

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM50444549 (CHEMBL3099695) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University and Helmholtz Institute for Pharmaceutical Research Saarland (HIPS) Curated by ChEMBL | Assay Description Inhibition of human CYP11B2 expressed in V79 MZh cells using [14C]-deoxycorticosterone substrate incubated for 6 hrs by HPTLC method | J Med Chem 58: 2530-7 (2015) Article DOI: 10.1021/acs.jmedchem.5b00079 BindingDB Entry DOI: 10.7270/Q20003S5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM8611 (4-{5H,6H,7H,8H-imidazo[1,5-a]pyridin-5-yl}benzonit...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University and Helmholtz Institute for Pharmaceutical Research Saarland (HIPS) Curated by ChEMBL | Assay Description Inhibition of human CYP11B2 expressed in V79 MZh cells using [14C]-deoxycorticosterone substrate incubated for 6 hrs by HPTLC method | J Med Chem 58: 2530-7 (2015) Article DOI: 10.1021/acs.jmedchem.5b00079 BindingDB Entry DOI: 10.7270/Q20003S5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM50078617 (CHEMBL3415172) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University and Helmholtz Institute for Pharmaceutical Research Saarland (HIPS) Curated by ChEMBL | Assay Description Inhibition of human CYP11B2 expressed in V79 MZh cells using [14C]-deoxycorticosterone substrate incubated for 6 hrs by HPTLC method | J Med Chem 58: 2530-7 (2015) Article DOI: 10.1021/acs.jmedchem.5b00079 BindingDB Entry DOI: 10.7270/Q20003S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B1, mitochondrial (Homo sapiens (Human)) | BDBM50444549 (CHEMBL3099695) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University and Helmholtz Institute for Pharmaceutical Research Saarland (HIPS) Curated by ChEMBL | Assay Description Inhibition of human CYP11B1 expressed in V79 MZh cells using [14C]-deoxycorticosterone substrate incubated for 6 hrs by HPTLC method | J Med Chem 58: 2530-7 (2015) Article DOI: 10.1021/acs.jmedchem.5b00079 BindingDB Entry DOI: 10.7270/Q20003S5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM50044143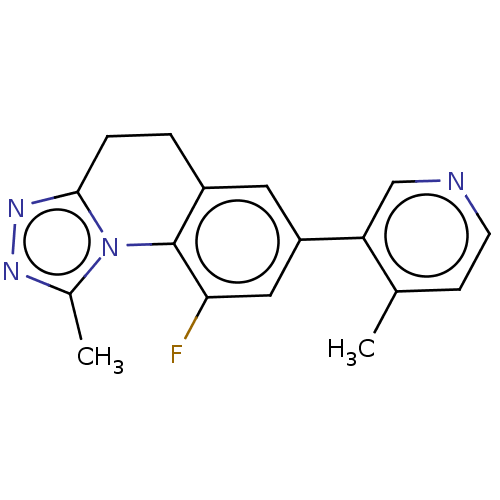 (CHEMBL3313969) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University and Helmholtz Institute for Pharmaceutical Research Saarland (HIPS) Curated by ChEMBL | Assay Description Inhibition of human CYP11B2 expressed in V79 MZh cells using [14C]-deoxycorticosterone substrate incubated for 6 hrs by HPTLC method | J Med Chem 58: 2530-7 (2015) Article DOI: 10.1021/acs.jmedchem.5b00079 BindingDB Entry DOI: 10.7270/Q20003S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B1, mitochondrial (Homo sapiens (Human)) | BDBM8611 (4-{5H,6H,7H,8H-imidazo[1,5-a]pyridin-5-yl}benzonit...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University and Helmholtz Institute for Pharmaceutical Research Saarland (HIPS) Curated by ChEMBL | Assay Description Inhibition of human CYP11B1 expressed in V79 MZh cells using [14C]-deoxycorticosterone substrate incubated for 6 hrs by HPTLC method | J Med Chem 58: 2530-7 (2015) Article DOI: 10.1021/acs.jmedchem.5b00079 BindingDB Entry DOI: 10.7270/Q20003S5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM50078616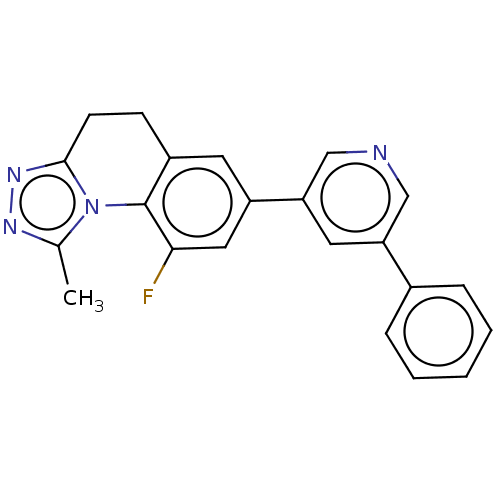 (CHEMBL3415171) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University and Helmholtz Institute for Pharmaceutical Research Saarland (HIPS) Curated by ChEMBL | Assay Description Inhibition of human CYP11B2 expressed in V79 MZh cells using [14C]-deoxycorticosterone substrate incubated for 6 hrs by HPTLC method | J Med Chem 58: 2530-7 (2015) Article DOI: 10.1021/acs.jmedchem.5b00079 BindingDB Entry DOI: 10.7270/Q20003S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM50078618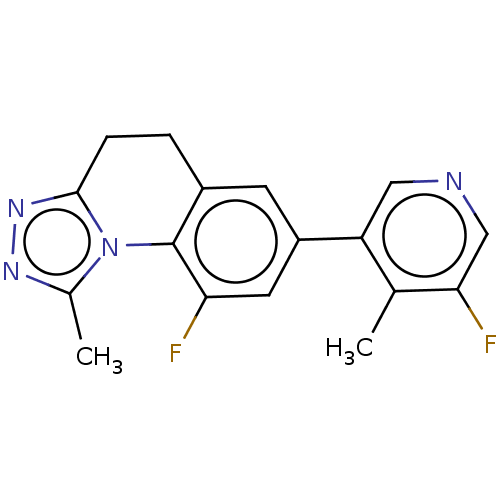 (CHEMBL3415173 | US9278093, 2) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University and Helmholtz Institute for Pharmaceutical Research Saarland (HIPS) Curated by ChEMBL | Assay Description Inhibition of human CYP11B2 expressed in V79 MZh cells using [14C]-deoxycorticosterone substrate incubated for 6 hrs by HPTLC method | J Med Chem 58: 2530-7 (2015) Article DOI: 10.1021/acs.jmedchem.5b00079 BindingDB Entry DOI: 10.7270/Q20003S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM50078611 (CHEMBL3415166) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University and Helmholtz Institute for Pharmaceutical Research Saarland (HIPS) Curated by ChEMBL | Assay Description Inhibition of human CYP11B2 expressed in V79 MZh cells using [14C]-deoxycorticosterone substrate incubated for 6 hrs by HPTLC method | J Med Chem 58: 2530-7 (2015) Article DOI: 10.1021/acs.jmedchem.5b00079 BindingDB Entry DOI: 10.7270/Q20003S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM50078612 (CHEMBL3415167) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University and Helmholtz Institute for Pharmaceutical Research Saarland (HIPS) Curated by ChEMBL | Assay Description Inhibition of human CYP11B2 expressed in V79 MZh cells using [14C]-deoxycorticosterone substrate incubated for 6 hrs by HPTLC method | J Med Chem 58: 2530-7 (2015) Article DOI: 10.1021/acs.jmedchem.5b00079 BindingDB Entry DOI: 10.7270/Q20003S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM50078620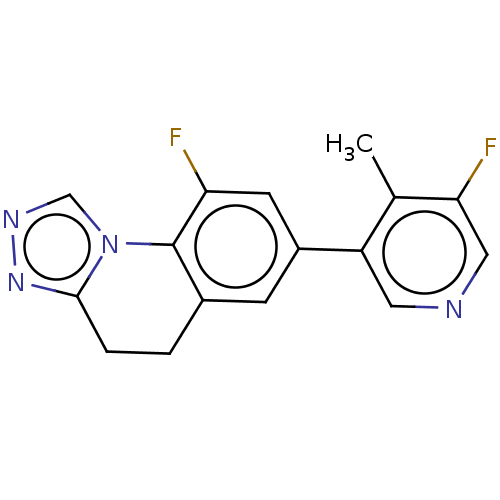 (CHEMBL3415175) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University and Helmholtz Institute for Pharmaceutical Research Saarland (HIPS) Curated by ChEMBL | Assay Description Inhibition of human CYP11B2 expressed in V79 MZh cells using [14C]-deoxycorticosterone substrate incubated for 6 hrs by HPTLC method | J Med Chem 58: 2530-7 (2015) Article DOI: 10.1021/acs.jmedchem.5b00079 BindingDB Entry DOI: 10.7270/Q20003S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM50078614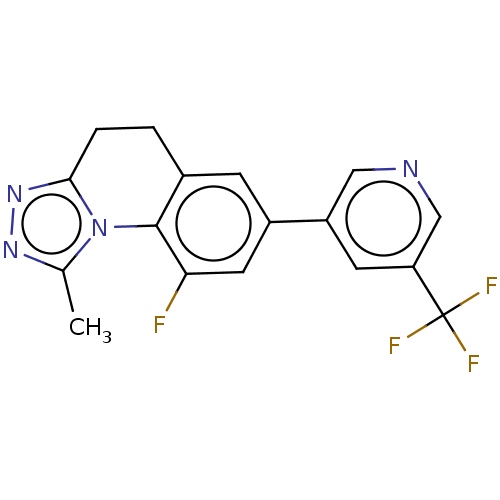 (CHEMBL3415169) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 57 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University and Helmholtz Institute for Pharmaceutical Research Saarland (HIPS) Curated by ChEMBL | Assay Description Inhibition of human CYP11B2 expressed in V79 MZh cells using [14C]-deoxycorticosterone substrate incubated for 6 hrs by HPTLC method | J Med Chem 58: 2530-7 (2015) Article DOI: 10.1021/acs.jmedchem.5b00079 BindingDB Entry DOI: 10.7270/Q20003S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM50078629 (CHEMBL3415155) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 63 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University and Helmholtz Institute for Pharmaceutical Research Saarland (HIPS) Curated by ChEMBL | Assay Description Inhibition of human CYP11B2 expressed in V79 MZh cells using [14C]-deoxycorticosterone substrate incubated for 6 hrs by HPTLC method | J Med Chem 58: 2530-7 (2015) Article DOI: 10.1021/acs.jmedchem.5b00079 BindingDB Entry DOI: 10.7270/Q20003S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM50078613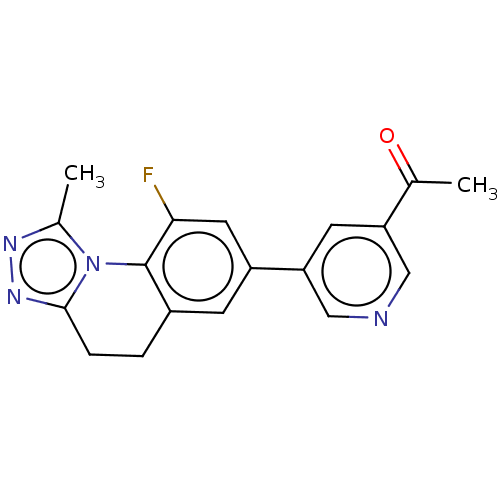 (CHEMBL3415168) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 88 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University and Helmholtz Institute for Pharmaceutical Research Saarland (HIPS) Curated by ChEMBL | Assay Description Inhibition of human CYP11B2 expressed in V79 MZh cells using [14C]-deoxycorticosterone substrate incubated for 6 hrs by HPTLC method | J Med Chem 58: 2530-7 (2015) Article