 Found 79 hits of Enzyme Inhibition Constant Data
Found 79 hits of Enzyme Inhibition Constant Data Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Aurora kinase B
(Homo sapiens (Human)) | BDBM50056188

(CHEMBL3329654)Show SMILES CN(CCO)CCCOc1ccc2c(Nc3cc(CC(=O)Nc4cccc(F)c4)n[nH]3)ncnc2c1 Show InChI InChI=1S/C25H28FN7O3/c1-33(9-10-34)8-3-11-36-20-6-7-21-22(15-20)27-16-28-25(21)30-23-13-19(31-32-23)14-24(35)29-18-5-2-4-17(26)12-18/h2,4-7,12-13,15-16,34H,3,8-11,14H2,1H3,(H,29,35)(H2,27,28,30,31,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University
Curated by ChEMBL
| Assay Description
Inhibition of Aurora B (unknown origin) using HLRRASLG substrate |
Eur J Med Chem 85: 268-88 (2014)
Article DOI: 10.1016/j.ejmech.2014.07.108
BindingDB Entry DOI: 10.7270/Q2028T6Z |
More data for this
Ligand-Target Pair | |
Aurora kinase B
(Homo sapiens (Human)) | BDBM50056199
(CHEMBL3329684)Show SMILES COc1ccc(C(=O)NC(=O)Nc2ccc3\C(=C\c4[nH]c(C)c(C(=O)NCCN5CCCC5)c4C)C(=O)Nc3c2)c(F)c1 Show InChI InChI=1S/C31H33FN6O5/c1-17-25(34-18(2)27(17)30(41)33-10-13-38-11-4-5-12-38)16-23-21-8-6-19(14-26(21)36-29(23)40)35-31(42)37-28(39)22-9-7-20(43-3)15-24(22)32/h6-9,14-16,34H,4-5,10-13H2,1-3H3,(H,33,41)(H,36,40)(H2,35,37,39,42)/b23-16- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University
Curated by ChEMBL
| Assay Description
Inhibition of Aurora B (unknown origin) using HLRRASLG substrate |
Eur J Med Chem 85: 268-88 (2014)
Article DOI: 10.1016/j.ejmech.2014.07.108
BindingDB Entry DOI: 10.7270/Q2028T6Z |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50056199

(CHEMBL3329684)Show SMILES COc1ccc(C(=O)NC(=O)Nc2ccc3\C(=C\c4[nH]c(C)c(C(=O)NCCN5CCCC5)c4C)C(=O)Nc3c2)c(F)c1 Show InChI InChI=1S/C31H33FN6O5/c1-17-25(34-18(2)27(17)30(41)33-10-13-38-11-4-5-12-38)16-23-21-8-6-19(14-26(21)36-29(23)40)35-31(42)37-28(39)22-9-7-20(43-3)15-24(22)32/h6-9,14-16,34H,4-5,10-13H2,1-3H3,(H,33,41)(H,36,40)(H2,35,37,39,42)/b23-16- | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University
Curated by ChEMBL
| Assay Description
Inhibition of FLT3 (unknown origin) using EAIYAAPFAKKK substrate |
Eur J Med Chem 85: 268-88 (2014)
Article DOI: 10.1016/j.ejmech.2014.07.108
BindingDB Entry DOI: 10.7270/Q2028T6Z |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University
Curated by ChEMBL
| Assay Description
Inhibition of FLT3 (unknown origin) using EAIYAAPFAKKK substrate |
Eur J Med Chem 85: 268-88 (2014)
Article DOI: 10.1016/j.ejmech.2014.07.108
BindingDB Entry DOI: 10.7270/Q2028T6Z |
More data for this
Ligand-Target Pair | |
Aurora kinase B
(Homo sapiens (Human)) | BDBM50056146
(CHEMBL3329669)Show SMILES COc1ccc(cc1)C(=O)NC(=O)Nc1ccc2\C(=C\c3[nH]c(C)c(CCC(O)=O)c3C)C(=O)Nc2c1 Show InChI InChI=1S/C27H26N4O6/c1-14-19(10-11-24(32)33)15(2)28-22(14)13-21-20-9-6-17(12-23(20)30-26(21)35)29-27(36)31-25(34)16-4-7-18(37-3)8-5-16/h4-9,12-13,28H,10-11H2,1-3H3,(H,30,35)(H,32,33)(H2,29,31,34,36)/b21-13- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University
Curated by ChEMBL
| Assay Description
Inhibition of Aurora B (unknown origin) using HLRRASLG substrate |
Eur J Med Chem 85: 268-88 (2014)
Article DOI: 10.1016/j.ejmech.2014.07.108
BindingDB Entry DOI: 10.7270/Q2028T6Z |
More data for this
Ligand-Target Pair | |
Aurora kinase B
(Homo sapiens (Human)) | BDBM50056142

(CHEMBL3329673)Show SMILES COc1ccc(C(=O)NC(=O)Nc2ccc3\C(=C\c4[nH]c(C)c(CCC(O)=O)c4C)C(=O)Nc3c2)c(F)c1 Show InChI InChI=1S/C27H25FN4O6/c1-13-17(8-9-24(33)34)14(2)29-22(13)12-20-18-6-4-15(10-23(18)31-26(20)36)30-27(37)32-25(35)19-7-5-16(38-3)11-21(19)28/h4-7,10-12,29H,8-9H2,1-3H3,(H,31,36)(H,33,34)(H2,30,32,35,37)/b20-12- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University
Curated by ChEMBL
| Assay Description
Inhibition of Aurora B (unknown origin) using HLRRASLG substrate |
Eur J Med Chem 85: 268-88 (2014)
Article DOI: 10.1016/j.ejmech.2014.07.108
BindingDB Entry DOI: 10.7270/Q2028T6Z |
More data for this
Ligand-Target Pair | |
Aurora kinase B
(Homo sapiens (Human)) | BDBM50056145

(CHEMBL3329670)Show SMILES COc1ccc(cc1)C(=O)NC(=O)Nc1cc2NC(=O)\C(=C/c3[nH]c(C)c(C(O)=O)c3C)c2cc1F Show InChI InChI=1S/C25H21FN4O6/c1-11-18(27-12(2)21(11)24(33)34)9-16-15-8-17(26)20(10-19(15)28-23(16)32)29-25(35)30-22(31)13-4-6-14(36-3)7-5-13/h4-10,27H,1-3H3,(H,28,32)(H,33,34)(H2,29,30,31,35)/b16-9- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University
Curated by ChEMBL
| Assay Description
Inhibition of Aurora B (unknown origin) using HLRRASLG substrate |
Eur J Med Chem 85: 268-88 (2014)
Article DOI: 10.1016/j.ejmech.2014.07.108
BindingDB Entry DOI: 10.7270/Q2028T6Z |
More data for this
Ligand-Target Pair | |
Aurora kinase B
(Homo sapiens (Human)) | BDBM50056140
(CHEMBL3329675)Show SMILES COc1ccc(C(=O)NC(=O)Nc2cc3NC(=O)\C(=C/c4[nH]c(C)c(CCC(O)=O)c4C)c3cc2F)c(F)c1 Show InChI InChI=1S/C27H24F2N4O6/c1-12-15(6-7-24(34)35)13(2)30-21(12)10-18-17-9-20(29)23(11-22(17)31-26(18)37)32-27(38)33-25(36)16-5-4-14(39-3)8-19(16)28/h4-5,8-11,30H,6-7H2,1-3H3,(H,31,37)(H,34,35)(H2,32,33,36,38)/b18-10- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University
Curated by ChEMBL
| Assay Description
Inhibition of Aurora B (unknown origin) using HLRRASLG substrate |
Eur J Med Chem 85: 268-88 (2014)
Article DOI: 10.1016/j.ejmech.2014.07.108
BindingDB Entry DOI: 10.7270/Q2028T6Z |
More data for this
Ligand-Target Pair | |
Aurora kinase B
(Homo sapiens (Human)) | BDBM50056151

(CHEMBL3329668)Show SMILES COc1ccc(cc1)C(=O)NC(=O)Nc1ccc2\C(=C\c3[nH]c(C)c(CC(O)=O)c3C)C(=O)Nc2c1 Show InChI InChI=1S/C26H24N4O6/c1-13-19(12-23(31)32)14(2)27-21(13)11-20-18-9-6-16(10-22(18)29-25(20)34)28-26(35)30-24(33)15-4-7-17(36-3)8-5-15/h4-11,27H,12H2,1-3H3,(H,29,34)(H,31,32)(H2,28,30,33,35)/b20-11- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University
Curated by ChEMBL
| Assay Description
Inhibition of Aurora B (unknown origin) using HLRRASLG substrate |
Eur J Med Chem 85: 268-88 (2014)
Article DOI: 10.1016/j.ejmech.2014.07.108
BindingDB Entry DOI: 10.7270/Q2028T6Z |
More data for this
Ligand-Target Pair | |
Aurora kinase B
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University
Curated by ChEMBL
| Assay Description
Inhibition of Aurora B (unknown origin) using HLRRASLG substrate |
Eur J Med Chem 85: 268-88 (2014)
Article DOI: 10.1016/j.ejmech.2014.07.108
BindingDB Entry DOI: 10.7270/Q2028T6Z |
More data for this
Ligand-Target Pair | |
Aurora kinase B
(Homo sapiens (Human)) | BDBM50056155

(CHEMBL3329664)Show SMILES COc1ccc(cc1)C(=O)NC(=O)Nc1ccc2\C(=C\c3[nH]c(C)c(C(O)=O)c3C)C(=O)Nc2c1 Show InChI InChI=1S/C25H22N4O6/c1-12-19(26-13(2)21(12)24(32)33)11-18-17-9-6-15(10-20(17)28-23(18)31)27-25(34)29-22(30)14-4-7-16(35-3)8-5-14/h4-11,26H,1-3H3,(H,28,31)(H,32,33)(H2,27,29,30,34)/b18-11- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University
Curated by ChEMBL
| Assay Description
Inhibition of Aurora B (unknown origin) using HLRRASLG substrate |
Eur J Med Chem 85: 268-88 (2014)
Article DOI: 10.1016/j.ejmech.2014.07.108
BindingDB Entry DOI: 10.7270/Q2028T6Z |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50056199

