

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50071073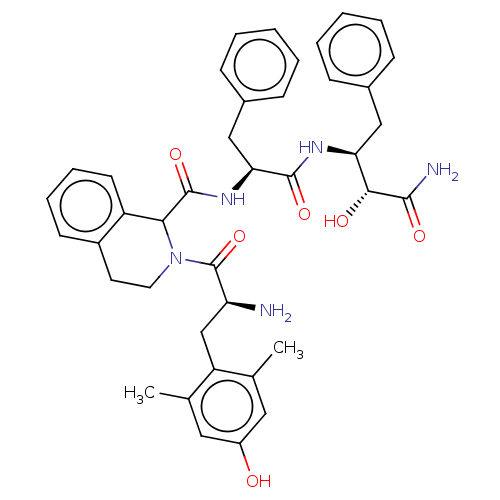 (CHEMBL3409763) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University Curated by ChEMBL | Assay Description Displacement of [3H]DPDPE from rat cortex delta opioid receptor after 2.5 hrs by liquid scintillation counting analysis | Eur J Med Chem 92: 270-81 (2015) Article DOI: 10.1016/j.ejmech.2014.12.049 BindingDB Entry DOI: 10.7270/Q22B90RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50071072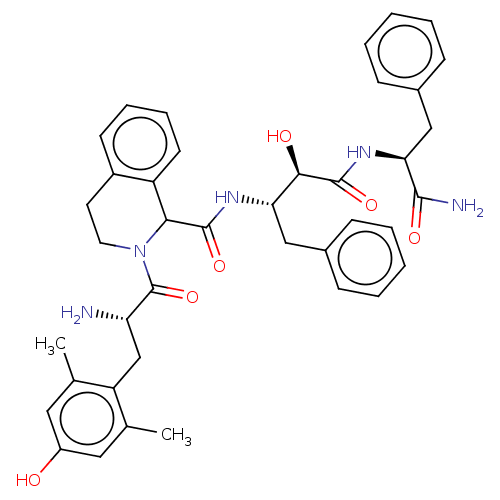 (CHEMBL3409762) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University Curated by ChEMBL | Assay Description Displacement of [3H]DPDPE from rat cortex delta opioid receptor after 2.5 hrs by liquid scintillation counting analysis | Eur J Med Chem 92: 270-81 (2015) Article DOI: 10.1016/j.ejmech.2014.12.049 BindingDB Entry DOI: 10.7270/Q22B90RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50071071 (CHEMBL3409761) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University Curated by ChEMBL | Assay Description Displacement of [3H]DPDPE from rat cortex delta opioid receptor after 2.5 hrs by liquid scintillation counting analysis | Eur J Med Chem 92: 270-81 (2015) Article DOI: 10.1016/j.ejmech.2014.12.049 BindingDB Entry DOI: 10.7270/Q22B90RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50071095 (CHEMBL3409766) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University Curated by ChEMBL | Assay Description Displacement of [3H]DPDPE from rat cortex delta opioid receptor after 2.5 hrs by liquid scintillation counting analysis | Eur J Med Chem 92: 270-81 (2015) Article DOI: 10.1016/j.ejmech.2014.12.049 BindingDB Entry DOI: 10.7270/Q22B90RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50071070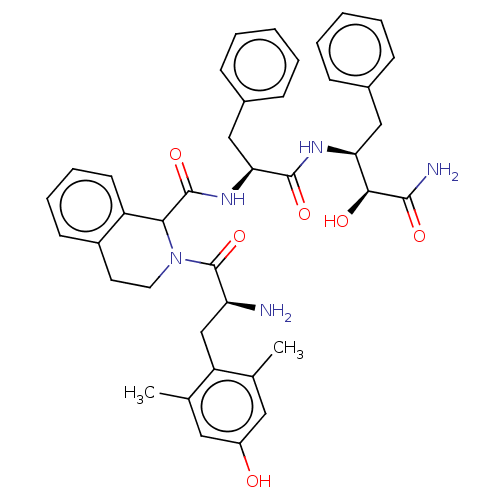 (CHEMBL3409760) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University Curated by ChEMBL | Assay Description Displacement of [3H]DPDPE from rat cortex delta opioid receptor after 2.5 hrs by liquid scintillation counting analysis | Eur J Med Chem 92: 270-81 (2015) Article DOI: 10.1016/j.ejmech.2014.12.049 BindingDB Entry DOI: 10.7270/Q22B90RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50071093 (CHEMBL3409764) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University Curated by ChEMBL | Assay Description Displacement of [3H]DPDPE from rat cortex delta opioid receptor after 2.5 hrs by liquid scintillation counting analysis | Eur J Med Chem 92: 270-81 (2015) Article DOI: 10.1016/j.ejmech.2014.12.049 BindingDB Entry DOI: 10.7270/Q22B90RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50071094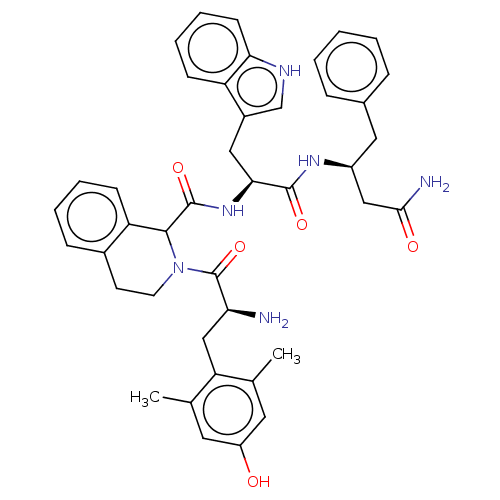 (CHEMBL3409765) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University Curated by ChEMBL | Assay Description Displacement of [3H]DPDPE from rat cortex delta opioid receptor after 2.5 hrs by liquid scintillation counting analysis | Eur J Med Chem 92: 270-81 (2015) Article DOI: 10.1016/j.ejmech.2014.12.049 BindingDB Entry DOI: 10.7270/Q22B90RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50071068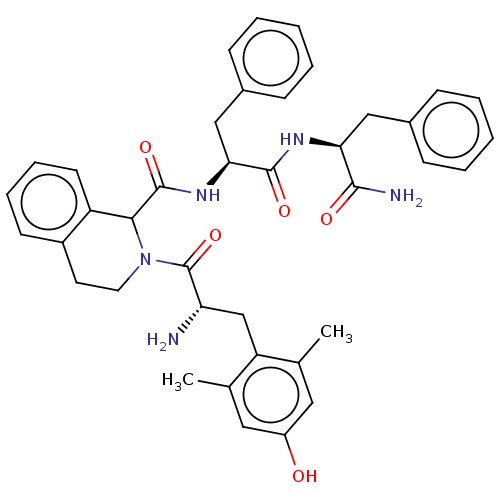 (CHEMBL3409758) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University Curated by ChEMBL | Assay Description Displacement of [3H]DPDPE from rat cortex delta opioid receptor after 2.5 hrs by liquid scintillation counting analysis | Eur J Med Chem 92: 270-81 (2015) Article DOI: 10.1016/j.ejmech.2014.12.049 BindingDB Entry DOI: 10.7270/Q22B90RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50071069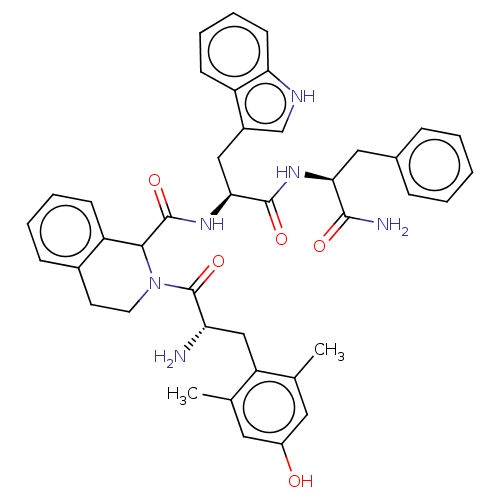 (CHEMBL3409759) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University Curated by ChEMBL | Assay Description Displacement of [3H]DPDPE from rat cortex delta opioid receptor after 2.5 hrs by liquid scintillation counting analysis | Eur J Med Chem 92: 270-81 (2015) Article DOI: 10.1016/j.ejmech.2014.12.049 BindingDB Entry DOI: 10.7270/Q22B90RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50071071 (CHEMBL3409761) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from rat cortex mu opioid receptor after 30 mins by liquid scintillation counting analysis | Eur J Med Chem 92: 270-81 (2015) Article DOI: 10.1016/j.ejmech.2014.12.049 BindingDB Entry DOI: 10.7270/Q22B90RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50139013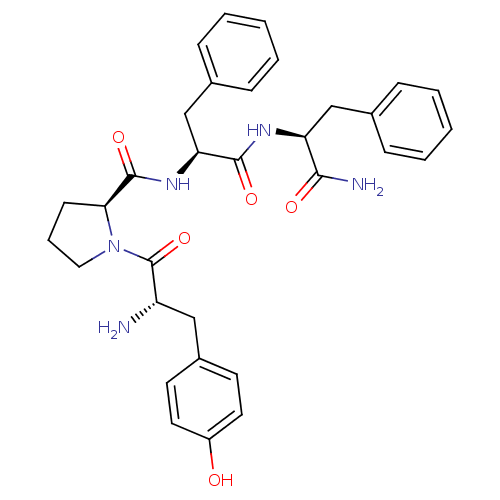 ((S)-1-[(S)-2-Amino-3-(4-hydroxy-phenyl)-propionyl]...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from rat cortex mu opioid receptor after 30 mins by liquid scintillation counting analysis | Eur J Med Chem 92: 270-81 (2015) Article DOI: 10.1016/j.ejmech.2014.12.049 BindingDB Entry DOI: 10.7270/Q22B90RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50095155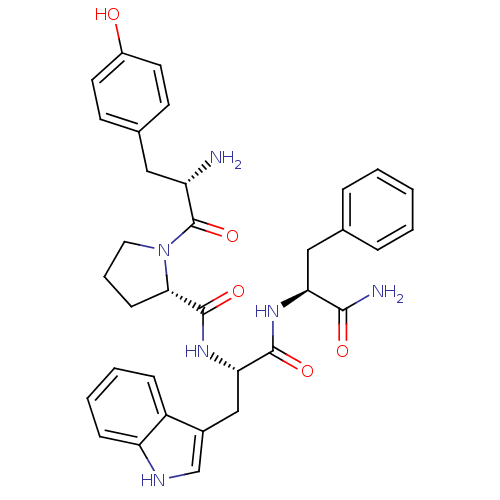 ((S)-1-[(S)-2-Amino-3-(4-hydroxy-phenyl)-propionyl]...