DOI: 10.1021/acs.jmedchem.5b00079 BindingDB Entry DOI: 10.7270/Q20003S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM50428331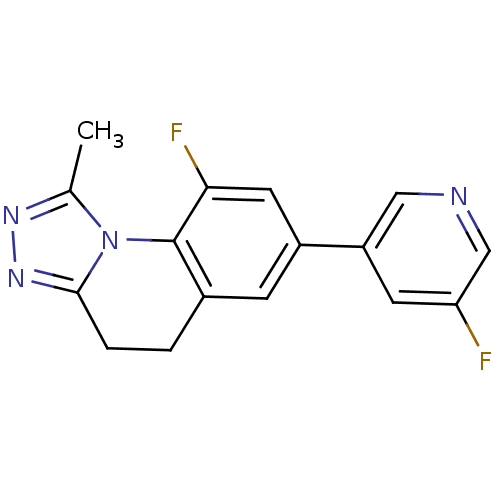 (CHEMBL2331699) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University and Helmholtz Institute for Pharmaceutical Research Saarland (HIPS) Curated by ChEMBL | Assay Description Inhibition of human CYP11B2 expressed in V79 MZh cells using [14C]-deoxycorticosterone substrate incubated for 6 hrs by HPTLC method | J Med Chem 58: 2530-7 (2015) Article DOI: 10.1021/acs.jmedchem.5b00079 BindingDB Entry DOI: 10.7270/Q20003S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B1, mitochondrial (Homo sapiens (Human)) | BDBM50078617 (CHEMBL3415172) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University and Helmholtz Institute for Pharmaceutical Research Saarland (HIPS) Curated by ChEMBL | Assay Description Inhibition of human CYP11B1 expressed in V79 MZh cells using [14C]-deoxycorticosterone substrate incubated for 6 hrs by HPTLC method | J Med Chem 58: 2530-7 (2015) Article DOI: 10.1021/acs.jmedchem.5b00079 BindingDB Entry DOI: 10.7270/Q20003S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM50078606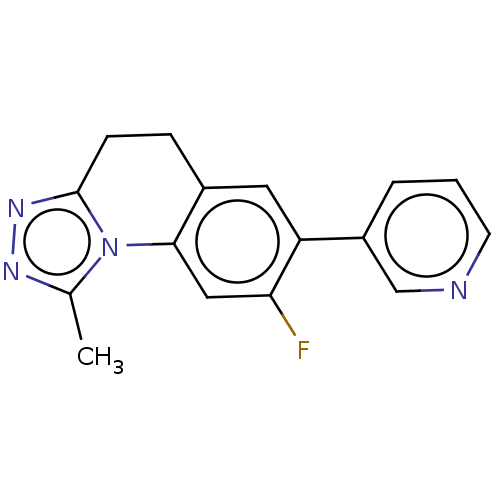 (CHEMBL3415154) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 131 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University and Helmholtz Institute for Pharmaceutical Research Saarland (HIPS) Curated by ChEMBL | Assay Description Inhibition of human CYP11B2 expressed in V79 MZh cells using [14C]-deoxycorticosterone substrate incubated for 6 hrs by HPTLC method | J Med Chem 58: 2530-7 (2015) Article DOI: 10.1021/acs.jmedchem.5b00079 BindingDB Entry DOI: 10.7270/Q20003S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM50078607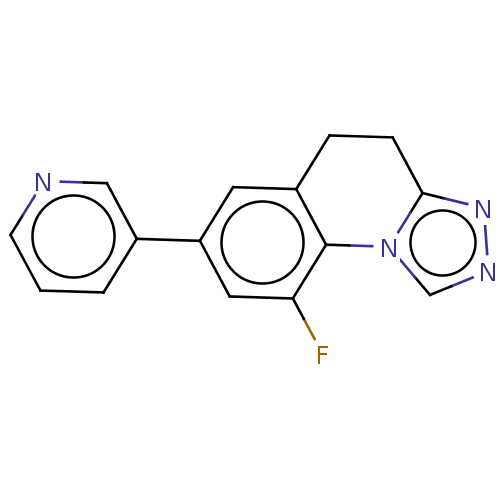 (CHEMBL3415153) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 172 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University and Helmholtz Institute for Pharmaceutical Research Saarland (HIPS) Curated by ChEMBL | Assay Description Inhibition of human CYP11B2 expressed in V79 MZh cells using [14C]-deoxycorticosterone substrate incubated for 6 hrs by HPTLC method | J Med Chem 58: 2530-7 (2015) Article DOI: 10.1021/acs.jmedchem.5b00079 BindingDB Entry DOI: 10.7270/Q20003S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM50078608 (CHEMBL3415152) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 244 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University and Helmholtz Institute for Pharmaceutical Research Saarland (HIPS) Curated by ChEMBL | Assay Description Inhibition of human CYP11B2 expressed in V79 MZh cells using [14C]-deoxycorticosterone substrate incubated for 6 hrs by HPTLC method | J Med Chem 58: 2530-7 (2015) Article DOI: 10.1021/acs.jmedchem.5b00079 BindingDB Entry DOI: 10.7270/Q20003S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM50078619 (CHEMBL3415174) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 335 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University and Helmholtz Institute for Pharmaceutical Research Saarland (HIPS) Curated by ChEMBL | Assay Description Inhibition of human CYP11B2 expressed in V79 MZh cells using [14C]-deoxycorticosterone substrate incubated for 6 hrs by HPTLC method | J Med Chem 58: 2530-7 (2015) Article DOI: 10.1021/acs.jmedchem.5b00079 BindingDB Entry DOI: 10.7270/Q20003S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B1, mitochondrial (Homo sapiens (Human)) | BDBM50078616 (CHEMBL3415171) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 428 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University and Helmholtz Institute for Pharmaceutical Research Saarland (HIPS) Curated by ChEMBL | Assay Description Inhibition of human CYP11B1 expressed in V79 MZh cells using [14C]-deoxycorticosterone substrate incubated for 6 hrs by HPTLC method | J Med Chem 58: 2530-7 (2015) Article DOI: 10.1021/acs.jmedchem.5b00079 BindingDB Entry DOI: 10.7270/Q20003S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM50078627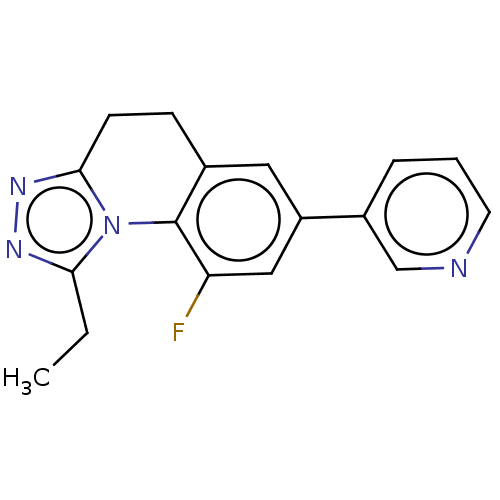 (CHEMBL3415157) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 542 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University and Helmholtz Institute for Pharmaceutical Research Saarland (HIPS) Curated by ChEMBL | Assay Description Inhibition of human CYP11B2 expressed in V79 MZh cells using [14C]-deoxycorticosterone substrate incubated for 6 hrs by HPTLC method | J Med Chem 58: 2530-7 (2015) Article DOI: 10.1021/acs.jmedchem.5b00079 BindingDB Entry DOI: 10.7270/Q20003S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM50078621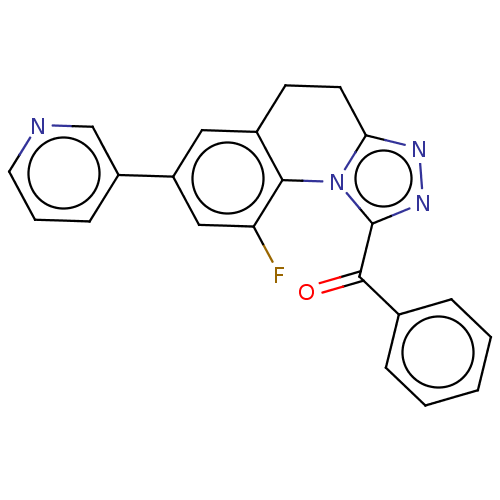 (CHEMBL3415163) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 708 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University and Helmholtz Institute for Pharmaceutical Research Saarland (HIPS) Curated by ChEMBL | Assay Description Inhibition of human CYP11B2 expressed in V79 MZh cells using [14C]-deoxycorticosterone substrate incubated for 6 hrs by HPTLC method | J Med Chem 58: 2530-7 (2015) Article DOI: 10.1021/acs.jmedchem.5b00079 BindingDB Entry DOI: 10.7270/Q20003S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM50078628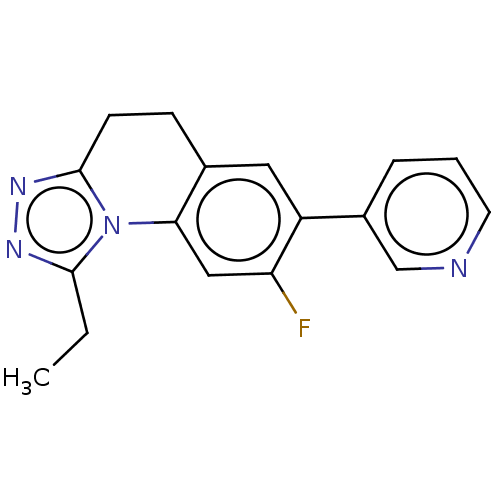 (CHEMBL3415156) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 979 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University and Helmholtz Institute for Pharmaceutical Research Saarland (HIPS) Curated by ChEMBL | Assay Description Inhibition of human CYP11B2 expressed in V79 MZh cells using [14C]-deoxycorticosterone substrate incubated for 6 hrs by HPTLC method | J Med Chem 58: 2530-7 (2015) Article DOI: 10.1021/acs.jmedchem.5b00079 BindingDB Entry DOI: 10.7270/Q20003S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM50078615 (CHEMBL3415170) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.18E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University and Helmholtz Institute for Pharmaceutical Research Saarland (HIPS) Curated by ChEMBL | Assay Description Inhibition of human CYP11B2 expressed in V79 MZh cells using [14C]-deoxycorticosterone substrate incubated for 6 hrs by HPTLC method | J Med Chem 58: 2530-7 (2015) Article DOI: 10.1021/acs.jmedchem.5b00079 BindingDB Entry DOI: 10.7270/Q20003S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B1, mitochondrial (Homo sapiens (Human)) | BDBM50044143 (CHEMBL3313969) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.77E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University and Helmholtz Institute for Pharmaceutical Research Saarland (HIPS) Curated by ChEMBL | Assay Description Inhibition of human CYP11B1 expressed in V79 MZh cells using [14C]-deoxycorticosterone substrate incubated for 6 hrs by HPTLC method | J Med Chem 58: 2530-7 (2015) Article DOI: 10.1021/acs.jmedchem.5b00079 BindingDB Entry DOI: 10.7270/Q20003S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B1, mitochondrial (Homo sapiens (Human)) | BDBM50078611 (CHEMBL3415166) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.81E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University and Helmholtz Institute for Pharmaceutical Research Saarland (HIPS) Curated by ChEMBL | Assay Description Inhibition of human CYP11B1 expressed in V79 MZh cells using [14C]-deoxycorticosterone substrate incubated for 6 hrs