(CHEMBL3329684)Show SMILES COc1ccc(C(=O)NC(=O)Nc2ccc3\C(=C\c4[nH]c(C)c(C(=O)NCCN5CCCC5)c4C)C(=O)Nc3c2)c(F)c1 Show InChI InChI=1S/C31H33FN6O5/c1-17-25(34-18(2)27(17)30(41)33-10-13-38-11-4-5-12-38)16-23-21-8-6-19(14-26(21)36-29(23)40)35-31(42)37-28(39)22-9-7-20(43-3)15-24(22)32/h6-9,14-16,34H,4-5,10-13H2,1-3H3,(H,33,41)(H,36,40)(H2,35,37,39,42)/b23-16- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University
Curated by ChEMBL
| Assay Description
Inhibition of Lck (unknown origin) |
Eur J Med Chem 85: 268-88 (2014)
Article DOI: 10.1016/j.ejmech.2014.07.108
BindingDB Entry DOI: 10.7270/Q2028T6Z |
More data for this
Ligand-Target Pair | |
Aurora kinase B
(Homo sapiens (Human)) | BDBM50056143

(CHEMBL3329672)Show SMILES COc1ccc(C(=O)NC(=O)Nc2ccc3\C(=C\c4[nH]c(C)c(C(O)=O)c4C)C(=O)Nc3c2)c(F)c1 Show InChI InChI=1S/C25H21FN4O6/c1-11-19(27-12(2)21(11)24(33)34)10-17-15-6-4-13(8-20(15)29-23(17)32)28-25(35)30-22(31)16-7-5-14(36-3)9-18(16)26/h4-10,27H,1-3H3,(H,29,32)(H,33,34)(H2,28,30,31,35)/b17-10- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University
Curated by ChEMBL
| Assay Description
Inhibition of Aurora B (unknown origin) using HLRRASLG substrate |
Eur J Med Chem 85: 268-88 (2014)
Article DOI: 10.1016/j.ejmech.2014.07.108
BindingDB Entry DOI: 10.7270/Q2028T6Z |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50056146

(CHEMBL3329669)Show SMILES COc1ccc(cc1)C(=O)NC(=O)Nc1ccc2\C(=C\c3[nH]c(C)c(CCC(O)=O)c3C)C(=O)Nc2c1 Show InChI InChI=1S/C27H26N4O6/c1-14-19(10-11-24(32)33)15(2)28-22(14)13-21-20-9-6-17(12-23(20)30-26(21)35)29-27(36)31-25(34)16-4-7-18(37-3)8-5-16/h4-9,12-13,28H,10-11H2,1-3H3,(H,30,35)(H,32,33)(H2,29,31,34,36)/b21-13- | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University
Curated by ChEMBL
| Assay Description
Inhibition of FLT3 (unknown origin) using EAIYAAPFAKKK substrate |
Eur J Med Chem 85: 268-88 (2014)
Article DOI: 10.1016/j.ejmech.2014.07.108
BindingDB Entry DOI: 10.7270/Q2028T6Z |
More data for this
Ligand-Target Pair | |
Aurora kinase B
(Homo sapiens (Human)) | BDBM50056141

(CHEMBL3329674)Show SMILES COc1ccc(C(=O)NC(=O)Nc2cc3NC(=O)\C(=C/c4[nH]c(C)c(C(O)=O)c4C)c3cc2F)c(F)c1 Show InChI InChI=1S/C25H20F2N4O6/c1-10-18(28-11(2)21(10)24(34)35)8-15-14-7-17(27)20(9-19(14)29-23(15)33)30-25(36)31-22(32)13-5-4-12(37-3)6-16(13)26/h4-9,28H,1-3H3,(H,29,33)(H,34,35)(H2,30,31,32,36)/b15-8- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University
Curated by ChEMBL
| Assay Description
Inhibition of Aurora B (unknown origin) using HLRRASLG substrate |
Eur J Med Chem 85: 268-88 (2014)
Article DOI: 10.1016/j.ejmech.2014.07.108
BindingDB Entry DOI: 10.7270/Q2028T6Z |
More data for this
Ligand-Target Pair | |
Aurora kinase B
(Homo sapiens (Human)) | BDBM50056144
(CHEMBL3329671)Show SMILES COc1ccc(cc1)C(=O)NC(=O)Nc1cc2NC(=O)\C(=C/c3[nH]c(C)c(CCC(O)=O)c3C)c2cc1F Show InChI InChI=1S/C27H25FN4O6/c1-13-17(8-9-24(33)34)14(2)29-21(13)11-19-18-10-20(28)23(12-22(18)30-26(19)36)31-27(37)32-25(35)15-4-6-16(38-3)7-5-15/h4-7,10-12,29H,8-9H2,1-3H3,(H,30,36)(H,33,34)(H2,31,32,35,37)/b19-11- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University
Curated by ChEMBL
| Assay Description
Inhibition of Aurora B (unknown origin) using HLRRASLG substrate |
Eur J Med Chem 85: 268-88 (2014)
Article DOI: 10.1016/j.ejmech.2014.07.108
BindingDB Entry DOI: 10.7270/Q2028T6Z |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50056198
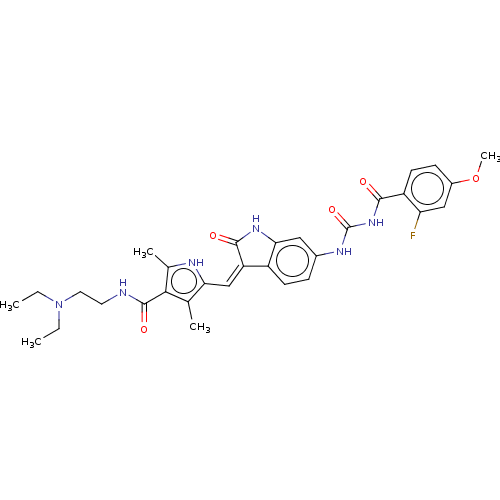
(CHEMBL3329683)Show SMILES CCN(CC)CCNC(=O)c1c(C)[nH]c(\C=C2/C(=O)Nc3cc(NC(=O)NC(=O)c4ccc(OC)cc4F)ccc23)c1C Show InChI InChI=1S/C31H35FN6O5/c1-6-38(7-2)13-12-33-30(41)27-17(3)25(34-18(27)4)16-23-21-10-8-19(14-26(21)36-29(23)40)35-31(42)37-28(39)22-11-9-20(43-5)15-24(22)32/h8-11,14-16,34H,6-7,12-13H2,1-5H3,(H,33,41)(H,36,40)(H2,35,37,39,42)/b23-16- | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University
Curated by ChEMBL
| Assay Description
Inhibition of FLT3 (unknown origin) using EAIYAAPFAKKK substrate |
Eur J Med Chem 85: 268-88 (2014)
Article DOI: 10.1016/j.ejmech.2014.07.108
BindingDB Entry DOI: 10.7270/Q2028T6Z |
More data for this
Ligand-Target Pair | |
Aurora kinase B
(Homo sapiens (Human)) | BDBM50056187

(CHEMBL3329655)Show SMILES O=C(NC(=O)c1ccccc1)Nc1ccc2\C(=C\c3ccc[nH]3)C(=O)Nc2c1 Show InChI InChI=1S/C21H16N4O3/c26-19(13-5-2-1-3-6-13)25-21(28)23-15-8-9-16-17(11-14-7-4-10-22-14)20(27)24-18(16)12-15/h1-12,22H,(H,24,27)(H2,23,25,26,28)/b17-11- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University
Curated by ChEMBL
| Assay Description
Inhibition of Aurora B (unknown origin) using HLRRASLG substrate |
Eur J Med Chem 85: 268-88 (2014)
Article DOI: 10.1016/j.ejmech.2014.07.108
BindingDB Entry DOI: 10.7270/Q2028T6Z |
More data for this
Ligand-Target Pair | |
Aurora kinase B
(Homo sapiens (Human)) | BDBM50056186

(CHEMBL3329656)Show SMILES Clc1ccc(cc1)C(=O)NC(=O)Nc1ccc2\C(=C\c3ccc[nH]3)C(=O)Nc2c1 Show InChI InChI=1S/C21H15ClN4O3/c22-13-5-3-12(4-6-13)19(27)26-21(29)24-15-7-8-16-17(10-14-2-1-9-23-14)20(28)25-18(16)11-15/h1-11,23H,(H,25,28)(H2,24,26,27,29)/b17-10- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University
Curated by ChEMBL
| Assay Description
Inhibition of Aurora B (unknown origin) using HLRRASLG substrate |
Eur J Med Chem 85: 268-88 (2014)
Article DOI: 10.1016/j.ejmech.2014.07.108
BindingDB Entry DOI: 10.7270/Q2028T6Z |
More data for this
Ligand-Target Pair | |
Aurora kinase B
(Homo sapiens (Human)) | BDBM50056184

(CHEMBL3329658)Show SMILES Fc1ccccc1C(=O)NC(=O)Nc1ccc2\C(=C\c3ccc[nH]3)C(=O)Nc2c1 Show InChI InChI=1S/C21H15FN4O3/c22-17-6-2-1-5-15(17)19(27)26-21(29)24-13-7-8-14-16(10-12-4-3-9-23-12)20(28)25-18(14)11-13/h1-11,23H,(H,25,28)(H2,24,26,27,29)/b16-10- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University
Curated by ChEMBL
| Assay Description
Inhibition of Aurora B (unknown origin) using HLRRASLG substrate |
Eur J Med Chem 85: 268-88 (2014)
Article DOI: 10.1016/j.ejmech.2014.07.108
BindingDB Entry DOI: 10.7270/Q2028T6Z |
More data for this
Ligand-Target Pair | |
Aurora kinase B
(Homo sapiens (Human)) | BDBM50056198

