

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cysteinyl leukotriene receptor 1/2 (Homo sapiens (Human)) | BDBM50009075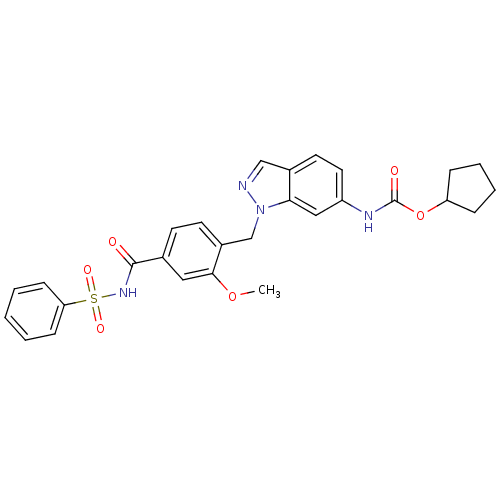 (CHEMBL22033 | ICI 198615 | ICI-198615 | [1-(4-Benz...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center Curated by ChEMBL | Assay Description Inhibitory activity to block binding of [3H]leukotriene D4 to LTD4 receptor sites in homogenized guinea pig lung | J Med Chem 32: 1842-60 (1989) BindingDB Entry DOI: 10.7270/Q28W3C8B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thromboxane-A synthase (Homo sapiens (Human)) | BDBM17638 (2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center Curated by ChEMBL | Assay Description Inhibitory activity of the compound to inhibit Inophore-induced arachidonic acid metabolism (inhibition of TXB2 formation) in rat | J Med Chem 32: 1842-60 (1989) BindingDB Entry DOI: 10.7270/Q28W3C8B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thromboxane-A synthase (Homo sapiens (Human)) | BDBM7962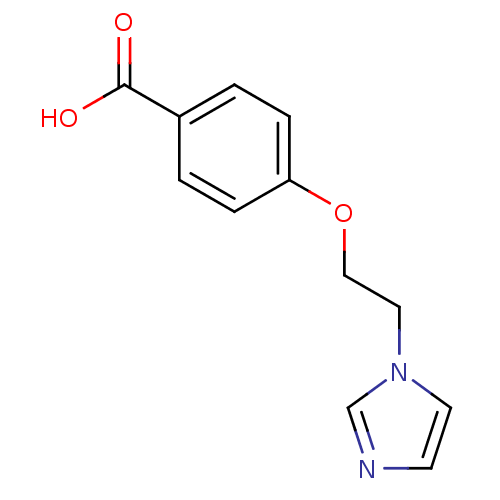 (4-(2-Imidazol-1-yl-ethoxy)-benzoic acid; hydrochlo...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center Curated by ChEMBL | Assay Description Inhibitory activity of the compound to inhibit Inophore-induced arachidonic acid metabolism (inhibition of TXB2 formation) in rat | J Med Chem 32: 1842-60 (1989) BindingDB Entry DOI: 10.7270/Q28W3C8B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteinyl leukotriene receptor 1/2 (Homo sapiens (Human)) | BDBM50228307 (CGP-35949) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center Curated by ChEMBL | Assay Description Inhibitory activity to block binding of [3H]leukotriene D4 to LTD4 receptor sites in homogenized guinea pig lung | J Med Chem 32: 1842-60 (1989) BindingDB Entry DOI: 10.7270/Q28W3C8B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thromboxane-A synthase (Homo sapiens (Human)) | BDBM50000838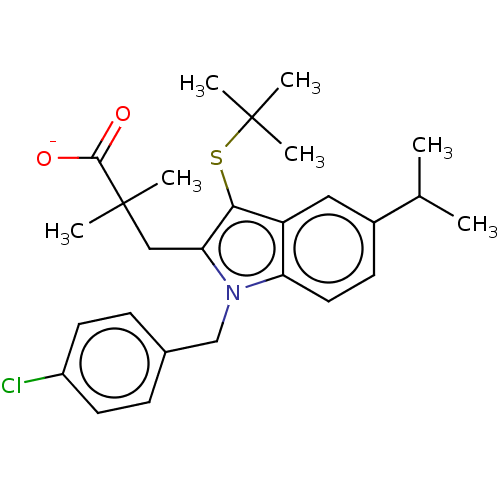 (CHEMBL416657 | Sodium; 3-[3-tert-butylsulfanyl-1-(...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center Curated by ChEMBL | Assay Description Inhibitory activity of the compound to inhibit Inophore-induced arachidonic acid metabolism (inhibition of TXB2 formation) in rat | J Med Chem 32: 1842-60 (1989) BindingDB Entry DOI: 10.7270/Q28W3C8B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteinyl leukotriene receptor 1/2 (Homo sapiens (Human)) | BDBM50228304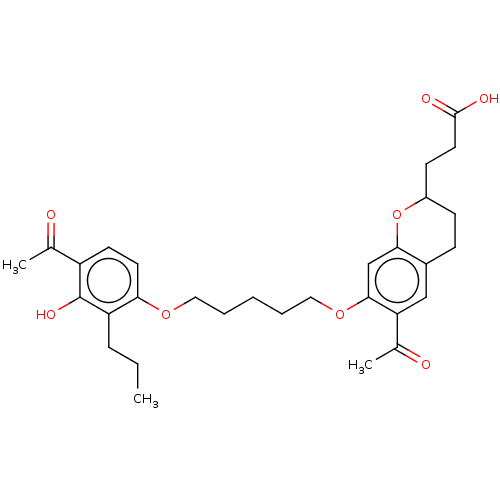 (CHEMBL417028) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center Curated by ChEMBL | Assay Description Inhibitory activity to block binding of [3H]leukotriene D4 to LTD4 receptor sites in homogenized guinea pig lung | J Med Chem 32: 1842-60 (1989) BindingDB Entry DOI: 10.7270/Q28W3C8B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteinyl leukotriene receptor 1/2 (Homo sapiens (Human)) | BDBM50228313 (CHEMBL55714) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center Curated by ChEMBL | Assay Description Inhibitory activity to block binding of [3H]leukotriene D4 to LTD4 receptor sites in homogenized guinea pig lung | J Med Chem 32: 1842-60 (1989) BindingDB Entry DOI: 10.7270/Q28W3C8B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteinyl leukotriene receptor 1 (GUINEA PIG) | BDBM50017208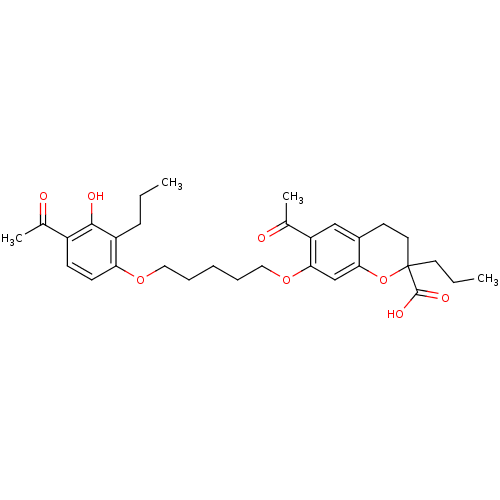 (6-Acetyl-7-[5-(4-acetyl-3-hydroxy-2-propyl-phenoxy...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center Curated by ChEMBL | Assay Description Inhibitory activity of compound to block binding of [3H]-leukotriene D4 to Cysteinyl leukotriene D4 receptor sites in homogenized guinea pig lung | J Med Chem 32: 1842-60 (1989) BindingDB Entry DOI: 10.7270/Q28W3C8B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteinyl leukotriene receptor 1/2 (Homo sapiens (Human)) | BDBM50228311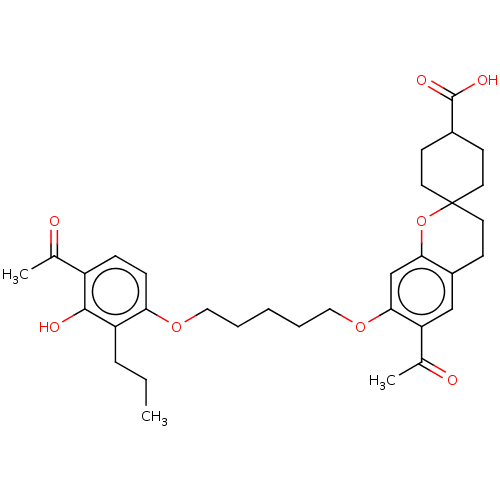 (CHEMBL53333) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center Curated by ChEMBL | Assay Description Inhibitory activity to block binding of [3H]leukotriene D4 to LTD4 receptor sites in homogenized guinea pig lung | J Med Chem 32: 1842-60 (1989) BindingDB Entry DOI: 10.7270/Q28W3C8B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteinyl leukotriene receptor 1 (GUINEA PIG) | BDBM50009071 (6-Acetyl-7-[5-(4-acetyl-3-hydroxy-2-propyl-phenoxy...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center Curated by ChEMBL | Assay Description Inhibitory activity of compound to block binding of [3H]-leukotriene D4 to Cysteinyl leukotriene D4 receptor sites in homogenized guinea pig lung | J Med Chem 32: 1842-60 (1989) BindingDB Entry DOI: 10.7270/Q28W3C8B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteinyl leukotriene receptor 1/2 (Homo sapiens (Human)) | BDBM50006812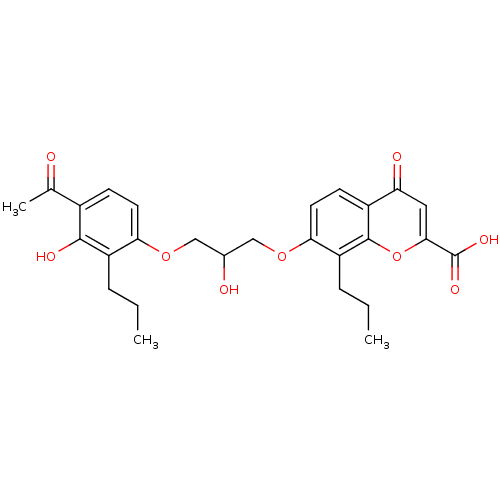 (7-[3-(4-Acetyl-3-hydroxy-2-propyl-phenoxy)-2-hydro...