 Found 44 hits of Enzyme Inhibition Constant Data
Found 44 hits of Enzyme Inhibition Constant Data Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50068115

(CHEMBL3402248)Show SMILES Cc1ccc(cc1)S(=O)(=O)NC(=O)C1[C@H](C(=O)OCc2ccccc2)[C@@]2(Cl)C(Cl)=C(Cl)[C@@]1(Cl)C2(Cl)Cl |r,t:30,TLB:15:14:27.29:33,THB:11:13:27.29:33,30:29:33:13.14,28:27:33:13.14| Show InChI InChI=1S/C23H17Cl6NO5S/c1-12-7-9-14(10-8-12)36(33,34)30-19(31)15-16(20(32)35-11-13-5-3-2-4-6-13)22(27)18(25)17(24)21(15,26)23(22,28)29/h2-10,15-16H,11H2,1H3,(H,30,31)/t15?,16-,21-,22-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 36 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Atat£rk University
Curated by ChEMBL
| Assay Description
Inhibition of human CA-1 using p-nitro-phenylacetate as substrate by Lineweaver-Burk plot analysis |
Bioorg Med Chem 23: 2598-605 (2015)
Article DOI: 10.1016/j.bmc.2014.12.054
BindingDB Entry DOI: 10.7270/Q2959K7Q |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50068115

(CHEMBL3402248)Show SMILES Cc1ccc(cc1)S(=O)(=O)NC(=O)C1[C@H](C(=O)OCc2ccccc2)[C@@]2(Cl)C(Cl)=C(Cl)[C@@]1(Cl)C2(Cl)Cl |r,t:30,TLB:15:14:27.29:33,THB:11:13:27.29:33,30:29:33:13.14,28:27:33:13.14| Show InChI InChI=1S/C23H17Cl6NO5S/c1-12-7-9-14(10-8-12)36(33,34)30-19(31)15-16(20(32)35-11-13-5-3-2-4-6-13)22(27)18(25)17(24)21(15,26)23(22,28)29/h2-10,15-16H,11H2,1H3,(H,30,31)/t15?,16-,21-,22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 48 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Atat£rk University
Curated by ChEMBL
| Assay Description
Inhibition of human CA-2 using p-nitro-phenylacetate as substrate by Lineweaver-Burk plot analysis |
Bioorg Med Chem 23: 2598-605 (2015)
Article DOI: 10.1016/j.bmc.2014.12.054
BindingDB Entry DOI: 10.7270/Q2959K7Q |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50068120
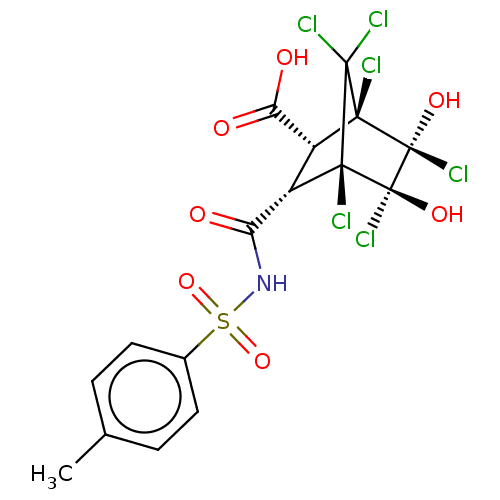
(CHEMBL3402253)Show SMILES Cc1ccc(cc1)S(=O)(=O)NC(=O)[C@@H]1[C@H](C(O)=O)[C@@]2(Cl)[C@@](O)(Cl)[C@](O)(Cl)[C@@]1(Cl)C2(Cl)Cl |r,TLB:15:14:20.23:28,21:20:28:13.14,THB:11:13:20.23:28,24:23:28:13.14| Show InChI InChI=1S/C16H13Cl6NO7S/c1-6-2-4-7(5-3-6)31(29,30)23-10(24)8-9(11(25)26)13(18)14(19,20)12(8,17)15(21,27)16(13,22)28/h2-5,8-9,27-28H,1H3,(H,23,24)(H,25,26)/t8-,9+,12-,13-,15-,16-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 52 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Atat£rk University
Curated by ChEMBL
| Assay Description
Inhibition of human CA-1 using p-nitro-phenylacetate as substrate by Lineweaver-Burk plot analysis |
Bioorg Med Chem 23: 2598-605 (2015)
Article DOI: 10.1016/j.bmc.2014.12.054
BindingDB Entry DOI: 10.7270/Q2959K7Q |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50068120

(CHEMBL3402253)Show SMILES Cc1ccc(cc1)S(=O)(=O)NC(=O)[C@@H]1[C@H](C(O)=O)[C@@]2(Cl)[C@@](O)(Cl)[C@](O)(Cl)[C@@]1(Cl)C2(Cl)Cl |r,TLB:15:14:20.23:28,21:20:28:13.14,THB:11:13:20.23:28,24:23:28:13.14| Show InChI InChI=1S/C16H13Cl6NO7S/c1-6-2-4-7(5-3-6)31(29,30)23-10(24)8-9(11(25)26)13(18)14(19,20)12(8,17)15(21,27)16(13,22)28/h2-5,8-9,27-28H,1H3,(H,23,24)(H,25,26)/t8-,9+,12-,13-,15-,16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 58 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Atat£rk University
Curated by ChEMBL
| Assay Description
Inhibition of human CA-2 using p-nitro-phenylacetate as substrate by Lineweaver-Burk plot analysis |
Bioorg Med Chem 23: 2598-605 (2015)
Article DOI: 10.1016/j.bmc.2014.12.054
BindingDB Entry DOI: 10.7270/Q2959K7Q |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM10880