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from rat cortex mu opioid receptor after 30 mins by liquid scintillation counting analysis | Eur J Med Chem 92: 270-81 (2015) Article DOI: 10.1016/j.ejmech.2014.12.049 BindingDB Entry DOI: 10.7270/Q22B90RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50071069 (CHEMBL3409759) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from rat cortex mu opioid receptor after 30 mins by liquid scintillation counting analysis | Eur J Med Chem 92: 270-81 (2015) Article DOI: 10.1016/j.ejmech.2014.12.049 BindingDB Entry DOI: 10.7270/Q22B90RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50071068 (CHEMBL3409758) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from rat cortex mu opioid receptor after 30 mins by liquid scintillation counting analysis | Eur J Med Chem 92: 270-81 (2015) Article DOI: 10.1016/j.ejmech.2014.12.049 BindingDB Entry DOI: 10.7270/Q22B90RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50071095 (CHEMBL3409766) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from rat cortex mu opioid receptor after 30 mins by liquid scintillation counting analysis | Eur J Med Chem 92: 270-81 (2015) Article DOI: 10.1016/j.ejmech.2014.12.049 BindingDB Entry DOI: 10.7270/Q22B90RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50071072 (CHEMBL3409762) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from rat cortex mu opioid receptor after 30 mins by liquid scintillation counting analysis | Eur J Med Chem 92: 270-81 (2015) Article DOI: 10.1016/j.ejmech.2014.12.049 BindingDB Entry DOI: 10.7270/Q22B90RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50071076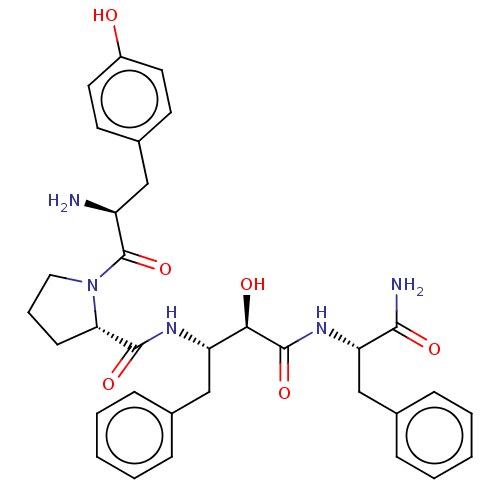 (CHEMBL3409749) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from rat cortex mu opioid receptor after 30 mins by liquid scintillation counting analysis | Eur J Med Chem 92: 270-81 (2015) Article DOI: 10.1016/j.ejmech.2014.12.049 BindingDB Entry DOI: 10.7270/Q22B90RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50114010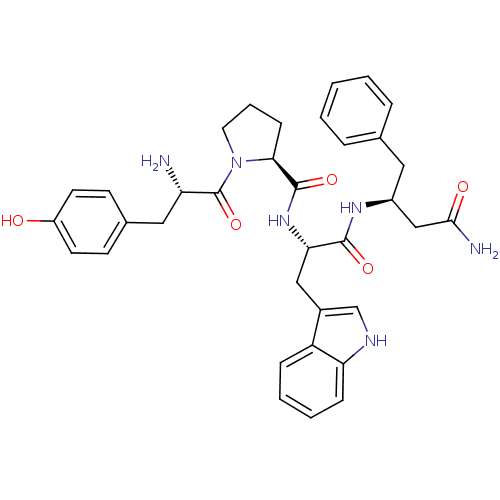 (1-[2-Amino-3-(4-hydroxy-phenyl)-propionyl]-pyrroli...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from rat cortex mu opioid receptor after 30 mins by liquid scintillation counting analysis | Eur J Med Chem 92: 270-81 (2015) Article DOI: 10.1016/j.ejmech.2014.12.049 BindingDB Entry DOI: 10.7270/Q22B90RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50071086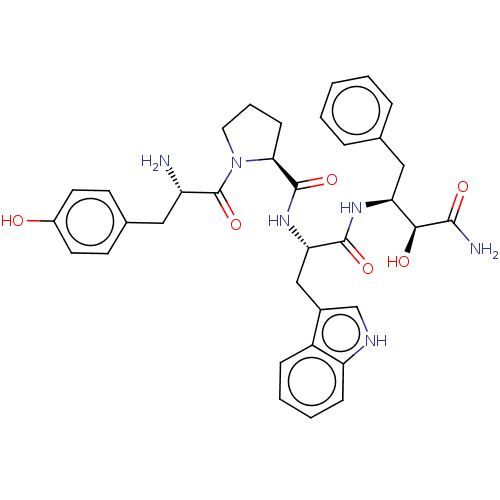 (CHEMBL3409741) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from rat cortex mu opioid receptor after 30 mins by liquid scintillation counting analysis | Eur J Med Chem 92: 270-81 (2015) Article DOI: 10.1016/j.ejmech.2014.12.049 BindingDB Entry DOI: 10.7270/Q22B90RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50071070 (CHEMBL3409760) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from rat cortex mu opioid receptor after 30 mins by liquid scintillation counting analysis | Eur J Med Chem 92: 270-81 (2015) Article DOI: 10.1016/j.ejmech.2014.12.049 BindingDB Entry DOI: 10.7270/Q22B90RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50071092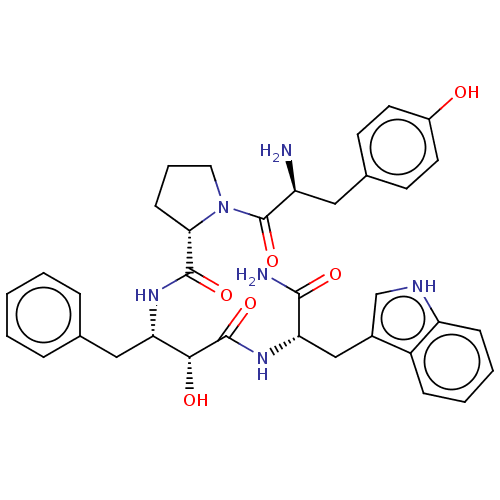 (CHEMBL3409755) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from rat cortex mu opioid receptor after 30 mins by liquid scintillation counting analysis | Eur J Med Chem 92: 270-81 (2015) Article DOI: 10.1016/j.ejmech.2014.12.049 BindingDB Entry DOI: 10.7270/Q22B90RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50071094 (CHEMBL3409765) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from rat cortex mu opioid receptor after 30 mins by liquid scintillation counting analysis | Eur J Med Chem 92: 270-81 (2015) Article DOI: 10.1016/j.ejmech.2014.12.049 BindingDB Entry DOI: 10.7270/Q22B90RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50071093 (CHEMBL3409764) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from rat cortex mu opioid receptor after 30 mins by liquid scintillation counting analysis | Eur J Med Chem 92: 270-81 (2015) Article DOI: 10.1016/j.ejmech.2014.12.049 BindingDB Entry DOI: 10.7270/Q22B90RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50071073 (CHEMBL3409763) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from rat cortex mu opioid receptor after 30 mins by liquid scintillation counting analysis | Eur J Med Chem 92: 270-81 (2015) Article DOI: 10.1016/j.ejmech.2014.12.049 BindingDB Entry DOI: 10.7270/Q22B90RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50071090 (CHEMBL3409745) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from rat cortex mu opioid receptor after 30 mins by liquid scintillation counting analysis | Eur J Med Chem 92: 270-81 (2015) Article DOI: 10.1016/j.ejmech.2014.12.049 BindingDB Entry DOI: 10.7270/Q22B90RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50071075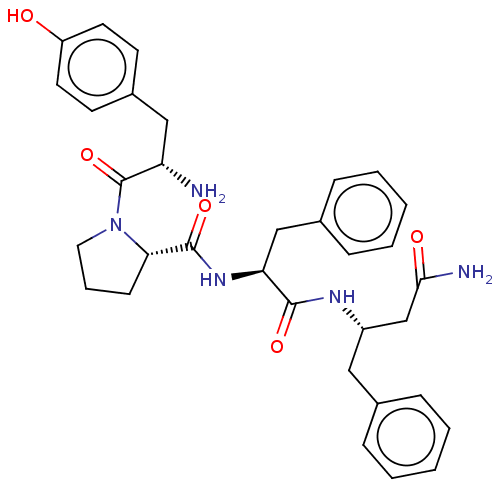 (CHEMBL3409748) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from rat cortex mu opioid receptor after 30 mins by liquid scintillation counting analysis | Eur J Med Chem 92: 270-81 (2015) Article DOI: 10.1016/j.ejmech.2014.12.049 BindingDB Entry DOI: 10.7270/Q22B90RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50071067 (CHEMBL3409757) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from