 Found 99 hits of Enzyme Inhibition Constant Data
Found 99 hits of Enzyme Inhibition Constant Data Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Nicotinamide phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50438939

(CHEMBL2420626)Show SMILES Fc1cc(F)cc(c1)S(=O)(=O)c1ccc(CNC(=O)c2cc3ccncc3s2)cc1 Show InChI InChI=1S/C21H14F2N2O3S2/c22-15-8-16(23)10-18(9-15)30(27,28)17-3-1-13(2-4-17)11-25-21(26)19-7-14-5-6-24-12-20(14)29-19/h1-10,12H,11H2,(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human C-terminal His6-tagged NAMPT expressed in Escherichia coli Rosetta(DE3) incubated for 15 mins prior to substrate addition measure... |
Bioorg Med Chem Lett 25: 529-41 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.026
BindingDB Entry DOI: 10.7270/Q2736SK7 |
More data for this
Ligand-Target Pair | |
Nicotinamide phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50060820
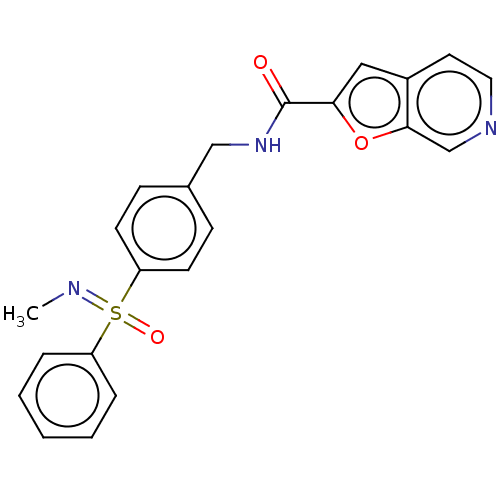
(CHEMBL3394727)Show SMILES CN=S(=O)(c1ccccc1)c1ccc(CNC(=O)c2cc3ccncc3o2)cc1 Show InChI InChI=1S/C22H19N3O3S/c1-23-29(27,18-5-3-2-4-6-18)19-9-7-16(8-10-19)14-25-22(26)20-13-17-11-12-24-15-21(17)28-20/h2-13,15H,14H2,1H3,(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human C-terminal His6-tagged NAMPT expressed in Escherichia coli Rosetta(DE3) incubated for 15 mins prior to substrate addition measure... |
Bioorg Med Chem Lett 25: 529-41 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.026
BindingDB Entry DOI: 10.7270/Q2736SK7 |
More data for this
Ligand-Target Pair | |
Nicotinamide phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50060764
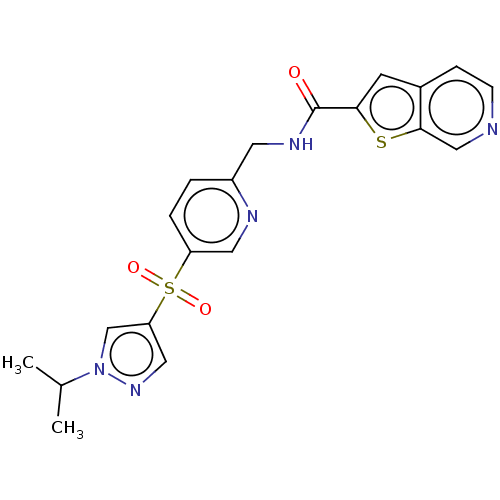
(CHEMBL3394740 | US9458172, 45)Show SMILES CC(C)n1cc(cn1)S(=O)(=O)c1ccc(CNC(=O)c2cc3ccncc3s2)nc1 Show InChI InChI=1S/C20H19N5O3S2/c1-13(2)25-12-17(10-24-25)30(27,28)16-4-3-15(22-9-16)8-23-20(26)18-7-14-5-6-21-11-19(14)29-18/h3-7,9-13H,8H2,1-2H3,(H,23,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human C-terminal His6-tagged NAMPT expressed in Escherichia coli Rosetta(DE3) incubated for 15 mins prior to substrate addition measure... |
Bioorg Med Chem Lett 25: 529-41 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.026
BindingDB Entry DOI: 10.7270/Q2736SK7 |
More data for this
Ligand-Target Pair | |
Nicotinamide phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50060763
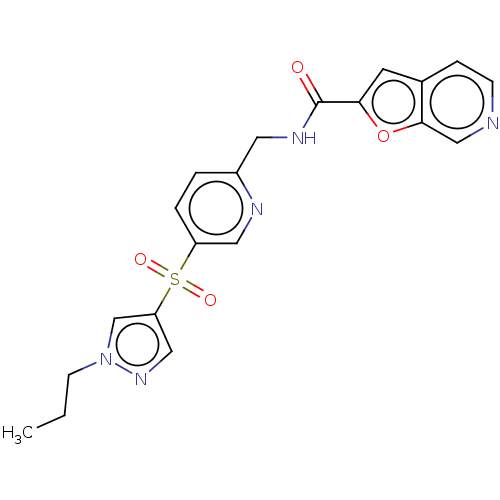
(CHEMBL3394741 | US9458172, 61)Show SMILES CCCn1cc(cn1)S(=O)(=O)c1ccc(CNC(=O)c2cc3ccncc3o2)nc1 Show InChI InChI=1S/C20H19N5O4S/c1-2-7-25-13-17(11-24-25)30(27,28)16-4-3-15(22-10-16)9-23-20(26)18-8-14-5-6-21-12-19(14)29-18/h3-6,8,10-13H,2,7,9H2,1H3,(H,23,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human C-terminal His6-tagged NAMPT expressed in Escherichia coli Rosetta(DE3) incubated for 15 mins prior to substrate addition measure... |
Bioorg Med Chem Lett 25: 529-41 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.026
BindingDB Entry DOI: 10.7270/Q2736SK7 |
More data for this
Ligand-Target Pair | |
Nicotinamide phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50060823

(CHEMBL3394724)Show SMILES CC(C)CN1CCC(CC1)[S+]([O-])c1ccc(CNC(=O)c2cc3ccncc3o2)cc1 Show InChI InChI=1S/C24H29N3O3S/c1-17(2)16-27-11-8-21(9-12-27)31(29)20-5-3-18(4-6-20)14-26-24(28)22-13-19-7-10-25-15-23(19)30-22/h3-7,10,13,15,17,21H,8-9,11-12,14,16H2,1-2H3,(H,26,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human C-terminal His6-tagged NAMPT expressed in Escherichia coli Rosetta(DE3) incubated for 15 mins prior to substrate addition measure... |
Bioorg Med Chem Lett 25: 529-41 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.026
BindingDB Entry DOI: 10.7270/Q2736SK7 |
More data for this
Ligand-Target Pair | |
Nicotinamide phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50060769
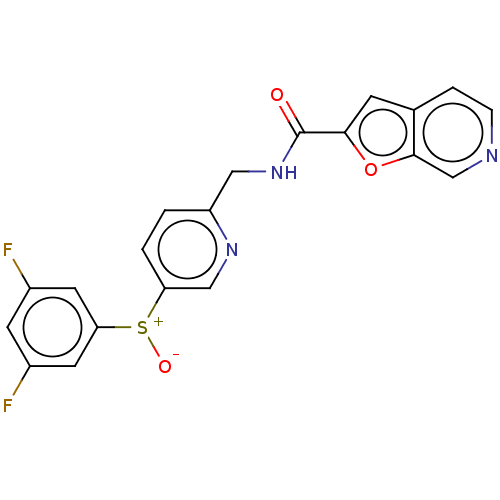
(CHEMBL3394736)Show SMILES [O-][S+](c1ccc(CNC(=O)c2cc3ccncc3o2)nc1)c1cc(F)cc(F)c1 Show InChI InChI=1S/C20H13F2N3O3S/c21-13-6-14(22)8-17(7-13)29(27)16-2-1-15(24-10-16)9-25-20(26)18-5-12-3-4-23-11-19(12)28-18/h1-8,10-11H,9H2,(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human C-terminal His6-tagged NAMPT expressed in Escherichia coli Rosetta(DE3) incubated for 15 mins prior to substrate addition measure... |
Bioorg Med Chem Lett 25: 529-41 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.026
BindingDB Entry DOI: 10.7270/Q2736SK7 |
More data for this
Ligand-Target Pair | |
Nicotinamide phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50060813
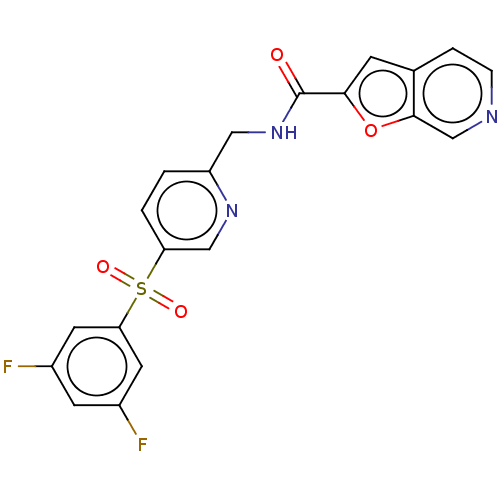
(CHEMBL3394734 | US9458172, 5)Show SMILES Fc1cc(F)cc(c1)S(=O)(=O)c1ccc(CNC(=O)c2cc3ccncc3o2)nc1 Show InChI InChI=1S/C20H13F2N3O4S/c21-13-6-14(22)8-17(7-13)30(27,28)16-2-1-15(24-10-16)9-25-20(26)18-5-12-3-4-23-11-19(12)29-18/h1-8,10-11H,9H2,(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human C-terminal His6-tagged NAMPT expressed in Escherichia coli Rosetta(DE3) incubated for 15 mins prior to substrate addition measure... |
Bioorg Med Chem Lett 25: 529-41 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.026
BindingDB Entry DOI: 10.7270/Q2736SK7 |
More data for this
Ligand-Target Pair | |
Nicotinamide phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50438945
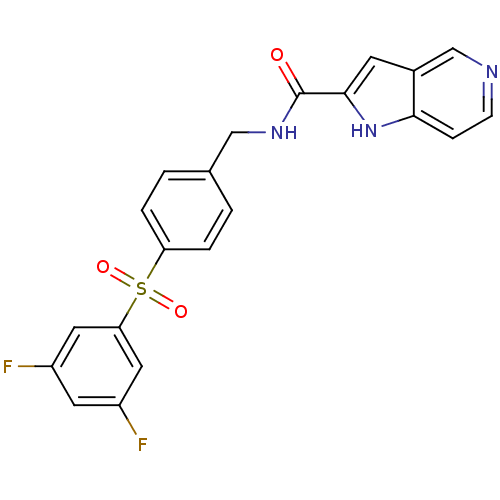
(CHEMBL2420620)Show SMILES Fc1cc(F)cc(c1)S(=O)(=O)c1ccc(CNC(=O)c2cc3cnccc3[nH]2)cc1 Show InChI InChI=1S/C21H15F2N3O3S/c22-15-8-16(23)10-18(9-15)30(28,29)17-3-1-13(2-4-17)11-25-21(27)20-7-14-12-24-6-5-19(14)26-20/h1-10,12,26H,11H2,(H,25,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human C-terminal His6-tagged NAMPT expressed in Escherichia coli Rosetta(DE3) incubated for 15 mins prior to substrate addition measure... |
Bioorg Med Chem Lett 25: 529-41 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.026
BindingDB Entry DOI: 10.7270/Q2736SK7 |
More data for this
Ligand-Target Pair | |
Nicotinamide phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50060817