by HPTLC method | J Med Chem 58: 2530-7 (2015) Article DOI: 10.1021/acs.jmedchem.5b00079 BindingDB Entry DOI: 10.7270/Q20003S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B1, mitochondrial (Homo sapiens (Human)) | BDBM50078614 (CHEMBL3415169) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.57E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University and Helmholtz Institute for Pharmaceutical Research Saarland (HIPS) Curated by ChEMBL | Assay Description Inhibition of human CYP11B1 expressed in V79 MZh cells using [14C]-deoxycorticosterone substrate incubated for 6 hrs by HPTLC method | J Med Chem 58: 2530-7 (2015) Article DOI: 10.1021/acs.jmedchem.5b00079 BindingDB Entry DOI: 10.7270/Q20003S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B1, mitochondrial (Homo sapiens (Human)) | BDBM50078612 (CHEMBL3415167) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.91E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University and Helmholtz Institute for Pharmaceutical Research Saarland (HIPS) Curated by ChEMBL | Assay Description Inhibition of human CYP11B1 expressed in V79 MZh cells using [14C]-deoxycorticosterone substrate incubated for 6 hrs by HPTLC method | J Med Chem 58: 2530-7 (2015) Article DOI: 10.1021/acs.jmedchem.5b00079 BindingDB Entry DOI: 10.7270/Q20003S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM50078622 (CHEMBL3415162) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.35E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University and Helmholtz Institute for Pharmaceutical Research Saarland (HIPS) Curated by ChEMBL | Assay Description Inhibition of human CYP11B2 expressed in V79 MZh cells using [14C]-deoxycorticosterone substrate incubated for 6 hrs by HPTLC method | J Med Chem 58: 2530-7 (2015) Article DOI: 10.1021/acs.jmedchem.5b00079 BindingDB Entry DOI: 10.7270/Q20003S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B1, mitochondrial (Homo sapiens (Human)) | BDBM50078618 (CHEMBL3415173 | US9278093, 2) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.52E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University and Helmholtz Institute for Pharmaceutical Research Saarland (HIPS) Curated by ChEMBL | Assay Description Inhibition of human CYP11B1 expressed in V79 MZh cells using [14C]-deoxycorticosterone substrate incubated for 6 hrs by HPTLC method | J Med Chem 58: 2530-7 (2015) Article DOI: 10.1021/acs.jmedchem.5b00079 BindingDB Entry DOI: 10.7270/Q20003S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B1, mitochondrial (Homo sapiens (Human)) | BDBM50078620 (CHEMBL3415175) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.71E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University and Helmholtz Institute for Pharmaceutical Research Saarland (HIPS) Curated by ChEMBL | Assay Description Inhibition of human CYP11B1 expressed in V79 MZh cells using [14C]-deoxycorticosterone substrate incubated for 6 hrs by HPTLC method | J Med Chem 58: 2530-7 (2015) Article DOI: 10.1021/acs.jmedchem.5b00079 BindingDB Entry DOI: 10.7270/Q20003S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B1, mitochondrial (Homo sapiens (Human)) | BDBM50078613 (CHEMBL3415168) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.97E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University and Helmholtz Institute for Pharmaceutical Research Saarland (HIPS) Curated by ChEMBL | Assay Description Inhibition of human CYP11B1 expressed in V79 MZh cells using [14C]-deoxycorticosterone substrate incubated for 6 hrs by HPTLC method | J Med Chem 58: 2530-7 (2015) Article DOI: 10.1021/acs.jmedchem.5b00079 BindingDB Entry DOI: 10.7270/Q20003S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B1, mitochondrial (Homo sapiens (Human)) | BDBM50078608 (CHEMBL3415152) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University and Helmholtz Institute for Pharmaceutical Research Saarland (HIPS) Curated by ChEMBL | Assay Description Inhibition of human CYP11B1 expressed in V79 MZh cells using [14C]-deoxycorticosterone substrate incubated for 6 hrs by HPTLC method | J Med Chem 58: 2530-7 (2015) Article DOI: 10.1021/acs.jmedchem.5b00079 BindingDB Entry DOI: 10.7270/Q20003S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B1, mitochondrial (Homo sapiens (Human)) | BDBM50078624 (CHEMBL3415160) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University and Helmholtz Institute for Pharmaceutical Research Saarland (HIPS) Curated by ChEMBL | Assay Description Inhibition of human CYP11B1 expressed in V79 MZh cells using [14C]-deoxycorticosterone substrate incubated for 6 hrs by HPTLC method | J Med Chem 58: 2530-7 (2015) Article DOI: 10.1021/acs.jmedchem.5b00079 BindingDB Entry DOI: 10.7270/Q20003S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B1, mitochondrial (Homo sapiens (Human)) | BDBM50078626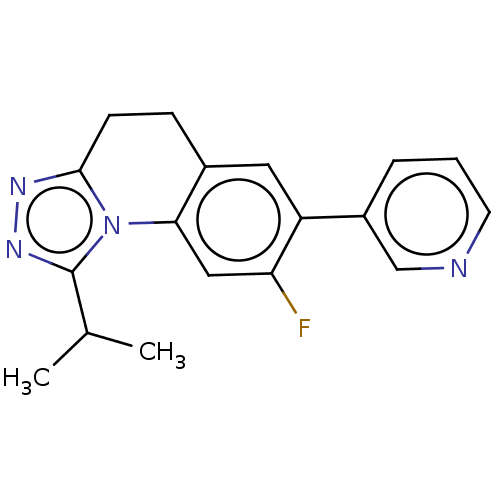 (CHEMBL3415158) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University and Helmholtz Institute for Pharmaceutical Research Saarland (HIPS) Curated by ChEMBL | Assay Description Inhibition of human CYP11B1 expressed in V79 MZh cells using [14C]-deoxycorticosterone substrate incubated for 6 hrs by HPTLC method | J Med Chem 58: 2530-7 (2015) Article DOI: 10.1021/acs.jmedchem.5b00079 BindingDB Entry DOI: 10.7270/Q20003S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM50078610 (CHEMBL3415165) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University and Helmholtz Institute for Pharmaceutical Research Saarland (HIPS) Curated by ChEMBL | Assay Description Inhibition of human CYP11B2 expressed in V79 MZh cells using [14C]-deoxycorticosterone substrate incubated for 6 hrs by HPTLC method | J Med Chem 58: 2530-7 (2015) Article DOI: 10.1021/acs.jmedchem.5b00079 BindingDB Entry DOI: 10.7270/Q20003S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM50078624 (CHEMBL3415160) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University and Helmholtz Institute for Pharmaceutical Research Saarland (HIPS) Curated by ChEMBL | Assay Description Inhibition of human CYP11B2 expressed in V79 MZh cells using [14C]-deoxycorticosterone substrate incubated for 6 hrs by HPTLC method | J Med Chem 58: 2530-7 (2015) Article DOI: 10.1021/acs.jmedchem.5b00079 BindingDB Entry DOI: 10.7270/Q20003S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM50078622 (CHEMBL3415162) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University and Helmholtz Institute for Pharmaceutical Research Saarland (HIPS) Curated by ChEMBL | Assay Description Inhibition of human CYP17 expressed in Escherichia coli coexpressing human CYP17 and NADPH-P450 reductase | J Med Chem 58: 2530-7 (2015) Article DOI: 10.1021/acs.jmedchem.5b00079 BindingDB Entry DOI: 10.7270/Q20003S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM50078621 (CHEMBL3415163) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University and Helmholtz Institute for Pharmaceutical Research Saarland (HIPS) Curated by ChEMBL | Assay Description Inhibition of human CYP17 expressed in Escherichia coli coexpressing human CYP17 and NADPH-P450 reductase | J Med Chem 58: 2530-7 (2015) Article DOI: 10.1021/acs.jmedchem.5b00079 BindingDB Entry DOI: 10.7270/Q20003S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM50078610 (CHEMBL3415165) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University and Helmholtz Institute for