

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ADP-ribosyl cyclase/cyclic ADP-ribose hydrolase 1 (Homo sapiens (Human)) | BDBM50027369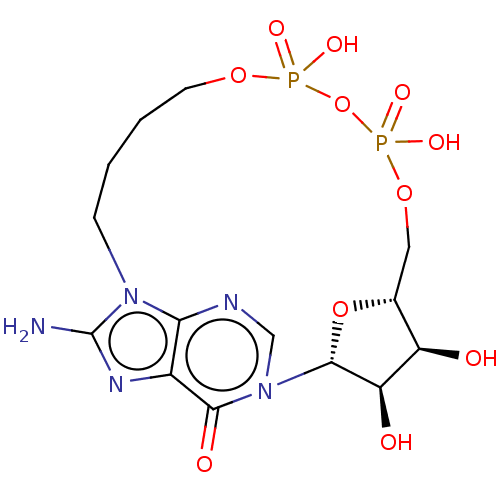 (CHEMBL3342444) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Inhibition of human CD38 catalytic domain assessed as reduction in cADPR hydrolysis incubated for 10 mins by fluorimetric cycling assay | J Med Chem 57: 8517-29 (2014) Article DOI: 10.1021/jm501037u BindingDB Entry DOI: 10.7270/Q26M38DH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ADP-ribosyl cyclase/cyclic ADP-ribose hydrolase 1 (Homo sapiens (Human)) | BDBM50027370 (CHEMBL3342440) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Inhibition of human CD38 catalytic domain assessed as reduction in cADPR hydrolysis incubated for 10 mins by fluorimetric cycling assay | J Med Chem 57: 8517-29 (2014) Article DOI: 10.1021/jm501037u BindingDB Entry DOI: 10.7270/Q26M38DH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ADP-ribosyl cyclase/cyclic ADP-ribose hydrolase 1 (Homo sapiens (Human)) | BDBM50027374 (CHEMBL3342443) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Inhibition of human CD38 catalytic domain assessed as reduction in cADPR hydrolysis incubated for 10 mins by fluorimetric cycling assay | J Med Chem 57: 8517-29 (2014) Article DOI: 10.1021/jm501037u BindingDB Entry DOI: 10.7270/Q26M38DH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ADP-ribosyl cyclase/cyclic ADP-ribose hydrolase 1 (Homo sapiens (Human)) | BDBM50027373 (CHEMBL3342442) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Inhibition of human CD38 catalytic domain assessed as reduction in cADPR hydrolysis incubated for 10 mins by fluorimetric cycling assay | J Med Chem 57: 8517-29 (2014) Article DOI: 10.1021/jm501037u BindingDB Entry DOI: 10.7270/Q26M38DH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ADP-ribosyl cyclase/cyclic ADP-ribose hydrolase 1 (Homo sapiens (Human)) | BDBM50027372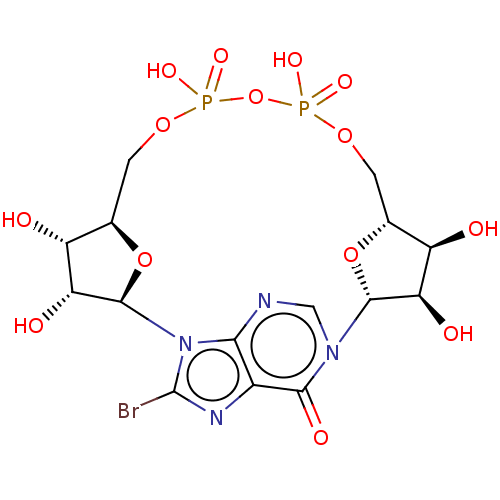 (CHEMBL3342441) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.58E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Inhibition of human CD38 catalytic domain assessed as reduction in cADPR hydrolysis incubated for 10 mins by fluorimetric cycling assay | J Med Chem 57: 8517-29 (2014) Article DOI: 10.1021/jm501037u BindingDB Entry DOI: 10.7270/Q26M38DH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ADP-ribosyl cyclase/cyclic ADP-ribose hydrolase 1 (Homo sapiens (Human)) | BDBM50027371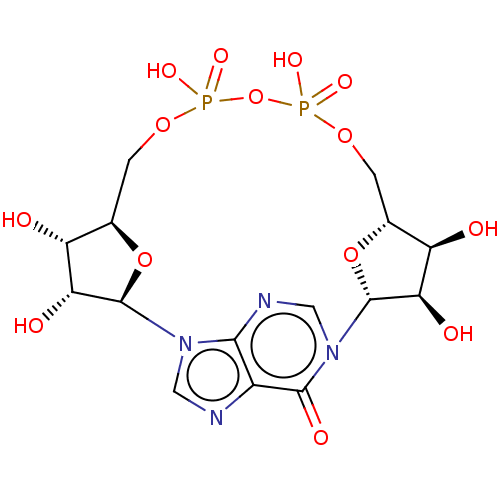 (CHEMBL1161863 | Cyclic Inosine 5''-Diphosphoribose) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.76E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Inhibition of human CD38 catalytic domain assessed as reduction in cADPR hydrolysis incubated for 10 mins by fluorimetric cycling assay | J Med Chem 57: 8517-29 (2014) Article DOI: 10.1021/jm501037u BindingDB Entry DOI: 10.7270/Q26M38DH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||