 Found 66 hits of Enzyme Inhibition Constant Data
Found 66 hits of Enzyme Inhibition Constant Data Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Carboxypeptidase B
(Homo sapiens (Human)) | BDBM50089691
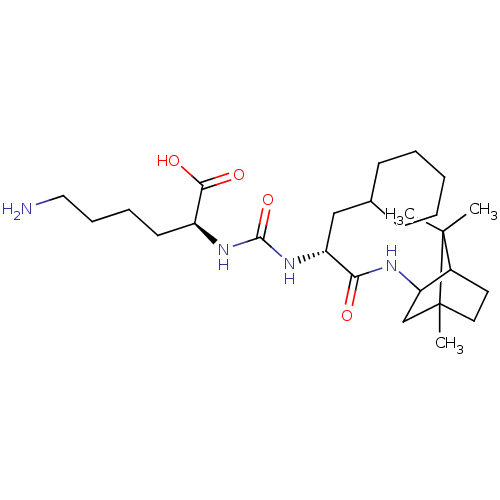
(CHEMBL3577425)Show SMILES CC1(C)C2CCC1(C)CC2NC(=O)[C@@H](CC1CCCCC1)NC(=O)N[C@@H](CCCCN)C(O)=O |r,TLB:10:9:5.4:1| Show InChI InChI=1S/C26H46N4O4/c1-25(2)18-12-13-26(25,3)16-21(18)28-22(31)20(15-17-9-5-4-6-10-17)30-24(34)29-19(23(32)33)11-7-8-14-27/h17-21H,4-16,27H2,1-3H3,(H,28,31)(H,32,33)(H2,29,30,34)/t18?,19-,20+,21?,26?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Infection Research
Curated by ChEMBL
| Assay Description
Inhibition of CBP (unknown origin) |
J Med Chem 58: 4839-44 (2015)
Article DOI: 10.1021/jm501840b
BindingDB Entry DOI: 10.7270/Q24B3314 |
More data for this
Ligand-Target Pair | |
Carboxypeptidase B2
(Homo sapiens (Human)) | BDBM50089758
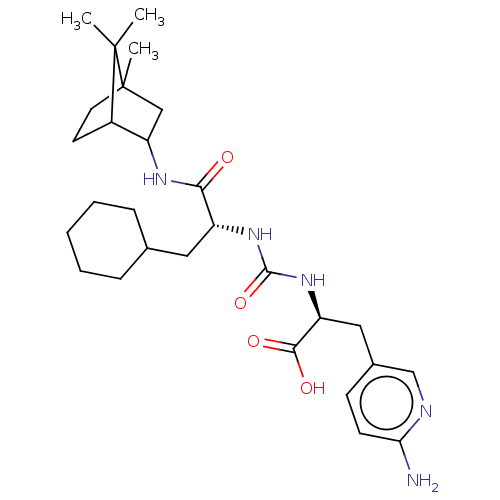
(CHEMBL3577442)Show SMILES CC12CCC(C(C1)NC(=O)[C@@H](CC1CCCCC1)NC(=O)N[C@@H](Cc1ccc(N)nc1)C(O)=O)C2(C)C |r,THB:7:5:34:2.3| Show InChI InChI=1S/C28H43N5O4/c1-27(2)19-11-12-28(27,3)15-22(19)31-24(34)20(13-17-7-5-4-6-8-17)32-26(37)33-21(25(35)36)14-18-9-10-23(29)30-16-18/h9-10,16-17,19-22H,4-8,11-15H2,1-3H3,(H2,29,30)(H,31,34)(H,35,36)(H2,32,33,37)/t19?,20-,21+,22?,28?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Infection Research
Curated by ChEMBL
| Assay Description
Inhibition of human activated TAFI incubated for 15 mins by microtiter plate reader based assay |
J Med Chem 58: 4839-44 (2015)
Article DOI: 10.1021/jm501840b
BindingDB Entry DOI: 10.7270/Q24B3314 |
More data for this
Ligand-Target Pair | |
Carboxypeptidase B2
(Homo sapiens (Human)) | BDBM50089687
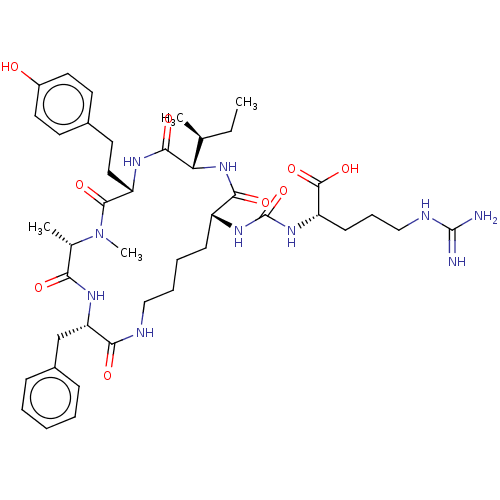
(Anabaenopeptin F)Show SMILES [H][C@]1(NC(=O)[C@@H](CCCCNC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](C)N(C)C(=O)[C@H](CCc2ccc(O)cc2)NC1=O)NC(=O)N[C@@H](CCCNC(N)=N)C(O)=O)[C@@H](C)CC |r| Show InChI InChI=1S/C42H62N10O9/c1-5-25(2)34-38(57)47-31(21-18-27-16-19-29(53)20-17-27)39(58)52(4)26(3)35(54)48-33(24-28-12-7-6-8-13-28)36(55)45-22-10-9-14-30(37(56)51-34)49-42(61)50-32(40(59)60)15-11-23-46-41(43)44/h6-8,12-13,16-17,19-20,25-26,30-34,53H,5,9-11,14-15,18,21-24H2,1-4H3,(H,45,55)(H,47,57)(H,48,54)(H,51,56)(H,59,60)(H4,43,44,46)(H2,49,50,61)/t25-,26-,30+,31-,32-,33-,34-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Infection Research