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center Curated by ChEMBL | Assay Description Inhibitory activity to block binding of [3H]leukotriene D4 to LTD4 receptor sites in homogenized guinea pig lung | J Med Chem 32: 1842-60 (1989) BindingDB Entry DOI: 10.7270/Q28W3C8B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteinyl leukotriene receptor 1 (GUINEA PIG) | BDBM50017202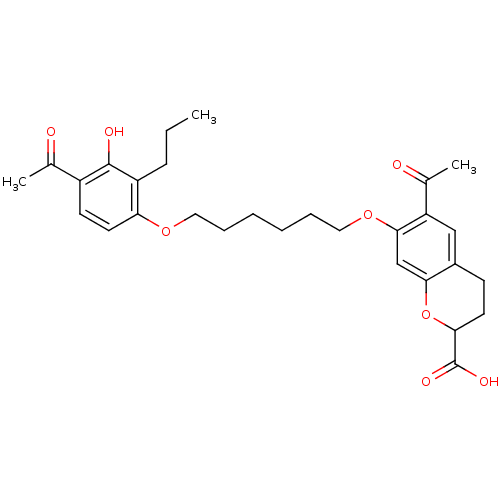 (6-Acetyl-7-[6-(4-acetyl-3-hydroxy-2-propyl-phenoxy...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center Curated by ChEMBL | Assay Description Inhibitory activity of compound to block binding of [3H]-leukotriene D4 to Cysteinyl leukotriene D4 receptor sites in homogenized guinea pig lung | J Med Chem 32: 1842-60 (1989) BindingDB Entry DOI: 10.7270/Q28W3C8B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteinyl leukotriene receptor 1/2 (Homo sapiens (Human)) | BDBM50228301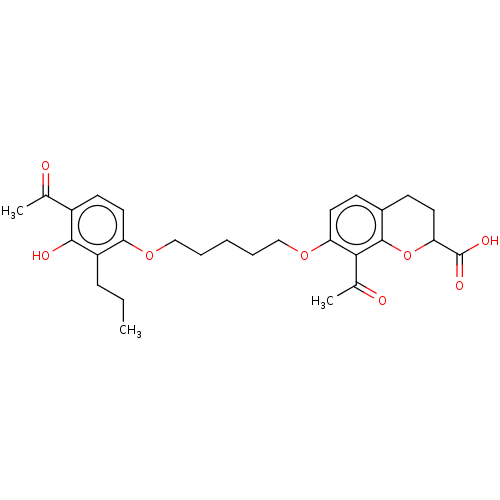 (CHEMBL54688) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center Curated by ChEMBL | Assay Description Inhibitory activity to block binding of [3H]leukotriene D4 to LTD4 receptor sites in homogenized guinea pig lung | J Med Chem 32: 1842-60 (1989) BindingDB Entry DOI: 10.7270/Q28W3C8B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteinyl leukotriene receptor 1/2 (Homo sapiens (Human)) | BDBM50228300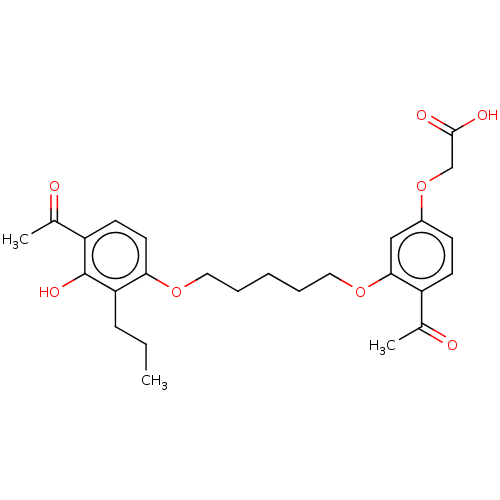 (CHEMBL293529) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center Curated by ChEMBL | Assay Description Inhibitory activity to block binding of [3H]leukotriene D4 to LTD4 receptor sites in homogenized guinea pig lung | J Med Chem 32: 1842-60 (1989) BindingDB Entry DOI: 10.7270/Q28W3C8B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteinyl leukotriene receptor 1 (GUINEA PIG) | BDBM50017201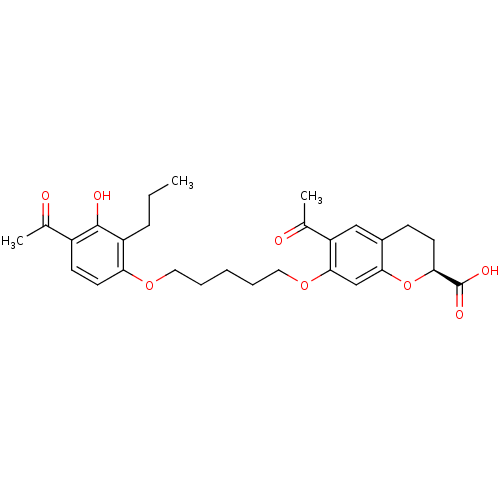 (6-Acetyl-7-[5-(4-acetyl-3-hydroxy-2-propyl-phenoxy...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center Curated by ChEMBL | Assay Description Invitro activity of the compound to inhibit binding of [3H]- leukotriene D4 to receptor sites in guinea pig lung membrane | J Med Chem 32: 1842-60 (1989) BindingDB Entry DOI: 10.7270/Q28W3C8B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thromboxane-A synthase (Homo sapiens (Human)) | BDBM50009071 (6-Acetyl-7-[5-(4-acetyl-3-hydroxy-2-propyl-phenoxy...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center Curated by ChEMBL | Assay Description Inhibitory activity of the compound to inhibit Inophore-induced arachidonic acid metabolism (inhibition of TXB2 formation) in rat | J Med Chem 32: 1842-60 (1989) BindingDB Entry DOI: 10.7270/Q28W3C8B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteinyl leukotriene receptor 1 (GUINEA PIG) | BDBM50017209 (6-Acetyl-7-[3-(4-acetyl-3-hydroxy-2-propyl-phenoxy...