rat cortex mu opioid receptor after 30 mins by liquid scintillation counting analysis | Eur J Med Chem 92: 270-81 (2015) Article DOI: 10.1016/j.ejmech.2014.12.049 BindingDB Entry DOI: 10.7270/Q22B90RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50071085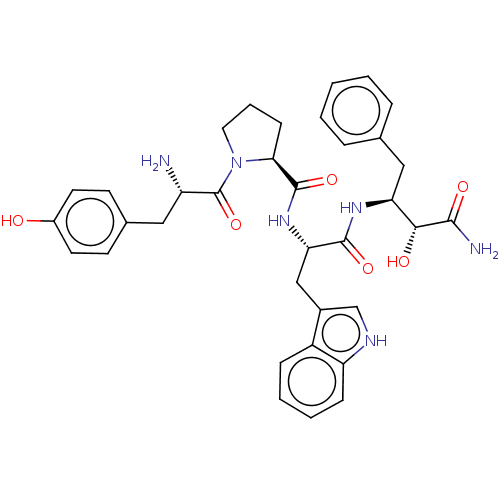 (CHEMBL3409740) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from rat cortex mu opioid receptor after 30 mins by liquid scintillation counting analysis | Eur J Med Chem 92: 270-81 (2015) Article DOI: 10.1016/j.ejmech.2014.12.049 BindingDB Entry DOI: 10.7270/Q22B90RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50071093 (CHEMBL3409764) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University Curated by ChEMBL | Assay Description Displacement of [3H]U69,593 from guinea pig cortex/cerebella kappa opioid receptor after 2 hrs by liquid scintillation counting analysis | Eur J Med Chem 92: 270-81 (2015) Article DOI: 10.1016/j.ejmech.2014.12.049 BindingDB Entry DOI: 10.7270/Q22B90RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50071065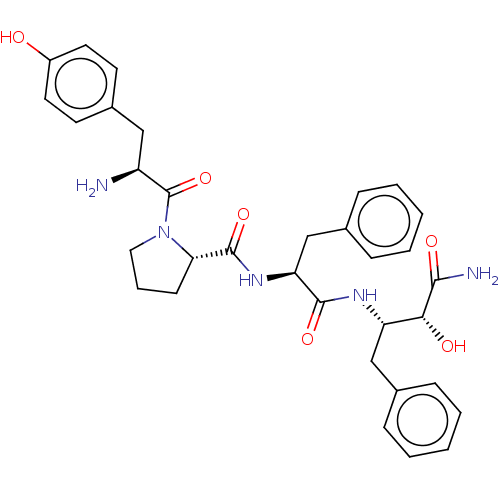 (CHEMBL3409744) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from rat cortex mu opioid receptor after 30 mins by liquid scintillation counting analysis | Eur J Med Chem 92: 270-81 (2015) Article DOI: 10.1016/j.ejmech.2014.12.049 BindingDB Entry DOI: 10.7270/Q22B90RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50071088 (CHEMBL3409753) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 39 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from rat cortex mu opioid receptor after 30 mins by liquid scintillation counting analysis | Eur J Med Chem 92: 270-81 (2015) Article DOI: 10.1016/j.ejmech.2014.12.049 BindingDB Entry DOI: 10.7270/Q22B90RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50071068 (CHEMBL3409758) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 51 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University Curated by ChEMBL | Assay Description Displacement of [3H]U69,593 from guinea pig cortex/cerebella kappa opioid receptor after 2 hrs by liquid scintillation counting analysis | Eur J Med Chem 92: 270-81 (2015) Article DOI: 10.1016/j.ejmech.2014.12.049 BindingDB Entry DOI: 10.7270/Q22B90RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50071072 (CHEMBL3409762) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 67 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University Curated by ChEMBL | Assay Description Displacement of [3H]U69,593 from guinea pig cortex/cerebella kappa opioid receptor after 2 hrs by liquid scintillation counting analysis | Eur J Med Chem 92: 270-81 (2015) Article DOI: 10.1016/j.ejmech.2014.12.049 BindingDB Entry DOI: 10.7270/Q22B90RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50071070 (CHEMBL3409760) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 82 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University Curated by ChEMBL | Assay Description Displacement of [3H]U69,593 from guinea pig cortex/cerebella kappa opioid receptor after 2 hrs by liquid scintillation counting analysis | Eur J Med Chem 92: 270-81 (2015) Article DOI: 10.1016/j.ejmech.2014.12.049 BindingDB Entry DOI: 10.7270/Q22B90RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50071073 (CHEMBL3409763) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 82 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University Curated by ChEMBL | Assay Description Displacement of [3H]U69,593 from guinea pig cortex/cerebella kappa opioid receptor after 2 hrs by liquid scintillation counting analysis | Eur J Med Chem 92: 270-81 (2015) Article DOI: 10.1016/j.ejmech.2014.12.049 BindingDB Entry DOI: 10.7270/Q22B90RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50071066 (CHEMBL3409756) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 86 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from rat cortex mu opioid receptor after 30 mins by liquid scintillation counting analysis | Eur J Med Chem 92: 270-81 (2015) Article DOI: 10.1016/j.ejmech.2014.12.049 BindingDB Entry DOI: 10.7270/Q22B90RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50071095 (CHEMBL3409766) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 112 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University Curated by ChEMBL | Assay Description Displacement of [3H]U69,593 from guinea pig cortex/cerebella kappa opioid receptor after 2 hrs by liquid scintillation counting analysis | Eur J Med Chem 92: 270-81 (2015) Article DOI: 10.1016/j.ejmech.2014.12.049 BindingDB Entry DOI: 10.7270/Q22B90RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50071071 (CHEMBL3409761) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 161 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University Curated by ChEMBL | Assay Description Displacement of [3H]U69,593 from guinea pig cortex/cerebella kappa opioid receptor after 2 hrs by liquid scintillation counting analysis | Eur J Med Chem 92: 270-81 (2015) Article DOI: 10.1016/j.ejmech.2014.12.049 BindingDB Entry DOI: 