Pharmaceutical Research Saarland (HIPS) Curated by ChEMBL | Assay Description Inhibition of human CYP17 expressed in Escherichia coli coexpressing human CYP17 and NADPH-P450 reductase | J Med Chem 58: 2530-7 (2015) Article DOI: 10.1021/acs.jmedchem.5b00079 BindingDB Entry DOI: 10.7270/Q20003S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM50078611 (CHEMBL3415166) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University and Helmholtz Institute for Pharmaceutical Research Saarland (HIPS) Curated by ChEMBL | Assay Description Inhibition of human CYP17 expressed in Escherichia coli coexpressing human CYP17 and NADPH-P450 reductase | J Med Chem 58: 2530-7 (2015) Article DOI: 10.1021/acs.jmedchem.5b00079 BindingDB Entry DOI: 10.7270/Q20003S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50078629 (CHEMBL3415155) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University and Helmholtz Institute for Pharmaceutical Research Saarland (HIPS) Curated by ChEMBL | Assay Description Inhibition of CYP19 in human placental microsomes using [1beta-3H]androstenedione substrate | J Med Chem 58: 2530-7 (2015) Article DOI: 10.1021/acs.jmedchem.5b00079 BindingDB Entry DOI: 10.7270/Q20003S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50078624 (CHEMBL3415160) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University and Helmholtz Institute for Pharmaceutical Research Saarland (HIPS) Curated by ChEMBL | Assay Description Inhibition of CYP19 in human placental microsomes using [1beta-3H]androstenedione substrate | J Med Chem 58: 2530-7 (2015) Article DOI: 10.1021/acs.jmedchem.5b00079 BindingDB Entry DOI: 10.7270/Q20003S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50078621 (CHEMBL3415163) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University and Helmholtz Institute for Pharmaceutical Research Saarland (HIPS) Curated by ChEMBL | Assay Description Inhibition of CYP19 in human placental microsomes using [1beta-3H]androstenedione substrate | J Med Chem 58: 2530-7 (2015) Article DOI: 10.1021/acs.jmedchem.5b00079 BindingDB Entry DOI: 10.7270/Q20003S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM50078625 (CHEMBL3415159) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University and Helmholtz Institute for Pharmaceutical Research Saarland (HIPS) Curated by ChEMBL | Assay Description Inhibition of human CYP17 expressed in Escherichia coli coexpressing human CYP17 and NADPH-P450 reductase | J Med Chem 58: 2530-7 (2015) Article DOI: 10.1021/acs.jmedchem.5b00079 BindingDB Entry DOI: 10.7270/Q20003S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM50428331 (CHEMBL2331699) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University and Helmholtz Institute for Pharmaceutical Research Saarland (HIPS) Curated by ChEMBL | Assay Description Inhibition of human CYP17 expressed in Escherichia coli coexpressing human CYP17 and NADPH-P450 reductase | J Med Chem 58: 2530-7 (2015) Article DOI: 10.1021/acs.jmedchem.5b00079 BindingDB Entry DOI: 10.7270/Q20003S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM50044143 (CHEMBL3313969) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University and Helmholtz Institute for Pharmaceutical Research Saarland (HIPS) Curated by ChEMBL | Assay Description Inhibition of human CYP17 expressed in Escherichia coli coexpressing human CYP17 and NADPH-P450 reductase | J Med Chem 58: 2530-7 (2015) Article DOI: 10.1021/acs.jmedchem.5b00079 BindingDB Entry DOI: 10.7270/Q20003S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B1, mitochondrial (Homo sapiens (Human)) | BDBM50078610 (CHEMBL3415165) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University and Helmholtz Institute for Pharmaceutical Research Saarland (HIPS) Curated by ChEMBL | Assay Description Inhibition of human CYP11B1 expressed in V79 MZh cells using [14C]-deoxycorticosterone substrate incubated for 6 hrs by HPTLC method | J Med Chem 58: 2530-7 (2015) Article DOI: 10.1021/acs.jmedchem.5b00079 BindingDB Entry DOI: 10.7270/Q20003S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B1, mitochondrial (Homo sapiens (Human)) | BDBM50078622 (CHEMBL3415162) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University and Helmholtz Institute for Pharmaceutical Research Saarland (HIPS) Curated by ChEMBL | Assay Description Inhibition of human CYP11B1 expressed in V79 MZh cells using [14C]-deoxycorticosterone substrate incubated for 6 hrs by HPTLC method | J Med Chem 58: 2530-7 (2015) Article DOI: 10.1021/acs.jmedchem.5b00079 BindingDB Entry DOI: 10.7270/Q20003S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM50078623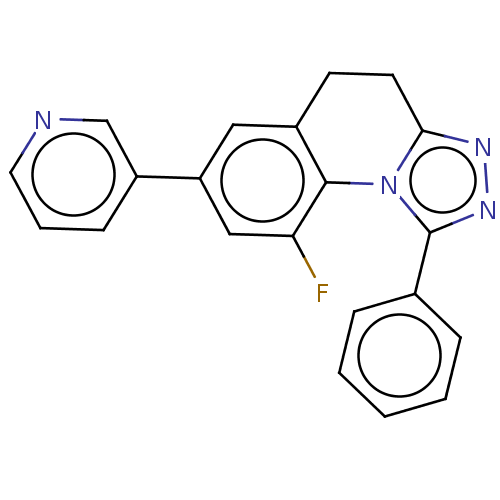 (CHEMBL3415161) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University and Helmholtz Institute for Pharmaceutical Research Saarland (HIPS) Curated by ChEMBL | Assay Description Inhibition of human CYP11B2 expressed in V79 MZh cells using [14C]-deoxycorticosterone substrate incubated for 6 hrs by HPTLC method | J Med Chem 58: 2530-7 (2015) Article DOI: 10.1021/acs.jmedchem.5b00079 BindingDB Entry DOI: 10.7270/Q20003S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50078608 (CHEMBL3415152) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University and Helmholtz Institute for Pharmaceutical Research Saarland (HIPS) Curated by ChEMBL | Assay Description Inhibition of CYP19 in human placental microsomes using [1beta-3H]androstenedione substrate | J Med Chem 58: 2530-7 (2015) Article DOI: 10.1021/acs.jmedchem.5b00079 BindingDB Entry DOI: 10.7270/Q20003S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50078607 (CHEMBL3415153) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University and Helmholtz Institute for Pharmaceutical Research Saarland (HIPS) Curated by ChEMBL | Assay Description Inhibition of CYP19 in human placental microsomes using [1beta-3H]androstenedione substrate | J Med Chem 58: 2530-7 (2015) Article DOI: 10.1021/acs.jmedchem.5b00079 BindingDB Entry DOI: 10.7270/Q20003S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50078626 (CHEMBL3415158) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University and Helmholtz Institute for Pharmaceutical Research Saarland (HIPS) Curated by ChEMBL | Assay Description Inhibition of CYP19 in human placental microsomes using [1beta-3H]androstenedione substrate | J Med Chem 58: 2530-7 (2015) Article DOI: 10.1021/acs.jmedchem.5b00079 BindingDB Entry DOI: 10.7270/Q20003S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50078613 (CHEMBL3415168) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University and Helmholtz Institute for Pharmaceutical Research Saarland (HIPS) Curated by ChEMBL | Assay Description Inhibition of CYP19 in human placental microsomes using [1beta-3H]androstenedione substrate | J Med Chem 58: 2530-7 (2015) Article DOI: 10.1021/acs.jmedchem.5b00079 BindingDB Entry DOI: 10.7270/Q20003S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50078614 (CHEMBL3415169) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University and Helmholtz Institute for Pharmaceutical Research Saarland (HIPS) Curated by ChEMBL | Assay Description Inhibition of CYP19 in human placental microsomes using [1beta-3H]androstenedione substrate | J Med Chem 58: 2530-7 (2015) Article DOI: 10.1021/acs.jmedchem.5b00079 BindingDB Entry DOI: 10.7270/Q20003S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50078620 (CHEMBL3415175) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University and Helmholtz Institute for Pharmaceutical Research Saarland (HIPS) Curated by ChEMBL | Assay Description Inhibition of CYP19 in human placental microsomes using [1beta-3H]androstenedione substrate | J Med Chem 58: 2530-7 (2015) Article DOI: 10.1021/acs.jmedchem.5b00079 BindingDB Entry DOI: 10.7270/Q20003S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM50078624 (CHEMBL3415160) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University and Helmholtz Institute for Pharmaceutical Research Saarland (HIPS) Curated by ChEMBL | Assay Description Inhibition of human CYP17 expressed in Escherichia coli coexpressing human CYP17 and NADPH-P450 reductase | J Med Chem 58: 2530-7 (2015) Article DOI: 10.1021/acs.jmedchem.5b00079 BindingDB Entry DOI: 10.7270/Q20003S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM50078616 (CHEMBL3415171) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University and Helmholtz Institute for Pharmaceutical Research Saarland (HIPS) Curated by ChEMBL | Assay Description Inhibition of human CYP17 expressed in Escherichia coli coexpressing human CYP17 and NADPH-P450 reductase | J Med Chem 58: 2530-7 (2015) Article DOI: 10.1021/acs.jmedchem.5b00079 BindingDB Entry DOI: 10.7270/Q20003S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50078628 (CHEMBL3415156) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University and Helmholtz Institute for Pharmaceutical Research Saarland (HIPS) Curated by ChEMBL | Assay Description Inhibition of CYP19 in human placental microsomes using [1beta-3H]androstenedione substrate | J Med Chem 58: 2530-7 (2015) Article DOI: 10.1021/acs.jmedchem.5b00079 BindingDB Entry DOI: 10.7270/Q20003S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50078627 (CHEMBL3415157) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University and Helmholtz Institute for Pharmaceutical Research Saarland (HIPS) Curated by ChEMBL | Assay Description Inhibition of CYP19 in human placental microsomes using [1beta-3H]androstenedione substrate | J Med Chem 58: 2530-7 (2015) Article DOI: 10.1021/acs.jmedchem.5b00079 BindingDB Entry DOI: 10.7270/Q20003S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50078617 (CHEMBL3415172) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University and Helmholtz Institute for Pharmaceutical Research Saarland (HIPS) Curated by ChEMBL | Assay Description Inhibition of CYP19 in human placental microsomes using [1beta-3H]androstenedione substrate | J Med Chem 58: 2530-7 (2015) Article DOI: 10.1021/acs.jmedchem.5b00079 BindingDB Entry DOI: 10.7270/Q20003S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM50078608 (CHEMBL3415152) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University and Helmholtz Institute for Pharmaceutical Research Saarland (HIPS) Curated by ChEMBL | Assay Description Inhibition of human CYP17 expressed in Escherichia coli coexpressing human CYP17 and NADPH-P450 reductase | J Med Chem 58: 2530-7 (2015) Article DOI: 10.1021/acs.jmedchem.5b00079 BindingDB Entry DOI: 10.7270/Q20003S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM50078626 (CHEMBL3415158) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University and Helmholtz Institute for Pharmaceutical Research Saarland (HIPS) Curated by ChEMBL | Assay Description Inhibition of human CYP17 expressed in Escherichia coli coexpressing human CYP17 and NADPH-P450 reductase | J Med Chem 58: 2530-7 (2015) Article DOI: 10.1021/acs.jmedchem.5b00079 BindingDB Entry DOI: 10.7270/Q20003S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM50078614 (CHEMBL3415169) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University and Helmholtz Institute for Pharmaceutical Research Saarland (HIPS) Curated by ChEMBL | Assay Description Inhibition of human CYP17 expressed in Escherichia coli coexpressing human CYP17 and NADPH-P450 reductase | J Med Chem 58: 2530-7 (2015) Article DOI: 10.1021/acs.jmedchem.5b00079 BindingDB Entry DOI: 10.7270/Q20003S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B1, mitochondrial (Homo sapiens (Human)) | BDBM50078615 (CHEMBL3415170) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University and Helmholtz Institute for Pharmaceutical Research Saarland (HIPS) Curated by ChEMBL | Assay Description Inhibition of human CYP11B1 expressed in V79 MZh cells using [14C]-deoxycorticosterone substrate incubated for 6 hrs by HPTLC method | J Med Chem 58: 2530-7 (2015) Article DOI: 10.1021/acs.jmedchem.5b00079 BindingDB Entry DOI: 10.7270/Q20003S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B1, mitochondrial (Homo sapiens (Human)) | BDBM50078623 (CHEMBL3415161) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University and Helmholtz Institute for Pharmaceutical Research Saarland (HIPS) Curated by ChEMBL | Assay Description Inhibition of human CYP11B1 expressed in V79 MZh cells using [14C]-deoxycorticosterone substrate incubated for 6 hrs by HPTLC method | J Med Chem 58: 2530-7 (2015) Article DOI: 10.1021/acs.jmedchem.5b00079 BindingDB Entry DOI: 10.7270/Q20003S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B1, mitochondrial (Homo sapiens (Human)) | BDBM50078625 (CHEMBL3415159) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University and Helmholtz Institute for Pharmaceutical Research Saarland (HIPS) Curated by ChEMBL | Assay Description Inhibition of human CYP11B1 expressed in V79 MZh cells using [14C]-deoxycorticosterone substrate incubated for 6 hrs by HPTLC method | J Med Chem 58: 2530-7 (2015) Article DOI: 10.1021/acs.jmedchem.5b00079 BindingDB Entry DOI: 10.7270/Q20003S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B1, mitochondrial (Homo sapiens (Human)) | BDBM50078627 (CHEMBL3415157) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University and Helmholtz Institute for Pharmaceutical Research Saarland (HIPS) Curated by ChEMBL | Assay Description Inhibition of human CYP11B1 expressed in V79 MZh cells using [14C]-deoxycorticosterone substrate incubated for 6 hrs by HPTLC method | J Med Chem 58: 2530-7 (2015) Article DOI: 10.1021/acs.jmedchem.5b00079 BindingDB Entry DOI: 10.7270/Q20003S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B1, mitochondrial (Homo sapiens (Human)) | BDBM50078628 (CHEMBL3415156) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University and Helmholtz Institute for Pharmaceutical Research Saarland (HIPS) Curated by ChEMBL | Assay Description Inhibition of human CYP11B1 expressed in V79 MZh cells using [14C]-deoxycorticosterone substrate incubated for 6 hrs by HPTLC method | J Med Chem 58: 2530-7 (2015) Article DOI: 10.1021/acs.jmedchem.5b00079 BindingDB Entry DOI: 10.7270/Q20003S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50078606 (CHEMBL3415154) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University and Helmholtz Institute for Pharmaceutical Research Saarland (HIPS) Curated by ChEMBL | Assay Description Inhibition of CYP19 in human placental microsomes using [1beta-3H]androstenedione substrate | J Med Chem 58: 2530-7 (2015) Article DOI: 10.1021/acs.jmedchem.5b00079 BindingDB Entry DOI: 10.7270/Q20003S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50078623 (CHEMBL3415161) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University and Helmholtz Institute for Pharmaceutical Research