Curated by ChEMBL
| Assay Description
Inhibition of human activated TAFI incubated for 15 mins by microtiter plate reader based assay |
J Med Chem 58: 4839-44 (2015)
Article DOI: 10.1021/jm501840b
BindingDB Entry DOI: 10.7270/Q24B3314 |
More data for this
Ligand-Target Pair | |
Carboxypeptidase B2
(Homo sapiens (Human)) | BDBM50089688
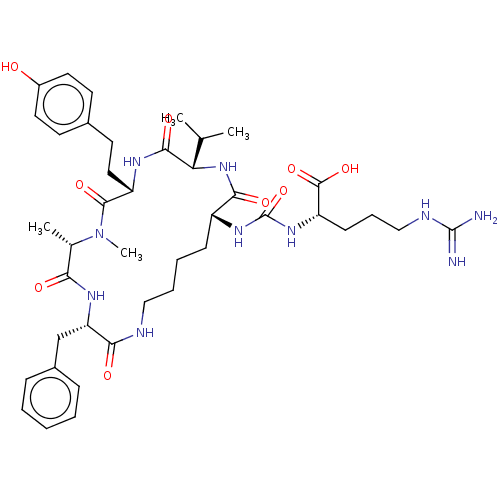
(ANABAENOPEPTIN B)Show SMILES CC(C)[C@@H]1NC(=O)[C@@H](CCCCNC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](C)N(C)C(=O)[C@H](CCc2ccc(O)cc2)NC1=O)NC(=O)N[C@@H](CCCNC(N)=N)C(O)=O |r| Show InChI InChI=1S/C41H60N10O9/c1-24(2)33-37(56)46-30(20-17-26-15-18-28(52)19-16-26)38(57)51(4)25(3)34(53)47-32(23-27-11-6-5-7-12-27)35(54)44-21-9-8-13-29(36(55)50-33)48-41(60)49-31(39(58)59)14-10-22-45-40(42)43/h5-7,11-12,15-16,18-19,24-25,29-33,52H,8-10,13-14,17,20-23H2,1-4H3,(H,44,54)(H,46,56)(H,47,53)(H,50,55)(H,58,59)(H4,42,43,45)(H2,48,49,60)/t25-,29+,30-,31-,32-,33-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Infection Research
Curated by ChEMBL
| Assay Description
Inhibition of human activated TAFI incubated for 15 mins by microtiter plate reader based assay |
J Med Chem 58: 4839-44 (2015)
Article DOI: 10.1021/jm501840b
BindingDB Entry DOI: 10.7270/Q24B3314 |
More data for this
Ligand-Target Pair | |
Carboxypeptidase B2
(Homo sapiens (Human)) | BDBM50089686
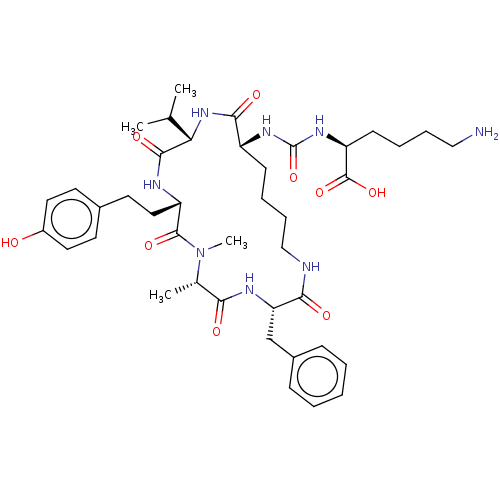
(CHEMBL3577334)Show SMILES CC(C)[C@@H]1NC(=O)[C@@H](CCCCNC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](C)N(C)C(=O)[C@H](CCc2ccc(O)cc2)NC1=O)NC(=O)N[C@@H](CCCCN)C(O)=O |r| Show InChI InChI=1S/C41H60N8O9/c1-25(2)34-38(54)44-31(21-18-27-16-19-29(50)20-17-27)39(55)49(4)26(3)35(51)45-33(24-28-12-6-5-7-13-28)36(52)43-23-11-9-14-30(37(53)48-34)46-41(58)47-32(40(56)57)15-8-10-22-42/h5-7,12-13,16-17,19-20,25-26,30-34,50H,8-11,14-15,18,21-24,42H2,1-4H3,(H,43,52)(H,44,54)(H,45,51)(H,48,53)(H,56,57)(H2,46,47,58)/t26-,30+,31-,32-,33-,34-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Infection Research