10.7270/Q22B90RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50071094 (CHEMBL3409765) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University Curated by ChEMBL | Assay Description Displacement of [3H]U69,593 from guinea pig cortex/cerebella kappa opioid receptor after 2 hrs by liquid scintillation counting analysis | Eur J Med Chem 92: 270-81 (2015) Article DOI: 10.1016/j.ejmech.2014.12.049 BindingDB Entry DOI: 10.7270/Q22B90RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50071087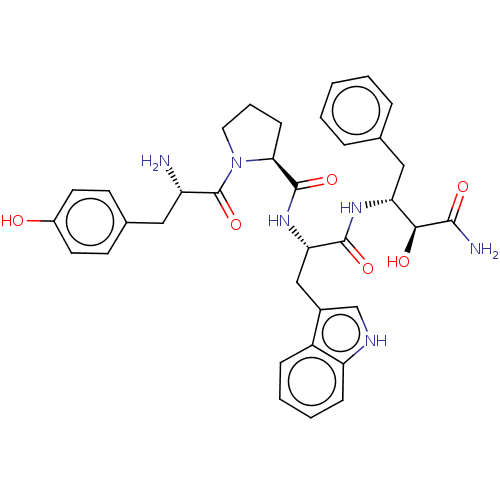 (CHEMBL3409742) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 176 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from rat cortex mu opioid receptor after 30 mins by liquid scintillation counting analysis | Eur J Med Chem 92: 270-81 (2015) Article DOI: 10.1016/j.ejmech.2014.12.049 BindingDB Entry DOI: 10.7270/Q22B90RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50071083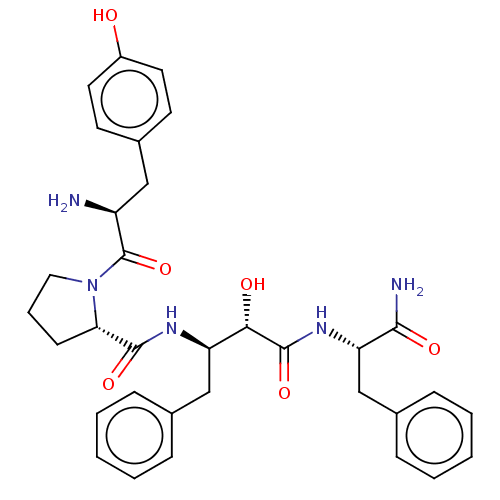 (CHEMBL3409751) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 202 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from rat cortex mu opioid receptor after 30 mins by liquid scintillation counting analysis | Eur J Med Chem 92: 270-81 (2015) Article DOI: 10.1016/j.ejmech.2014.12.049 BindingDB Entry DOI: 10.7270/Q22B90RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50071089 (CHEMBL3409754) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 207 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from rat cortex mu opioid receptor after 30 mins by liquid scintillation counting analysis | Eur J Med Chem 92: 270-81 (2015) Article DOI: 10.1016/j.ejmech.2014.12.049 BindingDB Entry DOI: 10.7270/Q22B90RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50071064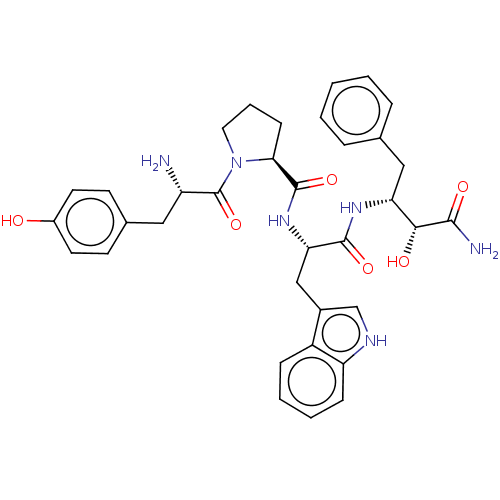 (CHEMBL3409743) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 243 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from rat cortex mu opioid receptor after 30 mins by liquid scintillation counting analysis | Eur J Med Chem 92: 270-81 (2015) Article DOI: 10.1016/j.ejmech.2014.12.049 BindingDB Entry DOI: 10.7270/Q22B90RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50071087 (CHEMBL3409742) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 287 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University Curated by ChEMBL | Assay Description Displacement of [3H]DPDPE from rat cortex delta opioid receptor after 2.5 hrs by liquid scintillation counting analysis | Eur J Med Chem 92: 270-81 (2015) Article DOI: 10.1016/j.ejmech.2014.12.049 BindingDB Entry DOI: 10.7270/Q22B90RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50071069 (CHEMBL3409759) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 289 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University Curated by ChEMBL | Assay Description Displacement of [3H]U69,593 from guinea pig cortex/cerebella kappa opioid receptor after 2 hrs by liquid scintillation counting analysis | Eur J Med Chem 92: 270-81 (2015) Article DOI: 10.1016/j.ejmech.2014.12.049 BindingDB Entry DOI: 10.7270/Q22B90RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50071091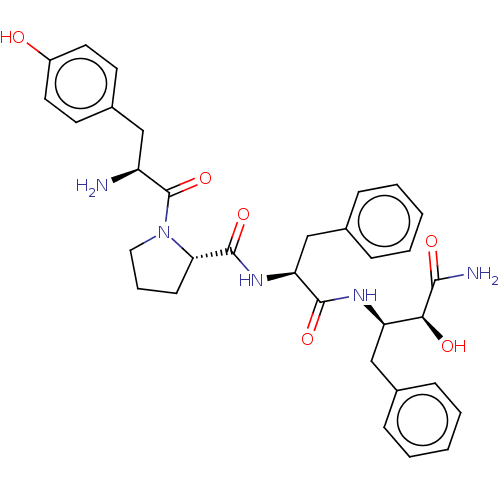 (CHEMBL3409746) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 324 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from rat cortex mu opioid receptor after 30 mins by liquid scintillation counting analysis | Eur J Med Chem 92: 270-81 (2015) Article DOI: 10.1016/j.ejmech.2014.12.049 BindingDB Entry DOI: 10.7270/Q22B90RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50071067 (CHEMBL3409757) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 385 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University Curated by ChEMBL | Assay Description Displacement of [3H]DPDPE from rat cortex delta opioid receptor after 2.5 hrs by liquid scintillation counting analysis | Eur J Med Chem 92: 270-81 (2015) Article DOI: 10.1016/j.ejmech.2014.12.049 BindingDB Entry DOI: 10.7270/Q22B90RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50071064 (CHEMBL3409743) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 441 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University Curated by ChEMBL | Assay Description Displacement of [3H]DPDPE from rat cortex delta opioid receptor after 2.5 hrs by liquid scintillation counting analysis | Eur J Med Chem 92: 270-81 (2015) Article DOI: 10.1016/j.ejmech.2014.12.049 BindingDB Entry DOI: 10.7270/Q22B90RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50071074 (CHEMBL3409747) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 447 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from rat cortex mu opioid receptor after 30 mins by liquid scintillation counting analysis | Eur J Med Chem 92: 270-81 (2015) Article DOI: 10.1016/j.ejmech.2014.12.049 BindingDB Entry DOI: 10.7270/Q22B90RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50071085 (CHEMBL3409740) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 453 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University Curated by ChEMBL | Assay Description Displacement