(CHEMBL3394730)Show SMILES CCCn1cc(cn1)S(=O)(=O)c1ccc(CNC(=O)c2cc3ccncc3o2)cc1 Show InChI InChI=1S/C21H20N4O4S/c1-2-9-25-14-18(12-24-25)30(27,28)17-5-3-15(4-6-17)11-23-21(26)19-10-16-7-8-22-13-20(16)29-19/h3-8,10,12-14H,2,9,11H2,1H3,(H,23,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human C-terminal His6-tagged NAMPT expressed in Escherichia coli Rosetta(DE3) incubated for 15 mins prior to substrate addition measure... |
Bioorg Med Chem Lett 25: 529-41 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.026
BindingDB Entry DOI: 10.7270/Q2736SK7 |
More data for this
Ligand-Target Pair | |
Nicotinamide phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50060818
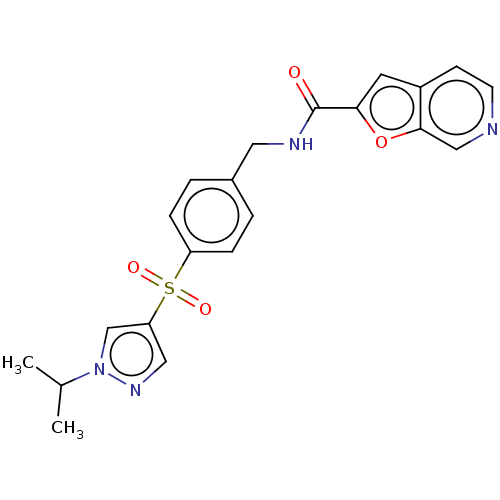
(CHEMBL3394729)Show SMILES CC(C)n1cc(cn1)S(=O)(=O)c1ccc(CNC(=O)c2cc3ccncc3o2)cc1 Show InChI InChI=1S/C21H20N4O4S/c1-14(2)25-13-18(11-24-25)30(27,28)17-5-3-15(4-6-17)10-23-21(26)19-9-16-7-8-22-12-20(16)29-19/h3-9,11-14H,10H2,1-2H3,(H,23,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human C-terminal His6-tagged NAMPT expressed in Escherichia coli Rosetta(DE3) incubated for 15 mins prior to substrate addition measure... |
Bioorg Med Chem Lett 25: 529-41 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.026
BindingDB Entry DOI: 10.7270/Q2736SK7 |
More data for this
Ligand-Target Pair | |
Nicotinamide phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50060819
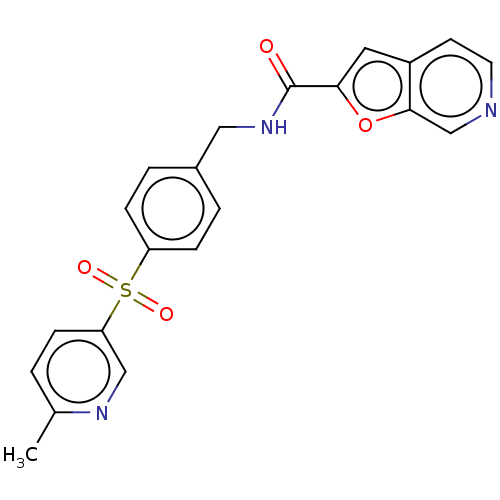
(CHEMBL3394728)Show SMILES Cc1ccc(cn1)S(=O)(=O)c1ccc(CNC(=O)c2cc3ccncc3o2)cc1 Show InChI InChI=1S/C21H17N3O4S/c1-14-2-5-18(12-23-14)29(26,27)17-6-3-15(4-7-17)11-24-21(25)19-10-16-8-9-22-13-20(16)28-19/h2-10,12-13H,11H2,1H3,(H,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human C-terminal His6-tagged NAMPT expressed in Escherichia coli Rosetta(DE3) incubated for 15 mins prior to substrate addition measure... |
Bioorg Med Chem Lett 25: 529-41 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.026
BindingDB Entry DOI: 10.7270/Q2736SK7 |
More data for this
Ligand-Target Pair | |
Nicotinamide phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50060825

(CHEMBL3394722 | US10696692, Example 213)Show SMILES O=C(NCc1ccc(cc1)S(=O)(=O)C1CCN(CC1)C1CCOCC1)c1cc2cnccc2[nH]1 Show InChI InChI=1S/C25H30N4O4S/c30-25(24-15-19-17-26-10-5-23(19)28-24)27-16-18-1-3-21(4-2-18)34(31,32)22-6-11-29(12-7-22)20-8-13-33-14-9-20/h1-5,10,15,17,20,22,28H,6-9,11-14,16H2,(H,27,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human C-terminal His6-tagged NAMPT expressed in Escherichia coli Rosetta(DE3) incubated for 15 mins prior to substrate addition measure... |
Bioorg Med Chem Lett 25: 529-41 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.026
BindingDB Entry DOI: 10.7270/Q2736SK7 |
More data for this
Ligand-Target Pair | |
Nicotinamide phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50438942

(CHEMBL2420623)Show SMILES Fc1cc(F)cc(c1)S(=O)(=O)c1ccc(CNC(=O)c2cc3ccncc3o2)cc1 Show InChI InChI=1S/C21H14F2N2O4S/c22-15-8-16(23)10-18(9-15)30(27,28)17-3-1-13(2-4-17)11-25-21(26)19-7-14-5-6-24-12-20(14)29-19/h1-10,12H,11H2,(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human C-terminal His6-tagged NAMPT expressed in Escherichia coli Rosetta(DE3) incubated for 15 mins prior to substrate addition measure... |
Bioorg Med Chem Lett 25: 529-41 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.026
BindingDB Entry DOI: 10.7270/Q2736SK7 |
More data for this
Ligand-Target Pair | |
Nicotinamide phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50438976

(CHEMBL2419504)Show SMILES O=C(NCc1ccc(cc1)S(=O)(=O)c1ccccc1)c1cc2ccncc2o1 Show InChI InChI=1S/C21H16N2O4S/c24-21(19-12-16-10-11-22-14-20(16)27-19)23-13-15-6-8-18(9-7-15)28(25,26)17-4-2-1-3-5-17/h1-12,14H,13H2,(H,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human C-terminal His6-tagged NAMPT expressed in Escherichia coli Rosetta(DE3) incubated for 15 mins prior to substrate addition measure... |
Bioorg Med Chem Lett 25: 529-41 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.026
BindingDB Entry DOI: 10.7270/Q2736SK7 |
More data for this
Ligand-Target Pair | |
Nicotinamide phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50060814
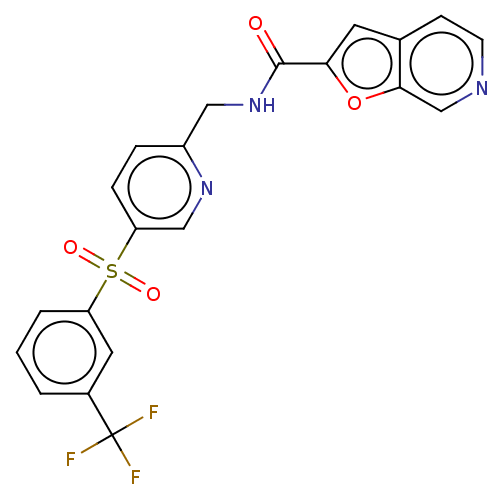
(CHEMBL3394733 | US9458172, 22)Show SMILES FC(F)(F)c1cccc(c1)S(=O)(=O)c1ccc(CNC(=O)c2cc3ccncc3o2)nc1 Show InChI InChI=1S/C21H14F3N3O4S/c22-21(23,24)14-2-1-3-16(9-14)32(29,30)17-5-4-15(26-11-17)10-27-20(28)18-8-13-6-7-25-12-19(13)31-18/h1-9,11-12H,10H2,(H,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human C-terminal His6-tagged NAMPT expressed in Escherichia coli Rosetta(DE3) incubated for 15 mins prior to substrate addition measure... |
Bioorg Med Chem Lett 25: 529-41 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.026
BindingDB Entry DOI: 10.7270/Q2736SK7 |
More data for this
Ligand-Target Pair | |
Nicotinamide phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50060827

(CHEMBL3394720 | US10696692, Example 250)Show SMILES O=C(NCc1ccc(cc1)S(=O)(=O)C1CCN(CC1)C1COC1)c1cc2ccncc2o1 Show InChI InChI=1S/C23H25N3O5S/c27-23(21-11-17-5-8-24-13-22(17)31-21)25-12-16-1-3-19(4-2-16)32(28,29)20-6-9-26(10-7-20)18-14-30-15-18/h1-5,8,11,13,18,20H,6-7,9-10,12,14-15H2,(H,25,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human C-terminal His6-tagged NAMPT expressed in Escherichia coli Rosetta(DE3) incubated for 15 mins prior to substrate addition measure... |
Bioorg Med Chem Lett 25: 529-41 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.026
BindingDB Entry DOI: 10.7270/Q2736SK7 |
More data for this
Ligand-Target Pair | |
Nicotinamide phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50060822
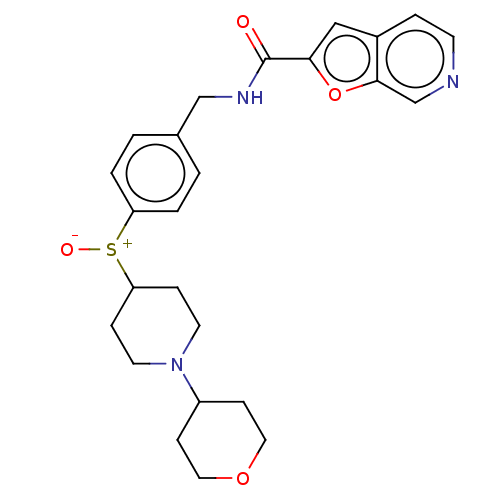
(CHEMBL3394725)Show SMILES [O-][S+](C1CCN(CC1)C1CCOCC1)c1ccc(CNC(=O)c2cc3ccncc3o2)cc1 Show InChI InChI=1S/C25H29N3O4S/c29-25(23-15-19-5-10-26-17-24(19)32-23)27-16-18-1-3-21(4-2-18)33(30)22-6-11-28(12-7-22)20-8-13-31-14-9-20/h1-5,10,15,17,20,22H,6-9,11-14,16H2,(H,27,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human C-terminal His6-tagged NAMPT expressed in Escherichia coli Rosetta(DE3) incubated for 15 mins prior to substrate addition measure... |
Bioorg Med Chem Lett 25: 529-41 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.026
BindingDB Entry DOI: 10.7270/Q2736SK7 |
More data for this
Ligand-Target Pair | |
Nicotinamide phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50060826

(CHEMBL3394721 | US10696692, Example 205)Show SMILES O=C(NCc1ccc(cc1)S(=O)(=O)C1CCN(CC1)C1CCOCC1)c1cc2ccncc2o1 Show InChI InChI=1S/C25H29N3O5S/c29-25(23-15-19-5-10-26-17-24(19)33-23)27-16-18-1-3-21(4-2-18)34(30,31)22-6-11-28(12-7-22)20-8-13-32-14-9-20/h1-5,10,15,17,20,22H,6-9,11-14,16H2,(H,27,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human C-terminal His6-tagged NAMPT expressed in Escherichia coli Rosetta(DE3) incubated for 15 mins prior to substrate addition measure... |
Bioorg Med Chem Lett 25: 529-41 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.026
BindingDB Entry DOI: 10.7270/Q2736SK7 |
More data for this
Ligand-Target Pair | |
Nicotinamide phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50060832