(AZA | AZA2 | AZM acetazolamide | Acerazolamide, AA...)Show InChI InChI=1S/C4H6N4O3S2/c1-2(9)6-3-7-8-4(12-3)13(5,10)11/h1H3,(H2,5,10,11)(H,6,7,9) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| 61 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Atat£rk University
Curated by ChEMBL
| Assay Description
Inhibition of human CA-2 using p-nitro-phenylacetate as substrate by Lineweaver-Burk plot analysis |
Bioorg Med Chem 23: 2598-605 (2015)
Article DOI: 10.1016/j.bmc.2014.12.054
BindingDB Entry DOI: 10.7270/Q2959K7Q |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50068113
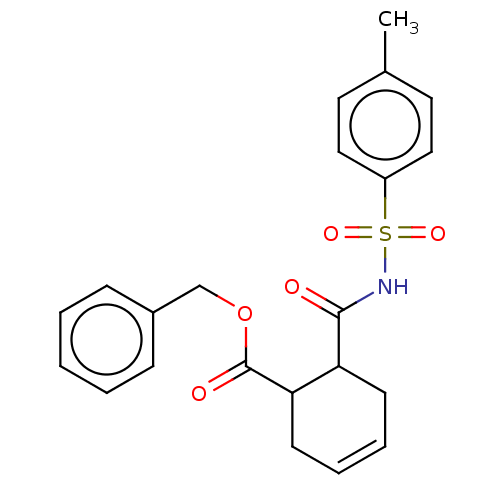
(CHEMBL3402246)Show SMILES Cc1ccc(cc1)S(=O)(=O)NC(=O)C1CC=CCC1C(=O)OCc1ccccc1 |c:16| Show InChI InChI=1S/C22H23NO5S/c1-16-11-13-18(14-12-16)29(26,27)23-21(24)19-9-5-6-10-20(19)22(25)28-15-17-7-3-2-4-8-17/h2-8,11-14,19-20H,9-10,15H2,1H3,(H,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Atat£rk University
Curated by ChEMBL
| Assay Description
Inhibition of human CA-2 using p-nitro-phenylacetate as substrate by Lineweaver-Burk plot analysis |
Bioorg Med Chem 23: 2598-605 (2015)
Article DOI: 10.1016/j.bmc.2014.12.054
BindingDB Entry DOI: 10.7270/Q2959K7Q |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50068116
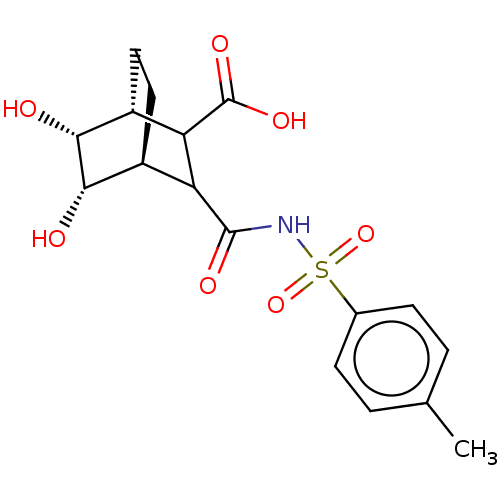
(CHEMBL3402249)Show SMILES [H][C@]12CC[C@]([H])([C@H](O)[C@@H]1O)C(C2C(O)=O)C(=O)NS(=O)(=O)c1ccc(C)cc1 |r,TLB:15:10:2.3:8.6,7:6:2.3:10.11,THB:12:11:2.3:8.6,9:8:2.3:10.11| Show InChI InChI=1S/C17H21NO7S/c1-8-2-4-9(5-3-8)26(24,25)18-16(21)12-10-6-7-11(13(12)17(22)23)15(20)14(10)19/h2-5,10-15,19-20H,6-7H2,1H3,(H,18,21)(H,22,23)/t10-,11+,12?,13?,14-,15+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 97 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Atat£rk University
Curated by ChEMBL
| Assay Description
Inhibition of human CA-1 using p-nitro-phenylacetate as substrate by Lineweaver-Burk plot analysis |
Bioorg Med Chem 23: 2598-605 (2015)
Article DOI: 10.1016/j.bmc.2014.12.054
BindingDB Entry DOI: 10.7270/Q2959K7Q |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50068119

(CHEMBL3402252)Show SMILES Cc1ccc(cc1)S(=O)(=O)NC(=O)C1CC(C)(O)C(O)CC1C(O)=O Show InChI InChI=1S/C16H21NO7S/c1-9-3-5-10(6-4-9)25(23,24)17-14(19)12-8-16(2,22)13(18)7-11(12)15(20)21/h3-6,11-13,18,22H,7-8H2,1-2H3,(H,17,19)(H,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 105 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Atat£rk University
Curated by ChEMBL
| Assay Description
Inhibition of human CA-1 using p-nitro-phenylacetate as substrate by Lineweaver-Burk plot analysis |
Bioorg Med Chem 23: 2598-605 (2015)
Article DOI: 10.1016/j.bmc.2014.12.054
BindingDB Entry DOI: 10.7270/Q2959K7Q |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50068112
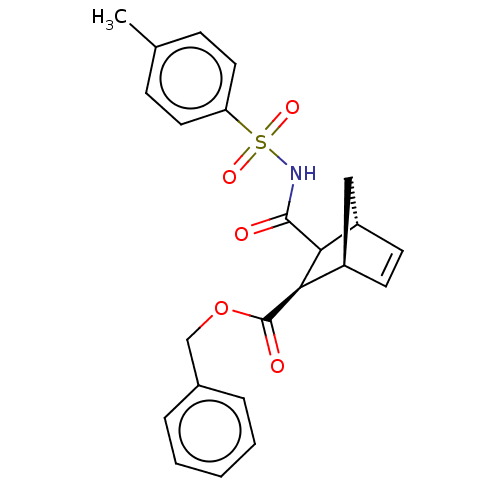
(CHEMBL3402245)Show SMILES [H][C@@]12C[C@@]([H])(C=C1)[C@H](C2C(=O)NS(=O)(=O)c1ccc(C)cc1)C(=O)OCc1ccccc1 |r,c:5| Show InChI InChI=1S/C23H23NO5S/c1-15-7-11-19(12-8-15)30(27,28)24-22(25)20-17-9-10-18(13-17)21(20)23(26)29-14-16-5-3-2-4-6-16/h2-12,17-18,20-21H,13-14H2,1H3,(H,24,25)/t17-,18+,20?,21+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 112 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Atat£rk University
Curated by ChEMBL
| Assay Description
Inhibition of human CA-2 using p-nitro-phenylacetate as substrate by Lineweaver-Burk plot analysis |
Bioorg Med Chem 23: 2598-605 (2015)
Article DOI: 10.1016/j.bmc.2014.12.054
BindingDB Entry DOI: 10.7270/Q2959K7Q |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50068119