(CHEMBL3329683)Show SMILES CCN(CC)CCNC(=O)c1c(C)[nH]c(\C=C2/C(=O)Nc3cc(NC(=O)NC(=O)c4ccc(OC)cc4F)ccc23)c1C Show InChI InChI=1S/C31H35FN6O5/c1-6-38(7-2)13-12-33-30(41)27-17(3)25(34-18(27)4)16-23-21-10-8-19(14-26(21)36-29(23)40)35-31(42)37-28(39)22-11-9-20(43-5)15-24(22)32/h8-11,14-16,34H,6-7,12-13H2,1-5H3,(H,33,41)(H,36,40)(H2,35,37,39,42)/b23-16- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7.60 | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University
Curated by ChEMBL
| Assay Description
Inhibition of Aurora B (unknown origin) using HLRRASLG substrate |
Eur J Med Chem 85: 268-88 (2014)
Article DOI: 10.1016/j.ejmech.2014.07.108
BindingDB Entry DOI: 10.7270/Q2028T6Z |
More data for this
Ligand-Target Pair | |
Aurora kinase B
(Homo sapiens (Human)) | BDBM50056185
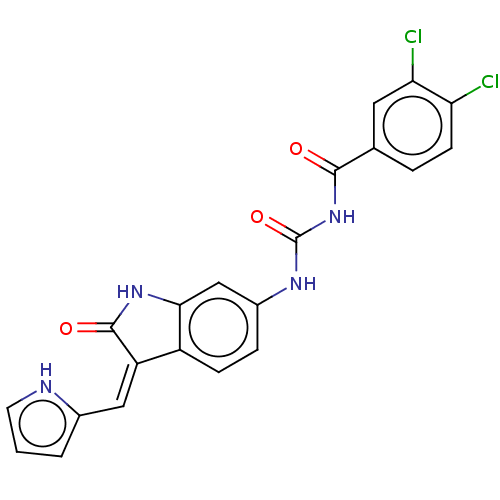
(CHEMBL3329657)Show SMILES Clc1ccc(cc1Cl)C(=O)NC(=O)Nc1ccc2\C(=C\c3ccc[nH]3)C(=O)Nc2c1 Show InChI InChI=1S/C21H14Cl2N4O3/c22-16-6-3-11(8-17(16)23)19(28)27-21(30)25-13-4-5-14-15(9-12-2-1-7-24-12)20(29)26-18(14)10-13/h1-10,24H,(H,26,29)(H2,25,27,28,30)/b15-9- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.70 | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University
Curated by ChEMBL
| Assay Description
Inhibition of Aurora B (unknown origin) using HLRRASLG substrate |
Eur J Med Chem 85: 268-88 (2014)
Article DOI: 10.1016/j.ejmech.2014.07.108
BindingDB Entry DOI: 10.7270/Q2028T6Z |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50056142

(CHEMBL3329673)Show SMILES COc1ccc(C(=O)NC(=O)Nc2ccc3\C(=C\c4[nH]c(C)c(CCC(O)=O)c4C)C(=O)Nc3c2)c(F)c1 Show InChI InChI=1S/C27H25FN4O6/c1-13-17(8-9-24(33)34)14(2)29-22(13)12-20-18-6-4-15(10-23(18)31-26(20)36)30-27(37)32-25(35)19-7-5-16(38-3)11-21(19)28/h4-7,10-12,29H,8-9H2,1-3H3,(H,31,36)(H,33,34)(H2,30,32,35,37)/b20-12- | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University
Curated by ChEMBL
| Assay Description
Inhibition of FLT3 (unknown origin) using EAIYAAPFAKKK substrate |
Eur J Med Chem 85: 268-88 (2014)
Article DOI: 10.1016/j.ejmech.2014.07.108
BindingDB Entry DOI: 10.7270/Q2028T6Z |
More data for this
Ligand-Target Pair | |
Aurora kinase B
(Homo sapiens (Human)) | BDBM50056197

(CHEMBL3329682)Show SMILES COc1ccc(C(=O)NC(=O)Nc2ccc3\C(=C\c4[nH]c(C)c(C(=O)NCCN(C)C)c4C)C(=O)Nc3c2)c(F)c1 Show InChI InChI=1S/C29H31FN6O5/c1-15-23(32-16(2)25(15)28(39)31-10-11-36(3)4)14-21-19-8-6-17(12-24(19)34-27(21)38)33-29(40)35-26(37)20-9-7-18(41-5)13-22(20)30/h6-9,12-14,32H,10-11H2,1-5H3,(H,31,39)(H,34,38)(H2,33,35,37,40)/b21-14- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University
Curated by ChEMBL
| Assay Description
Inhibition of Aurora B (unknown origin) using HLRRASLG substrate |
Eur J Med Chem 85: 268-88 (2014)
Article DOI: 10.1016/j.ejmech.2014.07.108
BindingDB Entry DOI: 10.7270/Q2028T6Z |
More data for this
Ligand-Target Pair | |
Mast/stem cell growth factor receptor Kit
(Homo sapiens (Human)) | BDBM50056199

(CHEMBL3329684)Show SMILES COc1ccc(C(=O)NC(=O)Nc2ccc3\C(=C\c4[nH]c(C)c(C(=O)NCCN5CCCC5)c4C)C(=O)Nc3c2)c(F)c1 Show InChI InChI=1S/C31H33FN6O5/c1-17-25(34-18(2)27(17)30(41)33-10-13-38-11-4-5-12-38)16-23-21-8-6-19(14-26(21)36-29(23)40)35-31(42)37-28(39)22-9-7-20(43-3)15-24(22)32/h6-9,14-16,34H,4-5,10-13H2,1-3H3,(H,33,41)(H,36,40)(H2,35,37,39,42)/b23-16- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University
Curated by ChEMBL
| Assay Description
Inhibition of c-Kit (unknown origin) |
Eur J Med Chem 85: 268-88 (2014)
Article DOI: 10.1016/j.ejmech.2014.07.108
BindingDB Entry DOI: 10.7270/Q2028T6Z |
More data for this
Ligand-Target Pair | |
Aurora kinase B
(Homo sapiens (Human)) | BDBM50056189
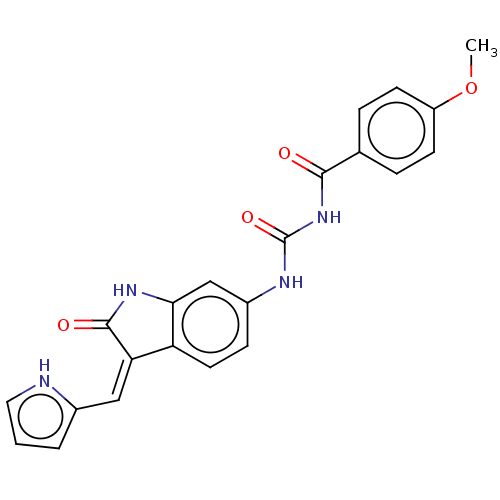
(CHEMBL3329652)Show SMILES COc1ccc(cc1)C(=O)NC(=O)Nc1ccc2\C(=C\c3ccc[nH]3)C(=O)Nc2c1 Show InChI InChI=1S/C22H18N4O4/c1-30-16-7-4-13(5-8-16)20(27)26-22(29)24-15-6-9-17-18(11-14-3-2-10-23-14)21(28)25-19(17)12-15/h2-12,23H,1H3,(H,25,28)(H2,24,26,27,29)/b18-11- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University
Curated by ChEMBL
| Assay Description
Inhibition of Aurora B (unknown origin) using HLRRASLG substrate |
Eur J Med Chem 85: 268-88 (2014)
Article DOI: 10.1016/j.ejmech.2014.07.108
BindingDB Entry DOI: 10.7270/Q2028T6Z |
More data for this
Ligand-Target Pair | |
Aurora kinase B
(Homo sapiens (Human)) | BDBM50056178

(CHEMBL3329660)Show SMILES FC(F)(F)c1cc(ccc1Cl)C(=O)NC(=O)Nc1ccc2\C(=C\c3ccc[nH]3)C(=O)Nc2c1 Show InChI InChI=1S/C22H14ClF3N4O3/c23-17-6-3-11(8-16(17)22(24,25)26)19(31)30-21(33)28-13-4-5-14-15(9-12-2-1-7-27-12)20(32)29-18(14)10-13/h1-10,27H,(H,29,32)(H2,28,30,31,33)/b15-9- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University
Curated by ChEMBL
| Assay Description
Inhibition of Aurora B (unknown origin) using HLRRASLG substrate |
Eur J Med Chem 85: 268-88 (2014)
Article DOI: 10.1016/j.ejmech.2014.07.108
BindingDB Entry DOI: 10.7270/Q2028T6Z |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50056197

(CHEMBL3329682)Show SMILES COc1ccc(C(=O)NC(=O)Nc2ccc3\C(=C\c4[nH]c(C)c(C(=O)NCCN(C)C)c4C)C(=O)Nc3c2)c(F)c1 Show InChI InChI=1S/C29H31FN6O5/c1-15-23(32-16(2)25(15)28(39)31-10-11-36(3)4)14-21-19-8-6-17(12-24(19)34-27(21)38)33-29(40)35-26(37)20-9-7-18(41-5)13-22(20)30/h6-9,12-14,32H,10-11H2,1-5H3,(H,31,39)(H,34,38)(H2,33,35,37,40)/b21-14- | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University
Curated by ChEMBL
| Assay Description
Inhibition of FLT3 (unknown origin) using EAIYAAPFAKKK substrate |
Eur J Med Chem 85: 268-88 (2014)
Article DOI: 10.1016/j.ejmech.2014.07.108
BindingDB Entry DOI: 10.7270/Q2028T6Z |
More data for this
Ligand-Target Pair | |
Aurora kinase B
(Homo sapiens (Human)) | BDBM50056196
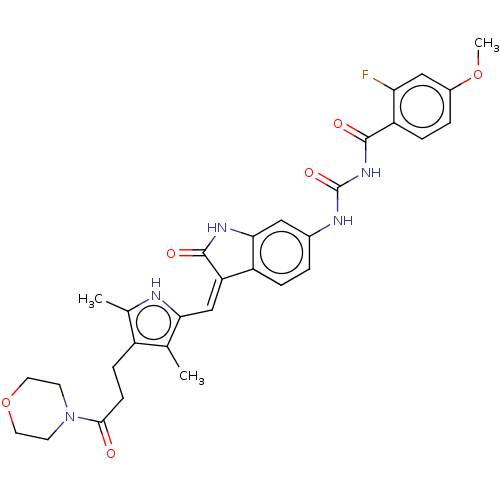
(CHEMBL3329681)Show SMILES COc1ccc(C(=O)NC(=O)Nc2ccc3\C(=C\c4[nH]c(C)c(CCC(=O)N5CCOCC5)c4C)C(=O)Nc3c2)c(F)c1 Show InChI InChI=1S/C31H32FN5O6/c1-17-21(8-9-28(38)37-10-12-43-13-11-37)18(2)33-26(17)16-24-22-6-4-19(14-27(22)35-30(24)40)34-31(41)36-29(39)23-7-5-20(42-3)15-25(23)32/h4-7,14-16,33H,8-13H2,1-3H3,(H,35,40)(H2,34,36,39,41)/b24-16- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University
Curated by ChEMBL
| Assay Description
Inhibition of Aurora B (unknown origin) using HLRRASLG substrate |
Eur J Med Chem 85: 268-88 (2014)
Article DOI: 10.1016/j.ejmech.2014.07.108
BindingDB Entry DOI: 10.7270/Q2028T6Z |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50056140