of [3H]DPDPE from rat cortex delta opioid receptor after 2.5 hrs by liquid scintillation counting analysis | Eur J Med Chem 92: 270-81 (2015) Article DOI: 10.1016/j.ejmech.2014.12.049 BindingDB Entry DOI: 10.7270/Q22B90RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50071074 (CHEMBL3409747) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 462 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University Curated by ChEMBL | Assay Description Displacement of [3H]DPDPE from rat cortex delta opioid receptor after 2.5 hrs by liquid scintillation counting analysis | Eur J Med Chem 92: 270-81 (2015) Article DOI: 10.1016/j.ejmech.2014.12.049 BindingDB Entry DOI: 10.7270/Q22B90RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50071066 (CHEMBL3409756) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 483 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University Curated by ChEMBL | Assay Description Displacement of [3H]DPDPE from rat cortex delta opioid receptor after 2.5 hrs by liquid scintillation counting analysis | Eur J Med Chem 92: 270-81 (2015) Article DOI: 10.1016/j.ejmech.2014.12.049 BindingDB Entry DOI: 10.7270/Q22B90RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50071082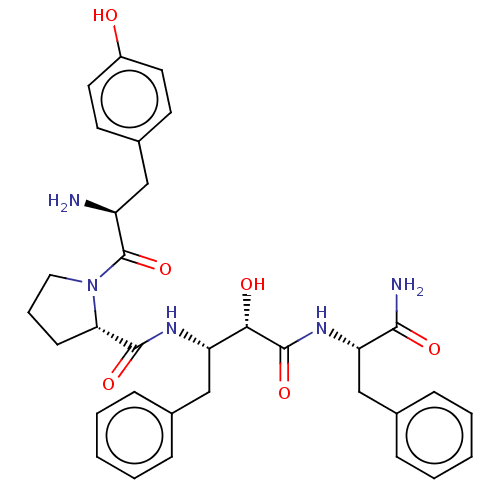 (CHEMBL3409750) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 497 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from rat cortex mu opioid receptor after 30 mins by liquid scintillation counting analysis | Eur J Med Chem 92: 270-81 (2015) Article DOI: 10.1016/j.ejmech.2014.12.049 BindingDB Entry DOI: 10.7270/Q22B90RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50071086 (CHEMBL3409741) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 508 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University Curated by ChEMBL | Assay Description Displacement of [3H]DPDPE from rat cortex delta opioid receptor after 2.5 hrs by liquid scintillation counting analysis | Eur J Med Chem 92: 270-81 (2015) Article DOI: 10.1016/j.ejmech.2014.12.049 BindingDB Entry DOI: 10.7270/Q22B90RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50071091 (CHEMBL3409746) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 563 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University Curated by ChEMBL | Assay Description Displacement of [3H]DPDPE from rat cortex delta opioid receptor after 2.5 hrs by liquid scintillation counting analysis | Eur J Med Chem 92: 270-81 (2015) Article DOI: 10.1016/j.ejmech.2014.12.049 BindingDB Entry DOI: 10.7270/Q22B90RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50071083 (CHEMBL3409751) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 593 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University Curated by ChEMBL | Assay Description Displacement of [3H]DPDPE from rat cortex delta opioid receptor after 2.5 hrs by liquid scintillation counting analysis | Eur J Med Chem 92: 270-81 (2015) Article DOI: 10.1016/j.ejmech.2014.12.049 BindingDB Entry DOI: 10.7270/Q22B90RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50095155 ((S)-1-[(S)-2-Amino-3-(4-hydroxy-phenyl)-propionyl]...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 729 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University Curated by ChEMBL | Assay Description Displacement of [3H]DPDPE from rat cortex delta opioid receptor after 2.5 hrs by liquid scintillation counting analysis | Eur J Med Chem 92: 270-81 (2015) Article DOI: 10.1016/j.ejmech.2014.12.049 BindingDB Entry DOI: 10.7270/Q22B90RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50071088 (CHEMBL3409753) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 757 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University Curated by ChEMBL | Assay Description Displacement of [3H]DPDPE from rat cortex delta opioid receptor after 2.5 hrs by liquid scintillation counting analysis | Eur J Med Chem 92: 270-81 (2015) Article DOI: 10.1016/j.ejmech.2014.12.049 BindingDB Entry DOI: 10.7270/Q22B90RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50071089 (CHEMBL3409754) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 829 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University Curated by ChEMBL | Assay Description Displacement of [3H]DPDPE from rat cortex delta opioid receptor after 2.5 hrs by liquid scintillation counting analysis | Eur J Med Chem 92: 270-81 (2015) Article DOI: 10.1016/j.ejmech.2014.12.049 BindingDB Entry DOI: 10.7270/Q22B90RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50071065 (CHEMBL3409744) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 967 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University Curated by ChEMBL | Assay Description Displacement of [3H]DPDPE from rat cortex delta opioid receptor after 2.5 hrs by liquid scintillation counting analysis | Eur J Med Chem 92: 270-81 (2015) Article DOI: 10.1016/j.ejmech.2014.12.049 BindingDB Entry DOI: 10.7270/Q22B90RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50071067 (CHEMBL3409757) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.13E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University