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center Curated by ChEMBL | Assay Description Inhibitory activity of compound to block binding of [3H]-leukotriene D4 to Cysteinyl leukotriene D4 receptor sites in homogenized guinea pig lung | J Med Chem 32: 1842-60 (1989) BindingDB Entry DOI: 10.7270/Q28W3C8B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteinyl leukotriene receptor 1/2 (Homo sapiens (Human)) | BDBM50006806 (C,C,C-Trifluoro-N-[3-(quinolin-2-ylmethoxy)-phenyl...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center Curated by ChEMBL | Assay Description Inhibitory activity to block binding of [3H]leukotriene D4 to LTD4 receptor sites in homogenized guinea pig lung | J Med Chem 32: 1842-60 (1989) BindingDB Entry DOI: 10.7270/Q28W3C8B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteinyl leukotriene receptor 1/2 (Homo sapiens (Human)) | BDBM50228302 (CHEMBL53512) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center Curated by ChEMBL | Assay Description Inhibitory activity to block binding of [3H]leukotriene D4 to LTD4 receptor sites in homogenized guinea pig lung | J Med Chem 32: 1842-60 (1989) BindingDB Entry DOI: 10.7270/Q28W3C8B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteinyl leukotriene receptor 1 (GUINEA PIG) | BDBM50017199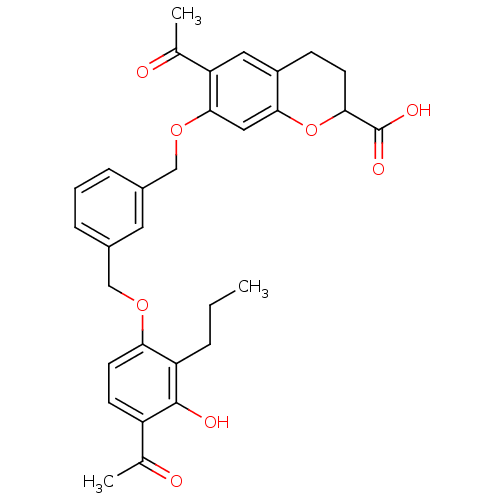 (6-Acetyl-7-[3-(4-acetyl-3-hydroxy-2-propyl-phenoxy...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center Curated by ChEMBL | Assay Description Inhibitory activity of compound to block binding of [3H]-leukotriene D4 to Cysteinyl leukotriene D4 receptor sites in homogenized guinea pig lung | J Med Chem 32: 1842-60 (1989) BindingDB Entry DOI: 10.7270/Q28W3C8B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteinyl leukotriene receptor 1/2 (Homo sapiens (Human)) | BDBM50228303 (CHEMBL52877) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center Curated by ChEMBL | Assay Description Inhibitory activity to block binding of [3H]leukotriene D4 to LTD4 receptor sites in homogenized guinea pig lung | J Med Chem 32: 1842-60 (1989) BindingDB Entry DOI: 10.7270/Q28W3C8B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteinyl leukotriene receptor 1 (GUINEA PIG) | BDBM50017200 (6-Acetyl-7-[5-(4-acetyl-3-hydroxy-2-propyl-phenoxy...