

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM167911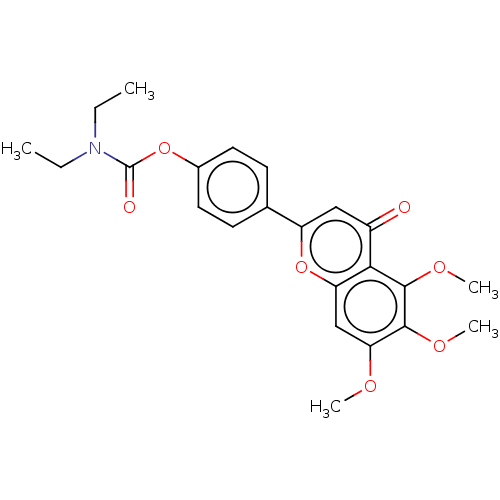 (N,N-diethylcarbamic acid-4-(5,6,7-trimethoxy-4-oxo...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 340 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University Curated by ChEMBL | Assay Description Inhibition of rat cortex AChE using acetylthiocholine iodide as substrate after 15 mins by Ellman method | Bioorg Med Chem 23: 668-80 (2015) Article DOI: 10.1016/j.bmc.2015.01.005 BindingDB Entry DOI: 10.7270/Q24T6M2R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM167912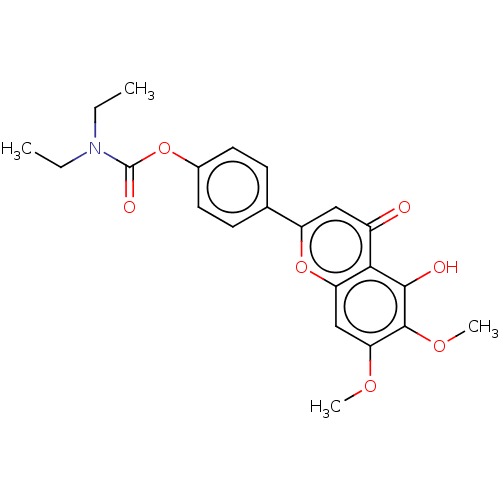 (N,N-diethylcarbamic acid-4-(5-hydroxy-6,7-dimethox...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 570 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University Curated by ChEMBL | Assay Description Inhibition of rat cortex AChE using acetylthiocholine iodide as substrate after 15 mins by Ellman method | Bioorg Med Chem 23: 668-80 (2015) Article DOI: 10.1016/j.bmc.2015.01.005 BindingDB Entry DOI: 10.7270/Q24T6M2R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50064040 (CHEMBL3401006) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.04E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University Curated by ChEMBL | Assay Description Inhibition of rat cortex AChE using acetylthiocholine iodide as substrate after 15 mins by Ellman method | Bioorg Med Chem 23: 668-80 (2015) Article DOI: 10.1016/j.bmc.2015.01.005 BindingDB Entry DOI: 10.7270/Q24T6M2R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50064054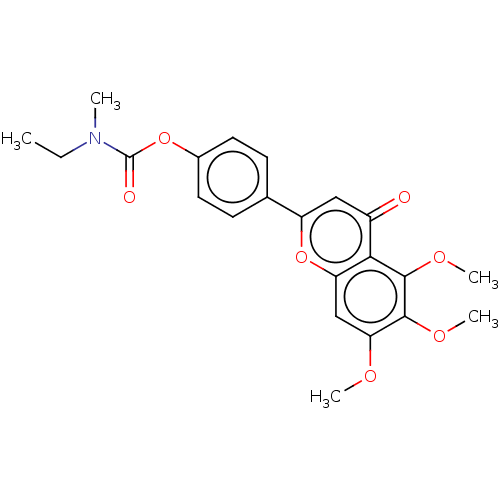 (CHEMBL3401001) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.54E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University Curated by ChEMBL | Assay Description Inhibition of rat cortex AChE using acetylthiocholine iodide as substrate after 15 mins by Ellman method | Bioorg Med Chem 23: 668-80 (2015) Article DOI: 10.1016/j.bmc.2015.01.005 BindingDB Entry DOI: 10.7270/Q24T6M2R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50064038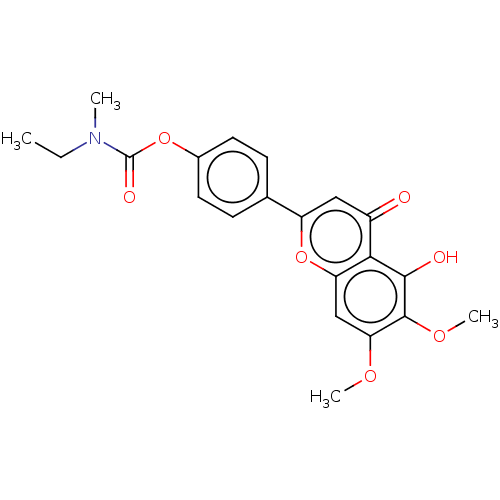 (CHEMBL3401008) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University