Saarland (HIPS) Curated by ChEMBL | Assay Description Inhibition of CYP19 in human placental microsomes using [1beta-3H]androstenedione substrate | J Med Chem 58: 2530-7 (2015) Article DOI: 10.1021/acs.jmedchem.5b00079 BindingDB Entry DOI: 10.7270/Q20003S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50078622 (CHEMBL3415162) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University and Helmholtz Institute for Pharmaceutical Research Saarland (HIPS) Curated by ChEMBL | Assay Description Inhibition of CYP19 in human placental microsomes using [1beta-3H]androstenedione substrate | J Med Chem 58: 2530-7 (2015) Article DOI: 10.1021/acs.jmedchem.5b00079 BindingDB Entry DOI: 10.7270/Q20003S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50078611 (CHEMBL3415166) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University and Helmholtz Institute for Pharmaceutical Research Saarland (HIPS) Curated by ChEMBL | Assay Description Inhibition of CYP19 in human placental microsomes using [1beta-3H]androstenedione substrate | J Med Chem 58: 2530-7 (2015) Article DOI: 10.1021/acs.jmedchem.5b00079 BindingDB Entry DOI: 10.7270/Q20003S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50428331 (CHEMBL2331699) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University and Helmholtz Institute for Pharmaceutical Research Saarland (HIPS) Curated by ChEMBL | Assay Description Inhibition of CYP19 in human placental microsomes using [1beta-3H]androstenedione substrate | J Med Chem 58: 2530-7 (2015) Article DOI: 10.1021/acs.jmedchem.5b00079 BindingDB Entry DOI: 10.7270/Q20003S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50078616 (CHEMBL3415171) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University and Helmholtz Institute for Pharmaceutical Research Saarland (HIPS) Curated by ChEMBL | Assay Description Inhibition of CYP19 in human placental microsomes using [1beta-3H]androstenedione substrate | J Med Chem 58: 2530-7 (2015) Article DOI: 10.1021/acs.jmedchem.5b00079 BindingDB Entry DOI: 10.7270/Q20003S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50078618 (CHEMBL3415173 | US9278093, 2) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University and Helmholtz Institute for Pharmaceutical Research Saarland (HIPS) Curated by ChEMBL | Assay Description Inhibition of CYP19 in human placental microsomes using [1beta-3H]androstenedione substrate | J Med Chem 58: 2530-7 (2015) Article DOI: 10.1021/acs.jmedchem.5b00079 BindingDB Entry DOI: 10.7270/Q20003S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM50078606 (CHEMBL3415154) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University and Helmholtz Institute for Pharmaceutical Research Saarland (HIPS) Curated by ChEMBL | Assay Description Inhibition of human CYP17 expressed in Escherichia coli coexpressing human CYP17 and NADPH-P450 reductase | J Med Chem 58: 2530-7 (2015) Article DOI: 10.1021/acs.jmedchem.5b00079 BindingDB Entry DOI: 10.7270/Q20003S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM50078618 (CHEMBL3415173 | US9278093, 2) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University and Helmholtz Institute for Pharmaceutical Research Saarland (HIPS) Curated by ChEMBL | Assay Description Inhibition of human CYP17 expressed in Escherichia coli coexpressing human CYP17 and NADPH-P450 reductase | J Med Chem 58: 2530-7 (2015) Article DOI: 10.1021/acs.jmedchem.5b00079 BindingDB Entry DOI: 10.7270/Q20003S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B1, mitochondrial (Homo sapiens (Human)) | BDBM50078609 (CHEMBL3415164) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University and Helmholtz Institute for Pharmaceutical Research Saarland (HIPS) Curated by ChEMBL | Assay Description Inhibition of human CYP11B1 expressed in V79 MZh cells using [14C]-deoxycorticosterone substrate incubated for 6 hrs by HPTLC method | J Med Chem 58: 2530-7 (2015) Article DOI: 10.1021/acs.jmedchem.5b00079 BindingDB Entry DOI: 10.7270/Q20003S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM50078625 (CHEMBL3415159) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University and Helmholtz Institute for Pharmaceutical Research Saarland (HIPS) Curated by ChEMBL | Assay Description Inhibition of human CYP11B2 expressed in V79 MZh cells using [14C]-deoxycorticosterone substrate incubated for 6 hrs by HPTLC method | J Med Chem 58: 2530-7 (2015) Article DOI: 10.1021/acs.jmedchem.5b00079 BindingDB Entry DOI: 10.7270/Q20003S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50078609 (CHEMBL3415164) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University and Helmholtz Institute for Pharmaceutical Research Saarland (HIPS) Curated by ChEMBL | Assay Description Inhibition of CYP19 in human placental microsomes using [1beta-3H]androstenedione substrate | J Med Chem 58: 2530-7 (2015) Article DOI: 10.1021/acs.jmedchem.5b00079 BindingDB Entry DOI: 10.7270/Q20003S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50078612 (CHEMBL3415167) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University and Helmholtz Institute for Pharmaceutical Research Saarland (HIPS) Curated by ChEMBL | Assay Description Inhibition of CYP19 in human placental microsomes using [1beta-3H]androstenedione substrate | J Med Chem 58: 2530-7 (2015) Article DOI: 10.1021/acs.jmedchem.5b00079 BindingDB Entry DOI: 10.7270/Q20003S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50078615 (CHEMBL3415170) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University and Helmholtz Institute for Pharmaceutical Research Saarland (HIPS) Curated by ChEMBL | Assay Description Inhibition of CYP19 in human placental microsomes using [1beta-3H]androstenedione substrate | J Med Chem 58: 2530-7 (2015) Article DOI: 10.1021/acs.jmedchem.5b00079 BindingDB Entry DOI: 10.7270/Q20003S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50044143 (CHEMBL3313969) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University and Helmholtz Institute for Pharmaceutical Research Saarland (HIPS) Curated by ChEMBL | Assay Description Inhibition of CYP19 in human placental microsomes using [1beta-3H]androstenedione substrate | J Med Chem 58: 2530-7 (2015) Article DOI: 10.1021/acs.jmedchem.5b00079 BindingDB Entry DOI: 10.7270/Q20003S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM50078607 (CHEMBL3415153) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University and Helmholtz Institute for Pharmaceutical Research Saarland (HIPS) Curated by ChEMBL | Assay Description Inhibition of human CYP17 expressed in Escherichia coli coexpressing human CYP17 and NADPH-P450 reductase | J Med Chem 58: 2530-7 (2015) Article DOI: 10.1021/acs.jmedchem.5b00079 BindingDB Entry DOI: 10.7270/Q20003S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM50078629 (CHEMBL3415155) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University and Helmholtz Institute for Pharmaceutical Research Saarland (HIPS) Curated by ChEMBL | Assay Description Inhibition of human CYP17 expressed in Escherichia coli coexpressing human CYP17 and NADPH-P450 reductase | J Med Chem 58: 2530-7 (2015) Article DOI: 10.1021/acs.jmedchem.5b00079 BindingDB Entry DOI: 10.7270/Q20003S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM50078627 (CHEMBL3415157) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University and Helmholtz Institute for Pharmaceutical