

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Carbonic anhydrase 2 (Bos taurus) | BDBM10880 (AZA | AZA2 | AZM acetazolamide | Acerazolamide, AA...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 137 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Martin-Luther-Universit£t Halle-Wittenberg Curated by ChEMBL | Assay Description Inhibition of bovine erythrocyte carbonic anhydrase 2 using 4-nitrophenyl acetate substrate by photometric assay | Eur J Med Chem 86: 95-102 (2014) Article DOI: 10.1016/j.ejmech.2014.08.051 BindingDB Entry DOI: 10.7270/Q2R78GWB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Bos taurus) | BDBM50058822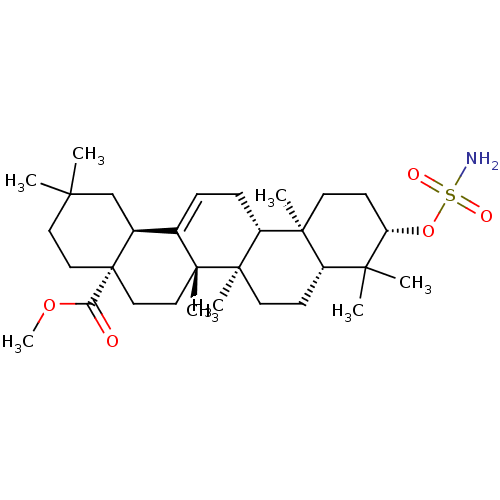 (CHEMBL3325725) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 308 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Martin-Luther-Universit£t Halle-Wittenberg Curated by ChEMBL | Assay Description Inhibition of bovine erythrocyte carbonic anhydrase 2 using 4-nitrophenyl acetate substrate by photometric assay | Eur J Med Chem 86: 95-102 (2014) Article DOI: 10.1016/j.ejmech.2014.08.051 BindingDB Entry DOI: 10.7270/Q2R78GWB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Bos taurus) | BDBM50058823 (CHEMBL3325726) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.11E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Martin-Luther-Universit£t Halle-Wittenberg Curated by ChEMBL | Assay Description Inhibition of bovine erythrocyte carbonic anhydrase 2 using 4-nitrophenyl acetate substrate by photometric assay | Eur J Med Chem 86: 95-102 (2014) Article DOI: 10.1016/j.ejmech.2014.08.051 BindingDB Entry DOI: 10.7270/Q2R78GWB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Bos taurus) | BDBM50058829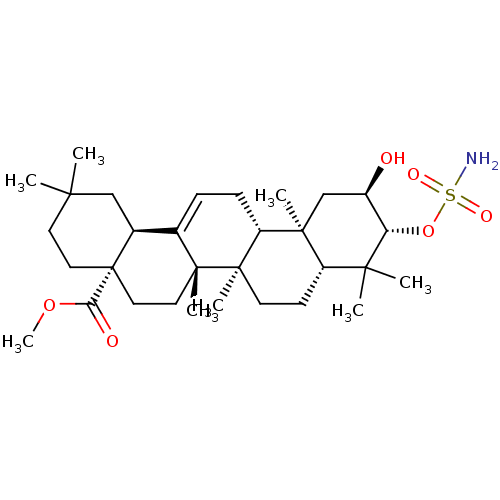 (CHEMBL3325867) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.41E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Martin-Luther-Universit£t Halle-Wittenberg Curated by ChEMBL | Assay Description Inhibition of bovine erythrocyte carbonic anhydrase 2 using 4-nitrophenyl acetate substrate by photometric assay | Eur J Med Chem 86: 95-102 (2014) Article DOI: 10.1016/j.ejmech.2014.08.051 BindingDB Entry DOI: 10.7270/Q2R78GWB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Bos taurus) | BDBM50058830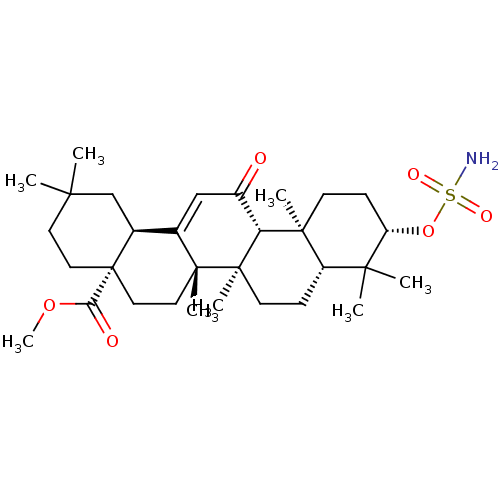 (CHEMBL3325868) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.49E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Martin-Luther-Universit£t Halle-Wittenberg Curated by ChEMBL | Assay Description Inhibition of bovine erythrocyte carbonic anhydrase 2 using 4-nitrophenyl