(CHEMBL3329675)Show SMILES COc1ccc(C(=O)NC(=O)Nc2cc3NC(=O)\C(=C/c4[nH]c(C)c(CCC(O)=O)c4C)c3cc2F)c(F)c1 Show InChI InChI=1S/C27H24F2N4O6/c1-12-15(6-7-24(34)35)13(2)30-21(12)10-18-17-9-20(29)23(11-22(17)31-26(18)37)32-27(38)33-25(36)16-5-4-14(39-3)8-19(16)28/h4-5,8-11,30H,6-7H2,1-3H3,(H,31,37)(H,34,35)(H2,32,33,36,38)/b18-10- | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University
Curated by ChEMBL
| Assay Description
Inhibition of FLT3 (unknown origin) using EAIYAAPFAKKK substrate |
Eur J Med Chem 85: 268-88 (2014)
Article DOI: 10.1016/j.ejmech.2014.07.108
BindingDB Entry DOI: 10.7270/Q2028T6Z |
More data for this
Ligand-Target Pair | |
Aurora kinase B
(Homo sapiens (Human)) | BDBM50056195
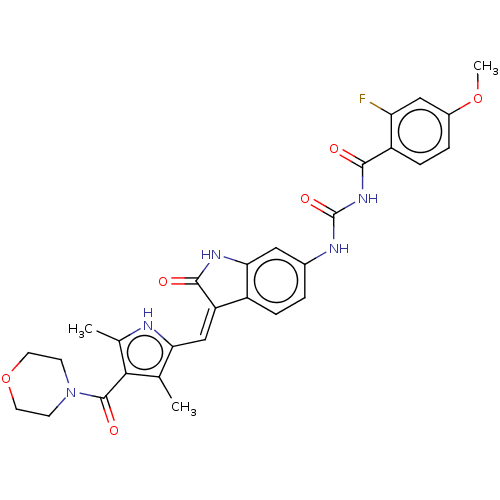
(CHEMBL3329680)Show SMILES COc1ccc(C(=O)NC(=O)Nc2ccc3\C(=C\c4[nH]c(C)c(C(=O)N5CCOCC5)c4C)C(=O)Nc3c2)c(F)c1 Show InChI InChI=1S/C29H28FN5O6/c1-15-23(31-16(2)25(15)28(38)35-8-10-41-11-9-35)14-21-19-6-4-17(12-24(19)33-27(21)37)32-29(39)34-26(36)20-7-5-18(40-3)13-22(20)30/h4-7,12-14,31H,8-11H2,1-3H3,(H,33,37)(H2,32,34,36,39)/b21-14- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University
Curated by ChEMBL
| Assay Description
Inhibition of Aurora B (unknown origin) using HLRRASLG substrate |
Eur J Med Chem 85: 268-88 (2014)
Article DOI: 10.1016/j.ejmech.2014.07.108
BindingDB Entry DOI: 10.7270/Q2028T6Z |
More data for this
Ligand-Target Pair | |
Aurora kinase B
(Homo sapiens (Human)) | BDBM50056158

(CHEMBL3329661)Show SMILES Cc1ccc(cc1)C(=O)NC(=O)Nc1ccc2\C(=C\c3ccc[nH]3)C(=O)Nc2c1 Show InChI InChI=1S/C22H18N4O3/c1-13-4-6-14(7-5-13)20(27)26-22(29)24-16-8-9-17-18(11-15-3-2-10-23-15)21(28)25-19(17)12-16/h2-12,23H,1H3,(H,25,28)(H2,24,26,27,29)/b18-11- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University
Curated by ChEMBL
| Assay Description
Inhibition of Aurora B (unknown origin) using HLRRASLG substrate |
Eur J Med Chem 85: 268-88 (2014)
Article DOI: 10.1016/j.ejmech.2014.07.108
BindingDB Entry DOI: 10.7270/Q2028T6Z |
More data for this
Ligand-Target Pair | |
Aurora kinase B
(Homo sapiens (Human)) | BDBM50056194

(CHEMBL3329679)Show SMILES COc1ccc(C(=O)NC(=O)Nc2ccc3\C(=C\c4[nH]c(C)c(CCC(=O)N5CCN(C)CC5)c4C)C(=O)Nc3c2)c(F)c1 Show InChI InChI=1S/C32H35FN6O5/c1-18-22(9-10-29(40)39-13-11-38(3)12-14-39)19(2)34-27(18)17-25-23-7-5-20(15-28(23)36-31(25)42)35-32(43)37-30(41)24-8-6-21(44-4)16-26(24)33/h5-8,15-17,34H,9-14H2,1-4H3,(H,36,42)(H2,35,37,41,43)/b25-17- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University
Curated by ChEMBL
| Assay Description
Inhibition of Aurora B (unknown origin) using HLRRASLG substrate |
Eur J Med Chem 85: 268-88 (2014)
Article DOI: 10.1016/j.ejmech.2014.07.108
BindingDB Entry DOI: 10.7270/Q2028T6Z |
More data for this
Ligand-Target Pair | |
Aurora kinase B
(Homo sapiens (Human)) | BDBM50056137

(CHEMBL3361346)Show SMILES COc1ccc(cc1)C(=O)NC(=O)Nc1ccc2\C(=C/c3cccs3)C(=O)Nc2c1 Show InChI InChI=1S/C22H17N3O4S/c1-29-15-7-4-13(5-8-15)20(26)25-22(28)23-14-6-9-17-18(12-16-3-2-10-30-16)21(27)24-19(17)11-14/h2-12H,1H3,(H,24,27)(H2,23,25,26,28)/b18-12+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University
Curated by ChEMBL
| Assay Description
Inhibition of Aurora B (unknown origin) using HLRRASLG substrate |
Eur J Med Chem 85: 268-88 (2014)
Article DOI: 10.1016/j.ejmech.2014.07.108
BindingDB Entry DOI: 10.7270/Q2028T6Z |
More data for this
Ligand-Target Pair | |
Aurora kinase B
(Homo sapiens (Human)) | BDBM50056157

(CHEMBL3329662)Show SMILES COc1cc(OC)cc(c1)C(=O)NC(=O)Nc1ccc2\C(=C\c3ccc[nH]3)C(=O)Nc2c1 Show InChI InChI=1S/C23H20N4O5/c1-31-16-8-13(9-17(12-16)32-2)21(28)27-23(30)25-15-5-6-18-19(10-14-4-3-7-24-14)22(29)26-20(18)11-15/h3-12,24H,1-2H3,(H,26,29)(H2,25,27,28,30)/b19-10- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University
Curated by ChEMBL
| Assay Description
Inhibition of Aurora B (unknown origin) using HLRRASLG substrate |
Eur J Med Chem 85: 268-88 (2014)
Article DOI: 10.1016/j.ejmech.2014.07.108
BindingDB Entry DOI: 10.7270/Q2028T6Z |
More data for this
Ligand-Target Pair | |
Aurora kinase B
(Homo sapiens (Human)) | BDBM50056193

(CHEMBL3329678)Show SMILES COc1ccc(C(=O)NC(=O)Nc2ccc3\C(=C\c4[nH]c(C)c(C(=O)N5CCN(C)CC5)c4C)C(=O)Nc3c2)c(F)c1 Show InChI InChI=1S/C30H31FN6O5/c1-16-24(32-17(2)26(16)29(40)37-11-9-36(3)10-12-37)15-22-20-7-5-18(13-25(20)34-28(22)39)33-30(41)35-27(38)21-8-6-19(42-4)14-23(21)31/h5-8,13-15,32H,9-12H2,1-4H3,(H,34,39)(H2,33,35,38,41)/b22-15- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University
Curated by ChEMBL
| Assay Description
Inhibition of Aurora B (unknown origin) using HLRRASLG substrate |
Eur J Med Chem 85: 268-88 (2014)
Article DOI: 10.1016/j.ejmech.2014.07.108
BindingDB Entry DOI: 10.7270/Q2028T6Z |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50056151

(CHEMBL3329668)Show SMILES COc1ccc(cc1)C(=O)NC(=O)Nc1ccc2\C(=C\c3[nH]c(C)c(CC(O)=O)c3C)C(=O)Nc2c1 Show InChI InChI=1S/C26H24N4O6/c1-13-19(12-23(31)32)14(2)27-21(13)11-20-18-9-6-16(10-22(18)29-25(20)34)28-26(35)30-24(33)15-4-7-17(36-3)8-5-15/h4-11,27H,12H2,1-3H3,(H,29,34)(H,31,32)(H2,28,30,33,35)/b20-11- | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University
Curated by ChEMBL
| Assay Description
Inhibition of FLT3 (unknown origin) using EAIYAAPFAKKK substrate |
Eur J Med Chem 85: 268-88 (2014)
Article DOI: 10.1016/j.ejmech.2014.07.108
BindingDB Entry DOI: 10.7270/Q2028T6Z |
More data for this
Ligand-Target Pair | |
Aurora kinase B
(Homo sapiens (Human)) | BDBM50056183
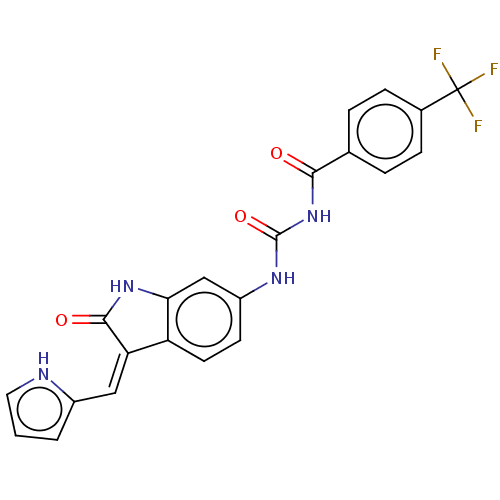
(CHEMBL3329659)Show SMILES FC(F)(F)c1ccc(cc1)C(=O)NC(=O)Nc1ccc2\C(=C\c3ccc[nH]3)C(=O)Nc2c1 Show InChI InChI=1S/C22H15F3N4O3/c23-22(24,25)13-5-3-12(4-6-13)19(30)29-21(32)27-15-7-8-16-17(10-14-2-1-9-26-14)20(31)28-18(16)11-15/h1-11,26H,(H,28,31)(H2,27,29,30,32)/b17-10- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University
Curated by ChEMBL
| Assay Description
Inhibition of Aurora B (unknown origin) using HLRRASLG substrate |
Eur J Med Chem 85: 268-88 (2014)
Article DOI: 10.1016/j.ejmech.2014.07.108
BindingDB Entry DOI: 10.7270/Q2028T6Z |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50056194