(CHEMBL3402252)Show SMILES Cc1ccc(cc1)S(=O)(=O)NC(=O)C1CC(C)(O)C(O)CC1C(O)=O Show InChI InChI=1S/C16H21NO7S/c1-9-3-5-10(6-4-9)25(23,24)17-14(19)12-8-16(2,22)13(18)7-11(12)15(20)21/h3-6,11-13,18,22H,7-8H2,1-2H3,(H,17,19)(H,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 122 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Atat£rk University
Curated by ChEMBL
| Assay Description
Inhibition of human CA-2 using p-nitro-phenylacetate as substrate by Lineweaver-Burk plot analysis |
Bioorg Med Chem 23: 2598-605 (2015)
Article DOI: 10.1016/j.bmc.2014.12.054
BindingDB Entry DOI: 10.7270/Q2959K7Q |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50068114
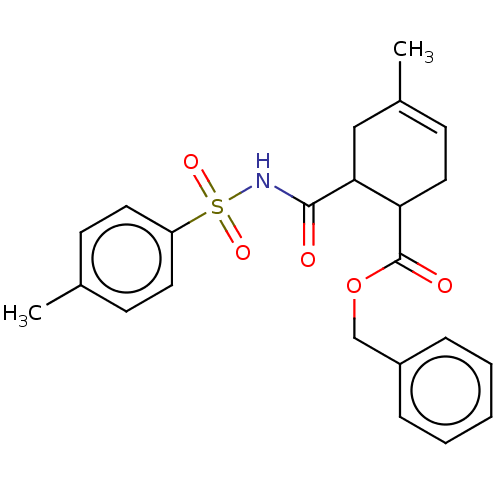
(CHEMBL3402247)Show SMILES CC1=CCC(C(C1)C(=O)NS(=O)(=O)c1ccc(C)cc1)C(=O)OCc1ccccc1 |t:1| Show InChI InChI=1S/C23H25NO5S/c1-16-8-11-19(12-9-16)30(27,28)24-22(25)21-14-17(2)10-13-20(21)23(26)29-15-18-6-4-3-5-7-18/h3-12,20-21H,13-15H2,1-2H3,(H,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 123 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Atat£rk University
Curated by ChEMBL
| Assay Description
Inhibition of human CA-1 using p-nitro-phenylacetate as substrate by Lineweaver-Burk plot analysis |
Bioorg Med Chem 23: 2598-605 (2015)
Article DOI: 10.1016/j.bmc.2014.12.054
BindingDB Entry DOI: 10.7270/Q2959K7Q |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50068111
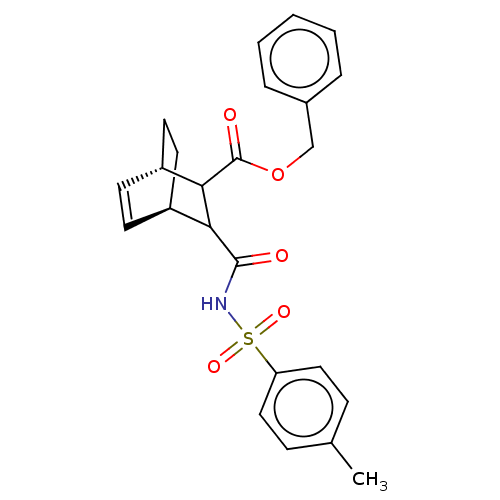
(CHEMBL3402244)Show SMILES [H][C@@]12CC[C@@]([H])(C=C1)C(C2C(=O)NS(=O)(=O)c1ccc(C)cc1)C(=O)OCc1ccccc1 |r,c:6| Show InChI InChI=1S/C24H25NO5S/c1-16-7-13-20(14-8-16)31(28,29)25-23(26)21-18-9-11-19(12-10-18)22(21)24(27)30-15-17-5-3-2-4-6-17/h2-9,11,13-14,18-19,21-22H,10,12,15H2,1H3,(H,25,26)/t18-,19+,21?,22?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 134 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Atat£rk University
Curated by ChEMBL
| Assay Description
Inhibition of human CA-2 using p-nitro-phenylacetate as substrate by Lineweaver-Burk plot analysis |
Bioorg Med Chem 23: 2598-605 (2015)
Article DOI: 10.1016/j.bmc.2014.12.054
BindingDB Entry DOI: 10.7270/Q2959K7Q |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50068118

(CHEMBL3402251)Show SMILES Cc1ccc(cc1)S(=O)(=O)NC(=O)C1CC(O)C(O)CC1C(O)=O Show InChI InChI=1S/C15H19NO7S/c1-8-2-4-9(5-3-8)24(22,23)16-14(19)10-6-12(17)13(18)7-11(10)15(20)21/h2-5,10-13,17-18H,6-7H2,1H3,(H,16,19)(H,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 138 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Atat£rk University
Curated by ChEMBL
| Assay Description
Inhibition of human CA-2 using p-nitro-phenylacetate as substrate by Lineweaver-Burk plot analysis |
Bioorg Med Chem 23: 2598-605 (2015)
Article DOI: 10.1016/j.bmc.2014.12.054
BindingDB Entry DOI: 10.7270/Q2959K7Q |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50068114

(CHEMBL3402247)Show SMILES CC1=CCC(C(C1)C(=O)NS(=O)(=O)c1ccc(C)cc1)C(=O)OCc1ccccc1 |t:1| Show InChI InChI=1S/C23H25NO5S/c1-16-8-11-19(12-9-16)30(27,28)24-22(25)21-14-17(2)10-13-20(21)23(26)29-15-18-6-4-3-5-7-18/h3-12,20-21H,13-15H2,1-2H3,(H,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 139 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Atat£rk University
Curated by ChEMBL
| Assay Description
Inhibition of human CA-2 using p-nitro-phenylacetate as substrate by Lineweaver-Burk plot analysis |
Bioorg Med Chem 23: 2598-605 (2015)
Article DOI: 10.1016/j.bmc.2014.12.054
BindingDB Entry DOI: 10.7270/Q2959K7Q |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50068118