acetate substrate by photometric assay | Eur J Med Chem 86: 95-102 (2014) Article DOI: 10.1016/j.ejmech.2014.08.051 BindingDB Entry DOI: 10.7270/Q2R78GWB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Bos taurus) | BDBM50058825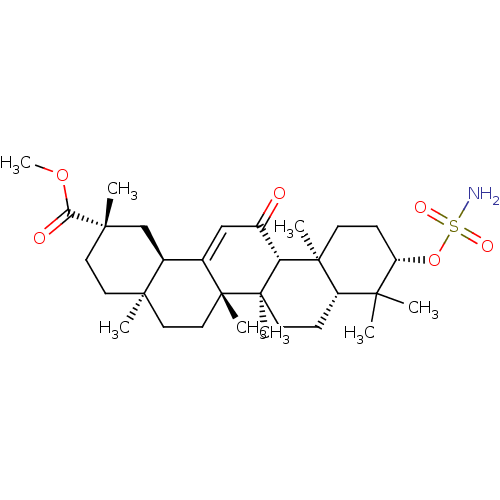 (CHEMBL3325728) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.51E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Martin-Luther-Universit£t Halle-Wittenberg Curated by ChEMBL | Assay Description Inhibition of bovine erythrocyte carbonic anhydrase 2 using 4-nitrophenyl acetate substrate by photometric assay | Eur J Med Chem 86: 95-102 (2014) Article DOI: 10.1016/j.ejmech.2014.08.051 BindingDB Entry DOI: 10.7270/Q2R78GWB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Bos taurus) | BDBM50058827 (CHEMBL3325865) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.74E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Martin-Luther-Universit£t Halle-Wittenberg Curated by ChEMBL | Assay Description Inhibition of bovine erythrocyte carbonic anhydrase 2 using 4-nitrophenyl acetate substrate by photometric assay | Eur J Med Chem 86: 95-102 (2014) Article DOI: 10.1016/j.ejmech.2014.08.051 BindingDB Entry DOI: 10.7270/Q2R78GWB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Bos taurus) | BDBM50058824 (CHEMBL3325727) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.16E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Martin-Luther-Universit£t Halle-Wittenberg Curated by ChEMBL | Assay Description Inhibition of bovine erythrocyte carbonic anhydrase 2 using 4-nitrophenyl acetate substrate by photometric assay | Eur J Med Chem 86: 95-102 (2014) Article DOI: 10.1016/j.ejmech.2014.08.051 BindingDB Entry DOI: 10.7270/Q2R78GWB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Bos taurus) | BDBM50058826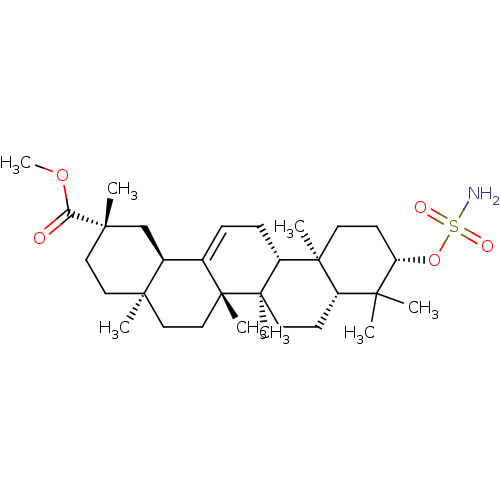 (CHEMBL3325864) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Martin-Luther-Universit£t Halle-Wittenberg Curated by ChEMBL | Assay Description Inhibition of bovine erythrocyte carbonic anhydrase 2 using 4-nitrophenyl acetate substrate by photometric assay | Eur J Med Chem 86: 95-102 (2014) Article DOI: 10.1016/j.ejmech.2014.08.051 BindingDB Entry DOI: 10.7270/Q2R78GWB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Bos taurus) | BDBM50058819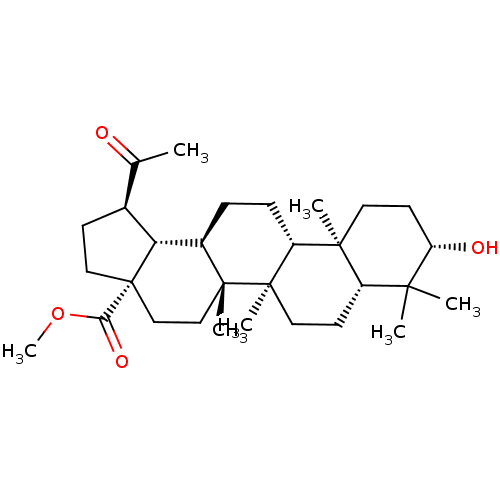 (CHEMBL3325723) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Martin-Luther-Universit£t Halle-Wittenberg Curated by ChEMBL | Assay Description Inhibition of bovine erythrocyte carbonic anhydrase 2 using 4-nitrophenyl acetate substrate by photometric assay | Eur J Med Chem 86: 95-102 (2014) Article DOI: 10.1016/j.ejmech.2014.08.051 BindingDB Entry DOI: 10.7270/Q2R78GWB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Bos taurus) | BDBM50242287 ((4aS,6aS,6bR,8aR,10S,12aR,12bR,14bS)-methyl 10-hyd...