

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prostaglandin G/H synthase 1 (Ovis aries (Sheep)) | BDBM152804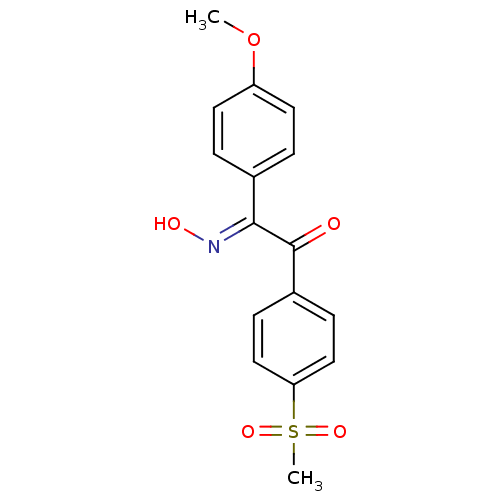 ((2E)-2-(N-hydroxyimino)-1-(4-methanesulfonylphenyl...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Mazandaran University of Medical Sciences | Assay Description Reaction mixtures were prepared in 100 mM Tris澦Cl buffer, pH 8.0 containing 1 然 heme and COX-1 or COX-2 and preincubated for 10 min in a waterbath ... | Chem Biol Drug Des 85: 494-503 (2015) Article DOI: 10.1111/cbdd.12435 BindingDB Entry DOI: 10.7270/Q21C1VK2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Ovis aries (Sheep)) | BDBM17638 (2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 600 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Mazandaran University of Medical Sciences | Assay Description Reaction mixtures were prepared in 100 mM Tris澦Cl buffer, pH 8.0 containing 1 然 heme and COX-1 or COX-2 and preincubated for 10 min in a waterbath ... | Chem Biol Drug Des 85: 494-503 (2015) Article DOI: 10.1111/cbdd.12435 BindingDB Entry DOI: 10.7270/Q21C1VK2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Ovis aries (Sheep)) | BDBM152806 ((2E)-2-(4-fluorophenyl)-2-(N-hydroxyimino)-1-(4-me...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Mazandaran University of Medical Sciences | Assay Description Reaction mixtures were prepared in 100 mM Tris澦Cl buffer, pH 8.0 containing 1 然 heme and COX-1 or COX-2 and preincubated for 10 min in a waterbath ... | Chem Biol Drug Des 85: 494-503 (2015) Article DOI: 10.1111/cbdd.12435 BindingDB Entry DOI: 10.7270/Q21C1VK2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50029593 (CHEMBL7162 | N-(2-(cyclohexyloxy)-4-nitrophenyl)me...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Mazandaran University of Medical Sciences | Assay Description Reaction mixtures were prepared in 100 mM Tris澦Cl buffer, pH 8.0 containing 1 然 heme and COX-1 or COX-2 and preincubated for 10 min in a waterbath ... | Chem Biol Drug Des 85: 494-503 (2015) Article DOI: 10.1111/cbdd.12435 BindingDB Entry DOI: 10.7270/Q21C1VK2 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM17638 (2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 1.85E+4 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Mazandaran University of Medical Sciences | Assay Description Reaction mixtures were prepared in 100 mM Tris澦Cl buffer, pH 8.0 containing 1 然 heme and COX-1 or COX-2 and preincubated for 10 min in a waterbath ... | Chem Biol Drug Des 85: 494-503 (2015) Article DOI: 10.1111/cbdd.12435 BindingDB Entry DOI: 10.7270/Q21C1VK2 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prostaglandin G/H synthase 1 (Ovis aries (Sheep)) | BDBM152802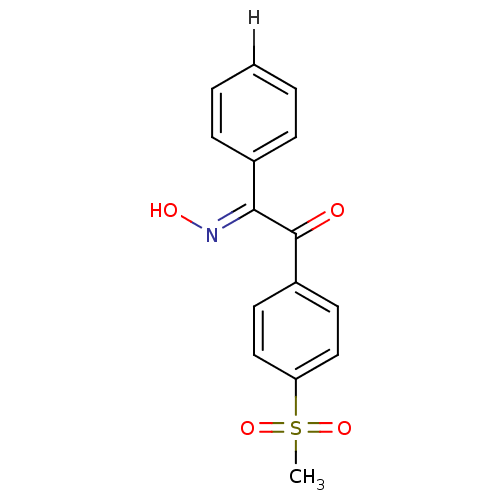 ((2E)-2-(N-hydroxyimino)-1-(4-methanesulfonylphenyl...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Mazandaran University of Medical Sciences | Assay Description Reaction mixtures were prepared in 100 mM Tris澦Cl buffer, pH 8.0 containing 1 然 heme and COX-1 or COX-2 and preincubated for 10 min in a waterbath ... | Chem Biol Drug Des 85: 494-503 (2015) Article DOI: 10.1111/cbdd.12435 BindingDB Entry DOI: 10.7270/Q21C1VK2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM152804 ((2E)-2-(N-hydroxyimino)-1-(4-methanesulfonylphenyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Mazandaran University of Medical Sciences | Assay Description Reaction mixtures were prepared in 100 mM Tris澦Cl buffer, pH 8.0 containing 1 然 heme and COX-1 or COX-2 and preincubated for 10 min in a waterbath ... | Chem Biol Drug Des 85: 494-503 (2015) Article DOI: 10.1111/cbdd.12435 BindingDB Entry DOI: 10.7270/Q21C1VK2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM152806 ((2E)-2-(4-fluorophenyl)-2-(N-hydroxyimino)-1-(4-me...