(CHEMBL3402251)Show SMILES Cc1ccc(cc1)S(=O)(=O)NC(=O)C1CC(O)C(O)CC1C(O)=O Show InChI InChI=1S/C15H19NO7S/c1-8-2-4-9(5-3-8)24(22,23)16-14(19)10-6-12(17)13(18)7-11(10)15(20)21/h2-5,10-13,17-18H,6-7H2,1H3,(H,16,19)(H,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 145 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Atat£rk University
Curated by ChEMBL
| Assay Description
Inhibition of human CA-1 using p-nitro-phenylacetate as substrate by Lineweaver-Burk plot analysis |
Bioorg Med Chem 23: 2598-605 (2015)
Article DOI: 10.1016/j.bmc.2014.12.054
BindingDB Entry DOI: 10.7270/Q2959K7Q |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50068116

(CHEMBL3402249)Show SMILES [H][C@]12CC[C@]([H])([C@H](O)[C@@H]1O)C(C2C(O)=O)C(=O)NS(=O)(=O)c1ccc(C)cc1 |r,TLB:15:10:2.3:8.6,7:6:2.3:10.11,THB:12:11:2.3:8.6,9:8:2.3:10.11| Show InChI InChI=1S/C17H21NO7S/c1-8-2-4-9(5-3-8)26(24,25)18-16(21)12-10-6-7-11(13(12)17(22)23)15(20)14(10)19/h2-5,10-15,19-20H,6-7H2,1H3,(H,18,21)(H,22,23)/t10-,11+,12?,13?,14-,15+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 151 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Atat£rk University
Curated by ChEMBL
| Assay Description
Inhibition of human CA-2 using p-nitro-phenylacetate as substrate by Lineweaver-Burk plot analysis |
Bioorg Med Chem 23: 2598-605 (2015)
Article DOI: 10.1016/j.bmc.2014.12.054
BindingDB Entry DOI: 10.7270/Q2959K7Q |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50068113

(CHEMBL3402246)Show SMILES Cc1ccc(cc1)S(=O)(=O)NC(=O)C1CC=CCC1C(=O)OCc1ccccc1 |c:16| Show InChI InChI=1S/C22H23NO5S/c1-16-11-13-18(14-12-16)29(26,27)23-21(24)19-9-5-6-10-20(19)22(25)28-15-17-7-3-2-4-8-17/h2-8,11-14,19-20H,9-10,15H2,1H3,(H,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 175 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Atat£rk University
Curated by ChEMBL
| Assay Description
Inhibition of human CA-1 using p-nitro-phenylacetate as substrate by Lineweaver-Burk plot analysis |
Bioorg Med Chem 23: 2598-605 (2015)
Article DOI: 10.1016/j.bmc.2014.12.054
BindingDB Entry DOI: 10.7270/Q2959K7Q |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM10880

(AZA | AZA2 | AZM acetazolamide | Acerazolamide, AA...)Show InChI InChI=1S/C4H6N4O3S2/c1-2(9)6-3-7-8-4(12-3)13(5,10)11/h1H3,(H2,5,10,11)(H,6,7,9) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 184 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Atat£rk University
Curated by ChEMBL
| Assay Description
Inhibition of human CA-1 using p-nitro-phenylacetate as substrate by Lineweaver-Burk plot analysis |
Bioorg Med Chem 23: 2598-605 (2015)
Article DOI: 10.1016/j.bmc.2014.12.054
BindingDB Entry DOI: 10.7270/Q2959K7Q |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50068111

(CHEMBL3402244)Show SMILES [H][C@@]12CC[C@@]([H])(C=C1)C(C2C(=O)NS(=O)(=O)c1ccc(C)cc1)C(=O)OCc1ccccc1 |r,c:6| Show InChI InChI=1S/C24H25NO5S/c1-16-7-13-20(14-8-16)31(28,29)25-23(26)21-18-9-11-19(12-10-18)22(21)24(27)30-15-17-5-3-2-4-6-17/h2-9,11,13-14,18-19,21-22H,10,12,15H2,1H3,(H,25,26)/t18-,19+,21?,22?/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 186 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Atat£rk University
Curated by ChEMBL
| Assay Description
Inhibition of human CA-1 using p-nitro-phenylacetate as substrate by Lineweaver-Burk plot analysis |
Bioorg Med Chem 23: 2598-605 (2015)
Article DOI: 10.1016/j.bmc.2014.12.054
BindingDB Entry DOI: 10.7270/Q2959K7Q |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50068112

(CHEMBL3402245)Show SMILES [H][C@@]12C[C@@]([H])(C=C1)[C@H](C2C(=O)NS(=O)(=O)c1ccc(C)cc1)C(=O)OCc1ccccc1 |r,c:5| Show InChI InChI=1S/C23H23NO5S/c1-15-7-11-19(12-8-15)30(27,28)24-22(25)20-17-9-10-18(13-17)21(20)23(26)29-14-16-5-3-2-4-6-16/h2-12,17-18,20-21H,13-14H2,1H3,(H,24,25)/t17-,18+,20?,21+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 191 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Atat£rk University
Curated by ChEMBL
| Assay Description
Inhibition of human CA-1 using p-nitro-phenylacetate as substrate by Lineweaver-Burk plot analysis |
Bioorg Med Chem 23: 2598-605 (2015)
Article DOI: 10.1016/j.bmc.2014.12.054
BindingDB Entry DOI: 10.7270/Q2959K7Q |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50068117