(CHEMBL3394715 | US10696692, Example 11)Show SMILES O=C(NCc1ccc(cc1)S(=O)(=O)N1CCOCC1)c1cc2ccncc2s1 Show InChI InChI=1S/C19H19N3O4S2/c23-19(17-11-15-5-6-20-13-18(15)27-17)21-12-14-1-3-16(4-2-14)28(24,25)22-7-9-26-10-8-22/h1-6,11,13H,7-10,12H2,(H,21,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human C-terminal His6-tagged NAMPT expressed in Escherichia coli Rosetta(DE3) incubated for 15 mins prior to substrate addition measure... |
Bioorg Med Chem Lett 25: 529-41 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.026
BindingDB Entry DOI: 10.7270/Q2736SK7 |
More data for this
Ligand-Target Pair | |
Nicotinamide phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50060824

(CHEMBL3394723)Show SMILES CC(C)N1CCC(CC1)[S+]([O-])c1ccc(CNC(=O)c2cc3ccncc3o2)cc1 Show InChI InChI=1S/C23H27N3O3S/c1-16(2)26-11-8-20(9-12-26)30(28)19-5-3-17(4-6-19)14-25-23(27)21-13-18-7-10-24-15-22(18)29-21/h3-7,10,13,15-16,20H,8-9,11-12,14H2,1-2H3,(H,25,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human C-terminal His6-tagged NAMPT expressed in Escherichia coli Rosetta(DE3) incubated for 15 mins prior to substrate addition measure... |
Bioorg Med Chem Lett 25: 529-41 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.026
BindingDB Entry DOI: 10.7270/Q2736SK7 |
More data for this
Ligand-Target Pair | |
Nicotinamide phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50060824

(CHEMBL3394723)Show SMILES CC(C)N1CCC(CC1)[S+]([O-])c1ccc(CNC(=O)c2cc3ccncc3o2)cc1 Show InChI InChI=1S/C23H27N3O3S/c1-16(2)26-11-8-20(9-12-26)30(28)19-5-3-17(4-6-19)14-25-23(27)21-13-18-7-10-24-15-22(18)29-21/h3-7,10,13,15-16,20H,8-9,11-12,14H2,1-2H3,(H,25,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human C-terminal His6-tagged NAMPT expressed in Escherichia coli Rosetta(DE3) incubated for 15 mins prior to substrate addition measure... |
Bioorg Med Chem Lett 25: 529-41 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.026
BindingDB Entry DOI: 10.7270/Q2736SK7 |
More data for this
Ligand-Target Pair | |
Nicotinamide phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50060834
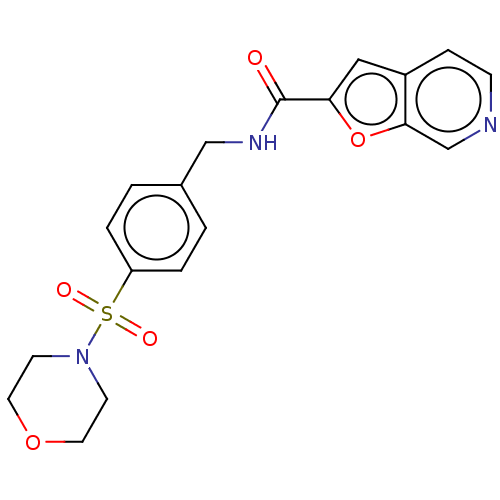
(CHEMBL3394713 | US10696692, Example 8)Show SMILES O=C(NCc1ccc(cc1)S(=O)(=O)N1CCOCC1)c1cc2ccncc2o1 Show InChI InChI=1S/C19H19N3O5S/c23-19(17-11-15-5-6-20-13-18(15)27-17)21-12-14-1-3-16(4-2-14)28(24,25)22-7-9-26-10-8-22/h1-6,11,13H,7-10,12H2,(H,21,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human C-terminal His6-tagged NAMPT expressed in Escherichia coli Rosetta(DE3) incubated for 15 mins prior to substrate addition measure... |
Bioorg Med Chem Lett 25: 529-41 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.026
BindingDB Entry DOI: 10.7270/Q2736SK7 |
More data for this
Ligand-Target Pair | |
Nicotinamide phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50060767

(CHEMBL3394737 | US9458172, 94)Show SMILES Cc1ccc(cn1)S(=O)(=O)c1ccc(CNC(=O)c2cc3ccncc3o2)nc1 Show InChI InChI=1S/C20H16N4O4S/c1-13-2-4-16(10-22-13)29(26,27)17-5-3-15(23-11-17)9-24-20(25)18-8-14-6-7-21-12-19(14)28-18/h2-8,10-12H,9H2,1H3,(H,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human C-terminal His6-tagged NAMPT expressed in Escherichia coli Rosetta(DE3) incubated for 15 mins prior to substrate addition measure... |
Bioorg Med Chem Lett 25: 529-41 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.026
BindingDB Entry DOI: 10.7270/Q2736SK7 |
More data for this
Ligand-Target Pair | |
Nicotinamide phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50060766
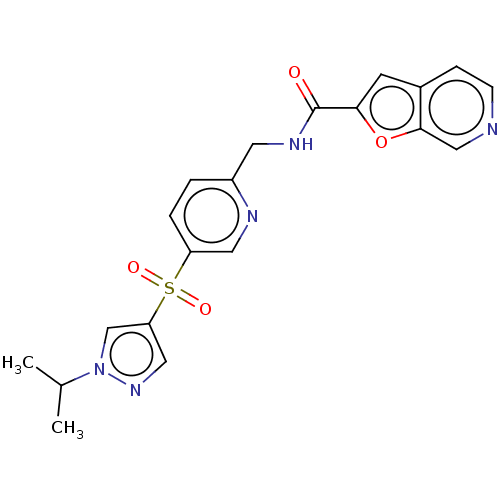
(CHEMBL3394738 | US9458172, 62)Show SMILES CC(C)n1cc(cn1)S(=O)(=O)c1ccc(CNC(=O)c2cc3ccncc3o2)nc1 Show InChI InChI=1S/C20H19N5O4S/c1-13(2)25-12-17(10-24-25)30(27,28)16-4-3-15(22-9-16)8-23-20(26)18-7-14-5-6-21-11-19(14)29-18/h3-7,9-13H,8H2,1-2H3,(H,23,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human C-terminal His6-tagged NAMPT expressed in Escherichia coli Rosetta(DE3) incubated for 15 mins prior to substrate addition measure... |
Bioorg Med Chem Lett 25: 529-41 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.026
BindingDB Entry DOI: 10.7270/Q2736SK7 |
More data for this
Ligand-Target Pair | |
Nicotinamide phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50060812

(CHEMBL3394735 | US9458172, 3)Show SMILES Fc1cc(F)cc(c1)S(=O)(=O)c1ccc(CNC(=O)c2cc3cnccc3[nH]2)nc1 Show InChI InChI=1S/C20H14F2N4O3S/c21-13-6-14(22)8-17(7-13)30(28,29)16-2-1-15(24-11-16)10-25-20(27)19-5-12-9-23-4-3-18(12)26-19/h1-9,11,26H,10H2,(H,25,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human C-terminal His6-tagged NAMPT expressed in Escherichia coli Rosetta(DE3) incubated for 15 mins prior to substrate addition measure... |
Bioorg Med Chem Lett 25: 529-41 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.026
BindingDB Entry DOI: 10.7270/Q2736SK7 |
More data for this
Ligand-Target Pair | |
Nicotinamide phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50060828

(CHEMBL3394719 | US10696692, Example 341)Show SMILES CC(C)CN1CCC(CC1)S(=O)(=O)c1ccc(CNC(=O)c2cc3ccncc3o2)cc1 Show InChI InChI=1S/C24H29N3O4S/c1-17(2)16-27-11-8-21(9-12-27)32(29,30)20-5-3-18(4-6-20)14-26-24(28)22-13-19-7-10-25-15-23(19)31-22/h3-7,10,13,15,17,21H,8-9,11-12,14,16H2,1-2H3,(H,26,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human C-terminal His6-tagged NAMPT expressed in Escherichia coli Rosetta(DE3) incubated for 15 mins prior to substrate addition measure... |
Bioorg Med Chem Lett 25: 529-41 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.026
BindingDB Entry DOI: 10.7270/Q2736SK7 |
More data for this
Ligand-Target Pair | |
Nicotinamide phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50060831

(CHEMBL3394716 | US10696692, Example 202)Show SMILES O=C(NCc1ccc(cc1)S(=O)(=O)C1CCOCC1)c1cc2ccncc2o1 Show InChI InChI=1S/C20H20N2O5S/c23-20(18-11-15-5-8-21-13-19(15)27-18)22-12-14-1-3-16(4-2-14)28(24,25)17-6-9-26-10-7-17/h1-5,8,11,13,17H,6-7,9-10,12H2,(H,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human C-terminal His6-tagged NAMPT expressed in Escherichia coli Rosetta(DE3) incubated for 15 mins prior to substrate addition measure... |
Bioorg Med Chem Lett 25: 529-41 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.026
BindingDB Entry DOI: 10.7270/Q2736SK7 |
More data for this
Ligand-Target Pair | |
Nicotinamide phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50060821
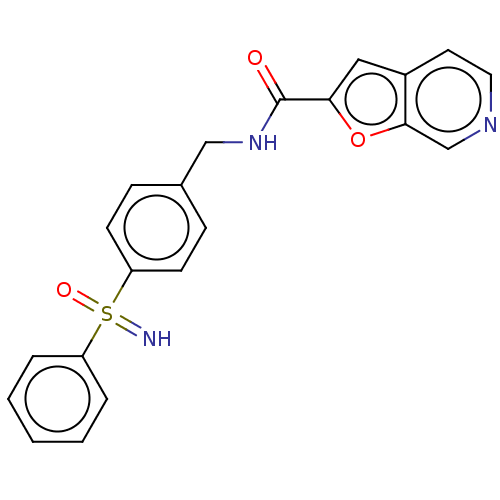
(CHEMBL3394726)Show SMILES N=S(=O)(c1ccccc1)c1ccc(CNC(=O)c2cc3ccncc3o2)cc1 Show InChI InChI=1S/C21H17N3O3S/c22-28(26,17-4-2-1-3-5-17)18-8-6-15(7-9-18)13-24-21(25)19-12-16-10-11-23-14-20(16)27-19/h1-12,14,22H,13H2,(H,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 49 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human C-terminal His6-tagged NAMPT expressed in Escherichia coli Rosetta(DE3) incubated for 15 mins prior to substrate addition measure... |
Bioorg Med Chem Lett 25: 529-41 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.026
BindingDB Entry DOI: 10.7270/Q2736SK7 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50060818

(CHEMBL3394729)Show SMILES CC(C)n1cc(cn1)S(=O)(=O)c1ccc(CNC(=O)c2cc3ccncc3o2)cc1 Show InChI InChI=1S/C21H20N4O4S/c1-14(2)25-13-18(11-24-25)30(27,28)17-5-3-15(4-6-17)10-23-21(26)19-9-16-7-8-22-12-20(16)29-19/h3-9,11-14H,10H2,1-2H3,(H,23,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc.
Curated by ChEMBL
| Assay Description
Reversible inhibition of CYP2C9 in human liver microsomes |
Bioorg Med Chem Lett 25: 529-41 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.026
BindingDB Entry DOI: 10.7270/Q2736SK7 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50060817