Curated by ChEMBL | Assay Description Inhibition of rat cortex AChE using acetylthiocholine iodide as substrate after 15 mins by Ellman method | Bioorg Med Chem 23: 668-80 (2015) Article DOI: 10.1016/j.bmc.2015.01.005 BindingDB Entry DOI: 10.7270/Q24T6M2R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50064028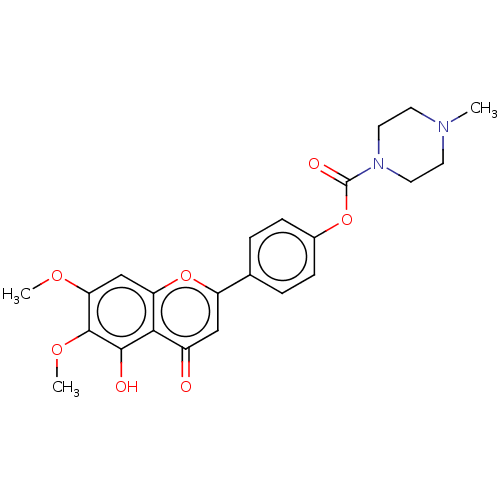 (CHEMBL3401013) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University Curated by ChEMBL | Assay Description Inhibition of rat cortex AChE using acetylthiocholine iodide as substrate after 15 mins by Ellman method | Bioorg Med Chem 23: 668-80 (2015) Article DOI: 10.1016/j.bmc.2015.01.005 BindingDB Entry DOI: 10.7270/Q24T6M2R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM10620 ((S)-3-(1-(dimethylamino)ethyl)phenyl ethyl(methyl)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University Curated by ChEMBL | Assay Description Inhibition of rat cortex AChE using acetylthiocholine iodide as substrate after 15 mins by Ellman method | Bioorg Med Chem 23: 668-80 (2015) Article DOI: 10.1016/j.bmc.2015.01.005 BindingDB Entry DOI: 10.7270/Q24T6M2R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50064002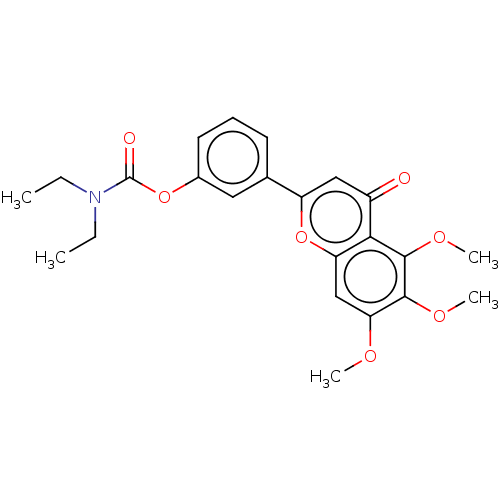 (CHEMBL3401017) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6.11E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University Curated by ChEMBL | Assay Description Inhibition of rat cortex AChE using acetylthiocholine iodide as substrate after 15 mins by Ellman method | Bioorg Med Chem 23: 668-80 (2015) Article DOI: 10.1016/j.bmc.2015.01.005 BindingDB Entry DOI: 10.7270/Q24T6M2R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50064001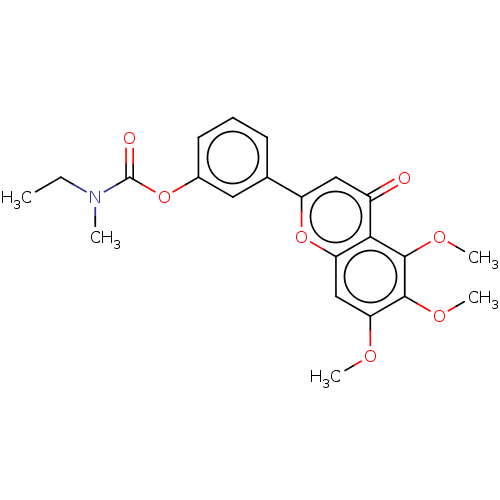 (CHEMBL3401016) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7.37E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University Curated by ChEMBL | Assay Description Inhibition of rat cortex AChE using acetylthiocholine iodide as substrate after 15 mins by Ellman method | Bioorg Med Chem 23: 668-80 (2015) Article DOI: 10.1016/j.bmc.2015.01.005 BindingDB Entry DOI: 10.7270/Q24T6M2R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50064056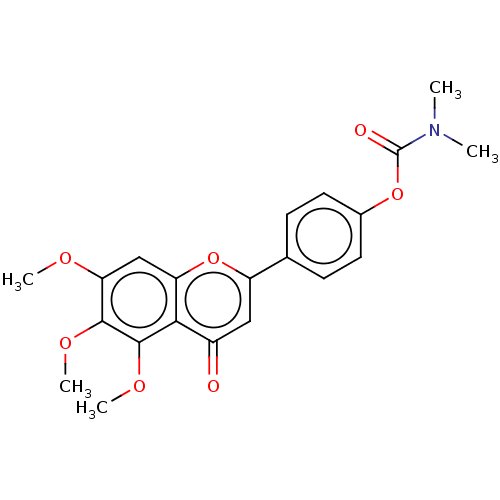 (CHEMBL3401000) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 8.24E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University Curated by ChEMBL | Assay Description Inhibition of rat cortex AChE using acetylthiocholine iodide as substrate after 15 mins by Ellman method | Bioorg Med Chem 23: 668-80 (2015) Article DOI: 10.1016/j.bmc.2015.01.005 BindingDB Entry DOI: 10.7270/Q24T6M2R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50064023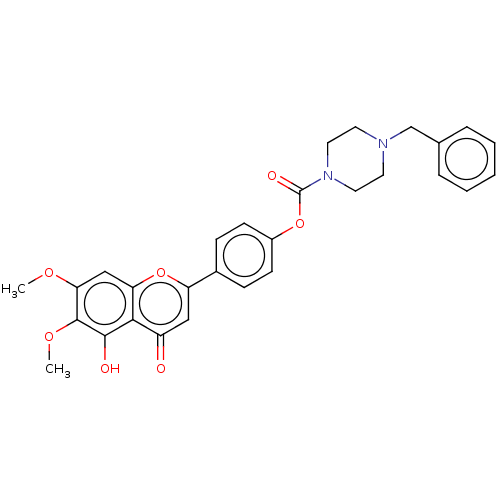 (CHEMBL3401014) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.25E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University Curated by ChEMBL | Assay Description Inhibition of rat cortex AChE using acetylthiocholine iodide as substrate after 15 mins by Ellman method | Bioorg Med Chem 23: 668-80 (2015) Article DOI: 10.1016/j.bmc.2015.01.005 BindingDB Entry DOI: 10.7270/Q24T6M2R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50064059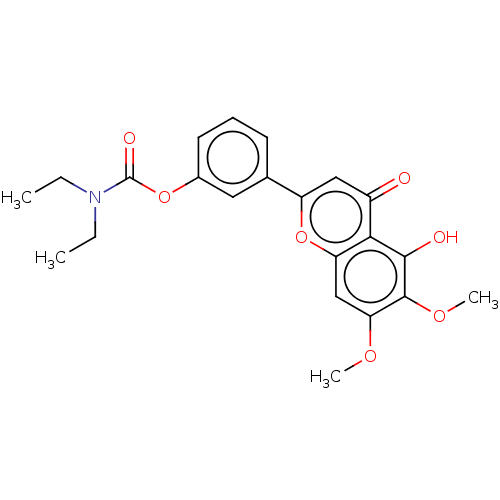 (CHEMBL3401025) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.03E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University Curated by ChEMBL | Assay Description Inhibition of rat cortex AChE using acetylthiocholine iodide as substrate after 15 mins by Ellman method | Bioorg Med Chem 23: 668-80 (2015) Article DOI: 10.1016/j.bmc.2015.01.005 BindingDB Entry DOI: 10.7270/Q24T6M2R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50064041 (CHEMBL3401005) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.14E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University Curated by ChEMBL | Assay Description Inhibition of rat cortex AChE using acetylthiocholine iodide as substrate after 15 mins by Ellman method | Bioorg Med Chem 23: 668-80 (2015) Article DOI: 10.1016/j.bmc.2015.01.005 BindingDB Entry DOI: 10.7270/Q24T6M2R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50064053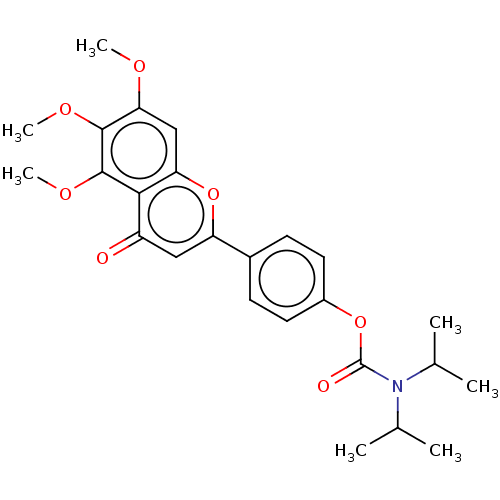 (CHEMBL3401003) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.37E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University Curated by ChEMBL | Assay Description Inhibition of rat cortex AChE using