(CHEMBL3402250)Show SMILES [H][C@]12C[C@]([H])([C@H](O)[C@@H]1O)[C@@H]([C@@H]2C(O)=O)C(=O)NS(=O)(=O)c1ccc(C)cc1 |r,TLB:14:9:7.5:2,11:10:7.5:2,6:5:2:9.10,8:7:2:9.10| Show InChI InChI=1S/C16H19NO7S/c1-7-2-4-8(5-3-7)25(23,24)17-15(20)11-9-6-10(12(11)16(21)22)14(19)13(9)18/h2-5,9-14,18-19H,6H2,1H3,(H,17,20)(H,21,22)/t9-,10+,11-,12+,13-,14+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 255 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Atat£rk University
Curated by ChEMBL
| Assay Description
Inhibition of human CA-1 using p-nitro-phenylacetate as substrate by Lineweaver-Burk plot analysis |
Bioorg Med Chem 23: 2598-605 (2015)
Article DOI: 10.1016/j.bmc.2014.12.054
BindingDB Entry DOI: 10.7270/Q2959K7Q |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50068117

(CHEMBL3402250)Show SMILES [H][C@]12C[C@]([H])([C@H](O)[C@@H]1O)[C@@H]([C@@H]2C(O)=O)C(=O)NS(=O)(=O)c1ccc(C)cc1 |r,TLB:14:9:7.5:2,11:10:7.5:2,6:5:2:9.10,8:7:2:9.10| Show InChI InChI=1S/C16H19NO7S/c1-7-2-4-8(5-3-7)25(23,24)17-15(20)11-9-6-10(12(11)16(21)22)14(19)13(9)18/h2-5,9-14,18-19H,6H2,1H3,(H,17,20)(H,21,22)/t9-,10+,11-,12+,13-,14+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 273 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Atat£rk University
Curated by ChEMBL
| Assay Description
Inhibition of human CA-2 using p-nitro-phenylacetate as substrate by Lineweaver-Burk plot analysis |
Bioorg Med Chem 23: 2598-605 (2015)
Article DOI: 10.1016/j.bmc.2014.12.054
BindingDB Entry DOI: 10.7270/Q2959K7Q |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50068115

(CHEMBL3402248)Show SMILES Cc1ccc(cc1)S(=O)(=O)NC(=O)C1[C@H](C(=O)OCc2ccccc2)[C@@]2(Cl)C(Cl)=C(Cl)[C@@]1(Cl)C2(Cl)Cl |r,t:30,TLB:15:14:27.29:33,THB:11:13:27.29:33,30:29:33:13.14,28:27:33:13.14| Show InChI InChI=1S/C23H17Cl6NO5S/c1-12-7-9-14(10-8-12)36(33,34)30-19(31)15-16(20(32)35-11-13-5-3-2-4-6-13)22(27)18(25)17(24)21(15,26)23(22,28)29/h2-10,15-16H,11H2,1H3,(H,30,31)/t15?,16-,21-,22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Atat£rk University
Curated by ChEMBL
| Assay Description
Inhibition of human CA-2 using p-nitro-phenylacetate as substrate after 3 mins by UV-Vis spectrophotometry |
Bioorg Med Chem 23: 2598-605 (2015)
Article DOI: 10.1016/j.bmc.2014.12.054
BindingDB Entry DOI: 10.7270/Q2959K7Q |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50068115

(CHEMBL3402248)Show SMILES Cc1ccc(cc1)S(=O)(=O)NC(=O)C1[C@H](C(=O)OCc2ccccc2)[C@@]2(Cl)C(Cl)=C(Cl)[C@@]1(Cl)C2(Cl)Cl |r,t:30,TLB:15:14:27.29:33,THB:11:13:27.29:33,30:29:33:13.14,28:27:33:13.14| Show InChI InChI=1S/C23H17Cl6NO5S/c1-12-7-9-14(10-8-12)36(33,34)30-19(31)15-16(20(32)35-11-13-5-3-2-4-6-13)22(27)18(25)17(24)21(15,26)23(22,28)29/h2-10,15-16H,11H2,1H3,(H,30,31)/t15?,16-,21-,22-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
Atat£rk University
Curated by ChEMBL
| Assay Description
Inhibition of human CA-1 using p-nitro-phenylacetate as substrate after 3 mins by UV-Vis spectrophotometry |
Bioorg Med Chem 23: 2598-605 (2015)
Article DOI: 10.1016/j.bmc.2014.12.054
BindingDB Entry DOI: 10.7270/Q2959K7Q |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50068112

(CHEMBL3402245)Show SMILES [H][C@@]12C[C@@]([H])(C=C1)[C@H](C2C(=O)NS(=O)(=O)c1ccc(C)cc1)C(=O)OCc1ccccc1 |r,c:5| Show InChI InChI=1S/C23H23NO5S/c1-15-7-11-19(12-8-15)30(27,28)24-22(25)20-17-9-10-18(13-17)21(20)23(26)29-14-16-5-3-2-4-6-16/h2-12,17-18,20-21H,13-14H2,1H3,(H,24,25)/t17-,18+,20?,21+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Atat£rk University
Curated by ChEMBL
| Assay Description
Inhibition of human CA-2 using p-nitro-phenylacetate as substrate after 3 mins by UV-Vis spectrophotometry |
Bioorg Med Chem 23: 2598-605 (2015)
Article DOI: 10.1016/j.bmc.2014.12.054
BindingDB Entry DOI: 10.7270/Q2959K7Q |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50068113

(CHEMBL3402246)Show SMILES Cc1ccc(cc1)S(=O)(=O)NC(=O)C1CC=CCC1C(=O)OCc1ccccc1 |c:16| Show InChI InChI=1S/C22H23NO5S/c1-16-11-13-18(14-12-16)29(26,27)23-21(24)19-9-5-6-10-20(19)22(25)28-15-17-7-3-2-4-8-17/h2-8,11-14,19-20H,9-10,15H2,1H3,(H,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 111 | n/a | n/a | n/a | n/a | n/a | n/a |
Atat£rk University
Curated by ChEMBL
| Assay Description
Inhibition of human CA-2 using p-nitro-phenylacetate as substrate after 3 mins by UV-Vis spectrophotometry |
Bioorg Med Chem 23: 2598-605 (2015)
Article DOI: 10.1016/j.bmc.2014.12.054
BindingDB Entry DOI: 10.7270/Q2959K7Q |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50068117

