

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Genome polyprotein (Hepatitis C virus) | BDBM50142916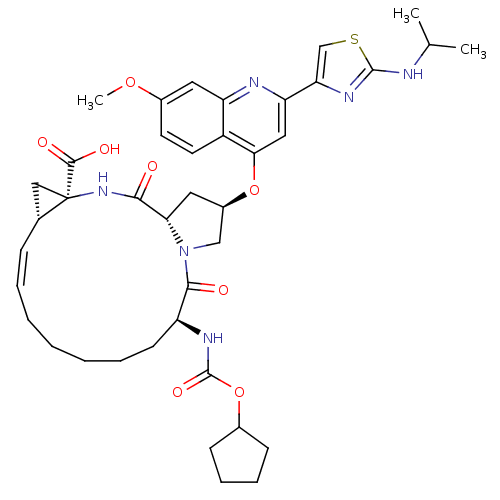 ((1S,4R,6S,14S,18R)-14-Cyclopentyloxycarbonylamino-...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
IDENIX Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of full length Hepatitis C virus genotype 1b Con1 NS3/4A by FRET assay | Bioorg Med Chem Lett 24: 4444-9 (2014) Article DOI: 10.1016/j.bmcl.2014.08.002 BindingDB Entry DOI: 10.7270/Q20C4XBK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50023510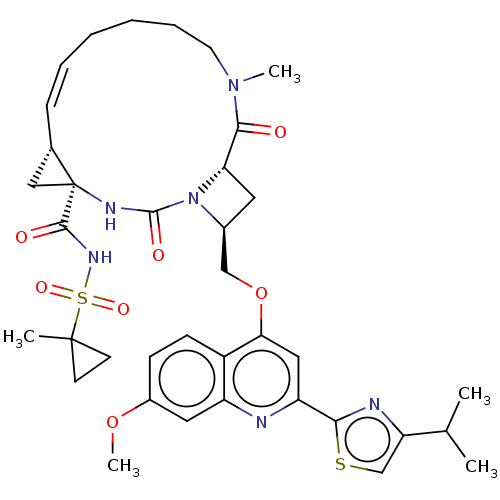 (CHEMBL3326826) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
IDENIX Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of full length Hepatitis C virus genotype 1b Con1 NS3/4A by FRET assay | Bioorg Med Chem Lett 24: 4444-9 (2014) Article DOI: 10.1016/j.bmcl.2014.08.002 BindingDB Entry DOI: 10.7270/Q20C4XBK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50023579 (CHEMBL3326830) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
IDENIX Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of full length Hepatitis C virus genotype 1b Con1 NS3/4A by FRET assay | Bioorg Med Chem Lett 24: 4444-9 (2014) Article DOI: 10.1016/j.bmcl.2014.08.002 BindingDB Entry DOI: 10.7270/Q20C4XBK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50023508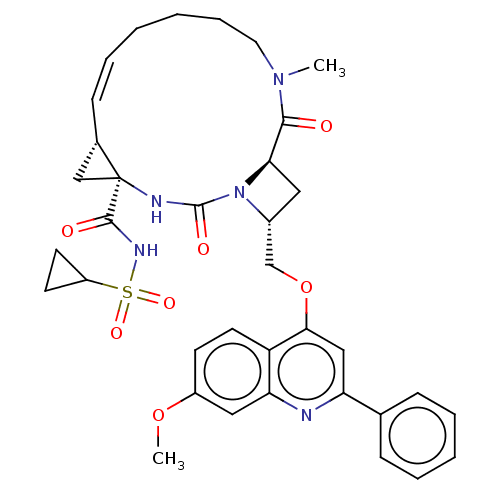 (CHEMBL3326539) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
IDENIX Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of full length Hepatitis C virus genotype 1b Con1 NS3/4A by FRET assay | Bioorg Med Chem Lett 24: 4444-9 (2014) Article DOI: 10.1016/j.bmcl.2014.08.002 BindingDB Entry DOI: 10.7270/Q20C4XBK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50023578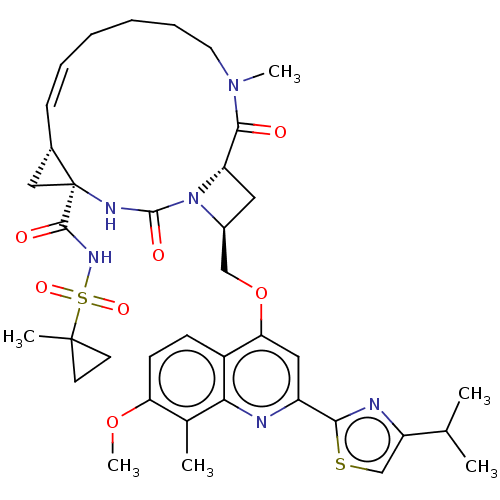 (CHEMBL3326829) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.60 | n/a | n/a | n/a | n/a | n/a | n/a |
IDENIX Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of full length Hepatitis C virus genotype 1b Con1 NS3/4A by FRET assay | Bioorg Med Chem Lett 24: 4444-9 (2014) Article DOI: 10.1016/j.bmcl.2014.08.002 BindingDB Entry DOI: 10.7270/Q20C4XBK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50023509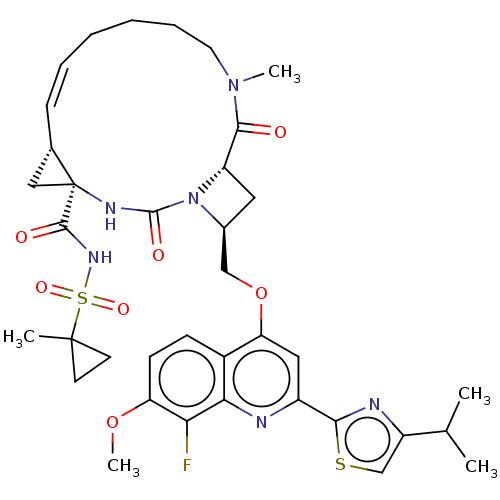 (CHEMBL3326540) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