(CHEMBL3329679)Show SMILES COc1ccc(C(=O)NC(=O)Nc2ccc3\C(=C\c4[nH]c(C)c(CCC(=O)N5CCN(C)CC5)c4C)C(=O)Nc3c2)c(F)c1 Show InChI InChI=1S/C32H35FN6O5/c1-18-22(9-10-29(40)39-13-11-38(3)12-14-39)19(2)34-27(18)17-25-23-7-5-20(15-28(23)36-31(25)42)35-32(43)37-30(41)24-8-6-21(44-4)16-26(24)33/h5-8,15-17,34H,9-14H2,1-4H3,(H,36,42)(H2,35,37,41,43)/b25-17- | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University
Curated by ChEMBL
| Assay Description
Inhibition of FLT3 (unknown origin) using EAIYAAPFAKKK substrate |
Eur J Med Chem 85: 268-88 (2014)
Article DOI: 10.1016/j.ejmech.2014.07.108
BindingDB Entry DOI: 10.7270/Q2028T6Z |
More data for this
Ligand-Target Pair | |
Aurora kinase B
(Homo sapiens (Human)) | BDBM50056154

(CHEMBL3329665)Show SMILES COc1ccc(cc1)C(=O)NC(=O)Nc1ccc2\C(=C\c3[nH]c(C)cc3C)C(=O)Nc2c1 Show InChI InChI=1S/C24H22N4O4/c1-13-10-14(2)25-20(13)12-19-18-9-6-16(11-21(18)27-23(19)30)26-24(31)28-22(29)15-4-7-17(32-3)8-5-15/h4-12,25H,1-3H3,(H,27,30)(H2,26,28,29,31)/b19-12- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 56 | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University
Curated by ChEMBL
| Assay Description
Inhibition of Aurora B (unknown origin) using HLRRASLG substrate |
Eur J Med Chem 85: 268-88 (2014)
Article DOI: 10.1016/j.ejmech.2014.07.108
BindingDB Entry DOI: 10.7270/Q2028T6Z |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50056193

(CHEMBL3329678)Show SMILES COc1ccc(C(=O)NC(=O)Nc2ccc3\C(=C\c4[nH]c(C)c(C(=O)N5CCN(C)CC5)c4C)C(=O)Nc3c2)c(F)c1 Show InChI InChI=1S/C30H31FN6O5/c1-16-24(32-17(2)26(16)29(40)37-11-9-36(3)10-12-37)15-22-20-7-5-18(13-25(20)34-28(22)39)33-30(41)35-27(38)21-8-6-19(42-4)14-23(21)31/h5-8,13-15,32H,9-12H2,1-4H3,(H,34,39)(H2,33,35,38,41)/b22-15- | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 57 | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University
Curated by ChEMBL
| Assay Description
Inhibition of FLT3 (unknown origin) using EAIYAAPFAKKK substrate |
Eur J Med Chem 85: 268-88 (2014)
Article DOI: 10.1016/j.ejmech.2014.07.108
BindingDB Entry DOI: 10.7270/Q2028T6Z |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50056200

(CHEMBL3329650)Show SMILES COc1ccc(cc1)C(=O)NC(=O)Nc1cccc2NC(=O)\C(=C/c3ccc[nH]3)c12 Show InChI InChI=1S/C22H18N4O4/c1-30-15-9-7-13(8-10-15)20(27)26-22(29)25-18-6-2-5-17-19(18)16(21(28)24-17)12-14-4-3-11-23-14/h2-12,23H,1H3,(H,24,28)(H2,25,26,27,29)/b16-12- | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 58 | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University
Curated by ChEMBL
| Assay Description
Inhibition of FLT3 (unknown origin) using EAIYAAPFAKKK substrate |
Eur J Med Chem 85: 268-88 (2014)
Article DOI: 10.1016/j.ejmech.2014.07.108
BindingDB Entry DOI: 10.7270/Q2028T6Z |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50056195

(CHEMBL3329680)Show SMILES COc1ccc(C(=O)NC(=O)Nc2ccc3\C(=C\c4[nH]c(C)c(C(=O)N5CCOCC5)c4C)C(=O)Nc3c2)c(F)c1 Show InChI InChI=1S/C29H28FN5O6/c1-15-23(31-16(2)25(15)28(38)35-8-10-41-11-9-35)14-21-19-6-4-17(12-24(19)33-27(21)37)32-29(39)34-26(36)20-7-5-18(40-3)13-22(20)30/h4-7,12-14,31H,8-11H2,1-3H3,(H,33,37)(H2,32,34,36,39)/b21-14- | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University
Curated by ChEMBL
| Assay Description
Inhibition of FLT3 (unknown origin) using EAIYAAPFAKKK substrate |
Eur J Med Chem 85: 268-88 (2014)
Article DOI: 10.1016/j.ejmech.2014.07.108
BindingDB Entry DOI: 10.7270/Q2028T6Z |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50056196

(CHEMBL3329681)Show SMILES COc1ccc(C(=O)NC(=O)Nc2ccc3\C(=C\c4[nH]c(C)c(CCC(=O)N5CCOCC5)c4C)C(=O)Nc3c2)c(F)c1 Show InChI InChI=1S/C31H32FN5O6/c1-17-21(8-9-28(38)37-10-12-43-13-11-37)18(2)33-26(17)16-24-22-6-4-19(14-27(22)35-30(24)40)34-31(41)36-29(39)23-7-5-20(42-3)15-25(23)32/h4-7,14-16,33H,8-13H2,1-3H3,(H,35,40)(H2,34,36,39,41)/b24-16- | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 106 | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University
Curated by ChEMBL
| Assay Description
Inhibition of FLT3 (unknown origin) using EAIYAAPFAKKK substrate |
Eur J Med Chem 85: 268-88 (2014)
Article DOI: 10.1016/j.ejmech.2014.07.108
BindingDB Entry DOI: 10.7270/Q2028T6Z |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50056143

(CHEMBL3329672)Show SMILES COc1ccc(C(=O)NC(=O)Nc2ccc3\C(=C\c4[nH]c(C)c(C(O)=O)c4C)C(=O)Nc3c2)c(F)c1 Show InChI InChI=1S/C25H21FN4O6/c1-11-19(27-12(2)21(11)24(33)34)10-17-15-6-4-13(8-20(15)29-23(17)32)28-25(35)30-22(31)16-7-5-14(36-3)9-18(16)26/h4-10,27H,1-3H3,(H,29,32)(H,33,34)(H2,28,30,31,35)/b17-10- | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 111 | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University
Curated by ChEMBL
| Assay Description
Inhibition of FLT3 (unknown origin) using EAIYAAPFAKKK substrate |
Eur J Med Chem 85: 268-88 (2014)
Article DOI: 10.1016/j.ejmech.2014.07.108
BindingDB Entry DOI: 10.7270/Q2028T6Z |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50056156
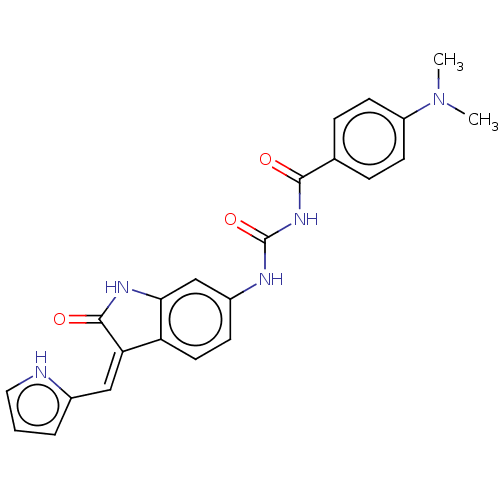
(CHEMBL3329663)Show SMILES CN(C)c1ccc(cc1)C(=O)NC(=O)Nc1ccc2\C(=C\c3ccc[nH]3)C(=O)Nc2c1 Show InChI InChI=1S/C23H21N5O3/c1-28(2)17-8-5-14(6-9-17)21(29)27-23(31)25-16-7-10-18-19(12-15-4-3-11-24-15)22(30)26-20(18)13-16/h3-13,24H,1-2H3,(H,26,30)(H2,25,27,29,31)/b19-12- | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 134 | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University
Curated by ChEMBL
| Assay Description
Inhibition of FLT3 (unknown origin) using EAIYAAPFAKKK substrate |
Eur J Med Chem 85: 268-88 (2014)
Article DOI: 10.1016/j.ejmech.2014.07.108
BindingDB Entry DOI: 10.7270/Q2028T6Z |
More data for this
Ligand-Target Pair | |
Aurora kinase B
(Homo sapiens (Human)) | BDBM50056156