(CHEMBL3402250)Show SMILES [H][C@]12C[C@]([H])([C@H](O)[C@@H]1O)[C@@H]([C@@H]2C(O)=O)C(=O)NS(=O)(=O)c1ccc(C)cc1 |r,TLB:14:9:7.5:2,11:10:7.5:2,6:5:2:9.10,8:7:2:9.10| Show InChI InChI=1S/C16H19NO7S/c1-7-2-4-8(5-3-7)25(23,24)17-15(20)11-9-6-10(12(11)16(21)22)14(19)13(9)18/h2-5,9-14,18-19H,6H2,1H3,(H,17,20)(H,21,22)/t9-,10+,11-,12+,13-,14+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 111 | n/a | n/a | n/a | n/a | n/a | n/a |
Atat£rk University
Curated by ChEMBL
| Assay Description
Inhibition of human CA-2 using p-nitro-phenylacetate as substrate after 3 mins by UV-Vis spectrophotometry |
Bioorg Med Chem 23: 2598-605 (2015)
Article DOI: 10.1016/j.bmc.2014.12.054
BindingDB Entry DOI: 10.7270/Q2959K7Q |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50068120

(CHEMBL3402253)Show SMILES Cc1ccc(cc1)S(=O)(=O)NC(=O)[C@@H]1[C@H](C(O)=O)[C@@]2(Cl)[C@@](O)(Cl)[C@](O)(Cl)[C@@]1(Cl)C2(Cl)Cl |r,TLB:15:14:20.23:28,21:20:28:13.14,THB:11:13:20.23:28,24:23:28:13.14| Show InChI InChI=1S/C16H13Cl6NO7S/c1-6-2-4-7(5-3-6)31(29,30)23-10(24)8-9(11(25)26)13(18)14(19,20)12(8,17)15(21,27)16(13,22)28/h2-5,8-9,27-28H,1H3,(H,23,24)(H,25,26)/t8-,9+,12-,13-,15-,16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Atat£rk University
Curated by ChEMBL
| Assay Description
Inhibition of human CA-2 using p-nitro-phenylacetate as substrate after 3 mins by UV-Vis spectrophotometry |
Bioorg Med Chem 23: 2598-605 (2015)
Article DOI: 10.1016/j.bmc.2014.12.054
BindingDB Entry DOI: 10.7270/Q2959K7Q |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50068111

(CHEMBL3402244)Show SMILES [H][C@@]12CC[C@@]([H])(C=C1)C(C2C(=O)NS(=O)(=O)c1ccc(C)cc1)C(=O)OCc1ccccc1 |r,c:6| Show InChI InChI=1S/C24H25NO5S/c1-16-7-13-20(14-8-16)31(28,29)25-23(26)21-18-9-11-19(12-10-18)22(21)24(27)30-15-17-5-3-2-4-6-17/h2-9,11,13-14,18-19,21-22H,10,12,15H2,1H3,(H,25,26)/t18-,19+,21?,22?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 122 | n/a | n/a | n/a | n/a | n/a | n/a |
Atat£rk University
Curated by ChEMBL
| Assay Description
Inhibition of human CA-2 using p-nitro-phenylacetate as substrate after 3 mins by UV-Vis spectrophotometry |
Bioorg Med Chem 23: 2598-605 (2015)
Article DOI: 10.1016/j.bmc.2014.12.054
BindingDB Entry DOI: 10.7270/Q2959K7Q |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM10880

(AZA | AZA2 | AZM acetazolamide | Acerazolamide, AA...)Show InChI InChI=1S/C4H6N4O3S2/c1-2(9)6-3-7-8-4(12-3)13(5,10)11/h1H3,(H2,5,10,11)(H,6,7,9) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | 124 | n/a | n/a | n/a | n/a | n/a | n/a |
Atat£rk University
Curated by ChEMBL
| Assay Description
Inhibition of human CA-2 using p-nitro-phenylacetate as substrate after 3 mins by UV-Vis spectrophotometry |
Bioorg Med Chem 23: 2598-605 (2015)
Article DOI: 10.1016/j.bmc.2014.12.054
BindingDB Entry DOI: 10.7270/Q2959K7Q |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50068116

(CHEMBL3402249)Show SMILES [H][C@]12CC[C@]([H])([C@H](O)[C@@H]1O)C(C2C(O)=O)C(=O)NS(=O)(=O)c1ccc(C)cc1 |r,TLB:15:10:2.3:8.6,7:6:2.3:10.11,THB:12:11:2.3:8.6,9:8:2.3:10.11| Show InChI InChI=1S/C17H21NO7S/c1-8-2-4-9(5-3-8)26(24,25)18-16(21)12-10-6-7-11(13(12)17(22)23)15(20)14(10)19/h2-5,10-15,19-20H,6-7H2,1H3,(H,18,21)(H,22,23)/t10-,11+,12?,13?,14-,15+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 127 | n/a | n/a | n/a | n/a | n/a | n/a |
Atat£rk University
Curated by ChEMBL
| Assay Description
Inhibition of human CA-2 using p-nitro-phenylacetate as substrate after 3 mins by UV-Vis spectrophotometry |
Bioorg Med Chem 23: 2598-605 (2015)
Article DOI: 10.1016/j.bmc.2014.12.054
BindingDB Entry DOI: 10.7270/Q2959K7Q |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50068114

(CHEMBL3402247)Show SMILES CC1=CCC(C(C1)C(=O)NS(=O)(=O)c1ccc(C)cc1)C(=O)OCc1ccccc1 |t:1| Show InChI InChI=1S/C23H25NO5S/c1-16-8-11-19(12-9-16)30(27,28)24-22(25)21-14-17(2)10-13-20(21)23(26)29-15-18-6-4-3-5-7-18/h3-12,20-21H,13-15H2,1-2H3,(H,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 131 | n/a | n/a | n/a | n/a | n/a | n/a |
Atat£rk University
Curated by ChEMBL
| Assay Description
Inhibition of human CA-2 using p-nitro-phenylacetate as substrate after 3 mins by UV-Vis spectrophotometry |
Bioorg Med Chem 23: 2598-605 (2015)
Article DOI: 10.1016/j.bmc.2014.12.054
BindingDB Entry DOI: 10.7270/Q2959K7Q |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50068112