(CHEMBL3394730)Show SMILES CCCn1cc(cn1)S(=O)(=O)c1ccc(CNC(=O)c2cc3ccncc3o2)cc1 Show InChI InChI=1S/C21H20N4O4S/c1-2-9-25-14-18(12-24-25)30(27,28)17-5-3-15(4-6-17)11-23-21(26)19-10-16-7-8-22-13-20(16)29-19/h3-8,10,12-14H,2,9,11H2,1H3,(H,23,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc.
Curated by ChEMBL
| Assay Description
Reversible inhibition of CYP2C9 in human liver microsomes |
Bioorg Med Chem Lett 25: 529-41 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.026
BindingDB Entry DOI: 10.7270/Q2736SK7 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50060763

(CHEMBL3394741 | US9458172, 61)Show SMILES CCCn1cc(cn1)S(=O)(=O)c1ccc(CNC(=O)c2cc3ccncc3o2)nc1 Show InChI InChI=1S/C20H19N5O4S/c1-2-7-25-13-17(11-24-25)30(27,28)16-4-3-15(22-10-16)9-23-20(26)18-8-14-5-6-21-12-19(14)29-18/h3-6,8,10-13H,2,7,9H2,1H3,(H,23,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc.
Curated by ChEMBL
| Assay Description
Reversible inhibition of CYP2C9 in human liver microsomes |
Bioorg Med Chem Lett 25: 529-41 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.026
BindingDB Entry DOI: 10.7270/Q2736SK7 |
More data for this
Ligand-Target Pair | |
Nicotinamide phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50060829
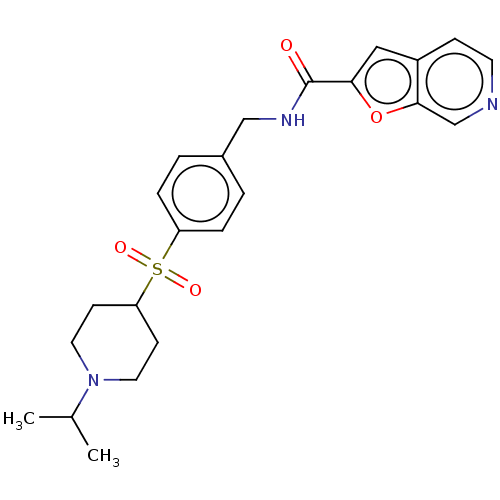
(CHEMBL3394718 | US10696692, Example 310)Show SMILES CC(C)N1CCC(CC1)S(=O)(=O)c1ccc(CNC(=O)c2cc3ccncc3o2)cc1 Show InChI InChI=1S/C23H27N3O4S/c1-16(2)26-11-8-20(9-12-26)31(28,29)19-5-3-17(4-6-19)14-25-23(27)21-13-18-7-10-24-15-22(18)30-21/h3-7,10,13,15-16,20H,8-9,11-12,14H2,1-2H3,(H,25,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 71 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human C-terminal His6-tagged NAMPT expressed in Escherichia coli Rosetta(DE3) incubated for 15 mins prior to substrate addition measure... |
Bioorg Med Chem Lett 25: 529-41 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.026
BindingDB Entry DOI: 10.7270/Q2736SK7 |
More data for this
Ligand-Target Pair | |
Nicotinamide phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50060765
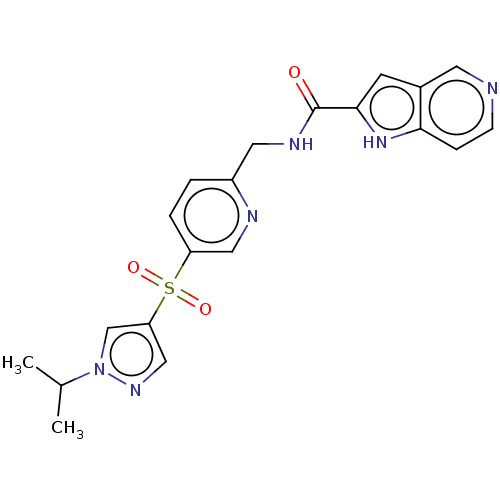
(CHEMBL3394739 | US9458172, 175)Show SMILES CC(C)n1cc(cn1)S(=O)(=O)c1ccc(CNC(=O)c2cc3cnccc3[nH]2)nc1 Show InChI InChI=1S/C20H20N6O3S/c1-13(2)26-12-17(11-24-26)30(28,29)16-4-3-15(22-10-16)9-23-20(27)19-7-14-8-21-6-5-18(14)25-19/h3-8,10-13,25H,9H2,1-2H3,(H,23,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 84 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human C-terminal His6-tagged NAMPT expressed in Escherichia coli Rosetta(DE3) incubated for 15 mins prior to substrate addition measure... |
Bioorg Med Chem Lett 25: 529-41 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.026
BindingDB Entry DOI: 10.7270/Q2736SK7 |
More data for this
Ligand-Target Pair | |
Nicotinamide phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50060833

(CHEMBL3394714 | US10696692, Example 14)Show SMILES O=C(NCc1ccc(cc1)S(=O)(=O)N1CCOCC1)c1cc2cnccc2[nH]1 Show InChI InChI=1S/C19H20N4O4S/c24-19(18-11-15-13-20-6-5-17(15)22-18)21-12-14-1-3-16(4-2-14)28(25,26)23-7-9-27-10-8-23/h1-6,11,13,22H,7-10,12H2,(H,21,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 88 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human C-terminal His6-tagged NAMPT expressed in Escherichia coli Rosetta(DE3) incubated for 15 mins prior to substrate addition measure... |
Bioorg Med Chem Lett 25: 529-41 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.026
BindingDB Entry DOI: 10.7270/Q2736SK7 |
More data for this
Ligand-Target Pair | |
Nicotinamide phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50060830

(CHEMBL3394717 | US10696692, Example 201)Show SMILES O=C(NCc1ccc(cc1)S(=O)(=O)C1CCOCC1)c1cc2cnccc2[nH]1 Show InChI InChI=1S/C20H21N3O4S/c24-20(19-11-15-13-21-8-5-18(15)23-19)22-12-14-1-3-16(4-2-14)28(25,26)17-6-9-27-10-7-17/h1-5,8,11,13,17,23H,6-7,9-10,12H2,(H,22,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 88 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human C-terminal His6-tagged NAMPT expressed in Escherichia coli Rosetta(DE3) incubated for 15 mins prior to substrate addition measure... |
Bioorg Med Chem Lett 25: 529-41 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.026
BindingDB Entry DOI: 10.7270/Q2736SK7 |
More data for this
Ligand-Target Pair | |
Nicotinamide phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50060815
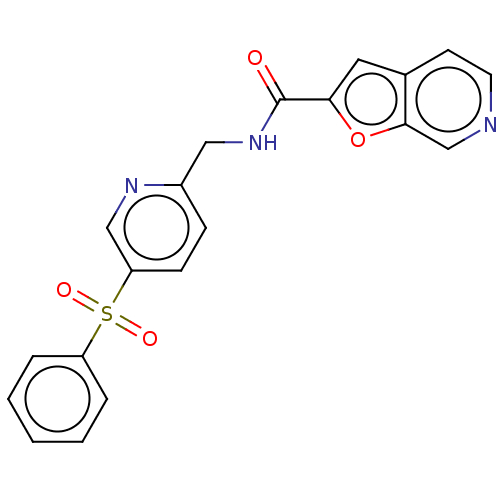
(CHEMBL3394732 | US9458172, 183)Show SMILES O=C(NCc1ccc(cn1)S(=O)(=O)c1ccccc1)c1cc2ccncc2o1 Show InChI InChI=1S/C20H15N3O4S/c24-20(18-10-14-8-9-21-13-19(14)27-18)23-11-15-6-7-17(12-22-15)28(25,26)16-4-2-1-3-5-16/h1-10,12-13H,11H2,(H,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human C-terminal His6-tagged NAMPT expressed in Escherichia coli Rosetta(DE3) incubated for 15 mins prior to substrate addition measure... |
Bioorg Med Chem Lett 25: 529-41 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.026
BindingDB Entry DOI: 10.7270/Q2736SK7 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50060766

(CHEMBL3394738 | US9458172, 62)Show SMILES CC(C)n1cc(cn1)S(=O)(=O)c1ccc(CNC(=O)c2cc3ccncc3o2)nc1 Show InChI InChI=1S/C20H19N5O4S/c1-13(2)25-12-17(10-24-25)30(27,28)16-4-3-15(22-9-16)8-23-20(26)18-7-14-5-6-21-11-19(14)29-18/h3-7,9-13H,8H2,1-2H3,(H,23,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc.
Curated by ChEMBL
| Assay Description
Reversible inhibition of CYP2C9 in human liver microsomes |
Bioorg Med Chem Lett 25: 529-41 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.026
BindingDB Entry DOI: 10.7270/Q2736SK7 |
More data for this
Ligand-Target Pair | |
Nicotinamide phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50060816
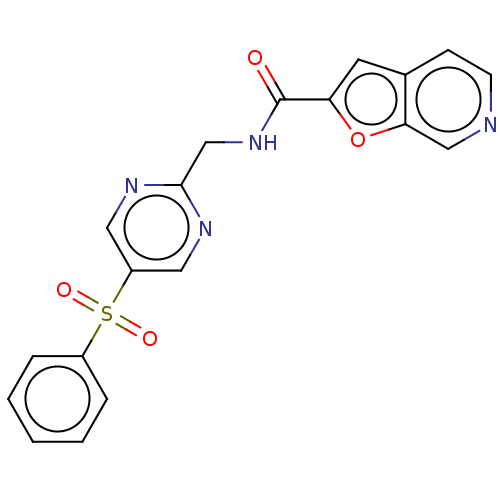
(CHEMBL3394731 | US9458172, 11)Show SMILES O=C(NCc1ncc(cn1)S(=O)(=O)c1ccccc1)c1cc2ccncc2o1 Show InChI InChI=1S/C19H14N4O4S/c24-19(16-8-13-6-7-20-11-17(13)27-16)23-12-18-21-9-15(10-22-18)28(25,26)14-4-2-1-3-5-14/h1-11H,12H2,(H,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human C-terminal His6-tagged NAMPT expressed in Escherichia coli Rosetta(DE3) incubated for 15 mins prior to substrate addition measure... |
Bioorg Med Chem Lett 25: 529-41 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.026
BindingDB Entry DOI: 10.7270/Q2736SK7 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50060828

(CHEMBL3394719 | US10696692, Example 341)Show SMILES CC(C)CN1CCC(CC1)S(=O)(=O)c1ccc(CNC(=O)c2cc3ccncc3o2)cc1 Show InChI InChI=1S/C24H29N3O4S/c1-17(2)16-27-11-8-21(9-12-27)32(29,30)20-5-3-18(4-6-20)14-26-24(28)22-13-19-7-10-25-15-23(19)31-22/h3-7,10,13,15,17,21H,8-9,11-12,14,16H2,1-2H3,(H,26,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc.
Curated by ChEMBL
| Assay Description
Reversible inhibition of CYP2C9 in human liver microsomes |
Bioorg Med Chem Lett 25: 529-41 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.026
BindingDB Entry DOI: 10.7270/Q2736SK7 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50060813