(CHEMBL3329663)Show SMILES CN(C)c1ccc(cc1)C(=O)NC(=O)Nc1ccc2\C(=C\c3ccc[nH]3)C(=O)Nc2c1 Show InChI InChI=1S/C23H21N5O3/c1-28(2)17-8-5-14(6-9-17)21(29)27-23(31)25-16-7-10-18-19(12-15-4-3-11-24-15)22(30)26-20(18)13-16/h3-13,24H,1-2H3,(H,26,30)(H2,25,27,29,31)/b19-12- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 159 | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University
Curated by ChEMBL
| Assay Description
Inhibition of Aurora B (unknown origin) using HLRRASLG substrate |
Eur J Med Chem 85: 268-88 (2014)
Article DOI: 10.1016/j.ejmech.2014.07.108
BindingDB Entry DOI: 10.7270/Q2028T6Z |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50056155

(CHEMBL3329664)Show SMILES COc1ccc(cc1)C(=O)NC(=O)Nc1ccc2\C(=C\c3[nH]c(C)c(C(O)=O)c3C)C(=O)Nc2c1 Show InChI InChI=1S/C25H22N4O6/c1-12-19(26-13(2)21(12)24(32)33)11-18-17-9-6-15(10-20(17)28-23(18)31)27-25(34)29-22(30)14-4-7-16(35-3)8-5-14/h4-11,26H,1-3H3,(H,28,31)(H,32,33)(H2,27,29,30,34)/b18-11- | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University
Curated by ChEMBL
| Assay Description
Inhibition of FLT3 (unknown origin) using EAIYAAPFAKKK substrate |
Eur J Med Chem 85: 268-88 (2014)
Article DOI: 10.1016/j.ejmech.2014.07.108
BindingDB Entry DOI: 10.7270/Q2028T6Z |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50056201

(CHEMBL3329651)Show SMILES COc1ccc(cc1)C(=O)NC(=O)Nc1ccc2NC(=O)\C(=C/c3ccc[nH]3)c2c1 Show InChI InChI=1S/C22H18N4O4/c1-30-16-7-4-13(5-8-16)20(27)26-22(29)24-15-6-9-19-17(12-15)18(21(28)25-19)11-14-3-2-10-23-14/h2-12,23H,1H3,(H,25,28)(H2,24,26,27,29)/b18-11- | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 201 | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University
Curated by ChEMBL
| Assay Description
Inhibition of FLT3 (unknown origin) using EAIYAAPFAKKK substrate |
Eur J Med Chem 85: 268-88 (2014)
Article DOI: 10.1016/j.ejmech.2014.07.108
BindingDB Entry DOI: 10.7270/Q2028T6Z |
More data for this
Ligand-Target Pair | |
Aurora kinase B
(Homo sapiens (Human)) | BDBM50056192

(CHEMBL3329677)Show SMILES CCOC(=O)CCc1c(C)[nH]c(\C=C2/C(=O)Nc3cc(NC(=O)NC(=O)c4ccc(OC)cc4F)ccc23)c1C Show InChI InChI=1S/C29H29FN4O6/c1-5-40-26(35)11-10-19-15(2)24(31-16(19)3)14-22-20-8-6-17(12-25(20)33-28(22)37)32-29(38)34-27(36)21-9-7-18(39-4)13-23(21)30/h6-9,12-14,31H,5,10-11H2,1-4H3,(H,33,37)(H2,32,34,36,38)/b22-14- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 218 | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University
Curated by ChEMBL
| Assay Description
Inhibition of Aurora B (unknown origin) using HLRRASLG substrate |
Eur J Med Chem 85: 268-88 (2014)
Article DOI: 10.1016/j.ejmech.2014.07.108
BindingDB Entry DOI: 10.7270/Q2028T6Z |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50056189

(CHEMBL3329652)Show SMILES COc1ccc(cc1)C(=O)NC(=O)Nc1ccc2\C(=C\c3ccc[nH]3)C(=O)Nc2c1 Show InChI InChI=1S/C22H18N4O4/c1-30-16-7-4-13(5-8-16)20(27)26-22(29)24-15-6-9-17-18(11-14-3-2-10-23-14)21(28)25-19(17)12-15/h2-12,23H,1H3,(H,25,28)(H2,24,26,27,29)/b18-11- | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 334 | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University
Curated by ChEMBL
| Assay Description
Inhibition of FLT3 (unknown origin) using EAIYAAPFAKKK substrate |
Eur J Med Chem 85: 268-88 (2014)
Article DOI: 10.1016/j.ejmech.2014.07.108
BindingDB Entry DOI: 10.7270/Q2028T6Z |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50056178

(CHEMBL3329660)Show SMILES FC(F)(F)c1cc(ccc1Cl)C(=O)NC(=O)Nc1ccc2\C(=C\c3ccc[nH]3)C(=O)Nc2c1 Show InChI InChI=1S/C22H14ClF3N4O3/c23-17-6-3-11(8-16(17)22(24,25)26)19(31)30-21(33)28-13-4-5-14-15(9-12-2-1-7-27-12)20(32)29-18(14)10-13/h1-10,27H,(H,29,32)(H2,28,30,31,33)/b15-9- | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 385 | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University
Curated by ChEMBL
| Assay Description
Inhibition of FLT3 (unknown origin) using EAIYAAPFAKKK substrate |
Eur J Med Chem 85: 268-88 (2014)
Article DOI: 10.1016/j.ejmech.2014.07.108
BindingDB Entry DOI: 10.7270/Q2028T6Z |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50056141

(CHEMBL3329674)Show SMILES COc1ccc(C(=O)NC(=O)Nc2cc3NC(=O)\C(=C/c4[nH]c(C)c(C(O)=O)c4C)c3cc2F)c(F)c1 Show InChI InChI=1S/C25H20F2N4O6/c1-10-18(28-11(2)21(10)24(34)35)8-15-14-7-17(27)20(9-19(14)29-23(15)33)30-25(36)31-22(32)13-5-4-12(37-3)6-16(13)26/h4-9,28H,1-3H3,(H,29,33)(H,34,35)(H2,30,31,32,36)/b15-8- | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 406 | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University
Curated by ChEMBL
| Assay Description
Inhibition of FLT3 (unknown origin) using EAIYAAPFAKKK substrate |
Eur J Med Chem 85: 268-88 (2014)
Article DOI: 10.1016/j.ejmech.2014.07.108
BindingDB Entry DOI: 10.7270/Q2028T6Z |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50056186

(CHEMBL3329656)Show SMILES Clc1ccc(cc1)C(=O)NC(=O)Nc1ccc2\C(=C\c3ccc[nH]3)C(=O)Nc2c1 Show InChI InChI=1S/C21H15ClN4O3/c22-13-5-3-12(4-6-13)19(27)26-21(29)24-15-7-8-16-17(10-14-2-1-9-23-14)20(28)25-18(16)11-15/h1-11,23H,(H,25,28)(H2,24,26,27,29)/b17-10- | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 676 | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University
Curated by ChEMBL
| Assay Description
Inhibition of FLT3 (unknown origin) using EAIYAAPFAKKK substrate |
Eur J Med Chem 85: 268-88 (2014)
Article DOI: 10.1016/j.ejmech.2014.07.108
BindingDB Entry DOI: 10.7270/Q2028T6Z |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50056145

(CHEMBL3329670)Show SMILES COc1ccc(cc1)C(=O)NC(=O)Nc1cc2NC(=O)\C(=C/c3[nH]c(C)c(C(O)=O)c3C)c2cc1F Show InChI InChI=1S/C25H21FN4O6/c1-11-18(27-12(2)21(11)24(33)34)9-16-15-8-17(26)20(10-19(15)28-23(16)32)29-25(35)30-22(31)13-4-6-14(36-3)7-5-13/h4-10,27H,1-3H3,(H,28,32)(H,33,34)(H2,29,30,31,35)/b16-9- | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 909 | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University
Curated by ChEMBL
| Assay Description
Inhibition of FLT3 (unknown origin) using EAIYAAPFAKKK substrate |
Eur J Med Chem 85: 268-88 (2014)
Article DOI: 10.1016/j.ejmech.2014.07.108
BindingDB Entry DOI: 10.7270/Q2028T6Z |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50056139
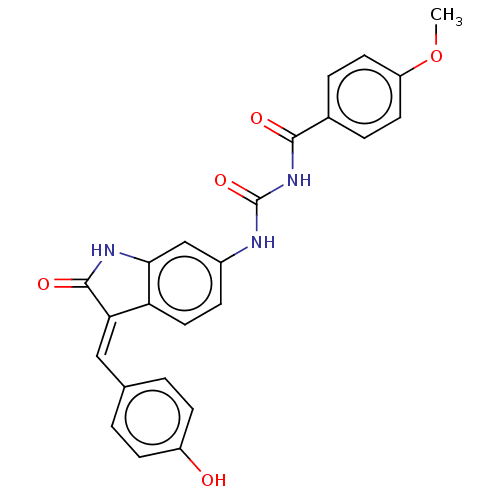
(CHEMBL3330969)Show SMILES COc1ccc(cc1)C(=O)NC(=O)Nc1ccc2\C(=C/c3ccc(O)cc3)C(=O)Nc2c1 Show InChI InChI=1S/C24H19N3O5/c1-32-18-9-4-15(5-10-18)22(29)27-24(31)25-16-6-11-19-20(23(30)26-21(19)13-16)12-14-2-7-17(28)8-3-14/h2-13,28H,1H3,(H,26,30)(H2,25,27,29,31)/b20-12+ | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University
Curated by ChEMBL
| Assay Description
Inhibition of FLT3 (unknown origin) using EAIYAAPFAKKK substrate |
Eur J Med Chem 85: 268-88 (2014)
Article DOI: 10.1016/j.ejmech.2014.07.108
BindingDB Entry DOI: 10.7270/Q2028T6Z |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50056157

(CHEMBL3329662)Show SMILES COc1cc(OC)cc(c1)C(=O)NC(=O)Nc1ccc2\C(=C\c3ccc[nH]3)C(=O)Nc2c1 Show InChI InChI=1S/C23H20N4O5/c1-31-16-8-13(9-17(12-16)32-2)21(28)27-23(30)25-15-5-6-18-19(10-14-4-3-7-24-14)22(29)26-20(18)11-15/h3-12,24H,1-2H3,(H,26,29)(H2,25,27,28,30)/b19-10- | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University
Curated by ChEMBL
| Assay Description
Inhibition of FLT3 (unknown origin) using EAIYAAPFAKKK substrate |
Eur J Med Chem 85: 268-88 (2014)
Article DOI: 10.1016/j.ejmech.2014.07.108
BindingDB Entry DOI: 10.7270/Q2028T6Z |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50056152