acetylthiocholine iodide as substrate after 15 mins by Ellman method | Bioorg Med Chem 23: 668-80 (2015) Article DOI: 10.1016/j.bmc.2015.01.005 BindingDB Entry DOI: 10.7270/Q24T6M2R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50064058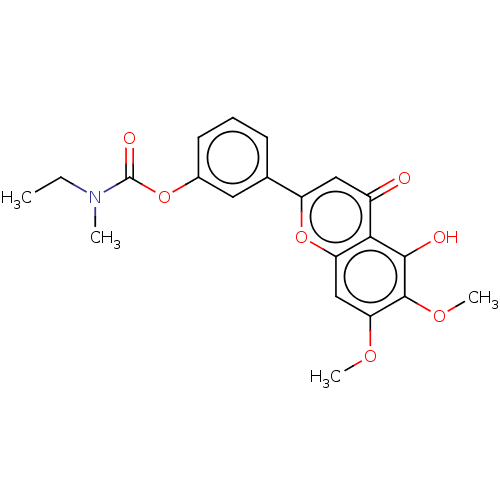 (CHEMBL3401024) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.48E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University Curated by ChEMBL | Assay Description Inhibition of rat cortex AChE using acetylthiocholine iodide as substrate after 15 mins by Ellman method | Bioorg Med Chem 23: 668-80 (2015) Article DOI: 10.1016/j.bmc.2015.01.005 BindingDB Entry DOI: 10.7270/Q24T6M2R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50064039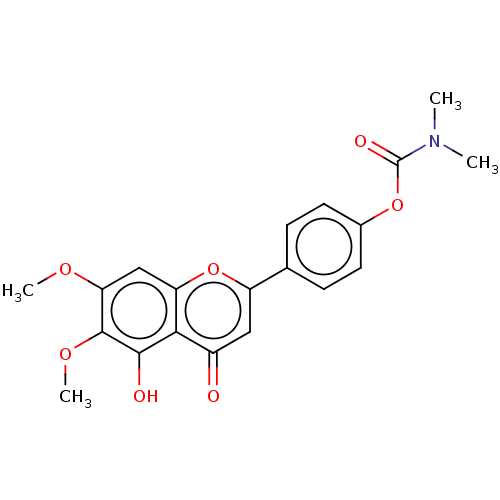 (CHEMBL3401007) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.73E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University Curated by ChEMBL | Assay Description Inhibition of rat cortex AChE using acetylthiocholine iodide as substrate after 15 mins by Ellman method | Bioorg Med Chem 23: 668-80 (2015) Article DOI: 10.1016/j.bmc.2015.01.005 BindingDB Entry DOI: 10.7270/Q24T6M2R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50064031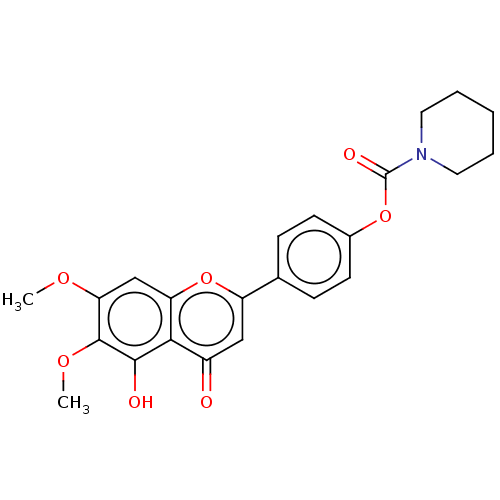 (CHEMBL3401012) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.78E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University Curated by ChEMBL | Assay Description Inhibition of rat cortex AChE using acetylthiocholine iodide as substrate after 15 mins by Ellman method | Bioorg Med Chem 23: 668-80 (2015) Article DOI: 10.1016/j.bmc.2015.01.005 BindingDB Entry DOI: 10.7270/Q24T6M2R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50064049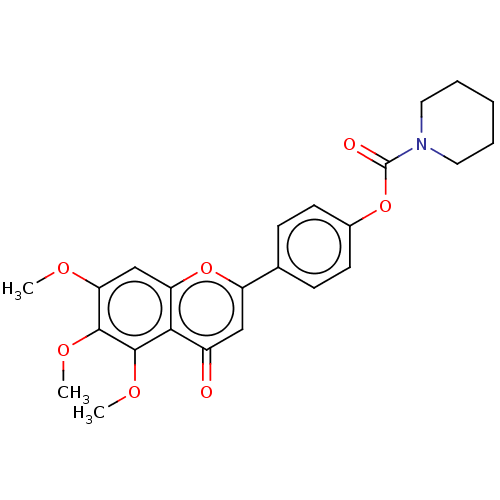 (CHEMBL3401004) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.12E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University Curated by ChEMBL | Assay Description Inhibition of rat cortex AChE using acetylthiocholine iodide as substrate after 15 mins by Ellman method | Bioorg Med Chem 23: 668-80 (2015) Article DOI: 10.1016/j.bmc.2015.01.005 BindingDB Entry DOI: 