IDENIX Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of full length Hepatitis C virus genotype 1b Con1 NS3/4A by FRET assay | Bioorg Med Chem Lett 24: 4444-9 (2014) Article DOI: 10.1016/j.bmcl.2014.08.002 BindingDB Entry DOI: 10.7270/Q20C4XBK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50023507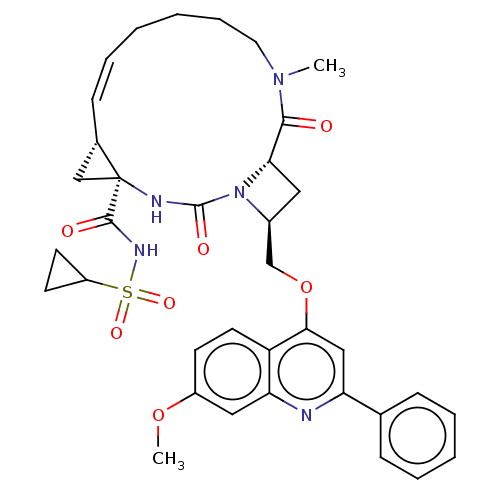 (CHEMBL3326538) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
IDENIX Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of full length Hepatitis C virus genotype 1b Con1 NS3/4A by FRET assay | Bioorg Med Chem Lett 24: 4444-9 (2014) Article DOI: 10.1016/j.bmcl.2014.08.002 BindingDB Entry DOI: 10.7270/Q20C4XBK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50023581 (CHEMBL3326537) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 278 | n/a | n/a | n/a | n/a | n/a | n/a |
IDENIX Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of full length Hepatitis C virus genotype 1b Con1 NS3/4A by FRET assay | Bioorg Med Chem Lett 24: 4444-9 (2014) Article DOI: 10.1016/j.bmcl.2014.08.002 BindingDB Entry DOI: 10.7270/Q20C4XBK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50023577 (CHEMBL3326828) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | <1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
IDENIX Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of full length Hepatitis C virus genotype 1b Con1 NS3/4A by FRET assay | Bioorg Med Chem Lett 24: 4444-9 (2014) Article DOI: 10.1016/j.bmcl.2014.08.002 BindingDB Entry DOI: 10.7270/Q20C4XBK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50023576 (CHEMBL3326827) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | <1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
IDENIX Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of full length Hepatitis C virus genotype 1b Con1 NS3/4A by FRET assay | Bioorg Med Chem Lett 24: 4444-9 (2014) Article DOI: 10.1016/j.bmcl.2014.08.002 BindingDB Entry DOI: 10.7270/Q20C4XBK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50023580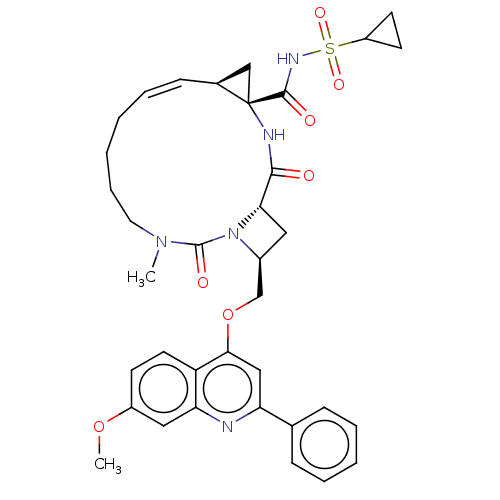 (CHEMBL3326536) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
IDENIX Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of full length Hepatitis C virus genotype 1b Con1 NS3/4A by FRET assay | Bioorg Med Chem Lett 24: 4444-9 (2014) Article DOI: 10.1016/j.bmcl.2014.08.002 BindingDB Entry DOI: 10.7270/Q20C4XBK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||