Curated by ChEMBL | Assay Description Displacement of [3H]U69,593 from guinea pig cortex/cerebella kappa opioid receptor after 2 hrs by liquid scintillation counting analysis | Eur J Med Chem 92: 270-81 (2015) Article DOI: 10.1016/j.ejmech.2014.12.049 BindingDB Entry DOI: 10.7270/Q22B90RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50071084 (CHEMBL3409752) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.22E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University Curated by ChEMBL | Assay Description Displacement of [3H]DPDPE from rat cortex delta opioid receptor after 2.5 hrs by liquid scintillation counting analysis | Eur J Med Chem 92: 270-81 (2015) Article DOI: 10.1016/j.ejmech.2014.12.049 BindingDB Entry DOI: 10.7270/Q22B90RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50071088 (CHEMBL3409753) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.38E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University Curated by ChEMBL | Assay Description Displacement of [3H]U69,593 from guinea pig cortex/cerebella kappa opioid receptor after 2 hrs by liquid scintillation counting analysis | Eur J Med Chem 92: 270-81 (2015) Article DOI: 10.1016/j.ejmech.2014.12.049 BindingDB Entry DOI: 10.7270/Q22B90RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50071066 (CHEMBL3409756) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.69E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University Curated by ChEMBL | Assay Description Displacement of [3H]U69,593 from guinea pig cortex/cerebella kappa opioid receptor after 2 hrs by liquid scintillation counting analysis | Eur J Med Chem 92: 270-81 (2015) Article DOI: 10.1016/j.ejmech.2014.12.049 BindingDB Entry DOI: 10.7270/Q22B90RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50071065 (CHEMBL3409744) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.73E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University Curated by ChEMBL | Assay Description Displacement of [3H]U69,593 from guinea pig cortex/cerebella kappa opioid receptor after 2 hrs by liquid scintillation counting analysis | Eur J Med Chem 92: 270-81 (2015) Article DOI: 10.1016/j.ejmech.2014.12.049 BindingDB Entry DOI: 10.7270/Q22B90RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50071082 (CHEMBL3409750) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.79E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University Curated by ChEMBL | Assay Description Displacement of [3H]DPDPE from rat cortex delta opioid receptor after 2.5 hrs by liquid scintillation counting analysis | Eur J Med Chem 92: 270-81 (2015) Article DOI: 10.1016/j.ejmech.2014.12.049 BindingDB Entry DOI: 10.7270/Q22B90RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50071090 (CHEMBL3409745) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.98E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University Curated by ChEMBL | Assay Description Displacement of [3H]U69,593 from guinea pig cortex/cerebella kappa opioid receptor after 2 hrs by liquid scintillation counting analysis | Eur J Med Chem 92: 270-81 (2015) Article DOI: 10.1016/j.ejmech.2014.12.049 BindingDB Entry DOI: 10.7270/Q22B90RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50071084 (CHEMBL3409752) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.08E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from rat cortex mu opioid receptor after 30 mins by liquid scintillation counting analysis | Eur J Med Chem 92: 270-81 (2015) Article DOI: 10.1016/j.ejmech.2014.12.049 BindingDB Entry DOI: 10.7270/Q22B90RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50071086 (CHEMBL3409741) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.09E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University Curated by ChEMBL | Assay Description Displacement of [3H]U69,593 from guinea pig cortex/cerebella kappa opioid receptor after 2 hrs by liquid scintillation counting analysis | Eur J Med Chem 92: 270-81 (2015) Article DOI: 10.1016/j.ejmech.2014.12.049 BindingDB Entry DOI: 10.7270/Q22B90RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50071084 (CHEMBL3409752) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.25E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University Curated by ChEMBL | Assay Description Displacement of [3H]U69,593 from guinea pig cortex/cerebella kappa opioid receptor after 2 hrs by liquid scintillation counting analysis | Eur J Med Chem 92: 270-81 (2015) Article DOI: 10.1016/j.ejmech.2014.12.049 BindingDB Entry DOI: 10.7270/Q22B90RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50071092 (CHEMBL3409755) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.32E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University Curated by ChEMBL | Assay Description Displacement of [3H]DPDPE from rat cortex delta opioid receptor after 2.5 hrs by liquid scintillation counting analysis | Eur J Med Chem 92: 270-81 (2015) Article DOI: 10.1016/j.ejmech.2014.12.049 BindingDB Entry DOI: 10.7270/Q22B90RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50095155 ((S)-1-[(S)-2-Amino-3-(4-hydroxy-phenyl)-propionyl]...