(CHEMBL3329667)Show SMILES COc1ccc(cc1)C(=O)NC(=O)Nc1ccc2\C(=C\c3ccc([nH]3)C(O)=O)C(=O)Nc2c1 Show InChI InChI=1S/C23H18N4O6/c1-33-15-6-2-12(3-7-15)20(28)27-23(32)25-14-4-8-16-17(21(29)26-19(16)11-14)10-13-5-9-18(24-13)22(30)31/h2-11,24H,1H3,(H,26,29)(H,30,31)(H2,25,27,28,32)/b17-10- | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University
Curated by ChEMBL
| Assay Description
Inhibition of FLT3 (unknown origin) using EAIYAAPFAKKK substrate |
Eur J Med Chem 85: 268-88 (2014)
Article DOI: 10.1016/j.ejmech.2014.07.108
BindingDB Entry DOI: 10.7270/Q2028T6Z |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50056185

(CHEMBL3329657)Show SMILES Clc1ccc(cc1Cl)C(=O)NC(=O)Nc1ccc2\C(=C\c3ccc[nH]3)C(=O)Nc2c1 Show InChI InChI=1S/C21H14Cl2N4O3/c22-16-6-3-11(8-17(16)23)19(28)27-21(30)25-13-4-5-14-15(9-12-2-1-7-24-12)20(29)26-18(14)10-13/h1-10,24H,(H,26,29)(H2,25,27,28,30)/b15-9- | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University
Curated by ChEMBL
| Assay Description
Inhibition of FLT3 (unknown origin) using EAIYAAPFAKKK substrate |
Eur J Med Chem 85: 268-88 (2014)
Article DOI: 10.1016/j.ejmech.2014.07.108
BindingDB Entry DOI: 10.7270/Q2028T6Z |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50056158

(CHEMBL3329661)Show SMILES Cc1ccc(cc1)C(=O)NC(=O)Nc1ccc2\C(=C\c3ccc[nH]3)C(=O)Nc2c1 Show InChI InChI=1S/C22H18N4O3/c1-13-4-6-14(7-5-13)20(27)26-22(29)24-16-8-9-17-18(11-15-3-2-10-23-15)21(28)25-19(17)12-16/h2-12,23H,1H3,(H,25,28)(H2,24,26,27,29)/b18-11- | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University
Curated by ChEMBL
| Assay Description
Inhibition of FLT3 (unknown origin) using EAIYAAPFAKKK substrate |
Eur J Med Chem 85: 268-88 (2014)
Article DOI: 10.1016/j.ejmech.2014.07.108
BindingDB Entry DOI: 10.7270/Q2028T6Z |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50056154

(CHEMBL3329665)Show SMILES COc1ccc(cc1)C(=O)NC(=O)Nc1ccc2\C(=C\c3[nH]c(C)cc3C)C(=O)Nc2c1 Show InChI InChI=1S/C24H22N4O4/c1-13-10-14(2)25-20(13)12-19-18-9-6-16(11-21(18)27-23(19)30)26-24(31)28-22(29)15-4-7-17(32-3)8-5-15/h4-12,25H,1-3H3,(H,27,30)(H2,26,28,29,31)/b19-12- | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University
Curated by ChEMBL
| Assay Description
Inhibition of FLT3 (unknown origin) using EAIYAAPFAKKK substrate |
Eur J Med Chem 85: 268-88 (2014)
Article DOI: 10.1016/j.ejmech.2014.07.108
BindingDB Entry DOI: 10.7270/Q2028T6Z |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50056153

(CHEMBL3329666)Show SMILES COc1ccc(cc1)C(=O)NC(=O)Nc1ccc2\C(=C\c3[nH]c(C)c(c3C)-c3ccccc3)C(=O)Nc2c1 Show InChI InChI=1S/C30H26N4O4/c1-17-25(31-18(2)27(17)19-7-5-4-6-8-19)16-24-23-14-11-21(15-26(23)33-29(24)36)32-30(37)34-28(35)20-9-12-22(38-3)13-10-20/h4-16,31H,1-3H3,(H,33,36)(H2,32,34,35,37)/b24-16- | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University
Curated by ChEMBL
| Assay Description
Inhibition of FLT3 (unknown origin) using EAIYAAPFAKKK substrate |
Eur J Med Chem 85: 268-88 (2014)
Article DOI: 10.1016/j.ejmech.2014.07.108
BindingDB Entry DOI: 10.7270/Q2028T6Z |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50056137

(CHEMBL3361346)Show SMILES COc1ccc(cc1)C(=O)NC(=O)Nc1ccc2\C(=C/c3cccs3)C(=O)Nc2c1 Show InChI InChI=1S/C22H17N3O4S/c1-29-15-7-4-13(5-8-15)20(26)25-22(28)23-14-6-9-17-18(12-16-3-2-10-30-16)21(27)24-19(17)11-14/h2-12H,1H3,(H,24,27)(H2,23,25,26,28)/b18-12+ | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University
Curated by ChEMBL
| Assay Description
Inhibition of FLT3 (unknown origin) using EAIYAAPFAKKK substrate |
Eur J Med Chem 85: 268-88 (2014)
Article DOI: 10.1016/j.ejmech.2014.07.108
BindingDB Entry DOI: 10.7270/Q2028T6Z |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50056187

(CHEMBL3329655)Show SMILES O=C(NC(=O)c1ccccc1)Nc1ccc2\C(=C\c3ccc[nH]3)C(=O)Nc2c1 Show InChI InChI=1S/C21H16N4O3/c26-19(13-5-2-1-3-6-13)25-21(28)23-15-8-9-16-17(11-14-7-4-10-22-14)20(27)24-18(16)12-15/h1-12,22H,(H,24,27)(H2,23,25,26,28)/b17-11- | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University
Curated by ChEMBL
| Assay Description
Inhibition of FLT3 (unknown origin) using EAIYAAPFAKKK substrate |
Eur J Med Chem 85: 268-88 (2014)
Article DOI: 10.1016/j.ejmech.2014.07.108
BindingDB Entry DOI: 10.7270/Q2028T6Z |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50056191

(CHEMBL3329676)Show SMILES CCOC(=O)c1c(C)[nH]c(\C=C2/C(=O)Nc3cc(NC(=O)NC(=O)c4ccc(OC)cc4F)ccc23)c1C Show InChI InChI=1S/C27H25FN4O6/c1-5-38-26(35)23-13(2)21(29-14(23)3)12-19-17-8-6-15(10-22(17)31-25(19)34)30-27(36)32-24(33)18-9-7-16(37-4)11-20(18)28/h6-12,29H,5H2,1-4H3,(H,31,34)(H2,30,32,33,36)/b19-12- | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University
Curated by ChEMBL
| Assay Description
Inhibition of FLT3 (unknown origin) using EAIYAAPFAKKK substrate |
Eur J Med Chem 85: 268-88 (2014)
Article DOI: 10.1016/j.ejmech.2014.07.108
BindingDB Entry DOI: 10.7270/Q2028T6Z |
More data for this
Ligand-Target Pair | |
Aurora kinase B
(Homo sapiens (Human)) | BDBM50056152

(CHEMBL3329667)Show SMILES COc1ccc(cc1)C(=O)NC(=O)Nc1ccc2\C(=C\c3ccc([nH]3)C(O)=O)C(=O)Nc2c1 Show InChI InChI=1S/C23H18N4O6/c1-33-15-6-2-12(3-7-15)20(28)27-23(32)25-14-4-8-16-17(21(29)26-19(16)11-14)10-13-5-9-18(24-13)22(30)31/h2-11,24H,1H3,(H,26,29)(H,30,31)(H2,25,27,28,32)/b17-10- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University
Curated by ChEMBL
| Assay Description
Inhibition of Aurora B (unknown origin) using HLRRASLG substrate |
Eur J Med Chem 85: 268-88 (2014)
Article DOI: 10.1016/j.ejmech.2014.07.108
BindingDB Entry DOI: 10.7270/Q2028T6Z |
More data for this
Ligand-Target Pair | |
Aurora kinase B
(Homo sapiens (Human)) | BDBM50056153

(CHEMBL3329666)Show SMILES COc1ccc(cc1)C(=O)NC(=O)Nc1ccc2\C(=C\c3[nH]c(C)c(c3C)-c3ccccc3)C(=O)Nc2c1 Show InChI InChI=1S/C30H26N4O4/c1-17-25(31-18(2)27(17)19-7-5-4-6-8-19)16-24-23-14-11-21(15-26(23)33-29(24)36)32-30(37)34-28(35)20-9-12-22(38-3)13-10-20/h4-16,31H,1-3H3,(H,33,36)(H2,32,34,35,37)/b24-16- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University
Curated by ChEMBL
| Assay Description
Inhibition of Aurora B (unknown origin) using HLRRASLG substrate |
Eur J Med Chem 85: 268-88 (2014)
Article DOI: 10.1016/j.ejmech.2014.07.108
BindingDB Entry DOI: 10.7270/Q2028T6Z |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50056144

(CHEMBL3329671)Show SMILES COc1ccc(cc1)C(=O)NC(=O)Nc1cc2NC(=O)\C(=C/c3[nH]c(C)c(CCC(O)=O)c3C)c2cc1F Show InChI InChI=1S/C27H25FN4O6/c1-13-17(8-9-24(33)34)14(2)29-21(13)11-19-18-10-20(28)23(12-22(18)30-26(19)36)31-27(37)32-25(35)15-4-6-16(38-3)7-5-15/h4-7,10-12,29H,8-9H2,1-3H3,(H,30,36)(H,33,34)(H2,31,32,35,37)/b19-11- | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University
Curated by ChEMBL
| Assay Description
Inhibition of FLT3 (unknown origin) using EAIYAAPFAKKK substrate |
Eur J Med Chem 85: 268-88 (2014)
Article DOI: 10.1016/j.ejmech.2014.07.108
BindingDB Entry DOI: 10.7270/Q2028T6Z |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50056184