(CHEMBL3394734 | US9458172, 5)Show SMILES Fc1cc(F)cc(c1)S(=O)(=O)c1ccc(CNC(=O)c2cc3ccncc3o2)nc1 Show InChI InChI=1S/C20H13F2N3O4S/c21-13-6-14(22)8-17(7-13)30(27,28)16-2-1-15(24-10-16)9-25-20(26)18-5-12-3-4-23-11-19(12)29-18/h1-8,10-11H,9H2,(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc.
Curated by ChEMBL
| Assay Description
Reversible inhibition of CYP2C9 in human liver microsomes |
Bioorg Med Chem Lett 25: 529-41 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.026
BindingDB Entry DOI: 10.7270/Q2736SK7 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50060825

(CHEMBL3394722 | US10696692, Example 213)Show SMILES O=C(NCc1ccc(cc1)S(=O)(=O)C1CCN(CC1)C1CCOCC1)c1cc2cnccc2[nH]1 Show InChI InChI=1S/C25H30N4O4S/c30-25(24-15-19-17-26-10-5-23(19)28-24)27-16-18-1-3-21(4-2-18)34(31,32)22-6-11-29(12-7-22)20-8-13-33-14-9-20/h1-5,10,15,17,20,22,28H,6-9,11-14,16H2,(H,27,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc.
Curated by ChEMBL
| Assay Description
Reversible inhibition of CYP2C9 in human liver microsomes |
Bioorg Med Chem Lett 25: 529-41 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.026
BindingDB Entry DOI: 10.7270/Q2736SK7 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50060826

(CHEMBL3394721 | US10696692, Example 205)Show SMILES O=C(NCc1ccc(cc1)S(=O)(=O)C1CCN(CC1)C1CCOCC1)c1cc2ccncc2o1 Show InChI InChI=1S/C25H29N3O5S/c29-25(23-15-19-5-10-26-17-24(19)33-23)27-16-18-1-3-21(4-2-18)34(30,31)22-6-11-28(12-7-22)20-8-13-32-14-9-20/h1-5,10,15,17,20,22H,6-9,11-14,16H2,(H,27,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc.
Curated by ChEMBL
| Assay Description
Reversible inhibition of CYP2C9 in human liver microsomes |
Bioorg Med Chem Lett 25: 529-41 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.026
BindingDB Entry DOI: 10.7270/Q2736SK7 |
More data for this
Ligand-Target Pair | |
Nicotinamide phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50060769

(CHEMBL3394736)Show SMILES [O-][S+](c1ccc(CNC(=O)c2cc3ccncc3o2)nc1)c1cc(F)cc(F)c1 Show InChI InChI=1S/C20H13F2N3O3S/c21-13-6-14(22)8-17(7-13)29(27)16-2-1-15(24-10-16)9-25-20(26)18-5-12-3-4-23-11-19(12)28-18/h1-8,10-11H,9H2,(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 330 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human C-terminal His6-tagged NAMPT expressed in Escherichia coli Rosetta(DE3) incubated for 15 mins prior to substrate addition measure... |
Bioorg Med Chem Lett 25: 529-41 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.026
BindingDB Entry DOI: 10.7270/Q2736SK7 |
More data for this
Ligand-Target Pair | |
Nicotinamide phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50060769

(CHEMBL3394736)Show SMILES [O-][S+](c1ccc(CNC(=O)c2cc3ccncc3o2)nc1)c1cc(F)cc(F)c1 Show InChI InChI=1S/C20H13F2N3O3S/c21-13-6-14(22)8-17(7-13)29(27)16-2-1-15(24-10-16)9-25-20(26)18-5-12-3-4-23-11-19(12)28-18/h1-8,10-11H,9H2,(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 330 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human C-terminal His6-tagged NAMPT expressed in Escherichia coli Rosetta(DE3) incubated for 15 mins prior to substrate addition measure... |
Bioorg Med Chem Lett 25: 529-41 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.026
BindingDB Entry DOI: 10.7270/Q2736SK7 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50060769

(CHEMBL3394736)Show SMILES [O-][S+](c1ccc(CNC(=O)c2cc3ccncc3o2)nc1)c1cc(F)cc(F)c1 Show InChI InChI=1S/C20H13F2N3O3S/c21-13-6-14(22)8-17(7-13)29(27)16-2-1-15(24-10-16)9-25-20(26)18-5-12-3-4-23-11-19(12)28-18/h1-8,10-11H,9H2,(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc.
Curated by ChEMBL
| Assay Description
Reversible inhibition of CYP2C9 in human liver microsomes |
Bioorg Med Chem Lett 25: 529-41 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.026
BindingDB Entry DOI: 10.7270/Q2736SK7 |
More data for this
Ligand-Target Pair | |
Nicotinamide phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50060823

(CHEMBL3394724)Show SMILES CC(C)CN1CCC(CC1)[S+]([O-])c1ccc(CNC(=O)c2cc3ccncc3o2)cc1 Show InChI InChI=1S/C24H29N3O3S/c1-17(2)16-27-11-8-21(9-12-27)31(29)20-5-3-18(4-6-20)14-26-24(28)22-13-19-7-10-25-15-23(19)30-22/h3-7,10,13,15,17,21H,8-9,11-12,14,16H2,1-2H3,(H,26,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human C-terminal His6-tagged NAMPT expressed in Escherichia coli Rosetta(DE3) incubated for 15 mins prior to substrate addition measure... |
Bioorg Med Chem Lett 25: 529-41 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.026
BindingDB Entry DOI: 10.7270/Q2736SK7 |
More data for this
Ligand-Target Pair | |
Nicotinamide phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50060823

(CHEMBL3394724)Show SMILES CC(C)CN1CCC(CC1)[S+]([O-])c1ccc(CNC(=O)c2cc3ccncc3o2)cc1 Show InChI InChI=1S/C24H29N3O3S/c1-17(2)16-27-11-8-21(9-12-27)31(29)20-5-3-18(4-6-20)14-26-24(28)22-13-19-7-10-25-15-23(19)30-22/h3-7,10,13,15,17,21H,8-9,11-12,14,16H2,1-2H3,(H,26,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human C-terminal His6-tagged NAMPT expressed in Escherichia coli Rosetta(DE3) incubated for 15 mins prior to substrate addition measure... |
Bioorg Med Chem Lett 25: 529-41 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.026
BindingDB Entry DOI: 10.7270/Q2736SK7 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50060823

(CHEMBL3394724)Show SMILES CC(C)CN1CCC(CC1)[S+]([O-])c1ccc(CNC(=O)c2cc3ccncc3o2)cc1 Show InChI InChI=1S/C24H29N3O3S/c1-17(2)16-27-11-8-21(9-12-27)31(29)20-5-3-18(4-6-20)14-26-24(28)22-13-19-7-10-25-15-23(19)30-22/h3-7,10,13,15,17,21H,8-9,11-12,14,16H2,1-2H3,(H,26,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc.
Curated by ChEMBL
| Assay Description
Reversible inhibition of CYP2C9 in human liver microsomes |
Bioorg Med Chem Lett 25: 529-41 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.026
BindingDB Entry DOI: 10.7270/Q2736SK7 |
More data for this
Ligand-Target Pair | |
Nicotinamide phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50060824

(CHEMBL3394723)Show SMILES CC(C)N1CCC(CC1)[S+]([O-])c1ccc(CNC(=O)c2cc3ccncc3o2)cc1 Show InChI InChI=1S/C23H27N3O3S/c1-16(2)26-11-8-20(9-12-26)30(28)19-5-3-17(4-6-19)14-25-23(27)21-13-18-7-10-24-15-22(18)29-21/h3-7,10,13,15-16,20H,8-9,11-12,14H2,1-2H3,(H,25,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human C-terminal His6-tagged NAMPT expressed in Escherichia coli Rosetta(DE3) incubated for 15 mins prior to substrate addition measure... |
Bioorg Med Chem Lett 25: 529-41 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.026
BindingDB Entry DOI: 10.7270/Q2736SK7 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50060818

(CHEMBL3394729)Show SMILES CC(C)n1cc(cn1)S(=O)(=O)c1ccc(CNC(=O)c2cc3ccncc3o2)cc1 Show InChI InChI=1S/C21H20N4O4S/c1-14(2)25-13-18(11-24-25)30(27,28)17-5-3-15(4-6-17)10-23-21(26)19-9-16-7-8-22-12-20(16)29-19/h3-9,11-14H,10H2,1-2H3,(H,23,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc.
Curated by ChEMBL
| Assay Description
Reversible inhibition of CYP2C19 in human liver microsomes |
Bioorg Med Chem Lett 25: 529-41 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.026
BindingDB Entry DOI: 10.7270/Q2736SK7 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50060817

(CHEMBL3394730)Show SMILES CCCn1cc(cn1)S(=O)(=O)c1ccc(CNC(=O)c2cc3ccncc3o2)cc1 Show InChI InChI=1S/C21H20N4O4S/c1-2-9-25-14-18(12-24-25)30(27,28)17-5-3-15(4-6-17)11-23-21(26)19-10-16-7-8-22-13-20(16)29-19/h3-8,10,12-14H,2,9,11H2,1H3,(H,23,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc.
Curated by ChEMBL
| Assay Description
Reversible inhibition of CYP2C19 in human liver microsomes |
Bioorg Med Chem Lett 25: 529-41 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.026
BindingDB Entry DOI: 10.7270/Q2736SK7 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50060817

(CHEMBL3394730)Show SMILES CCCn1cc(cn1)S(=O)(=O)c1ccc(CNC(=O)c2cc3ccncc3o2)cc1 Show InChI InChI=1S/C21H20N4O4S/c1-2-9-25-14-18(12-24-25)30(27,28)17-5-3-15(4-6-17)11-23-21(26)19-10-16-7-8-22-13-20(16)29-19/h3-8,10,12-14H,2,9,11H2,1H3,(H,23,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc.
Curated by ChEMBL
| Assay Description
Reversible inhibition of CYP3A4 in human liver microsomes using midazolam as substrate |
Bioorg Med Chem Lett 25: 529-41 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.026
BindingDB Entry DOI: 10.7270/Q2736SK7 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50060763

(CHEMBL3394741 | US9458172, 61)Show SMILES CCCn1cc(cn1)S(=O)(=O)c1ccc(CNC(=O)c2cc3ccncc3o2)nc1 Show InChI InChI=1S/C20H19N5O4S/c1-2-7-25-13-17(11-24-25)30(27,28)16-4-3-15(22-10-16)9-23-20(26)18-8-14-5-6-21-12-19(14)29-18/h3-6,8,10-13H,2,7,9H2,1H3,(H,23,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc.
Curated by ChEMBL
| Assay Description
Reversible inhibition of CYP3A4 in human liver microsomes using midazolam as substrate |
Bioorg Med Chem Lett 25: 529-41 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.026
BindingDB Entry DOI: 10.7270/Q2736SK7 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50060766

(CHEMBL3394738 | US9458172, 62)Show SMILES CC(C)n1cc(cn1)S(=O)(=O)c1ccc(CNC(=O)c2cc3ccncc3o2)nc1 Show InChI InChI=1S/C20H19N5O4S/c1-13(2)25-12-17(10-24-25)30(27,28)16-4-3-15(22-9-16)8-23-20(26)18-7-14-5-6-21-11-19(14)29-18/h3-7,9-13H,8H2,1-2H3,(H,23,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc.
Curated by ChEMBL
| Assay Description
Reversible inhibition of CYP1A2 in human liver microsomes |
Bioorg Med Chem Lett 25: 529-41 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.026
BindingDB Entry DOI: 10.7270/Q2736SK7 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50060769