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Mazandaran University of Medical Sciences | Assay Description Reaction mixtures were prepared in 100 mM Tris澦Cl buffer, pH 8.0 containing 1 然 heme and COX-1 or COX-2 and preincubated for 10 min in a waterbath ... | Chem Biol Drug Des 85: 494-503 (2015) Article DOI: 10.1111/cbdd.12435 BindingDB Entry DOI: 10.7270/Q21C1VK2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM152805 ((2E)-2-(4-chlorophenyl)-2-(N-hydroxyimino)-1-(4-me...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Mazandaran University of Medical Sciences | Assay Description Reaction mixtures were prepared in 100 mM Tris澦Cl buffer, pH 8.0 containing 1 然 heme and COX-1 or COX-2 and preincubated for 10 min in a waterbath ... | Chem Biol Drug Des 85: 494-503 (2015) Article DOI: 10.1111/cbdd.12435 BindingDB Entry DOI: 10.7270/Q21C1VK2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Ovis aries (Sheep)) | BDBM152803 ((2E)-2-(N-hydroxyimino)-1-(4-methanesulfonylphenyl...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Mazandaran University of Medical Sciences | Assay Description Reaction mixtures were prepared in 100 mM Tris澦Cl buffer, pH 8.0 containing 1 然 heme and COX-1 or COX-2 and preincubated for 10 min in a waterbath ... | Chem Biol Drug Des 85: 494-503 (2015) Article DOI: 10.1111/cbdd.12435 BindingDB Entry DOI: 10.7270/Q21C1VK2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM152803 ((2E)-2-(N-hydroxyimino)-1-(4-methanesulfonylphenyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Mazandaran University of Medical Sciences | Assay Description Reaction mixtures were prepared in 100 mM Tris澦Cl buffer, pH 8.0 containing 1 然 heme and COX-1 or COX-2 and preincubated for 10 min in a waterbath ... | Chem Biol Drug Des 85: 494-503 (2015) Article DOI: 10.1111/cbdd.12435 BindingDB Entry DOI: 10.7270/Q21C1VK2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Ovis aries (Sheep)) | BDBM152805 ((2E)-2-(4-chlorophenyl)-2-(N-hydroxyimino)-1-(4-me...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Mazandaran University of Medical Sciences | Assay Description Reaction mixtures were prepared in 100 mM Tris澦Cl buffer, pH 8.0 containing 1 然 heme and COX-1 or COX-2 and preincubated for 10 min in a waterbath ... | Chem Biol Drug Des 85: 494-503 (2015) Article DOI: 10.1111/cbdd.12435 BindingDB Entry DOI: 10.7270/Q21C1VK2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM152802 ((2E)-2-(N-hydroxyimino)-1-(4-methanesulfonylphenyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Mazandaran University of Medical Sciences | Assay Description Reaction mixtures were prepared in 100 mM Tris澦Cl buffer, pH 8.0 containing 1 然 heme and COX-1 or COX-2 and preincubated for 10 min in a waterbath ... | Chem Biol Drug Des 85: 494-503 (2015) Article DOI: 10.1111/cbdd.12435 BindingDB Entry DOI: 10.7270/Q21C1VK2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Ovis aries (Sheep)) | BDBM50029593 (CHEMBL7162 | N-(2-(cyclohexyloxy)-4-nitrophenyl)me...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 2.13E+5 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Mazandaran University of Medical Sciences | Assay Description Reaction mixtures were prepared in 100 mM Tris澦Cl buffer, pH 8.0 containing 1 然 heme and COX-1 or COX-2 and preincubated for 10 min in a waterbath ... | Chem Biol Drug Des 85: 494-503 (2015) Article DOI: 10.1111/cbdd.12435 BindingDB Entry DOI: 10.7270/Q21C1VK2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||