Research Saarland (HIPS) Curated by ChEMBL | Assay Description Inhibition of human CYP17 expressed in Escherichia coli coexpressing human CYP17 and NADPH-P450 reductase | J Med Chem 58: 2530-7 (2015) Article DOI: 10.1021/acs.jmedchem.5b00079 BindingDB Entry DOI: 10.7270/Q20003S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM50078615 (CHEMBL3415170) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University and Helmholtz Institute for Pharmaceutical Research Saarland (HIPS) Curated by ChEMBL | Assay Description Inhibition of human CYP17 expressed in Escherichia coli coexpressing human CYP17 and NADPH-P450 reductase | J Med Chem 58: 2530-7 (2015) Article DOI: 10.1021/acs.jmedchem.5b00079 BindingDB Entry DOI: 10.7270/Q20003S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM50078617 (CHEMBL3415172) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University and Helmholtz Institute for Pharmaceutical Research Saarland (HIPS) Curated by ChEMBL | Assay Description Inhibition of human CYP17 expressed in Escherichia coli coexpressing human CYP17 and NADPH-P450 reductase | J Med Chem 58: 2530-7 (2015) Article DOI: 10.1021/acs.jmedchem.5b00079 BindingDB Entry DOI: 10.7270/Q20003S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM50078619 (CHEMBL3415174) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University and Helmholtz Institute for Pharmaceutical Research Saarland (HIPS) Curated by ChEMBL | Assay Description Inhibition of human CYP17 expressed in Escherichia coli coexpressing human CYP17 and NADPH-P450 reductase | J Med Chem 58: 2530-7 (2015) Article DOI: 10.1021/acs.jmedchem.5b00079 BindingDB Entry DOI: 10.7270/Q20003S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM50078626 (CHEMBL3415158) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University and Helmholtz Institute for Pharmaceutical Research Saarland (HIPS) Curated by ChEMBL | Assay Description Inhibition of human CYP11B2 expressed in V79 MZh cells using [14C]-deoxycorticosterone substrate incubated for 6 hrs by HPTLC method | J Med Chem 58: 2530-7 (2015) Article DOI: 10.1021/acs.jmedchem.5b00079 BindingDB Entry DOI: 10.7270/Q20003S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50078610 (CHEMBL3415165) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University and Helmholtz Institute for Pharmaceutical Research Saarland (HIPS) Curated by ChEMBL | Assay Description Inhibition of CYP19 in human placental microsomes using [1beta-3H]androstenedione substrate | J Med Chem 58: 2530-7 (2015) Article DOI: 10.1021/acs.jmedchem.5b00079 BindingDB Entry DOI: 10.7270/Q20003S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50078619 (CHEMBL3415174) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University and Helmholtz Institute for Pharmaceutical Research Saarland (HIPS) Curated by ChEMBL | Assay Description Inhibition of CYP19 in human placental microsomes using [1beta-3H]androstenedione substrate | J Med Chem 58: 2530-7 (2015) Article DOI: 10.1021/acs.jmedchem.5b00079 BindingDB Entry DOI: 10.7270/Q20003S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM50078623 (CHEMBL3415161) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University and Helmholtz Institute for Pharmaceutical Research Saarland (HIPS) Curated by ChEMBL | Assay Description Inhibition of human CYP17 expressed in Escherichia coli coexpressing human CYP17 and NADPH-P450 reductase | J Med Chem 58: 2530-7 (2015) Article DOI: 10.1021/acs.jmedchem.5b00079 BindingDB Entry DOI: 10.7270/Q20003S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM50078609 (CHEMBL3415164) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University and Helmholtz Institute for Pharmaceutical Research Saarland (HIPS) Curated by ChEMBL | Assay Description Inhibition of human CYP17 expressed in Escherichia coli coexpressing human CYP17 and NADPH-P450 reductase | J Med Chem 58: 2530-7 (2015) Article DOI: 10.1021/acs.jmedchem.5b00079 BindingDB Entry DOI: 10.7270/Q20003S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM50078612 (CHEMBL3415167) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University and Helmholtz Institute for Pharmaceutical Research Saarland (HIPS) Curated by ChEMBL | Assay Description Inhibition of human CYP17 expressed in Escherichia coli coexpressing human CYP17 and NADPH-P450 reductase | J Med Chem 58: 2530-7 (2015) Article DOI: 10.1021/acs.jmedchem.5b00079 BindingDB Entry DOI: 10.7270/Q20003S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM50078620 (CHEMBL3415175) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University and Helmholtz Institute for Pharmaceutical Research Saarland (HIPS) Curated by ChEMBL | Assay Description Inhibition of human CYP17 expressed in Escherichia coli coexpressing human CYP17 and NADPH-P450 reductase | J Med Chem 58: 2530-7 (2015) Article DOI: 10.1021/acs.jmedchem.5b00079 BindingDB Entry DOI: 10.7270/Q20003S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B1, mitochondrial (Homo sapiens (Human)) | BDBM50078619 (CHEMBL3415174) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University and Helmholtz Institute for Pharmaceutical Research Saarland (HIPS) Curated by ChEMBL | Assay Description Inhibition of human CYP11B1 expressed in V79 MZh cells using [14C]-deoxycorticosterone substrate incubated for 6 hrs by HPTLC method | J Med Chem 58: 2530-7 (2015) Article DOI: 10.1021/acs.jmedchem.5b00079 BindingDB Entry DOI: 10.7270/Q20003S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B1, mitochondrial (Homo sapiens (Human)) | BDBM50078621 (CHEMBL3415163) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University and Helmholtz Institute for Pharmaceutical Research Saarland (HIPS) Curated by ChEMBL | Assay Description Inhibition of human CYP11B1 expressed in V79 MZh cells using [14C]-deoxycorticosterone substrate incubated for 6 hrs by HPTLC method | J Med Chem 58: 2530-7 (2015) Article DOI: 10.1021/acs.jmedchem.5b00079 BindingDB Entry DOI: 10.7270/Q20003S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM50078609 (CHEMBL3415164) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University and Helmholtz Institute for Pharmaceutical Research Saarland (HIPS) Curated by ChEMBL | Assay Description Inhibition of human CYP11B2 expressed in V79 MZh cells using [14C]-deoxycorticosterone substrate incubated for 6 hrs by HPTLC method | J Med Chem 58: 2530-7 (2015) Article DOI: 10.1021/acs.jmedchem.5b00079 BindingDB Entry DOI: 10.7270/Q20003S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50078625 (CHEMBL3415159) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University and Helmholtz Institute for Pharmaceutical Research Saarland (HIPS) Curated by ChEMBL | Assay Description Inhibition of CYP19 in human placental microsomes using [1beta-3H]androstenedione substrate | J Med Chem 58: 2530-7 (2015) Article DOI: 10.1021/acs.jmedchem.5b00079 BindingDB Entry DOI: 10.7270/Q20003S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM50078628 (CHEMBL3415156) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University and Helmholtz Institute for Pharmaceutical Research Saarland (HIPS) Curated by ChEMBL | Assay Description Inhibition of human CYP17 expressed in Escherichia coli coexpressing human CYP17 and NADPH-P450 reductase | J Med Chem 58: 2530-7 (2015) Article DOI: 10.1021/acs.jmedchem.5b00079 BindingDB Entry DOI: 