(CHEMBL3402245)Show SMILES [H][C@@]12C[C@@]([H])(C=C1)[C@H](C2C(=O)NS(=O)(=O)c1ccc(C)cc1)C(=O)OCc1ccccc1 |r,c:5| Show InChI InChI=1S/C23H23NO5S/c1-15-7-11-19(12-8-15)30(27,28)24-22(25)20-17-9-10-18(13-17)21(20)23(26)29-14-16-5-3-2-4-6-16/h2-12,17-18,20-21H,13-14H2,1H3,(H,24,25)/t17-,18+,20?,21+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 133 | n/a | n/a | n/a | n/a | n/a | n/a |
Atat£rk University
Curated by ChEMBL
| Assay Description
Inhibition of human CA-1 using p-nitro-phenylacetate as substrate after 3 mins by UV-Vis spectrophotometry |
Bioorg Med Chem 23: 2598-605 (2015)
Article DOI: 10.1016/j.bmc.2014.12.054
BindingDB Entry DOI: 10.7270/Q2959K7Q |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50068116

(CHEMBL3402249)Show SMILES [H][C@]12CC[C@]([H])([C@H](O)[C@@H]1O)C(C2C(O)=O)C(=O)NS(=O)(=O)c1ccc(C)cc1 |r,TLB:15:10:2.3:8.6,7:6:2.3:10.11,THB:12:11:2.3:8.6,9:8:2.3:10.11| Show InChI InChI=1S/C17H21NO7S/c1-8-2-4-9(5-3-8)26(24,25)18-16(21)12-10-6-7-11(13(12)17(22)23)15(20)14(10)19/h2-5,10-15,19-20H,6-7H2,1H3,(H,18,21)(H,22,23)/t10-,11+,12?,13?,14-,15+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 134 | n/a | n/a | n/a | n/a | n/a | n/a |
Atat£rk University
Curated by ChEMBL
| Assay Description
Inhibition of human CA-1 using p-nitro-phenylacetate as substrate after 3 mins by UV-Vis spectrophotometry |
Bioorg Med Chem 23: 2598-605 (2015)
Article DOI: 10.1016/j.bmc.2014.12.054
BindingDB Entry DOI: 10.7270/Q2959K7Q |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50068119

(CHEMBL3402252)Show SMILES Cc1ccc(cc1)S(=O)(=O)NC(=O)C1CC(C)(O)C(O)CC1C(O)=O Show InChI InChI=1S/C16H21NO7S/c1-9-3-5-10(6-4-9)25(23,24)17-14(19)12-8-16(2,22)13(18)7-11(12)15(20)21/h3-6,11-13,18,22H,7-8H2,1-2H3,(H,17,19)(H,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 144 | n/a | n/a | n/a | n/a | n/a | n/a |
Atat£rk University
Curated by ChEMBL
| Assay Description
Inhibition of human CA-2 using p-nitro-phenylacetate as substrate after 3 mins by UV-Vis spectrophotometry |
Bioorg Med Chem 23: 2598-605 (2015)
Article DOI: 10.1016/j.bmc.2014.12.054
BindingDB Entry DOI: 10.7270/Q2959K7Q |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50068113

(CHEMBL3402246)Show SMILES Cc1ccc(cc1)S(=O)(=O)NC(=O)C1CC=CCC1C(=O)OCc1ccccc1 |c:16| Show InChI InChI=1S/C22H23NO5S/c1-16-11-13-18(14-12-16)29(26,27)23-21(24)19-9-5-6-10-20(19)22(25)28-15-17-7-3-2-4-8-17/h2-8,11-14,19-20H,9-10,15H2,1H3,(H,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 153 | n/a | n/a | n/a | n/a | n/a | n/a |
Atat£rk University
Curated by ChEMBL
| Assay Description
Inhibition of human CA-1 using p-nitro-phenylacetate as substrate after 3 mins by UV-Vis spectrophotometry |
Bioorg Med Chem 23: 2598-605 (2015)
Article DOI: 10.1016/j.bmc.2014.12.054
BindingDB Entry DOI: 10.7270/Q2959K7Q |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50068118

(CHEMBL3402251)Show SMILES Cc1ccc(cc1)S(=O)(=O)NC(=O)C1CC(O)C(O)CC1C(O)=O Show InChI InChI=1S/C15H19NO7S/c1-8-2-4-9(5-3-8)24(22,23)16-14(19)10-6-12(17)13(18)7-11(10)15(20)21/h2-5,10-13,17-18H,6-7H2,1H3,(H,16,19)(H,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 154 | n/a | n/a | n/a | n/a | n/a | n/a |
Atat£rk University
Curated by ChEMBL
| Assay Description
Inhibition of human CA-2 using p-nitro-phenylacetate as substrate after 3 mins by UV-Vis spectrophotometry |
Bioorg Med Chem 23: 2598-605 (2015)
Article DOI: 10.1016/j.bmc.2014.12.054
BindingDB Entry DOI: 10.7270/Q2959K7Q |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50068120

(CHEMBL3402253)Show SMILES Cc1ccc(cc1)S(=O)(=O)NC(=O)[C@@H]1[C@H](C(O)=O)[C@@]2(Cl)[C@@](O)(Cl)[C@](O)(Cl)[C@@]1(Cl)C2(Cl)Cl |r,TLB:15:14:20.23:28,21:20:28:13.14,THB:11:13:20.23:28,24:23:28:13.14| Show InChI InChI=1S/C16H13Cl6NO7S/c1-6-2-4-7(5-3-6)31(29,30)23-10(24)8-9(11(25)26)13(18)14(19,20)12(8,17)15(21,27)16(13,22)28/h2-5,8-9,27-28H,1H3,(H,23,24)(H,25,26)/t8-,9+,12-,13-,15-,16-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 171 | n/a | n/a | n/a | n/a | n/a | n/a |
Atat£rk University
Curated by ChEMBL
| Assay Description
Inhibition of human CA-1 using p-nitro-phenylacetate as substrate after 3 mins by UV-Vis spectrophotometry |
Bioorg Med Chem 23: 2598-605 (2015)
Article DOI: 10.1016/j.bmc.2014.12.054
BindingDB Entry DOI: 10.7270/Q2959K7Q |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50068114

