 (2014) Article DOI: 10.1016/j.ejmech.2014.05.056 BindingDB Entry DOI: 10.7270/Q27D2WQV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50020738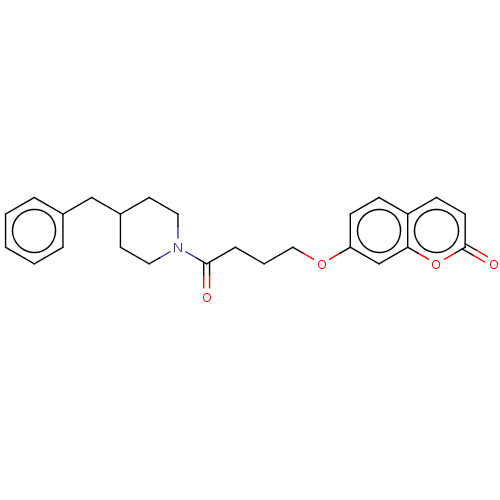 (CHEMBL3286726) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate by spectrophotometer analysis | Eur J Med Chem 82: 536-44 (2014) Article DOI: 10.1016/j.ejmech.2014.05.056 BindingDB Entry DOI: 10.7270/Q27D2WQV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50020734 (CHEMBL3286722) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate by spectrophotometer analysis | Eur J Med Chem 82: 536-44 (2014) Article DOI: 10.1016/j.ejmech.2014.05.056 BindingDB Entry DOI: 10.7270/Q27D2WQV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM8960 ((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 7.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using butyrylthiocholine iodide as substrate by spectrophotometer analysis | Eur J Med Chem 82: 536-44 (2014) Article DOI: 10.1016/j.ejmech.2014.05.056 BindingDB Entry DOI: 10.7270/Q27D2WQV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50020731 (CHEMBL3286948) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate by spectrophotometer analysis | Eur J Med Chem 82: 536-44 (2014) Article DOI: 10.1016/j.ejmech.2014.05.056 BindingDB Entry DOI: 10.7270/Q27D2WQV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50020735 (CHEMBL3286723) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using butyrylthiocholine iodide as substrate by spectrophotometer analysis | Eur J Med Chem 82: 536-44 (2014) Article DOI: 10.1016/j.ejmech.2014.05.056 BindingDB Entry DOI: 10.7270/Q27D2WQV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50020742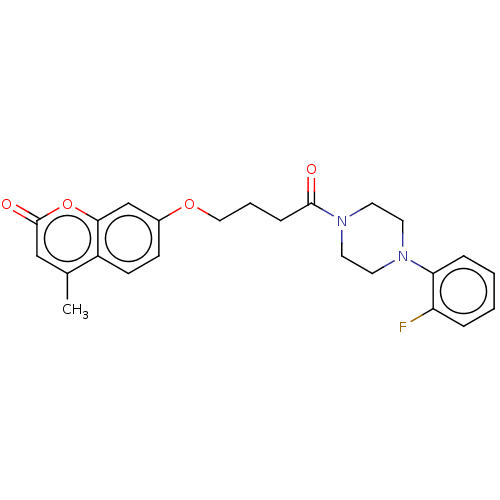 (CHEMBL3286942) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.64E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate by spectrophotometer analysis | Eur J Med Chem 82: 536-44 (2014) Article DOI: 10.1016/j.ejmech.2014.05.056 BindingDB Entry DOI: 10.7270/Q27D2WQV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50020740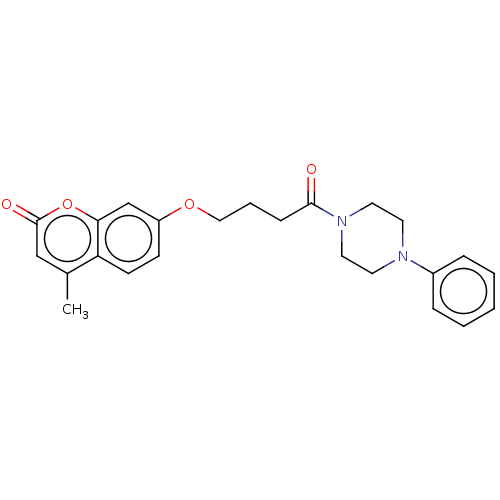 (CHEMBL3286728) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.08E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate by spectrophotometer analysis | Eur J Med Chem 82: 536-44 (2014) Article DOI: 10.1016/j.ejmech.2014.05.056 BindingDB Entry DOI: 10.7270/Q27D2WQV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50020744 (CHEMBL3286943) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using butyrylthiocholine iodide as substrate by spectrophotometer analysis | Eur J Med Chem 82: 536-44 (2014) Article DOI: 10.1016/j.ejmech.2014.05.056 BindingDB Entry DOI: 10.7270/Q27D2WQV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50020737 (CHEMBL3286725) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate by spectrophotometer