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center Curated by ChEMBL | Assay Description Inhibitory activity of compound to block binding of [3H]-leukotriene D4 toCysteinyl leukotriene D4 receptor sites in homogenized guinea pig lung | J Med Chem 32: 1842-60 (1989) BindingDB Entry DOI: 10.7270/Q28W3C8B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteinyl leukotriene receptor 1/2 (Homo sapiens (Human)) | BDBM50228306 (CHEMBL55164) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem | PubMed | n/a | n/a | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center Curated by ChEMBL | Assay Description In vitro activity to inhibit binding of [3H]- leukotriene D4 to receptor sites in guinea pig lung membrane | J Med Chem 32: 1842-60 (1989) BindingDB Entry DOI: 10.7270/Q28W3C8B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity calcium/calmodulin-dependent 3',5'-cyclic nucleotide phosphodiesterase 1A (BOVINE) | BDBM50009071 (6-Acetyl-7-[5-(4-acetyl-3-hydroxy-2-propyl-phenoxy...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 3.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center Curated by ChEMBL | Assay Description Inhibition of bovine heart Phosphodiesterase 1A | J Med Chem 32: 1842-60 (1989) BindingDB Entry DOI: 10.7270/Q28W3C8B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity calcium/calmodulin-dependent 3',5'-cyclic nucleotide phosphodiesterase 1A (BOVINE) | BDBM50006812 (7-[3-(4-Acetyl-3-hydroxy-2-propyl-phenoxy)-2-hydro...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center Curated by ChEMBL | Assay Description Inhibition of bovine heart Phosphodiesterase 1A | J Med Chem 32: 1842-60 (1989) BindingDB Entry DOI: 10.7270/Q28W3C8B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity calcium/calmodulin-dependent 3',5'-cyclic nucleotide phosphodiesterase 1A (BOVINE) | BDBM50017210 (8-(1,2-Dimethyl-propyl)-purine-2,6-dione | CHEMBL5...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center Curated by ChEMBL | Assay Description Inhibition of bovine heart Phosphodiesterase 1A | J Med Chem 32: 1842-60 (1989) BindingDB Entry DOI: 10.7270/Q28W3C8B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity calcium/calmodulin-dependent 3',5'-cyclic nucleotide phosphodiesterase 1A (BOVINE) | BDBM10847 (1,3-dimethyl-2,3,6,7-tetrahydro-1H-purine-2,6-dion...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 5.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center Curated by ChEMBL | Assay Description Inhibition of bovine heart Phosphodiesterase 1A | J Med Chem 32: 1842-60 (1989) BindingDB Entry DOI: 10.7270/Q28W3C8B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||