10.7270/Q24T6M2R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50064134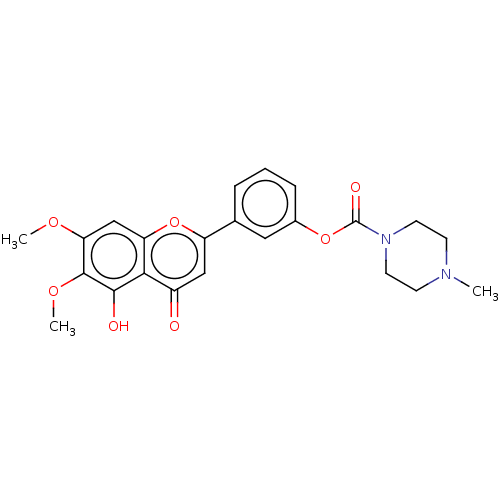 (CHEMBL3401029) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.33E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University Curated by ChEMBL | Assay Description Inhibition of rat cortex AChE using acetylthiocholine iodide as substrate after 15 mins by Ellman method | Bioorg Med Chem 23: 668-80 (2015) Article DOI: 10.1016/j.bmc.2015.01.005 BindingDB Entry DOI: 10.7270/Q24T6M2R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50064057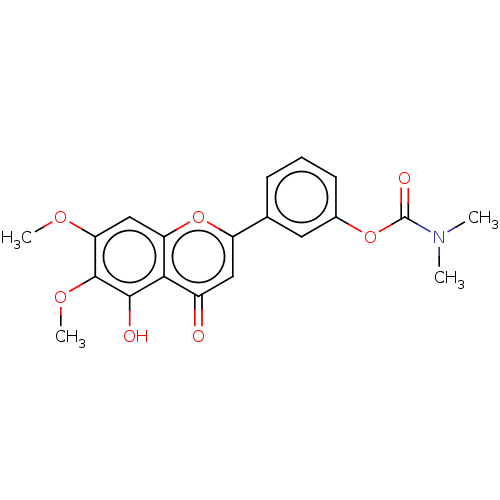 (CHEMBL3401023) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.57E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University Curated by ChEMBL | Assay Description Inhibition of rat cortex AChE using acetylthiocholine iodide as substrate after 15 mins by Ellman method | Bioorg Med Chem 23: 668-80 (2015) Article DOI: 10.1016/j.bmc.2015.01.005 BindingDB Entry DOI: 10.7270/Q24T6M2R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50064006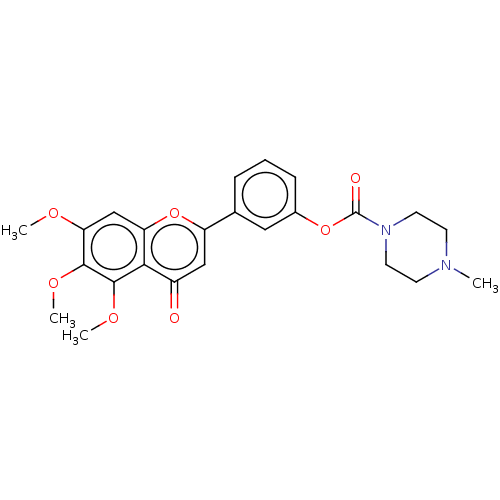 (CHEMBL3401021) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.12E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University Curated by ChEMBL | Assay Description Inhibition of rat cortex AChE using acetylthiocholine iodide as substrate after 15 mins by Ellman method | Bioorg Med Chem 23: 668-80 (2015) Article DOI: 10.1016/j.bmc.2015.01.005 BindingDB Entry DOI: 10.7270/Q24T6M2R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50064022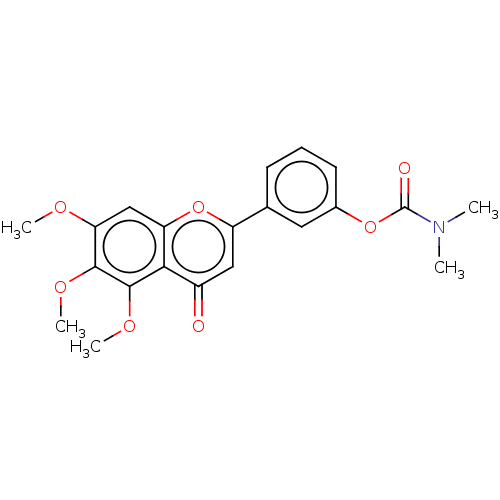 (CHEMBL3401015) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.73E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University Curated by ChEMBL | Assay Description Inhibition of rat cortex AChE using acetylthiocholine iodide as substrate after 15 mins by Ellman method | Bioorg Med Chem 23: 668-80 (2015) Article DOI: 10.1016/j.bmc.2015.01.005 BindingDB Entry DOI: 10.7270/Q24T6M2R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50064005 (CHEMBL3401020) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.57E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University Curated by ChEMBL | Assay Description Inhibition