analysis | Eur J Med Chem 82: 536-44 (2014) Article DOI: 10.1016/j.ejmech.2014.05.056 BindingDB Entry DOI: 10.7270/Q27D2WQV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50020749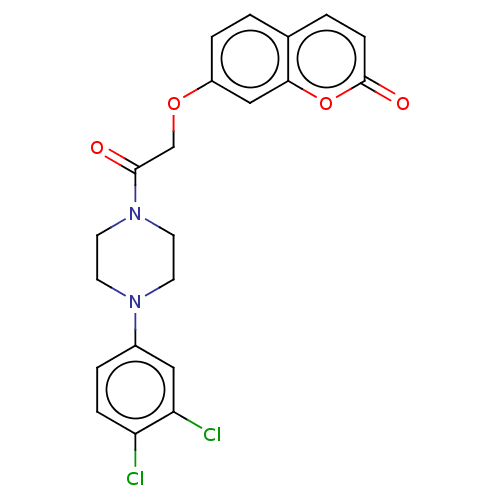 (CHEMBL3286947) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.68E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate by spectrophotometer analysis | Eur J Med Chem 82: 536-44 (2014) Article DOI: 10.1016/j.ejmech.2014.05.056 BindingDB Entry DOI: 10.7270/Q27D2WQV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50020742 (CHEMBL3286942) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using butyrylthiocholine iodide as substrate by spectrophotometer analysis | Eur J Med Chem 82: 536-44 (2014) Article DOI: 10.1016/j.ejmech.2014.05.056 BindingDB Entry DOI: 10.7270/Q27D2WQV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50020747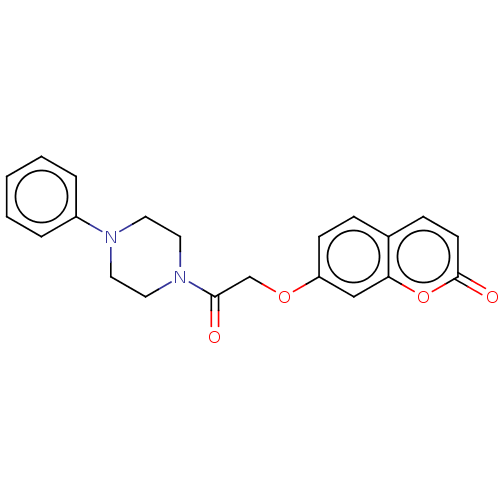 (CHEMBL1618958) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate by spectrophotometer analysis | Eur J Med Chem 82: 536-44 (2014) Article DOI: 10.1016/j.ejmech.2014.05.056 BindingDB Entry DOI: 10.7270/Q27D2WQV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50020748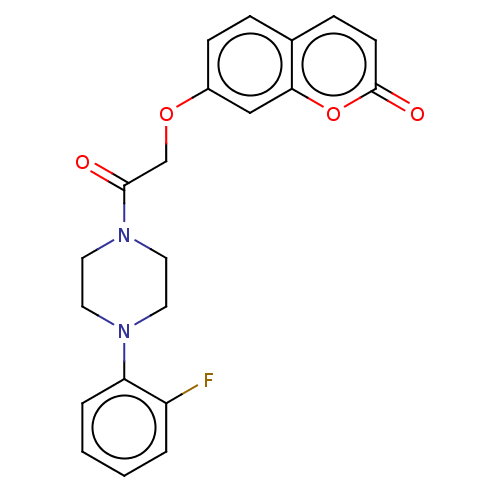 (CHEMBL3286946) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.45E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate by spectrophotometer analysis | Eur J Med Chem 82: 536-44 (2014) Article DOI: 10.1016/j.ejmech.2014.05.056 BindingDB Entry DOI: 10.7270/Q27D2WQV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50020746 (CHEMBL3286945) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.46E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate by spectrophotometer analysis | Eur J Med Chem 82: 536-44 (2014) Article DOI: 10.1016/j.ejmech.2014.05.056 BindingDB Entry DOI: 10.7270/Q27D2WQV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50020744 (CHEMBL3286943) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.16E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate by spectrophotometer analysis | Eur J Med Chem 82: 536-44 (2014) Article DOI: 10.1016/j.ejmech.2014.05.056 BindingDB Entry DOI: 10.7270/Q27D2WQV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50020730 (CHEMBL1469070) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using butyrylthiocholine iodide as substrate by spectrophotometer analysis | Eur J Med Chem 82: 536-44 (2014) Article DOI: 10.1016/j.ejmech.2014.05.056 BindingDB Entry DOI: 10.7270/Q27D2WQV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50020731 (CHEMBL3286948) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using butyrylthiocholine iodide as substrate by spectrophotometer analysis | Eur J Med Chem 82: 536-44 (2014) Article DOI: 10.1016/j.ejmech.2014.05.056 BindingDB Entry DOI: 10.7270/Q27D2WQV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50020736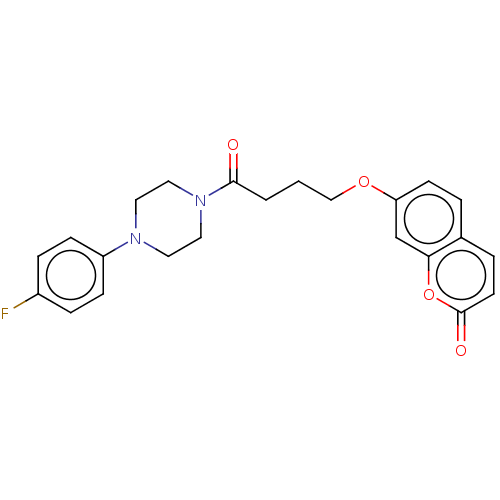 (CHEMBL3286724) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate by spectrophotometer analysis | Eur J Med Chem 82: 536-44 (2014) Article DOI: 10.1016/j.ejmech.2014.05.056 BindingDB Entry DOI: 10.7270/Q27D2WQV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50020747 (CHEMBL1618958) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using butyrylthiocholine iodide as substrate by spectrophotometer analysis | Eur J Med Chem 82: 536-44 (2014) Article DOI: 10.1016/j.ejmech.2014.05.056 BindingDB Entry DOI: 10.7270/Q27D2WQV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50020740 (CHEMBL3286728) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using butyrylthiocholine iodide as substrate by spectrophotometer analysis | Eur J Med Chem 82: 536-44 (2014) Article DOI: 10.1016/j.ejmech.2014.05.056 BindingDB Entry DOI: 10.7270/Q27D2WQV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50020739 (CHEMBL3286727) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate by spectrophotometer analysis | Eur J Med Chem 82: 536-44 (2014) Article DOI: 10.1016/j.ejmech.2014.05.056 BindingDB Entry DOI: 10.7270/Q27D2WQV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50020732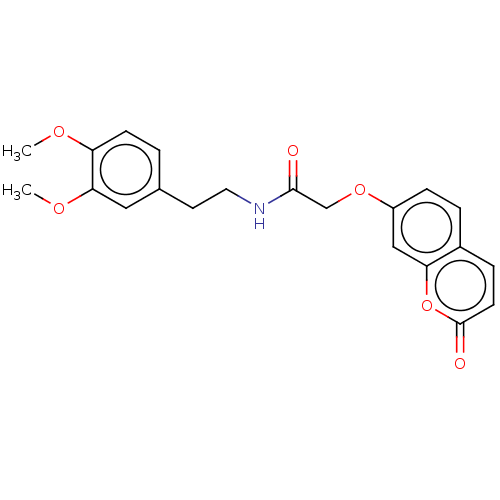 (CHEMBL3286949) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate by spectrophotometer analysis | Eur J Med Chem 82: 536-44 (2014) Article DOI: 10.1016/j.ejmech.2014.05.056 BindingDB Entry DOI: 10.7270/Q27D2WQV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50020736 (CHEMBL3286724) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using butyrylthiocholine iodide as substrate by spectrophotometer analysis | Eur J Med Chem 82: 536-44 (2014) Article DOI: 10.1016/j.ejmech.2014.05.056 BindingDB Entry DOI: 10.7270/Q27D2WQV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50020737 (CHEMBL3286725) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using butyrylthiocholine iodide as substrate by spectrophotometer analysis | Eur J Med Chem 82: 536-44 (2014) Article DOI: 10.1016/j.ejmech.2014.05.056 BindingDB Entry DOI: 10.7270/Q27D2WQV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50020748 (CHEMBL3286946) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using butyrylthiocholine iodide as substrate by spectrophotometer analysis | Eur J Med Chem 82: 536-44 (2014) Article DOI: 10.1016/j.ejmech.2014.05.056 BindingDB Entry DOI: 10.7270/Q27D2WQV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50020749 (CHEMBL3286947) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using butyrylthiocholine iodide as substrate by spectrophotometer analysis | Eur J Med Chem 82: 536-44 (2014) Article DOI: 10.1016/j.ejmech.2014.05.056 BindingDB Entry DOI: 10.7270/Q27D2WQV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50020733 (CHEMBL3286721) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using butyrylthiocholine iodide as substrate by spectrophotometer analysis | Eur J Med Chem 82: 536-44 (2014) Article DOI: 10.1016/j.ejmech.2014.05.056 BindingDB Entry DOI: 10.7270/Q27D2WQV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50020746 (CHEMBL3286945) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.41E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using butyrylthiocholine iodide as substrate by spectrophotometer analysis | Eur J Med Chem 82: 536-44 (2014) Article DOI: 10.1016/j.ejmech.2014.05.056 BindingDB Entry DOI: 10.7270/Q27D2WQV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50020732 (CHEMBL3286949) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.63E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using butyrylthiocholine iodide as substrate by spectrophotometer analysis | Eur J Med Chem 82: 536-44 (2014) Article DOI: 10.1016/j.ejmech.2014.05.056 BindingDB Entry DOI: 10.7270/Q27D2WQV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50020739 (CHEMBL3286727) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.80E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using butyrylthiocholine iodide as substrate by spectrophotometer analysis | Eur J Med Chem 82: 536-44 (2014) Article DOI: 10.1016/j.ejmech.2014.05.056 BindingDB Entry DOI: 10.7270/Q27D2WQV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50020741 (CHEMBL3286941) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.84E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using butyrylthiocholine iodide as substrate by spectrophotometer analysis | Eur J Med Chem 82: 536-44 (2014) Article DOI: 10.1016/j.ejmech.2014.05.056 BindingDB Entry DOI: 10.7270/Q27D2WQV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50020745 (CHEMBL3286944) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using butyrylthiocholine iodide as substrate by spectrophotometer analysis | Eur J Med Chem 82: 536-44 (2014) Article DOI: 10.1016/j.ejmech.2014.05.056 BindingDB Entry DOI: 10.7270/Q27D2WQV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50020734 (CHEMBL3286722) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.80E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using butyrylthiocholine iodide as substrate by spectrophotometer analysis | Eur J Med Chem 82: 536-44 (2014) Article DOI: 10.1016/j.ejmech.2014.05.056 BindingDB Entry DOI: 10.7270/Q27D2WQV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50020738 (CHEMBL3286726) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.87E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using butyrylthiocholine iodide as substrate by spectrophotometer analysis | Eur J Med Chem 82: 536-44 (2014) Article DOI: 10.1016/j.ejmech.2014.05.056 BindingDB Entry DOI: 10.7270/Q27D2WQV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||