

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tissue-type plasminogen activator (Homo sapiens (Human)) | BDBM50089084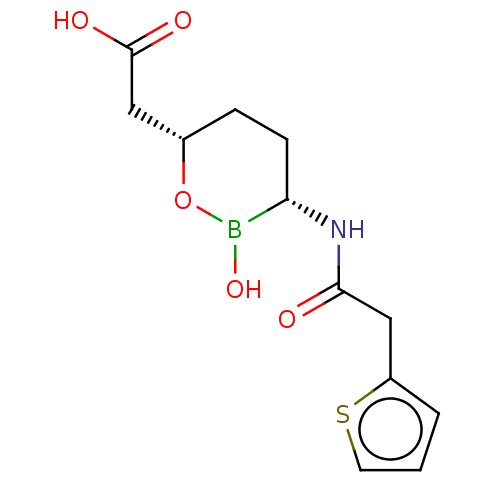 (CHEMBL3317857 | Vaborbactam) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents | Article PubMed | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Rempex Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of tissue plasminogen activator (unknown origin) using GK-pNA as substrate preincubated for 10 mins followed by substrate addition measure... | J Med Chem 58: 3682-92 (2015) Article DOI: 10.1021/acs.jmedchem.5b00127 BindingDB Entry DOI: 10.7270/Q2765H2V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM50089084 (CHEMBL3317857 | Vaborbactam) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents | Article PubMed | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Rempex Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of chymase (unknown origin) using Suc-AAPF-AMC as substrate preincubated for 10 mins followed by substrate addition measured for 30 mins b... | J Med Chem 58: 3682-92 (2015) Article DOI: 10.1021/acs.jmedchem.5b00127 BindingDB Entry DOI: 10.7270/Q2765H2V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50089084 (CHEMBL3317857 | Vaborbactam) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents | Article PubMed | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Rempex Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of neutrophil elastase (unknown origin) using MeOSuc-AAVP-AMC as substrate preincubated for 10 mins followed by substrate addition measure... | J Med Chem 58: 3682-92 (2015) Article DOI: 10.1021/acs.jmedchem.5b00127 BindingDB Entry DOI: 10.7270/Q2765H2V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypsin (Homo sapiens (Human)) | BDBM50089084 (CHEMBL3317857 | Vaborbactam) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents | Article PubMed | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Rempex Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of trypsin (unknown origin) using N-Bz-R-AMC as substrate preincubated for 10 mins followed by substrate addition measured for 30 mins by ... | J Med Chem 58: 3682-92 (2015) Article DOI: 10.1021/acs.jmedchem.5b00127 BindingDB Entry DOI: 10.7270/Q2765H2V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 2 (Homo sapiens (Human)) | BDBM50089084 (CHEMBL3317857 | Vaborbactam) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents | Article PubMed | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Rempex Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of dipeptidyl peptidase 7 (unknown origin) using H-Lys-Pro-AMC as substrate preincubated for 10 mins followed by substrate addition measur... | J Med Chem 58: 3682-92 (2015) Article DOI: 10.1021/acs.jmedchem.5b00127 BindingDB Entry DOI: 10.7270/Q2765H2V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Homo sapiens (Human)) | BDBM50089084 (CHEMBL3317857 | Vaborbactam) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents | Article PubMed | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Rempex Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of plasmin (unknown origin) using H-D-VLK-pNA as substrate preincubated for 10 mins followed by substrate addition measured for 30 mins by... | J Med Chem 58: 3682-92 (2015) Article DOI: 10.1021/acs.jmedchem.5b00127 BindingDB Entry DOI: 10.7270/Q2765H2V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50089084 (CHEMBL3317857 | Vaborbactam) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents | Article PubMed | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Rempex Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of thrombin (unknown origin) using Benz-FVR-AMC as substrate preincubated for 10 mins followed by substrate addition measured for 30 mins ... | J Med Chem 58: 3682-92 (2015) Article DOI: 10.1021/acs.jmedchem.5b00127 BindingDB Entry DOI: 10.7270/Q2765H2V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal protective protein (Homo sapiens (Human)) | BDBM50089084 (CHEMBL3317857 | Vaborbactam) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents | Article PubMed | n/a | n/a | 1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Rempex Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of cathepsin A (unknown origin) using MCA-RPPGFSAFK-Dnp as substrate preincubated for 10 mins followed by substrate addition measured for ... | J Med Chem 58: 3682-92 (2015) Article DOI: 10.1021/acs.jmedchem.5b00127 BindingDB Entry DOI: 10.7270/Q2765H2V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM50089084 (CHEMBL3317857 | Vaborbactam) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents | Article PubMed | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Rempex Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of urokinase (unknown origin) using NGK-pNA as substrate preincubated for 10 mins followed by substrate addition measured for 30 mins by s... | J Med Chem 58: 3682-92 (2015) Article DOI: 10.1021/acs.jmedchem.5b00127 BindingDB Entry DOI: 10.7270/Q2765H2V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||