(CHEMBL3402247)Show SMILES CC1=CCC(C(C1)C(=O)NS(=O)(=O)c1ccc(C)cc1)C(=O)OCc1ccccc1 |t:1| Show InChI InChI=1S/C23H25NO5S/c1-16-8-11-19(12-9-16)30(27,28)24-22(25)21-14-17(2)10-13-20(21)23(26)29-15-18-6-4-3-5-7-18/h3-12,20-21H,13-15H2,1-2H3,(H,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 179 | n/a | n/a | n/a | n/a | n/a | n/a |
Atat£rk University
Curated by ChEMBL
| Assay Description
Inhibition of human CA-1 using p-nitro-phenylacetate as substrate after 3 mins by UV-Vis spectrophotometry |
Bioorg Med Chem 23: 2598-605 (2015)
Article DOI: 10.1016/j.bmc.2014.12.054
BindingDB Entry DOI: 10.7270/Q2959K7Q |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50068111

(CHEMBL3402244)Show SMILES [H][C@@]12CC[C@@]([H])(C=C1)C(C2C(=O)NS(=O)(=O)c1ccc(C)cc1)C(=O)OCc1ccccc1 |r,c:6| Show InChI InChI=1S/C24H25NO5S/c1-16-7-13-20(14-8-16)31(28,29)25-23(26)21-18-9-11-19(12-10-18)22(21)24(27)30-15-17-5-3-2-4-6-17/h2-9,11,13-14,18-19,21-22H,10,12,15H2,1H3,(H,25,26)/t18-,19+,21?,22?/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 179 | n/a | n/a | n/a | n/a | n/a | n/a |
Atat£rk University
Curated by ChEMBL
| Assay Description
Inhibition of human CA-1 using p-nitro-phenylacetate as substrate after 3 mins by UV-Vis spectrophotometry |
Bioorg Med Chem 23: 2598-605 (2015)
Article DOI: 10.1016/j.bmc.2014.12.054
BindingDB Entry DOI: 10.7270/Q2959K7Q |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50068118

(CHEMBL3402251)Show SMILES Cc1ccc(cc1)S(=O)(=O)NC(=O)C1CC(O)C(O)CC1C(O)=O Show InChI InChI=1S/C15H19NO7S/c1-8-2-4-9(5-3-8)24(22,23)16-14(19)10-6-12(17)13(18)7-11(10)15(20)21/h2-5,10-13,17-18H,6-7H2,1H3,(H,16,19)(H,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
Atat£rk University
Curated by ChEMBL
| Assay Description
Inhibition of human CA-1 using p-nitro-phenylacetate as substrate after 3 mins by UV-Vis spectrophotometry |
Bioorg Med Chem 23: 2598-605 (2015)
Article DOI: 10.1016/j.bmc.2014.12.054
BindingDB Entry DOI: 10.7270/Q2959K7Q |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50068117

(CHEMBL3402250)Show SMILES [H][C@]12C[C@]([H])([C@H](O)[C@@H]1O)[C@@H]([C@@H]2C(O)=O)C(=O)NS(=O)(=O)c1ccc(C)cc1 |r,TLB:14:9:7.5:2,11:10:7.5:2,6:5:2:9.10,8:7:2:9.10| Show InChI InChI=1S/C16H19NO7S/c1-7-2-4-8(5-3-7)25(23,24)17-15(20)11-9-6-10(12(11)16(21)22)14(19)13(9)18/h2-5,9-14,18-19H,6H2,1H3,(H,17,20)(H,21,22)/t9-,10+,11-,12+,13-,14+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
Atat£rk University
Curated by ChEMBL
| Assay Description
Inhibition of human CA-1 using p-nitro-phenylacetate as substrate after 3 mins by UV-Vis spectrophotometry |
Bioorg Med Chem 23: 2598-605 (2015)
Article DOI: 10.1016/j.bmc.2014.12.054
BindingDB Entry DOI: 10.7270/Q2959K7Q |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50068119

(CHEMBL3402252)Show SMILES Cc1ccc(cc1)S(=O)(=O)NC(=O)C1CC(C)(O)C(O)CC1C(O)=O Show InChI InChI=1S/C16H21NO7S/c1-9-3-5-10(6-4-9)25(23,24)17-14(19)12-8-16(2,22)13(18)7-11(12)15(20)21/h3-6,11-13,18,22H,7-8H2,1-2H3,(H,17,19)(H,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 215 | n/a | n/a | n/a | n/a | n/a | n/a |
Atat£rk University
Curated by ChEMBL
| Assay Description
Inhibition of human CA-1 using p-nitro-phenylacetate as substrate after 3 mins by UV-Vis spectrophotometry |
Bioorg Med Chem 23: 2598-605 (2015)
Article DOI: 10.1016/j.bmc.2014.12.054
BindingDB Entry DOI: 10.7270/Q2959K7Q |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM10880

(AZA | AZA2 | AZM acetazolamide | Acerazolamide, AA...)Show InChI InChI=1S/C4H6N4O3S2/c1-2(9)6-3-7-8-4(12-3)13(5,10)11/h1H3,(H2,5,10,11)(H,6,7,9) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 316 | n/a | n/a | n/a | n/a | n/a | n/a |
Atat£rk University
Curated by ChEMBL
| Assay Description
Inhibition of human CA-1 using p-nitro-phenylacetate as substrate after 3 mins by UV-Vis spectrophotometry |
Bioorg Med Chem 23: 2598-605 (2015)
Article DOI: 10.1016/j.bmc.2014.12.054
BindingDB Entry DOI: 10.7270/Q2959K7Q |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data