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 2.35E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University Curated by ChEMBL | Assay Description Displacement of [3H]U69,593 from guinea pig cortex/cerebella kappa opioid receptor after 2 hrs by liquid scintillation counting analysis | Eur J Med Chem 92: 270-81 (2015) Article DOI: 10.1016/j.ejmech.2014.12.049 BindingDB Entry DOI: 10.7270/Q22B90RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50071092 (CHEMBL3409755) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.38E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University Curated by ChEMBL | Assay Description Displacement of [3H]U69,593 from guinea pig cortex/cerebella kappa opioid receptor after 2 hrs by liquid scintillation counting analysis | Eur J Med Chem 92: 270-81 (2015) Article DOI: 10.1016/j.ejmech.2014.12.049 BindingDB Entry DOI: 10.7270/Q22B90RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50071075 (CHEMBL3409748) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University Curated by ChEMBL | Assay Description Displacement of [3H]DPDPE from rat cortex delta opioid receptor after 2.5 hrs by liquid scintillation counting analysis | Eur J Med Chem 92: 270-81 (2015) Article DOI: 10.1016/j.ejmech.2014.12.049 BindingDB Entry DOI: 10.7270/Q22B90RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50139013 ((S)-1-[(S)-2-Amino-3-(4-hydroxy-phenyl)-propionyl]...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 2.71E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University Curated by ChEMBL | Assay Description Displacement of [3H]U69,593 from guinea pig cortex/cerebella kappa opioid receptor after 2 hrs by liquid scintillation counting analysis | Eur J Med Chem 92: 270-81 (2015) Article DOI: 10.1016/j.ejmech.2014.12.049 BindingDB Entry DOI: 10.7270/Q22B90RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50071089 (CHEMBL3409754) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.71E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University Curated by ChEMBL | Assay Description Displacement of [3H]U69,593 from guinea pig cortex/cerebella kappa opioid receptor after 2 hrs by liquid scintillation counting analysis | Eur J Med Chem 92: 270-81 (2015) Article DOI: 10.1016/j.ejmech.2014.12.049 BindingDB Entry DOI: 10.7270/Q22B90RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50071076 (CHEMBL3409749) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.71E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University Curated by ChEMBL | Assay Description Displacement of [3H]U69,593 from guinea pig cortex/cerebella kappa opioid receptor after 2 hrs by liquid scintillation counting analysis | Eur J Med Chem 92: 270-81 (2015) Article DOI: 10.1016/j.ejmech.2014.12.049 BindingDB Entry DOI: 10.7270/Q22B90RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50071085 (CHEMBL3409740) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.78E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University Curated by ChEMBL | Assay Description Displacement of [3H]U69,593 from guinea pig cortex/cerebella kappa opioid receptor after 2 hrs by liquid scintillation counting analysis | Eur J Med Chem 92: 270-81 (2015) Article DOI: 10.1016/j.ejmech.2014.12.049 BindingDB Entry DOI: 10.7270/Q22B90RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50071076 (CHEMBL3409749) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 3.29E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University Curated by ChEMBL | Assay Description Displacement of [3H]DPDPE from rat cortex delta opioid receptor after 2.5 hrs by liquid scintillation counting analysis | Eur J Med Chem 92: 270-81 (2015) Article DOI: 10.1016/j.ejmech.2014.12.049 BindingDB Entry DOI: 10.7270/Q22B90RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50071087 (CHEMBL3409742) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University Curated by ChEMBL | Assay Description Displacement of [3H]U69,593 from guinea pig cortex/cerebella kappa opioid receptor after 2 hrs by liquid scintillation counting analysis | Eur J Med Chem 92: 270-81 (2015) Article DOI: 10.1016/j.ejmech.2014.12.049 BindingDB Entry DOI: 10.7270/Q22B90RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50071090 (CHEMBL3409745) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.84E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University Curated by ChEMBL | Assay Description Displacement of [3H]DPDPE from rat cortex delta opioid receptor after 2.5 hrs by liquid scintillation counting analysis | Eur J Med Chem 92: 270-81 (2015) Article DOI: 10.1016/j.ejmech.2014.12.049 BindingDB Entry DOI: 10.7270/Q22B90RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50071064 (CHEMBL3409743) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.05E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University Curated by ChEMBL | Assay Description Displacement of [3H]U69,593 from guinea pig cortex/cerebella kappa opioid receptor after 2 hrs by liquid scintillation counting analysis | Eur J Med Chem 92: 270-81 (2015) Article DOI: 10.1016/j.ejmech.2014.12.049 BindingDB Entry DOI: 10.7270/Q22B90RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50114010 (1-[2-Amino-3-(4-hydroxy-phenyl)-propionyl]-pyrroli...