(CHEMBL3394736)Show SMILES [O-][S+](c1ccc(CNC(=O)c2cc3ccncc3o2)nc1)c1cc(F)cc(F)c1 Show InChI InChI=1S/C20H13F2N3O3S/c21-13-6-14(22)8-17(7-13)29(27)16-2-1-15(24-10-16)9-25-20(26)18-5-12-3-4-23-11-19(12)28-18/h1-8,10-11H,9H2,(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc.
Curated by ChEMBL
| Assay Description
Reversible inhibition of CYP2C19 in human liver microsomes |
Bioorg Med Chem Lett 25: 529-41 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.026
BindingDB Entry DOI: 10.7270/Q2736SK7 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50060825

(CHEMBL3394722 | US10696692, Example 213)Show SMILES O=C(NCc1ccc(cc1)S(=O)(=O)C1CCN(CC1)C1CCOCC1)c1cc2cnccc2[nH]1 Show InChI InChI=1S/C25H30N4O4S/c30-25(24-15-19-17-26-10-5-23(19)28-24)27-16-18-1-3-21(4-2-18)34(31,32)22-6-11-29(12-7-22)20-8-13-33-14-9-20/h1-5,10,15,17,20,22,28H,6-9,11-14,16H2,(H,27,30) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc.
Curated by ChEMBL
| Assay Description
Reversible inhibition of CYP2D6 in human liver microsomes |
Bioorg Med Chem Lett 25: 529-41 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.026
BindingDB Entry DOI: 10.7270/Q2736SK7 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50060823

(CHEMBL3394724)Show SMILES CC(C)CN1CCC(CC1)[S+]([O-])c1ccc(CNC(=O)c2cc3ccncc3o2)cc1 Show InChI InChI=1S/C24H29N3O3S/c1-17(2)16-27-11-8-21(9-12-27)31(29)20-5-3-18(4-6-20)14-26-24(28)22-13-19-7-10-25-15-23(19)30-22/h3-7,10,13,15,17,21H,8-9,11-12,14,16H2,1-2H3,(H,26,28) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc.
Curated by ChEMBL
| Assay Description
Reversible inhibition of CYP2D6 in human liver microsomes |
Bioorg Med Chem Lett 25: 529-41 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.026
BindingDB Entry DOI: 10.7270/Q2736SK7 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50060763

(CHEMBL3394741 | US9458172, 61)Show SMILES CCCn1cc(cn1)S(=O)(=O)c1ccc(CNC(=O)c2cc3ccncc3o2)nc1 Show InChI InChI=1S/C20H19N5O4S/c1-2-7-25-13-17(11-24-25)30(27,28)16-4-3-15(22-10-16)9-23-20(26)18-8-14-5-6-21-12-19(14)29-18/h3-6,8,10-13H,2,7,9H2,1H3,(H,23,26) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc.
Curated by ChEMBL
| Assay Description
Reversible inhibition of CYP2D6 in human liver microsomes |
Bioorg Med Chem Lett 25: 529-41 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.026
BindingDB Entry DOI: 10.7270/Q2736SK7 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50060826

(CHEMBL3394721 | US10696692, Example 205)Show SMILES O=C(NCc1ccc(cc1)S(=O)(=O)C1CCN(CC1)C1CCOCC1)c1cc2ccncc2o1 Show InChI InChI=1S/C25H29N3O5S/c29-25(23-15-19-5-10-26-17-24(19)33-23)27-16-18-1-3-21(4-2-18)34(30,31)22-6-11-28(12-7-22)20-8-13-32-14-9-20/h1-5,10,15,17,20,22H,6-9,11-14,16H2,(H,27,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc.
Curated by ChEMBL
| Assay Description
Reversible inhibition of CYP3A4 in human liver microsomes using midazolam as substrate |
Bioorg Med Chem Lett 25: 529-41 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.026
BindingDB Entry DOI: 10.7270/Q2736SK7 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50060818

(CHEMBL3394729)Show SMILES CC(C)n1cc(cn1)S(=O)(=O)c1ccc(CNC(=O)c2cc3ccncc3o2)cc1 Show InChI InChI=1S/C21H20N4O4S/c1-14(2)25-13-18(11-24-25)30(27,28)17-5-3-15(4-6-17)10-23-21(26)19-9-16-7-8-22-12-20(16)29-19/h3-9,11-14H,10H2,1-2H3,(H,23,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc.
Curated by ChEMBL
| Assay Description
Reversible inhibition of CYP3A4 in human liver microsomes using midazolam as substrate |
Bioorg Med Chem Lett 25: 529-41 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.026
BindingDB Entry DOI: 10.7270/Q2736SK7 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50060813

(CHEMBL3394734 | US9458172, 5)Show SMILES Fc1cc(F)cc(c1)S(=O)(=O)c1ccc(CNC(=O)c2cc3ccncc3o2)nc1 Show InChI InChI=1S/C20H13F2N3O4S/c21-13-6-14(22)8-17(7-13)30(27,28)16-2-1-15(24-10-16)9-25-20(26)18-5-12-3-4-23-11-19(12)29-18/h1-8,10-11H,9H2,(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc.
Curated by ChEMBL
| Assay Description
Reversible inhibition of CYP3A4 in human liver microsomes using midazolam as substrate |
Bioorg Med Chem Lett 25: 529-41 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.026
BindingDB Entry DOI: 10.7270/Q2736SK7 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50060769

(CHEMBL3394736)Show SMILES [O-][S+](c1ccc(CNC(=O)c2cc3ccncc3o2)nc1)c1cc(F)cc(F)c1 Show InChI InChI=1S/C20H13F2N3O3S/c21-13-6-14(22)8-17(7-13)29(27)16-2-1-15(24-10-16)9-25-20(26)18-5-12-3-4-23-11-19(12)28-18/h1-8,10-11H,9H2,(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc.
Curated by ChEMBL
| Assay Description
Reversible inhibition of CYP3A4 in human liver microsomes using midazolam as substrate |
Bioorg Med Chem Lett 25: 529-41 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.026
BindingDB Entry DOI: 10.7270/Q2736SK7 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50060766

(CHEMBL3394738 | US9458172, 62)Show SMILES CC(C)n1cc(cn1)S(=O)(=O)c1ccc(CNC(=O)c2cc3ccncc3o2)nc1 Show InChI InChI=1S/C20H19N5O4S/c1-13(2)25-12-17(10-24-25)30(27,28)16-4-3-15(22-9-16)8-23-20(26)18-7-14-5-6-21-11-19(14)29-18/h3-7,9-13H,8H2,1-2H3,(H,23,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc.
Curated by ChEMBL
| Assay Description
Reversible inhibition of CYP3A4 in human liver microsomes using midazolam as substrate |
Bioorg Med Chem Lett 25: 529-41 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.026
BindingDB Entry DOI: 10.7270/Q2736SK7 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50060826

(CHEMBL3394721 | US10696692, Example 205)Show SMILES O=C(NCc1ccc(cc1)S(=O)(=O)C1CCN(CC1)C1CCOCC1)c1cc2ccncc2o1 Show InChI InChI=1S/C25H29N3O5S/c29-25(23-15-19-5-10-26-17-24(19)33-23)27-16-18-1-3-21(4-2-18)34(30,31)22-6-11-28(12-7-22)20-8-13-32-14-9-20/h1-5,10,15,17,20,22H,6-9,11-14,16H2,(H,27,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc.
Curated by ChEMBL
| Assay Description
Reversible inhibition of CYP3A4 in human liver microsomes using testosterone as substrate |
Bioorg Med Chem Lett 25: 529-41 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.026
BindingDB Entry DOI: 10.7270/Q2736SK7 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50060818

(CHEMBL3394729)Show SMILES CC(C)n1cc(cn1)S(=O)(=O)c1ccc(CNC(=O)c2cc3ccncc3o2)cc1 Show InChI InChI=1S/C21H20N4O4S/c1-14(2)25-13-18(11-24-25)30(27,28)17-5-3-15(4-6-17)10-23-21(26)19-9-16-7-8-22-12-20(16)29-19/h3-9,11-14H,10H2,1-2H3,(H,23,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc.
Curated by ChEMBL
| Assay Description
Reversible inhibition of CYP3A4 in human liver microsomes using testosterone as substrate |
Bioorg Med Chem Lett 25: 529-41 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.026
BindingDB Entry DOI: 10.7270/Q2736SK7 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50060766

(CHEMBL3394738 | US9458172, 62)Show SMILES CC(C)n1cc(cn1)S(=O)(=O)c1ccc(CNC(=O)c2cc3ccncc3o2)nc1 Show InChI InChI=1S/C20H19N5O4S/c1-13(2)25-12-17(10-24-25)30(27,28)16-4-3-15(22-9-16)8-23-20(26)18-7-14-5-6-21-11-19(14)29-18/h3-7,9-13H,8H2,1-2H3,(H,23,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc.
Curated by ChEMBL
| Assay Description
Reversible inhibition of CYP3A4 in human liver microsomes using testosterone as substrate |
Bioorg Med Chem Lett 25: 529-41 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.026
BindingDB Entry DOI: 10.7270/Q2736SK7 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50060818

(CHEMBL3394729)Show SMILES CC(C)n1cc(cn1)S(=O)(=O)c1ccc(CNC(=O)c2cc3ccncc3o2)cc1 Show InChI InChI=1S/C21H20N4O4S/c1-14(2)25-13-18(11-24-25)30(27,28)17-5-3-15(4-6-17)10-23-21(26)19-9-16-7-8-22-12-20(16)29-19/h3-9,11-14H,10H2,1-2H3,(H,23,26) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc.
Curated by ChEMBL
| Assay Description
Reversible inhibition of CYP2D6 in human liver microsomes |
Bioorg Med Chem Lett 25: 529-41 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.026
BindingDB Entry DOI: 10.7270/Q2736SK7 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50060828

(CHEMBL3394719 | US10696692, Example 341)Show SMILES CC(C)CN1CCC(CC1)S(=O)(=O)c1ccc(CNC(=O)c2cc3ccncc3o2)cc1 Show InChI InChI=1S/C24H29N3O4S/c1-17(2)16-27-11-8-21(9-12-27)32(29,30)20-5-3-18(4-6-20)14-26-24(28)22-13-19-7-10-25-15-23(19)31-22/h3-7,10,13,15,17,21H,8-9,11-12,14,16H2,1-2H3,(H,26,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc.
Curated by ChEMBL
| Assay Description
Reversible inhibition of CYP1A2 in human liver microsomes |
Bioorg Med Chem Lett 25: 529-41 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.026
BindingDB Entry DOI: 10.7270/Q2736SK7 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50060825

(CHEMBL3394722 | US10696692, Example 213)Show SMILES O=C(NCc1ccc(cc1)S(=O)(=O)C1CCN(CC1)C1CCOCC1)c1cc2cnccc2[nH]1 Show InChI InChI=1S/C25H30N4O4S/c30-25(24-15-19-17-26-10-5-23(19)28-24)27-16-18-1-3-21(4-2-18)34(31,32)22-6-11-29(12-7-22)20-8-13-33-14-9-20/h1-5,10,15,17,20,22,28H,6-9,11-14,16H2,(H,27,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc.
Curated by ChEMBL
| Assay Description
Reversible inhibition of CYP1A2 in human liver microsomes |
Bioorg Med Chem Lett 25: 529-41 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.026
BindingDB Entry DOI: 10.7270/Q2736SK7 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50060828