(CHEMBL3329658)Show SMILES Fc1ccccc1C(=O)NC(=O)Nc1ccc2\C(=C\c3ccc[nH]3)C(=O)Nc2c1 Show InChI InChI=1S/C21H15FN4O3/c22-17-6-2-1-5-15(17)19(27)26-21(29)24-13-7-8-14-16(10-12-4-3-9-23-12)20(28)25-18(14)11-13/h1-11,23H,(H,25,28)(H2,24,26,27,29)/b16-10- | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University
Curated by ChEMBL
| Assay Description
Inhibition of FLT3 (unknown origin) using EAIYAAPFAKKK substrate |
Eur J Med Chem 85: 268-88 (2014)
Article DOI: 10.1016/j.ejmech.2014.07.108
BindingDB Entry DOI: 10.7270/Q2028T6Z |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50056183

(CHEMBL3329659)Show SMILES FC(F)(F)c1ccc(cc1)C(=O)NC(=O)Nc1ccc2\C(=C\c3ccc[nH]3)C(=O)Nc2c1 Show InChI InChI=1S/C22H15F3N4O3/c23-22(24,25)13-5-3-12(4-6-13)19(30)29-21(32)27-15-7-8-16-17(10-14-2-1-9-26-14)20(31)28-18(16)11-15/h1-11,26H,(H,28,31)(H2,27,29,30,32)/b17-10- | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University
Curated by ChEMBL
| Assay Description
Inhibition of FLT3 (unknown origin) using EAIYAAPFAKKK substrate |
Eur J Med Chem 85: 268-88 (2014)
Article DOI: 10.1016/j.ejmech.2014.07.108
BindingDB Entry DOI: 10.7270/Q2028T6Z |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50056138

(CHEMBL3330975)Show SMILES COc1ccc(cc1)C(=O)NC(=O)Nc1ccc2\C(=C/c3ccccn3)C(=O)Nc2c1 Show InChI InChI=1S/C23H18N4O4/c1-31-17-8-5-14(6-9-17)21(28)27-23(30)25-16-7-10-18-19(22(29)26-20(18)13-16)12-15-4-2-3-11-24-15/h2-13H,1H3,(H,26,29)(H2,25,27,28,30)/b19-12+ | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University
Curated by ChEMBL
| Assay Description
Inhibition of FLT3 (unknown origin) using EAIYAAPFAKKK substrate |
Eur J Med Chem 85: 268-88 (2014)
Article DOI: 10.1016/j.ejmech.2014.07.108
BindingDB Entry DOI: 10.7270/Q2028T6Z |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50056192

(CHEMBL3329677)Show SMILES CCOC(=O)CCc1c(C)[nH]c(\C=C2/C(=O)Nc3cc(NC(=O)NC(=O)c4ccc(OC)cc4F)ccc23)c1C Show InChI InChI=1S/C29H29FN4O6/c1-5-40-26(35)11-10-19-15(2)24(31-16(19)3)14-22-20-8-6-17(12-25(20)33-28(22)37)32-29(38)34-27(36)21-9-7-18(39-4)13-23(21)30/h6-9,12-14,31H,5,10-11H2,1-4H3,(H,33,37)(H2,32,34,36,38)/b22-14- | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University
Curated by ChEMBL
| Assay Description
Inhibition of FLT3 (unknown origin) using EAIYAAPFAKKK substrate |
Eur J Med Chem 85: 268-88 (2014)
Article DOI: 10.1016/j.ejmech.2014.07.108
BindingDB Entry DOI: 10.7270/Q2028T6Z |
More data for this
Ligand-Target Pair | |
Aurora kinase B
(Homo sapiens (Human)) | BDBM50056191

(CHEMBL3329676)Show SMILES CCOC(=O)c1c(C)[nH]c(\C=C2/C(=O)Nc3cc(NC(=O)NC(=O)c4ccc(OC)cc4F)ccc23)c1C Show InChI InChI=1S/C27H25FN4O6/c1-5-38-26(35)23-13(2)21(29-14(23)3)12-19-17-8-6-15(10-22(17)31-25(19)34)30-27(36)32-24(33)18-9-7-16(37-4)11-20(18)28/h6-12,29H,5H2,1-4H3,(H,31,34)(H2,30,32,33,36)/b19-12- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University
Curated by ChEMBL
| Assay Description
Inhibition of Aurora B (unknown origin) using HLRRASLG substrate |
Eur J Med Chem 85: 268-88 (2014)
Article DOI: 10.1016/j.ejmech.2014.07.108
BindingDB Entry DOI: 10.7270/Q2028T6Z |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50056199

(CHEMBL3329684)Show SMILES COc1ccc(C(=O)NC(=O)Nc2ccc3\C(=C\c4[nH]c(C)c(C(=O)NCCN5CCCC5)c4C)C(=O)Nc3c2)c(F)c1 Show InChI InChI=1S/C31H33FN6O5/c1-17-25(34-18(2)27(17)30(41)33-10-13-38-11-4-5-12-38)16-23-21-8-6-19(14-26(21)36-29(23)40)35-31(42)37-28(39)22-9-7-20(43-3)15-24(22)32/h6-9,14-16,34H,4-5,10-13H2,1-3H3,(H,33,41)(H,36,40)(H2,35,37,39,42)/b23-16- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 9.67E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University
Curated by ChEMBL
| Assay Description
Inhibition of human ERG channel after 3 hrs by fluorescence assay |
Eur J Med Chem 85: 268-88 (2014)
Article DOI: 10.1016/j.ejmech.2014.07.108
BindingDB Entry DOI: 10.7270/Q2028T6Z |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50056199

(CHEMBL3329684)Show SMILES COc1ccc(C(=O)NC(=O)Nc2ccc3\C(=C\c4[nH]c(C)c(C(=O)NCCN5CCCC5)c4C)C(=O)Nc3c2)c(F)c1 Show InChI InChI=1S/C31H33FN6O5/c1-17-25(34-18(2)27(17)30(41)33-10-13-38-11-4-5-12-38)16-23-21-8-6-19(14-26(21)36-29(23)40)35-31(42)37-28(39)22-9-7-20(43-3)15-24(22)32/h6-9,14-16,34H,4-5,10-13H2,1-3H3,(H,33,41)(H,36,40)(H2,35,37,39,42)/b23-16- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C19 (unknown origin) using 3-cyano-7-ethoxycoumarin substrate |
Eur J Med Chem 85: 268-88 (2014)
Article DOI: 10.1016/j.ejmech.2014.07.108
BindingDB Entry DOI: 10.7270/Q2028T6Z |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50056199

(CHEMBL3329684)Show SMILES COc1ccc(C(=O)NC(=O)Nc2ccc3\C(=C\c4[nH]c(C)c(C(=O)NCCN5CCCC5)c4C)C(=O)Nc3c2)c(F)c1 Show InChI InChI=1S/C31H33FN6O5/c1-17-25(34-18(2)27(17)30(41)33-10-13-38-11-4-5-12-38)16-23-21-8-6-19(14-26(21)36-29(23)40)35-31(42)37-28(39)22-9-7-20(43-3)15-24(22)32/h6-9,14-16,34H,4-5,10-13H2,1-3H3,(H,33,41)(H,36,40)(H2,35,37,39,42)/b23-16- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) using Vivid OOMR substrate |
Eur J Med Chem 85: 268-88 (2014)
Article DOI: 10.1016/j.ejmech.2014.07.108
BindingDB Entry DOI: 10.7270/Q2028T6Z |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50056199

(CHEMBL3329684)Show SMILES COc1ccc(C(=O)NC(=O)Nc2ccc3\C(=C\c4[nH]c(C)c(C(=O)NCCN5CCCC5)c4C)C(=O)Nc3c2)c(F)c1 Show InChI InChI=1S/C31H33FN6O5/c1-17-25(34-18(2)27(17)30(41)33-10-13-38-11-4-5-12-38)16-23-21-8-6-19(14-26(21)36-29(23)40)35-31(42)37-28(39)22-9-7-20(43-3)15-24(22)32/h6-9,14-16,34H,4-5,10-13H2,1-3H3,(H,33,41)(H,36,40)(H2,35,37,39,42)/b23-16- | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 (unknown origin) using 3-cyano-7-ethoxycoumarin substrate |
Eur J Med Chem 85: 268-88 (2014)
Article DOI: 10.1016/j.ejmech.2014.07.108
BindingDB Entry DOI: 10.7270/Q2028T6Z |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50056199

(CHEMBL3329684)Show SMILES COc1ccc(C(=O)NC(=O)Nc2ccc3\C(=C\c4[nH]c(C)c(C(=O)NCCN5CCCC5)c4C)C(=O)Nc3c2)c(F)c1 Show InChI InChI=1S/C31H33FN6O5/c1-17-25(34-18(2)27(17)30(41)33-10-13-38-11-4-5-12-38)16-23-21-8-6-19(14-26(21)36-29(23)40)35-31(42)37-28(39)22-9-7-20(43-3)15-24(22)32/h6-9,14-16,34H,4-5,10-13H2,1-3H3,(H,33,41)(H,36,40)(H2,35,37,39,42)/b23-16- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 (unknown origin) using Vivid OOMR substrate |
Eur J Med Chem 85: 268-88 (2014)
Article DOI: 10.1016/j.ejmech.2014.07.108
BindingDB Entry DOI: 10.7270/Q2028T6Z |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A1
(Homo sapiens (Human)) | BDBM50056199

(CHEMBL3329684)Show SMILES COc1ccc(C(=O)NC(=O)Nc2ccc3\C(=C\c4[nH]c(C)c(C(=O)NCCN5CCCC5)c4C)C(=O)Nc3c2)c(F)c1 Show InChI InChI=1S/C31H33FN6O5/c1-17-25(34-18(2)27(17)30(41)33-10-13-38-11-4-5-12-38)16-23-21-8-6-19(14-26(21)36-29(23)40)35-31(42)37-28(39)22-9-7-20(43-3)15-24(22)32/h6-9,14-16,34H,4-5,10-13H2,1-3H3,(H,33,41)(H,36,40)(H2,35,37,39,42)/b23-16- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University
Curated by ChEMBL
| Assay Description
Inhibition of CYP1A2 (unknown origin) using 3-cyano-7-ethoxycoumarin substrate |
Eur J Med Chem 85: 268-88 (2014)
Article DOI: 10.1016/j.ejmech.2014.07.108
BindingDB Entry DOI: 10.7270/Q2028T6Z |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data