of rat cortex AChE using acetylthiocholine iodide as substrate after 15 mins by Ellman method | Bioorg Med Chem 23: 668-80 (2015) Article DOI: 10.1016/j.bmc.2015.01.005 BindingDB Entry DOI: 10.7270/Q24T6M2R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50064135 (CHEMBL3401030) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.17E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University Curated by ChEMBL | Assay Description Inhibition of rat cortex AChE using acetylthiocholine iodide as substrate after 15 mins by Ellman method | Bioorg Med Chem 23: 668-80 (2015) Article DOI: 10.1016/j.bmc.2015.01.005 BindingDB Entry DOI: 10.7270/Q24T6M2R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50064052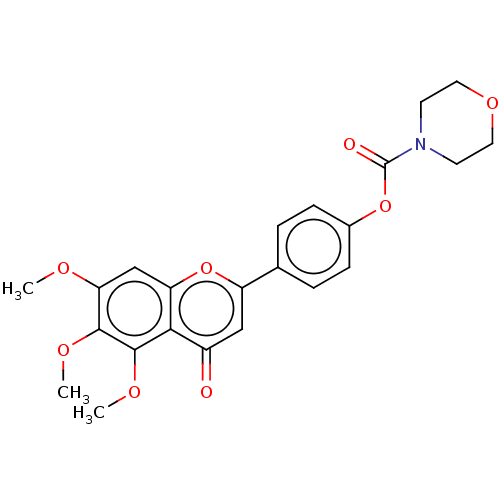 (CHEMBL3400158) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.83E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University Curated by ChEMBL | Assay Description Inhibition of rat cortex AChE using acetylthiocholine iodide as substrate after 15 mins by Ellman method | Bioorg Med Chem 23: 668-80 (2015) Article DOI: 10.1016/j.bmc.2015.01.005 BindingDB Entry DOI: 10.7270/Q24T6M2R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50064021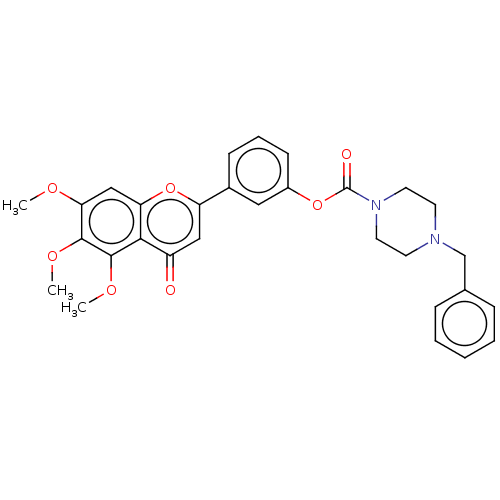 (CHEMBL3401022) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.71E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University Curated by ChEMBL | Assay Description Inhibition of rat cortex AChE using acetylthiocholine iodide as substrate after 15 mins by Ellman method | Bioorg Med Chem 23: 668-80 (2015) Article DOI: 10.1016/j.bmc.2015.01.005 BindingDB Entry DOI: 10.7270/Q24T6M2R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50064003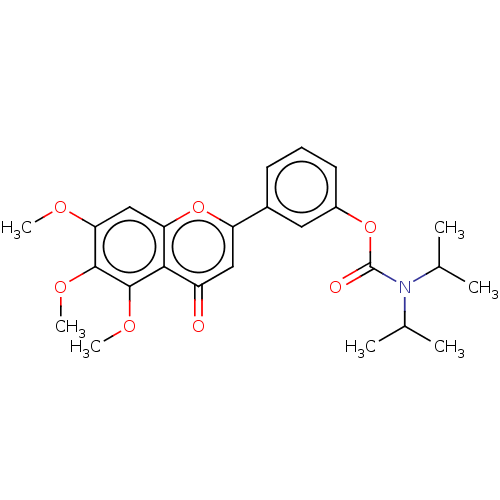 (CHEMBL3401018) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 8.75E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University Curated by ChEMBL | Assay Description Inhibition of rat cortex AChE using acetylthiocholine iodide as substrate after 15 mins by Ellman method | Bioorg Med Chem 23: 668-80 (2015) Article DOI: 10.1016/j.bmc.2015.01.005 BindingDB Entry DOI: 10.7270/Q24T6M2R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50064087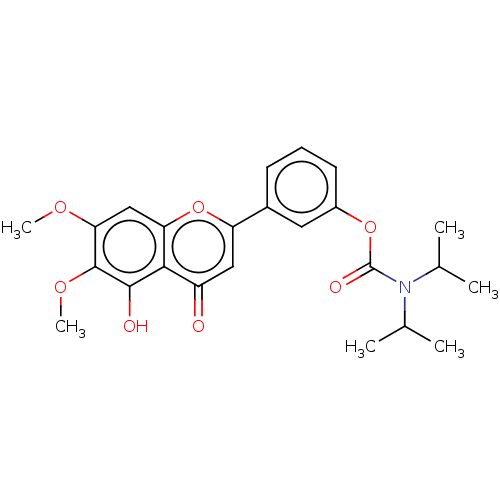 (CHEMBL3401026) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.65E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University Curated by ChEMBL | Assay Description Inhibition of rat cortex AChE using acetylthiocholine iodide as substrate after 15 mins by Ellman method | Bioorg Med Chem 23: 668-80 (2015) Article DOI: 10.1016/j.bmc.2015.01.005 BindingDB Entry DOI: 10.7270/Q24T6M2R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50064136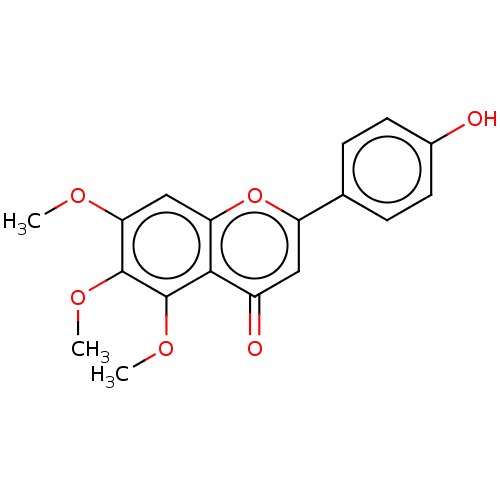 (CHEBI:79510 | CHEMBL74490 | NSC-53906) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University Curated by ChEMBL | Assay Description Inhibition of rat cortex AChE using acetylthiocholine iodide as substrate after 15 mins by Ellman method | Bioorg Med Chem 23: 668-80 (2015) Article DOI: 10.1016/j.bmc.2015.01.005 BindingDB Entry DOI: 10.7270/Q24T6M2R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50064102 (CHEMBL3401027) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University Curated by ChEMBL | Assay Description Inhibition of rat cortex AChE using acetylthiocholine iodide as substrate after 15 mins by Ellman method | Bioorg Med Chem 23: 668-80 (2015) Article DOI: 10.1016/j.bmc.2015.01.005 BindingDB Entry DOI: 10.7270/Q24T6M2R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50064004 (CHEMBL3401019) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University Curated by ChEMBL | Assay Description Inhibition of rat cortex AChE using acetylthiocholine iodide as substrate after 15 mins by Ellman method | Bioorg Med Chem 23: 668-80 (2015) Article DOI: 10.1016/j.bmc.2015.01.005 BindingDB Entry DOI: 10.7270/Q24T6M2R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50064133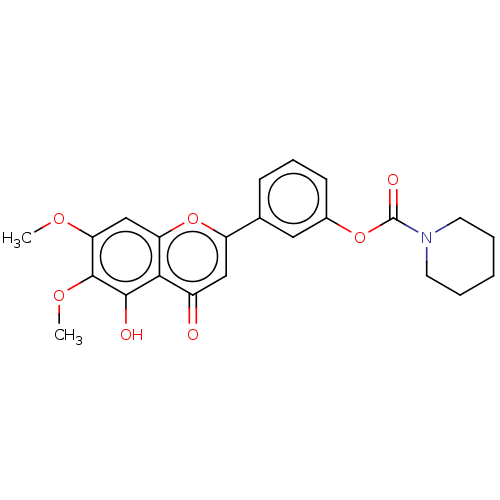 (CHEMBL3401028) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University Curated by ChEMBL | Assay Description Inhibition of rat cortex AChE using acetylthiocholine iodide as substrate after 15 mins by Ellman method | Bioorg Med Chem 23: 668-80 (2015) Article DOI: 10.1016/j.bmc.2015.01.005 BindingDB Entry DOI: 10.7270/Q24T6M2R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50064033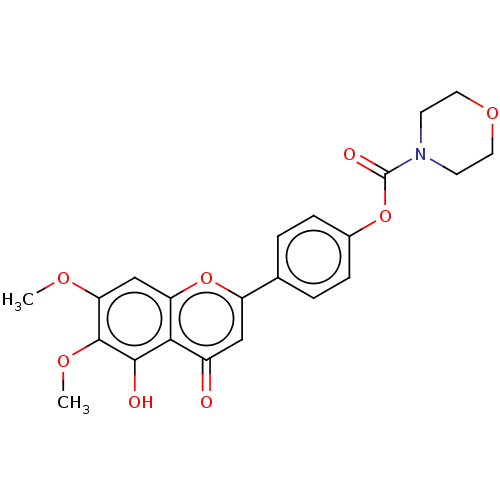 (CHEMBL3401011) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University Curated by ChEMBL | Assay Description Inhibition of rat cortex AChE using acetylthiocholine iodide as substrate after 15 mins by Ellman method | Bioorg Med Chem 23: 668-80 (2015) Article DOI: 10.1016/j.bmc.2015.01.005 BindingDB Entry DOI: 10.7270/Q24T6M2R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50064037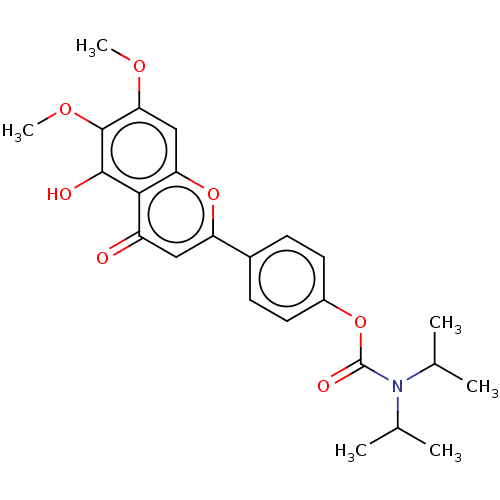 (CHEMBL3401010) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University Curated by ChEMBL | Assay Description Inhibition of rat cortex AChE using acetylthiocholine iodide as substrate after 15 mins by Ellman method | Bioorg Med Chem 23: 668-80 (2015) Article DOI: 10.1016/j.bmc.2015.01.005 BindingDB Entry DOI: 10.7270/Q24T6M2R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50064137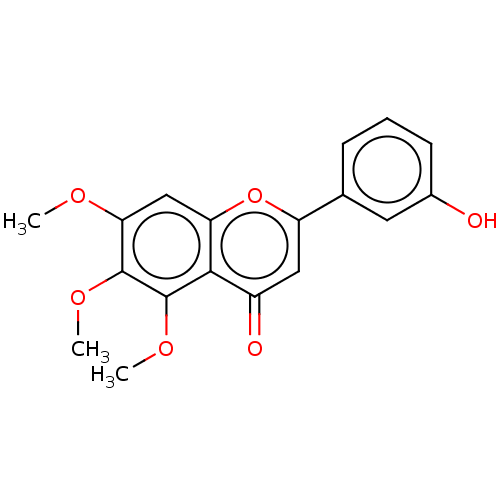 (CHEMBL3401031) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University Curated by ChEMBL | Assay Description Inhibition of rat cortex AChE using acetylthiocholine iodide as substrate after 15 mins by Ellman method | Bioorg Med Chem 23: 668-80 (2015) Article DOI: 10.1016/j.bmc.2015.01.005 BindingDB Entry DOI: 10.7270/Q24T6M2R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50049394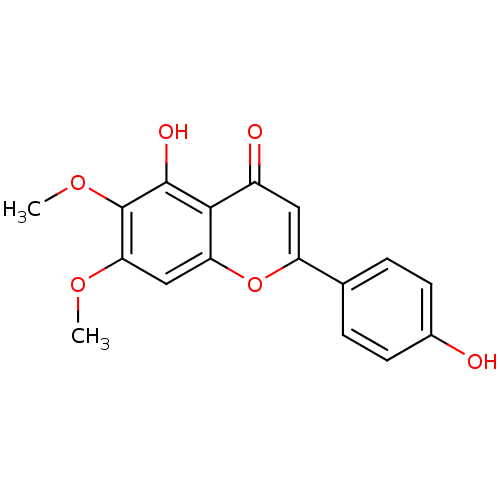 (5,4'-Dihydroxy-6,7-dimethoxyflavone | 5-Hydroxy-2-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University Curated by ChEMBL | Assay Description Inhibition of rat cortex AChE using acetylthiocholine iodide as substrate after 15 mins by Ellman method | Bioorg Med Chem 23: 668-80 (2015) Article DOI: 10.1016/j.bmc.2015.01.005 BindingDB Entry DOI: 10.7270/Q24T6M2R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50064138 (CHEMBL3401032) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University Curated by ChEMBL | Assay Description Inhibition of rat cortex AChE using acetylthiocholine iodide as substrate after 15 mins by Ellman method | Bioorg Med Chem 23: 668-80 (2015) Article DOI: 10.1016/j.bmc.2015.01.005 BindingDB Entry DOI: 10.7270/Q24T6M2R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||