(CHEMBL3394719 | US10696692, Example 341)Show SMILES CC(C)CN1CCC(CC1)S(=O)(=O)c1ccc(CNC(=O)c2cc3ccncc3o2)cc1 Show InChI InChI=1S/C24H29N3O4S/c1-17(2)16-27-11-8-21(9-12-27)32(29,30)20-5-3-18(4-6-20)14-26-24(28)22-13-19-7-10-25-15-23(19)31-22/h3-7,10,13,15,17,21H,8-9,11-12,14,16H2,1-2H3,(H,26,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc.
Curated by ChEMBL
| Assay Description
Reversible inhibition of CYP2C19 in human liver microsomes |
Bioorg Med Chem Lett 25: 529-41 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.026
BindingDB Entry DOI: 10.7270/Q2736SK7 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50060813

(CHEMBL3394734 | US9458172, 5)Show SMILES Fc1cc(F)cc(c1)S(=O)(=O)c1ccc(CNC(=O)c2cc3ccncc3o2)nc1 Show InChI InChI=1S/C20H13F2N3O4S/c21-13-6-14(22)8-17(7-13)30(27,28)16-2-1-15(24-10-16)9-25-20(26)18-5-12-3-4-23-11-19(12)29-18/h1-8,10-11H,9H2,(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc.
Curated by ChEMBL
| Assay Description
Reversible inhibition of CYP2C19 in human liver microsomes |
Bioorg Med Chem Lett 25: 529-41 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.026
BindingDB Entry DOI: 10.7270/Q2736SK7 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50060828

(CHEMBL3394719 | US10696692, Example 341)Show SMILES CC(C)CN1CCC(CC1)S(=O)(=O)c1ccc(CNC(=O)c2cc3ccncc3o2)cc1 Show InChI InChI=1S/C24H29N3O4S/c1-17(2)16-27-11-8-21(9-12-27)32(29,30)20-5-3-18(4-6-20)14-26-24(28)22-13-19-7-10-25-15-23(19)31-22/h3-7,10,13,15,17,21H,8-9,11-12,14,16H2,1-2H3,(H,26,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc.
Curated by ChEMBL
| Assay Description
Reversible inhibition of CYP3A4 in human liver microsomes using midazolam as substrate |
Bioorg Med Chem Lett 25: 529-41 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.026
BindingDB Entry DOI: 10.7270/Q2736SK7 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50060825

(CHEMBL3394722 | US10696692, Example 213)Show SMILES O=C(NCc1ccc(cc1)S(=O)(=O)C1CCN(CC1)C1CCOCC1)c1cc2cnccc2[nH]1 Show InChI InChI=1S/C25H30N4O4S/c30-25(24-15-19-17-26-10-5-23(19)28-24)27-16-18-1-3-21(4-2-18)34(31,32)22-6-11-29(12-7-22)20-8-13-33-14-9-20/h1-5,10,15,17,20,22,28H,6-9,11-14,16H2,(H,27,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc.
Curated by ChEMBL
| Assay Description
Reversible inhibition of CYP3A4 in human liver microsomes using testosterone as substrate |
Bioorg Med Chem Lett 25: 529-41 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.026
BindingDB Entry DOI: 10.7270/Q2736SK7 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50060817

(CHEMBL3394730)Show SMILES CCCn1cc(cn1)S(=O)(=O)c1ccc(CNC(=O)c2cc3ccncc3o2)cc1 Show InChI InChI=1S/C21H20N4O4S/c1-2-9-25-14-18(12-24-25)30(27,28)17-5-3-15(4-6-17)11-23-21(26)19-10-16-7-8-22-13-20(16)29-19/h3-8,10,12-14H,2,9,11H2,1H3,(H,23,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc.
Curated by ChEMBL
| Assay Description
Reversible inhibition of CYP3A4 in human liver microsomes using testosterone as substrate |
Bioorg Med Chem Lett 25: 529-41 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.026
BindingDB Entry DOI: 10.7270/Q2736SK7 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50060826

(CHEMBL3394721 | US10696692, Example 205)Show SMILES O=C(NCc1ccc(cc1)S(=O)(=O)C1CCN(CC1)C1CCOCC1)c1cc2ccncc2o1 Show InChI InChI=1S/C25H29N3O5S/c29-25(23-15-19-5-10-26-17-24(19)33-23)27-16-18-1-3-21(4-2-18)34(30,31)22-6-11-28(12-7-22)20-8-13-32-14-9-20/h1-5,10,15,17,20,22H,6-9,11-14,16H2,(H,27,29) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc.
Curated by ChEMBL
| Assay Description
Reversible inhibition of CYP2D6 in human liver microsomes |
Bioorg Med Chem Lett 25: 529-41 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.026
BindingDB Entry DOI: 10.7270/Q2736SK7 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50060817

(CHEMBL3394730)Show SMILES CCCn1cc(cn1)S(=O)(=O)c1ccc(CNC(=O)c2cc3ccncc3o2)cc1 Show InChI InChI=1S/C21H20N4O4S/c1-2-9-25-14-18(12-24-25)30(27,28)17-5-3-15(4-6-17)11-23-21(26)19-10-16-7-8-22-13-20(16)29-19/h3-8,10,12-14H,2,9,11H2,1H3,(H,23,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc.
Curated by ChEMBL
| Assay Description
Reversible inhibition of CYP1A2 in human liver microsomes |
Bioorg Med Chem Lett 25: 529-41 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.026
BindingDB Entry DOI: 10.7270/Q2736SK7 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50060769

(CHEMBL3394736)Show SMILES [O-][S+](c1ccc(CNC(=O)c2cc3ccncc3o2)nc1)c1cc(F)cc(F)c1 Show InChI InChI=1S/C20H13F2N3O3S/c21-13-6-14(22)8-17(7-13)29(27)16-2-1-15(24-10-16)9-25-20(26)18-5-12-3-4-23-11-19(12)28-18/h1-8,10-11H,9H2,(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc.
Curated by ChEMBL
| Assay Description
Reversible inhibition of CYP1A2 in human liver microsomes |
Bioorg Med Chem Lett 25: 529-41 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.026
BindingDB Entry DOI: 10.7270/Q2736SK7 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50060766

(CHEMBL3394738 | US9458172, 62)Show SMILES CC(C)n1cc(cn1)S(=O)(=O)c1ccc(CNC(=O)c2cc3ccncc3o2)nc1 Show InChI InChI=1S/C20H19N5O4S/c1-13(2)25-12-17(10-24-25)30(27,28)16-4-3-15(22-9-16)8-23-20(26)18-7-14-5-6-21-11-19(14)29-18/h3-7,9-13H,8H2,1-2H3,(H,23,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc.
Curated by ChEMBL
| Assay Description
Reversible inhibition of CYP2C19 in human liver microsomes |
Bioorg Med Chem Lett 25: 529-41 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.026
BindingDB Entry DOI: 10.7270/Q2736SK7 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50060825

(CHEMBL3394722 | US10696692, Example 213)Show SMILES O=C(NCc1ccc(cc1)S(=O)(=O)C1CCN(CC1)C1CCOCC1)c1cc2cnccc2[nH]1 Show InChI InChI=1S/C25H30N4O4S/c30-25(24-15-19-17-26-10-5-23(19)28-24)27-16-18-1-3-21(4-2-18)34(31,32)22-6-11-29(12-7-22)20-8-13-33-14-9-20/h1-5,10,15,17,20,22,28H,6-9,11-14,16H2,(H,27,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc.
Curated by ChEMBL
| Assay Description
Reversible inhibition of CYP3A4 in human liver microsomes using midazolam as substrate |
Bioorg Med Chem Lett 25: 529-41 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.026
BindingDB Entry DOI: 10.7270/Q2736SK7 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50060813

(CHEMBL3394734 | US9458172, 5)Show SMILES Fc1cc(F)cc(c1)S(=O)(=O)c1ccc(CNC(=O)c2cc3ccncc3o2)nc1 Show InChI InChI=1S/C20H13F2N3O4S/c21-13-6-14(22)8-17(7-13)30(27,28)16-2-1-15(24-10-16)9-25-20(26)18-5-12-3-4-23-11-19(12)29-18/h1-8,10-11H,9H2,(H,25,26) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc.
Curated by ChEMBL
| Assay Description
Reversible inhibition of CYP2D6 in human liver microsomes |
Bioorg Med Chem Lett 25: 529-41 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.026
BindingDB Entry DOI: 10.7270/Q2736SK7 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50060823

(CHEMBL3394724)Show SMILES CC(C)CN1CCC(CC1)[S+]([O-])c1ccc(CNC(=O)c2cc3ccncc3o2)cc1 Show InChI InChI=1S/C24H29N3O3S/c1-17(2)16-27-11-8-21(9-12-27)31(29)20-5-3-18(4-6-20)14-26-24(28)22-13-19-7-10-25-15-23(19)30-22/h3-7,10,13,15,17,21H,8-9,11-12,14,16H2,1-2H3,(H,26,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc.
Curated by ChEMBL
| Assay Description
Reversible inhibition of CYP1A2 in human liver microsomes |
Bioorg Med Chem Lett 25: 529-41 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.026
BindingDB Entry DOI: 10.7270/Q2736SK7 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50060763

(CHEMBL3394741 | US9458172, 61)Show SMILES CCCn1cc(cn1)S(=O)(=O)c1ccc(CNC(=O)c2cc3ccncc3o2)nc1 Show InChI InChI=1S/C20H19N5O4S/c1-2-7-25-13-17(11-24-25)30(27,28)16-4-3-15(22-10-16)9-23-20(26)18-8-14-5-6-21-12-19(14)29-18/h3-6,8,10-13H,2,7,9H2,1H3,(H,23,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc.
Curated by ChEMBL
| Assay Description
Reversible inhibition of CYP1A2 in human liver microsomes |
Bioorg Med Chem Lett 25: 529-41 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.026
BindingDB Entry DOI: 10.7270/Q2736SK7 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50060825

(CHEMBL3394722 | US10696692, Example 213)Show SMILES O=C(NCc1ccc(cc1)S(=O)(=O)C1CCN(CC1)C1CCOCC1)c1cc2cnccc2[nH]1 Show InChI InChI=1S/C25H30N4O4S/c30-25(24-15-19-17-26-10-5-23(19)28-24)27-16-18-1-3-21(4-2-18)34(31,32)22-6-11-29(12-7-22)20-8-13-33-14-9-20/h1-5,10,15,17,20,22,28H,6-9,11-14,16H2,(H,27,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc.
Curated by ChEMBL
| Assay Description
Reversible inhibition of CYP2C19 in human liver microsomes |
Bioorg Med Chem Lett 25: 529-41 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.026
BindingDB Entry DOI: 10.7270/Q2736SK7 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50060813

(CHEMBL3394734 | US9458172, 5)Show SMILES Fc1cc(F)cc(c1)S(=O)(=O)c1ccc(CNC(=O)c2cc3ccncc3o2)nc1 Show InChI InChI=1S/C20H13F2N3O4S/c21-13-6-14(22)8-17(7-13)30(27,28)16-2-1-15(24-10-16)9-25-20(26)18-5-12-3-4-23-11-19(12)29-18/h1-8,10-11H,9H2,(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc.
Curated by ChEMBL
| Assay Description
Reversible inhibition of CYP3A4 in human liver microsomes using testosterone as substrate |
Bioorg Med Chem Lett 25: 529-41 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.026
BindingDB Entry DOI: 10.7270/Q2736SK7 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50060828