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Martin-Luther-Universit£t Halle-Wittenberg Curated by ChEMBL | Assay Description Inhibition of bovine erythrocyte carbonic anhydrase 2 using 4-nitrophenyl acetate substrate by photometric assay | Eur J Med Chem 86: 95-102 (2014) Article DOI: 10.1016/j.ejmech.2014.08.051 BindingDB Entry DOI: 10.7270/Q2R78GWB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Bos taurus) | BDBM50058818 (CHEMBL3100032) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Martin-Luther-Universit£t Halle-Wittenberg Curated by ChEMBL | Assay Description Inhibition of bovine erythrocyte carbonic anhydrase 2 using 4-nitrophenyl acetate substrate by photometric assay | Eur J Med Chem 86: 95-102 (2014) Article DOI: 10.1016/j.ejmech.2014.08.051 BindingDB Entry DOI: 10.7270/Q2R78GWB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Bos taurus) | BDBM50058817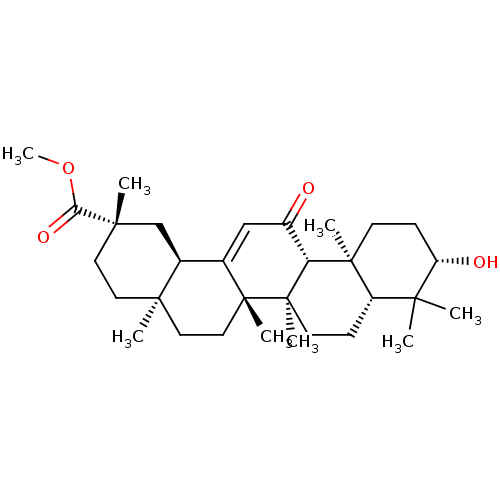 (CHEMBL1271483) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Martin-Luther-Universit£t Halle-Wittenberg Curated by ChEMBL | Assay Description Inhibition of bovine erythrocyte carbonic anhydrase 2 using 4-nitrophenyl acetate substrate by photometric assay | Eur J Med Chem 86: 95-102 (2014) Article DOI: 10.1016/j.ejmech.2014.08.051 BindingDB Entry DOI: 10.7270/Q2R78GWB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Bos taurus) | BDBM50058810 (Methyl Ursolate | Ursolic Acid Methyl Ester) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Martin-Luther-Universit£t Halle-Wittenberg Curated by ChEMBL | Assay Description Inhibition of bovine erythrocyte carbonic anhydrase 2 using 4-nitrophenyl acetate substrate by photometric assay | Eur J Med Chem 86: 95-102 (2014) Article DOI: 10.1016/j.ejmech.2014.08.051 BindingDB Entry DOI: 10.7270/Q2R78GWB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Bos taurus) | BDBM50058831 (CHEMBL3325869) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | >1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Martin-Luther-Universit£t Halle-Wittenberg Curated by ChEMBL | Assay Description Inhibition of bovine erythrocyte carbonic anhydrase 2 using 4-nitrophenyl acetate substrate by photometric assay | Eur J Med Chem 86: 95-102 (2014) Article DOI: 10.1016/j.ejmech.2014.08.051 BindingDB Entry DOI: 