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.11E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University Curated by ChEMBL | Assay Description Displacement of [3H]DPDPE from rat cortex delta opioid receptor after 2.5 hrs by liquid scintillation counting analysis | Eur J Med Chem 92: 270-81 (2015) Article DOI: 10.1016/j.ejmech.2014.12.049 BindingDB Entry DOI: 10.7270/Q22B90RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50071091 (CHEMBL3409746) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.13E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University Curated by ChEMBL | Assay Description Displacement of [3H]U69,593 from guinea pig cortex/cerebella kappa opioid receptor after 2 hrs by liquid scintillation counting analysis | Eur J Med Chem 92: 270-81 (2015) Article DOI: 10.1016/j.ejmech.2014.12.049 BindingDB Entry DOI: 10.7270/Q22B90RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50071083 (CHEMBL3409751) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.82E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University Curated by ChEMBL | Assay Description Displacement of [3H]U69,593 from guinea pig cortex/cerebella kappa opioid receptor after 2 hrs by liquid scintillation counting analysis | Eur J Med Chem 92: 270-81 (2015) Article DOI: 10.1016/j.ejmech.2014.12.049 BindingDB Entry DOI: 10.7270/Q22B90RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50071082 (CHEMBL3409750) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.78E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University Curated by ChEMBL | Assay Description Displacement of [3H]U69,593 from guinea pig cortex/cerebella kappa opioid receptor after 2 hrs by liquid scintillation counting analysis | Eur J Med Chem 92: 270-81 (2015) Article DOI: 10.1016/j.ejmech.2014.12.049 BindingDB Entry DOI: 10.7270/Q22B90RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50139013 ((S)-1-[(S)-2-Amino-3-(4-hydroxy-phenyl)-propionyl]...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 6.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University Curated by ChEMBL | Assay Description Displacement of [3H]DPDPE from rat cortex delta opioid receptor after 2.5 hrs by liquid scintillation counting analysis | Eur J Med Chem 92: 270-81 (2015) Article DOI: 10.1016/j.ejmech.2014.12.049 BindingDB Entry DOI: 10.7270/Q22B90RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50071074 (CHEMBL3409747) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8.87E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University Curated by ChEMBL | Assay Description Displacement of [3H]U69,593 from guinea pig cortex/cerebella kappa opioid receptor after 2 hrs by liquid scintillation counting analysis | Eur J Med Chem 92: 270-81 (2015) Article DOI: 10.1016/j.ejmech.2014.12.049 BindingDB Entry DOI: 10.7270/Q22B90RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50114010 (1-[2-Amino-3-(4-hydroxy-phenyl)-propionyl]-pyrroli...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University Curated by ChEMBL | Assay Description Displacement of [3H]U69,593 from guinea pig cortex/cerebella kappa opioid receptor after 2 hrs by liquid scintillation counting analysis | Eur J Med Chem 92: 270-81 (2015) Article DOI: 10.1016/j.ejmech.2014.12.049 BindingDB Entry DOI: 10.7270/Q22B90RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50071075 (CHEMBL3409748) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University Curated by ChEMBL | Assay Description Displacement of [3H]U69,593 from guinea pig cortex/cerebella kappa opioid receptor after 2 hrs by liquid scintillation counting analysis | Eur J Med Chem 92: 270-81 (2015) Article DOI: 10.1016/j.ejmech.2014.12.049 BindingDB Entry DOI: 10.7270/Q22B90RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50001683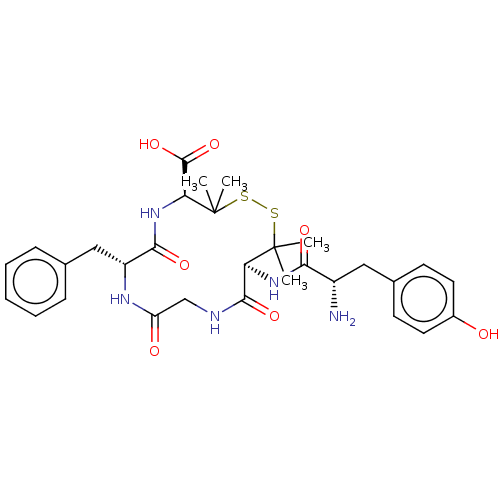 (13-[2-Amino-3-(4-hydroxy-phenyl)-propionylamino]-7...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a |
Zhejiang University Curated by ChEMBL | Assay Description Agonist activity at delta opioid receptor (unknown origin) expressed in CHO cells assessed as change in cell morphology measured over 30 mins by labe... | Eur J Med Chem 92: 270-81 (2015) Article DOI: 10.1016/j.ejmech.2014.12.049 BindingDB Entry DOI: 10.7270/Q22B90RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50071072 (CHEMBL3409762) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 23 | n/a | n/a | n/a | n/a |
Zhejiang University Curated by ChEMBL | Assay Description Agonist activity at delta opioid receptor (unknown origin) expressed in CHO cells assessed as change in cell morphology measured over 30 mins by labe... | Eur J Med Chem 92: 270-81 (2015) Article DOI: 10.1016/j.ejmech.2014.12.049 BindingDB Entry DOI: 10.7270/Q22B90RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||