(CHEMBL3394719 | US10696692, Example 341)Show SMILES CC(C)CN1CCC(CC1)S(=O)(=O)c1ccc(CNC(=O)c2cc3ccncc3o2)cc1 Show InChI InChI=1S/C24H29N3O4S/c1-17(2)16-27-11-8-21(9-12-27)32(29,30)20-5-3-18(4-6-20)14-26-24(28)22-13-19-7-10-25-15-23(19)31-22/h3-7,10,13,15,17,21H,8-9,11-12,14,16H2,1-2H3,(H,26,28) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc.
Curated by ChEMBL
| Assay Description
Reversible inhibition of CYP2D6 in human liver microsomes |
Bioorg Med Chem Lett 25: 529-41 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.026
BindingDB Entry DOI: 10.7270/Q2736SK7 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50060817

(CHEMBL3394730)Show SMILES CCCn1cc(cn1)S(=O)(=O)c1ccc(CNC(=O)c2cc3ccncc3o2)cc1 Show InChI InChI=1S/C21H20N4O4S/c1-2-9-25-14-18(12-24-25)30(27,28)17-5-3-15(4-6-17)11-23-21(26)19-10-16-7-8-22-13-20(16)29-19/h3-8,10,12-14H,2,9,11H2,1H3,(H,23,26) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc.
Curated by ChEMBL
| Assay Description
Reversible inhibition of CYP2D6 in human liver microsomes |
Bioorg Med Chem Lett 25: 529-41 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.026
BindingDB Entry DOI: 10.7270/Q2736SK7 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50060826

(CHEMBL3394721 | US10696692, Example 205)Show SMILES O=C(NCc1ccc(cc1)S(=O)(=O)C1CCN(CC1)C1CCOCC1)c1cc2ccncc2o1 Show InChI InChI=1S/C25H29N3O5S/c29-25(23-15-19-5-10-26-17-24(19)33-23)27-16-18-1-3-21(4-2-18)34(30,31)22-6-11-28(12-7-22)20-8-13-32-14-9-20/h1-5,10,15,17,20,22H,6-9,11-14,16H2,(H,27,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc.
Curated by ChEMBL
| Assay Description
Reversible inhibition of CYP2C19 in human liver microsomes |
Bioorg Med Chem Lett 25: 529-41 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.026
BindingDB Entry DOI: 10.7270/Q2736SK7 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50060763

(CHEMBL3394741 | US9458172, 61)Show SMILES CCCn1cc(cn1)S(=O)(=O)c1ccc(CNC(=O)c2cc3ccncc3o2)nc1 Show InChI InChI=1S/C20H19N5O4S/c1-2-7-25-13-17(11-24-25)30(27,28)16-4-3-15(22-10-16)9-23-20(26)18-8-14-5-6-21-12-19(14)29-18/h3-6,8,10-13H,2,7,9H2,1H3,(H,23,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc.
Curated by ChEMBL
| Assay Description
Reversible inhibition of CYP2C19 in human liver microsomes |
Bioorg Med Chem Lett 25: 529-41 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.026
BindingDB Entry DOI: 10.7270/Q2736SK7 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50060823

(CHEMBL3394724)Show SMILES CC(C)CN1CCC(CC1)[S+]([O-])c1ccc(CNC(=O)c2cc3ccncc3o2)cc1 Show InChI InChI=1S/C24H29N3O3S/c1-17(2)16-27-11-8-21(9-12-27)31(29)20-5-3-18(4-6-20)14-26-24(28)22-13-19-7-10-25-15-23(19)30-22/h3-7,10,13,15,17,21H,8-9,11-12,14,16H2,1-2H3,(H,26,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc.
Curated by ChEMBL
| Assay Description
Reversible inhibition of CYP2C19 in human liver microsomes |
Bioorg Med Chem Lett 25: 529-41 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.026
BindingDB Entry DOI: 10.7270/Q2736SK7 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50060813

(CHEMBL3394734 | US9458172, 5)Show SMILES Fc1cc(F)cc(c1)S(=O)(=O)c1ccc(CNC(=O)c2cc3ccncc3o2)nc1 Show InChI InChI=1S/C20H13F2N3O4S/c21-13-6-14(22)8-17(7-13)30(27,28)16-2-1-15(24-10-16)9-25-20(26)18-5-12-3-4-23-11-19(12)29-18/h1-8,10-11H,9H2,(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc.
Curated by ChEMBL
| Assay Description
Reversible inhibition of CYP1A2 in human liver microsomes |
Bioorg Med Chem Lett 25: 529-41 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.026
BindingDB Entry DOI: 10.7270/Q2736SK7 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50060818

(CHEMBL3394729)Show SMILES CC(C)n1cc(cn1)S(=O)(=O)c1ccc(CNC(=O)c2cc3ccncc3o2)cc1 Show InChI InChI=1S/C21H20N4O4S/c1-14(2)25-13-18(11-24-25)30(27,28)17-5-3-15(4-6-17)10-23-21(26)19-9-16-7-8-22-12-20(16)29-19/h3-9,11-14H,10H2,1-2H3,(H,23,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc.
Curated by ChEMBL
| Assay Description
Reversible inhibition of CYP1A2 in human liver microsomes |
Bioorg Med Chem Lett 25: 529-41 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.026
BindingDB Entry DOI: 10.7270/Q2736SK7 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50060826

(CHEMBL3394721 | US10696692, Example 205)Show SMILES O=C(NCc1ccc(cc1)S(=O)(=O)C1CCN(CC1)C1CCOCC1)c1cc2ccncc2o1 Show InChI InChI=1S/C25H29N3O5S/c29-25(23-15-19-5-10-26-17-24(19)33-23)27-16-18-1-3-21(4-2-18)34(30,31)22-6-11-28(12-7-22)20-8-13-32-14-9-20/h1-5,10,15,17,20,22H,6-9,11-14,16H2,(H,27,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc.
Curated by ChEMBL
| Assay Description
Reversible inhibition of CYP1A2 in human liver microsomes |
Bioorg Med Chem Lett 25: 529-41 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.026
BindingDB Entry DOI: 10.7270/Q2736SK7 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50060766

(CHEMBL3394738 | US9458172, 62)Show SMILES CC(C)n1cc(cn1)S(=O)(=O)c1ccc(CNC(=O)c2cc3ccncc3o2)nc1 Show InChI InChI=1S/C20H19N5O4S/c1-13(2)25-12-17(10-24-25)30(27,28)16-4-3-15(22-9-16)8-23-20(26)18-7-14-5-6-21-11-19(14)29-18/h3-7,9-13H,8H2,1-2H3,(H,23,26) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc.
Curated by ChEMBL
| Assay Description
Reversible inhibition of CYP2D6 in human liver microsomes |
Bioorg Med Chem Lett 25: 529-41 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.026
BindingDB Entry DOI: 10.7270/Q2736SK7 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50060769

(CHEMBL3394736)Show SMILES [O-][S+](c1ccc(CNC(=O)c2cc3ccncc3o2)nc1)c1cc(F)cc(F)c1 Show InChI InChI=1S/C20H13F2N3O3S/c21-13-6-14(22)8-17(7-13)29(27)16-2-1-15(24-10-16)9-25-20(26)18-5-12-3-4-23-11-19(12)28-18/h1-8,10-11H,9H2,(H,25,26) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc.
Curated by ChEMBL
| Assay Description
Reversible inhibition of CYP2D6 in human liver microsomes |
Bioorg Med Chem Lett 25: 529-41 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.026
BindingDB Entry DOI: 10.7270/Q2736SK7 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50060763

(CHEMBL3394741 | US9458172, 61)Show SMILES CCCn1cc(cn1)S(=O)(=O)c1ccc(CNC(=O)c2cc3ccncc3o2)nc1 Show InChI InChI=1S/C20H19N5O4S/c1-2-7-25-13-17(11-24-25)30(27,28)16-4-3-15(22-10-16)9-23-20(26)18-8-14-5-6-21-12-19(14)29-18/h3-6,8,10-13H,2,7,9H2,1H3,(H,23,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc.
Curated by ChEMBL
| Assay Description
Reversible inhibition of CYP3A4 in human liver microsomes using testosterone as substrate |
Bioorg Med Chem Lett 25: 529-41 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.026
BindingDB Entry DOI: 10.7270/Q2736SK7 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50060823

(CHEMBL3394724)Show SMILES CC(C)CN1CCC(CC1)[S+]([O-])c1ccc(CNC(=O)c2cc3ccncc3o2)cc1 Show InChI InChI=1S/C24H29N3O3S/c1-17(2)16-27-11-8-21(9-12-27)31(29)20-5-3-18(4-6-20)14-26-24(28)22-13-19-7-10-25-15-23(19)30-22/h3-7,10,13,15,17,21H,8-9,11-12,14,16H2,1-2H3,(H,26,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc.
Curated by ChEMBL
| Assay Description
Reversible inhibition of CYP3A4 in human liver microsomes using midazolam as substrate |
Bioorg Med Chem Lett 25: 529-41 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.026
BindingDB Entry DOI: 10.7270/Q2736SK7 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50060769

(CHEMBL3394736)Show SMILES [O-][S+](c1ccc(CNC(=O)c2cc3ccncc3o2)nc1)c1cc(F)cc(F)c1 Show InChI InChI=1S/C20H13F2N3O3S/c21-13-6-14(22)8-17(7-13)29(27)16-2-1-15(24-10-16)9-25-20(26)18-5-12-3-4-23-11-19(12)28-18/h1-8,10-11H,9H2,(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc.
Curated by ChEMBL
| Assay Description
Reversible inhibition of CYP3A4 in human liver microsomes using testosterone as substrate |
Bioorg Med Chem Lett 25: 529-41 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.026
BindingDB Entry DOI: 10.7270/Q2736SK7 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50060823

(CHEMBL3394724)Show SMILES CC(C)CN1CCC(CC1)[S+]([O-])c1ccc(CNC(=O)c2cc3ccncc3o2)cc1 Show InChI InChI=1S/C24H29N3O3S/c1-17(2)16-27-11-8-21(9-12-27)31(29)20-5-3-18(4-6-20)14-26-24(28)22-13-19-7-10-25-15-23(19)30-22/h3-7,10,13,15,17,21H,8-9,11-12,14,16H2,1-2H3,(H,26,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc.
Curated by ChEMBL
| Assay Description
Reversible inhibition of CYP3A4 in human liver microsomes using testosterone as substrate |
Bioorg Med Chem Lett 25: 529-41 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.026
BindingDB Entry DOI: 10.7270/Q2736SK7 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50060828

(CHEMBL3394719 | US10696692, Example 341)Show SMILES CC(C)CN1CCC(CC1)S(=O)(=O)c1ccc(CNC(=O)c2cc3ccncc3o2)cc1 Show InChI InChI=1S/C24H29N3O4S/c1-17(2)16-27-11-8-21(9-12-27)32(29,30)20-5-3-18(4-6-20)14-26-24(28)22-13-19-7-10-25-15-23(19)31-22/h3-7,10,13,15,17,21H,8-9,11-12,14,16H2,1-2H3,(H,26,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc.
Curated by ChEMBL
| Assay Description
Reversible inhibition of CYP3A4 in human liver microsomes using testosterone as substrate |
Bioorg Med Chem Lett 25: 529-41 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.026
BindingDB Entry DOI: 10.7270/Q2736SK7 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data