10.7270/Q20003S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM50078613 (CHEMBL3415168) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University and Helmholtz Institute for Pharmaceutical Research Saarland (HIPS) Curated by ChEMBL | Assay Description Inhibition of human CYP17 expressed in Escherichia coli coexpressing human CYP17 and NADPH-P450 reductase | J Med Chem 58: 2530-7 (2015) Article DOI: 10.1021/acs.jmedchem.5b00079 BindingDB Entry DOI: 10.7270/Q20003S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B1, mitochondrial (Homo sapiens (Human)) | BDBM50078606 (CHEMBL3415154) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.26E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University and Helmholtz Institute for Pharmaceutical Research Saarland (HIPS) Curated by ChEMBL | Assay Description Inhibition of human CYP11B1 expressed in V79 MZh cells using [14C]-deoxycorticosterone substrate incubated for 6 hrs by HPTLC method | J Med Chem 58: 2530-7 (2015) Article DOI: 10.1021/acs.jmedchem.5b00079 BindingDB Entry DOI: 10.7270/Q20003S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B1, mitochondrial (Homo sapiens (Human)) | BDBM50078607 (CHEMBL3415153) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.61E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University and Helmholtz Institute for Pharmaceutical Research Saarland (HIPS) Curated by ChEMBL | Assay Description Inhibition of human CYP11B1 expressed in V79 MZh cells using [14C]-deoxycorticosterone substrate incubated for 6 hrs by HPTLC method | J Med Chem 58: 2530-7 (2015) Article DOI: 10.1021/acs.jmedchem.5b00079 BindingDB Entry DOI: 10.7270/Q20003S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B1, mitochondrial (Homo sapiens (Human)) | BDBM50078629 (CHEMBL3415155) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University and Helmholtz Institute for Pharmaceutical Research Saarland (HIPS) Curated by ChEMBL | Assay Description Inhibition of human CYP11B1 expressed in V79 MZh cells using [14C]-deoxycorticosterone substrate incubated for 6 hrs by HPTLC method | J Med Chem 58: 2530-7 (2015) Article DOI: 10.1021/acs.jmedchem.5b00079 BindingDB Entry DOI: 10.7270/Q20003S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B1, mitochondrial (Homo sapiens (Human)) | BDBM50428331 (CHEMBL2331699) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.95E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University and Helmholtz Institute for Pharmaceutical Research Saarland (HIPS) Curated by ChEMBL | Assay Description Inhibition of human CYP11B1 expressed in V79 MZh cells using [14C]-deoxycorticosterone substrate incubated for 6 hrs by HPTLC method | J Med Chem 58: 2530-7 (2015) Article DOI: 10.1021/acs.jmedchem.5b00079 BindingDB Entry DOI: 10.7270/Q20003S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50428331 (CHEMBL2331699) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University and Helmholtz Institute for Pharmaceutical Research Saarland (HIPS) Curated by ChEMBL | Assay Description Inhibition of human recombinant CYP2C9 using diclofenac substrate by LC-MS/MS method | J Med Chem 58: 2530-7 (2015) Article DOI: 10.1021/acs.jmedchem.5b00079 BindingDB Entry DOI: 10.7270/Q20003S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50428331 (CHEMBL2331699) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University and Helmholtz Institute for Pharmaceutical Research Saarland (HIPS) Curated by ChEMBL | Assay Description Inhibition of human recombinant CYP3A4 using midazolam substrate by LC-MS/MS method | J Med Chem 58: 2530-7 (2015) Article DOI: 10.1021/acs.jmedchem.5b00079 BindingDB Entry DOI: 10.7270/Q20003S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50044143 (CHEMBL3313969) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University and Helmholtz Institute for Pharmaceutical Research Saarland (HIPS) Curated by ChEMBL | Assay Description Inhibition of human recombinant CYP3A4 using midazolam substrate by LC-MS/MS method | J Med Chem 58: 2530-7 (2015) Article DOI: 10.1021/acs.jmedchem.5b00079 BindingDB Entry DOI: 10.7270/Q20003S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50044143 (CHEMBL3313969) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University and Helmholtz Institute for Pharmaceutical Research Saarland (HIPS) Curated by ChEMBL | Assay Description Inhibition of human recombinant CYP2C9 using diclofenac substrate by LC-MS/MS method | J Med Chem 58: 2530-7 (2015) Article DOI: 10.1021/acs.jmedchem.5b00079 BindingDB Entry DOI: 10.7270/Q20003S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50428331 (CHEMBL2331699) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University and Helmholtz Institute for Pharmaceutical Research Saarland (HIPS) Curated by ChEMBL | Assay Description Inhibition of human recombinant CYP2D6 using bufuralol substrate by LC-MS/MS method | J Med Chem 58: 2530-7 (2015) Article DOI: 10.1021/acs.jmedchem.5b00079 BindingDB Entry DOI: 10.7270/Q20003S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A2 (Homo sapiens (Human)) | BDBM50044143 (CHEMBL3313969) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University and Helmholtz Institute for Pharmaceutical Research Saarland (HIPS) Curated by ChEMBL | Assay Description Inhibition of human recombinant CYP1A2 using phenacetin substrate by LC-MS/MS method | J Med Chem 58: 2530-7 (2015) Article DOI: 10.1021/acs.jmedchem.5b00079 BindingDB Entry DOI: 10.7270/Q20003S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C19 (Homo sapiens (Human)) | BDBM50044143 (CHEMBL3313969) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University and Helmholtz Institute for Pharmaceutical Research Saarland (HIPS) Curated by ChEMBL | Assay Description Inhibition of human recombinant CYP2C19 using smephenytoin substrate by LC-MS/MS method | J Med Chem 58: 2530-7 (2015) Article DOI: 10.1021/acs.jmedchem.5b00079 BindingDB Entry DOI: 10.7270/Q20003S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C19 (Homo sapiens (Human)) | BDBM50428331 (CHEMBL2331699) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University and Helmholtz Institute for Pharmaceutical Research Saarland (HIPS) Curated by ChEMBL | Assay Description Inhibition of human recombinant CYP2C19 using smephenytoin substrate by LC-MS/MS method | J Med Chem 58: 2530-7 (2015) Article DOI: 10.1021/acs.jmedchem.5b00079 BindingDB Entry DOI: 10.7270/Q20003S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A2 (Homo sapiens (Human)) | BDBM50428331 (CHEMBL2331699) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University and Helmholtz Institute for Pharmaceutical Research Saarland (HIPS) Curated by ChEMBL | Assay Description Inhibition of human recombinant CYP1A2 using phenacetin substrate by LC-MS/MS method | J Med Chem 58: 2530-7 (2015) Article DOI: 10.1021/acs.jmedchem.5b00079 BindingDB Entry DOI: 10.7270/Q20003S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50044143 (CHEMBL3313969) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University and Helmholtz Institute for Pharmaceutical Research Saarland (HIPS) Curated by ChEMBL | Assay Description Inhibition of human recombinant CYP2D6 using bufuralol substrate by LC-MS/MS method | J Med Chem 58: 2530-7 (2015) Article DOI: 10.1021/acs.jmedchem.5b00079 BindingDB Entry DOI: 10.7270/Q20003S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||