10.7270/Q2R78GWB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Bos taurus) | BDBM50058828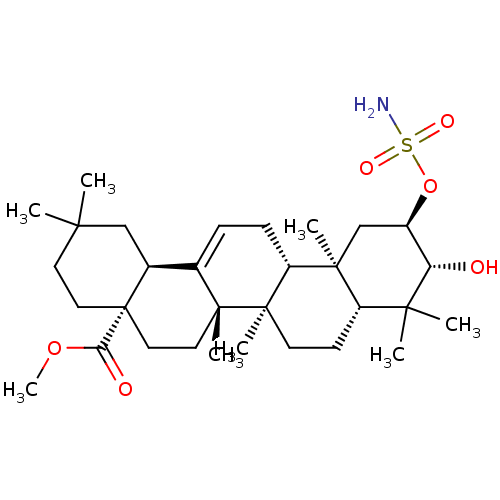 (CHEMBL3325866) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Martin-Luther-Universit£t Halle-Wittenberg Curated by ChEMBL | Assay Description Inhibition of bovine erythrocyte carbonic anhydrase 2 using 4-nitrophenyl acetate substrate by photometric assay | Eur J Med Chem 86: 95-102 (2014) Article DOI: 10.1016/j.ejmech.2014.08.051 BindingDB Entry DOI: 10.7270/Q2R78GWB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Bos taurus) | BDBM50175875 ((4aS,6aS,6bR,8aR,10R,11R,12aR,12bR,14bS)-methyl 10...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | >2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Martin-Luther-Universit£t Halle-Wittenberg Curated by ChEMBL | Assay Description Inhibition of bovine erythrocyte carbonic anhydrase 2 using 4-nitrophenyl acetate substrate by photometric assay | Eur J Med Chem 86: 95-102 (2014) Article DOI: 10.1016/j.ejmech.2014.08.051 BindingDB Entry DOI: 10.7270/Q2R78GWB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Bos taurus) | BDBM50058820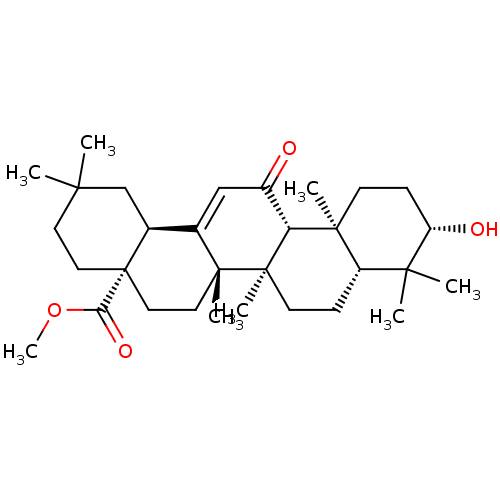 (CHEMBL3109256) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Martin-Luther-Universit£t Halle-Wittenberg Curated by ChEMBL | Assay Description Inhibition of bovine erythrocyte carbonic anhydrase 2 using 4-nitrophenyl acetate substrate by photometric assay | Eur J Med Chem 86: 95-102 (2014) Article DOI: 10.1016/j.ejmech.2014.08.051 BindingDB Entry DOI: 10.7270/Q2R78GWB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Bos taurus) | BDBM50240501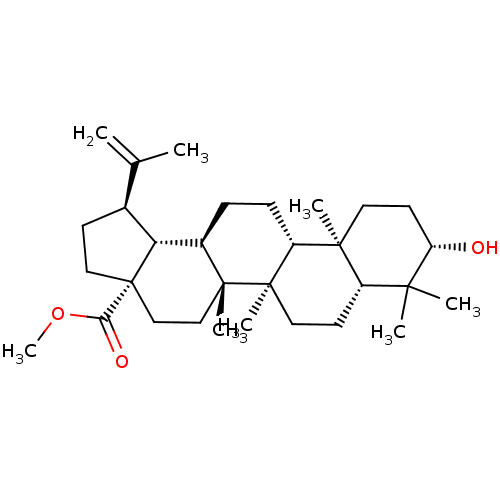 ((1R,3aS,5aR,5bR,7aR,9S,11aR,11bR,13aR)-methyl 9-hy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Martin-Luther-Universit£t Halle-Wittenberg Curated by ChEMBL | Assay Description Inhibition of bovine erythrocyte carbonic anhydrase 2 using 4-nitrophenyl acetate substrate by photometric assay | Eur J Med Chem 86: 95-102 (2014) Article DOI: 10.1016/j.ejmech.2014.08.051 BindingDB Entry DOI: 10.7270/Q2R78GWB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Bos taurus) | BDBM50058821 (CHEMBL3325724) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Martin-Luther-Universit£t Halle-Wittenberg Curated by ChEMBL | Assay Description Inhibition of bovine erythrocyte carbonic anhydrase 2 using 4-nitrophenyl acetate substrate by photometric assay | Eur J Med Chem 86: 95-102 (2014) Article DOI: 10.1016/j.ejmech.2014.08.051 BindingDB Entry DOI: 10.7270/Q2R78GWB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||