Curated by ChEMBL
| Assay Description
Inhibition of human activated TAFI incubated for 15 mins by microtiter plate reader based assay |
J Med Chem 58: 4839-44 (2015)
Article DOI: 10.1021/jm501840b
BindingDB Entry DOI: 10.7270/Q24B3314 |
More data for this
Ligand-Target Pair | |
Carboxypeptidase B2
(Homo sapiens (Human)) | BDBM50089691

(CHEMBL3577425)Show SMILES CC1(C)C2CCC1(C)CC2NC(=O)[C@@H](CC1CCCCC1)NC(=O)N[C@@H](CCCCN)C(O)=O |r,TLB:10:9:5.4:1| Show InChI InChI=1S/C26H46N4O4/c1-25(2)18-12-13-26(25,3)16-21(18)28-22(31)20(15-17-9-5-4-6-10-17)30-24(34)29-19(23(32)33)11-7-8-14-27/h17-21H,4-16,27H2,1-3H3,(H,28,31)(H,32,33)(H2,29,30,34)/t18?,19-,20+,21?,26?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Infection Research
Curated by ChEMBL
| Assay Description
Inhibition of human activated TAFI incubated for 15 mins by microtiter plate reader based assay |
J Med Chem 58: 4839-44 (2015)
Article DOI: 10.1021/jm501840b
BindingDB Entry DOI: 10.7270/Q24B3314 |
More data for this
Ligand-Target Pair | |
Carboxypeptidase B2
(Homo sapiens (Human)) | BDBM50089751
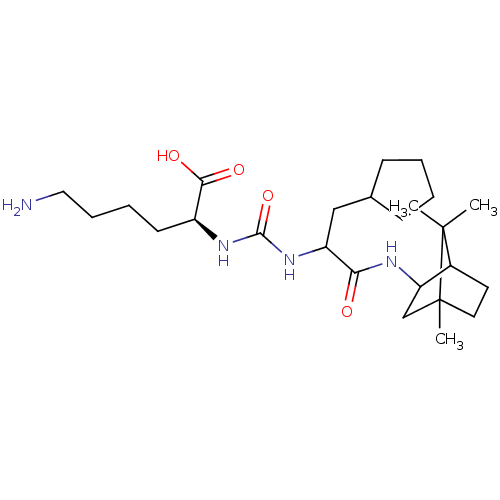
(CHEMBL3577435)Show SMILES CC1(C)C2CCC1(C)CC2NC(=O)C(CC1CCCC1)NC(=O)N[C@@H](CCCCN)C(O)=O |r,TLB:10:9:1:5.4| Show InChI InChI=1S/C25H44N4O4/c1-24(2)17-11-12-25(24,3)15-20(17)27-21(30)19(14-16-8-4-5-9-16)29-23(33)28-18(22(31)32)10-6-7-13-26/h16-20H,4-15,26H2,1-3H3,(H,27,30)(H,31,32)(H2,28,29,33)/t17?,18-,19?,20?,25?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Infection Research
Curated by ChEMBL
| Assay Description
Inhibition of human activated TAFI incubated for 15 mins by microtiter plate reader based assay |
J Med Chem 58: 4839-44 (2015)
Article DOI: 10.1021/jm501840b
BindingDB Entry DOI: 10.7270/Q24B3314 |
More data for this
Ligand-Target Pair | |
Carboxypeptidase B2
(Homo sapiens (Human)) | BDBM50089755
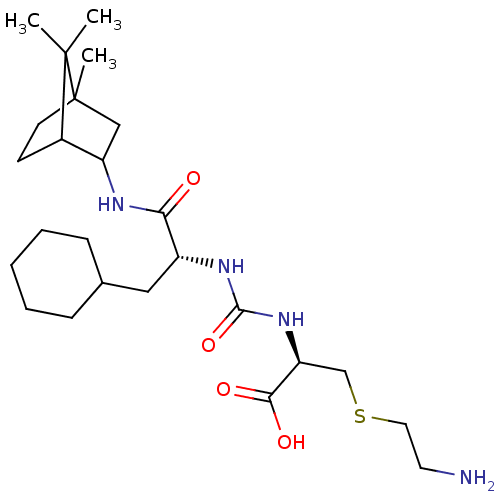
(CHEMBL3577439)Show SMILES CC12CCC(C(C1)NC(=O)[C@@H](CC1CCCCC1)NC(=O)N[C@@H](CSCCN)C(O)=O)C2(C)C |r,THB:7:5:31:2.3| Show InChI InChI=1S/C25H44N4O4S/c1-24(2)17-9-10-25(24,3)14-19(17)27-21(30)18(13-16-7-5-4-6-8-16)28-23(33)29-20(22(31)32)15-34-12-11-26/h16-20H,4-15,26H2,1-3H3,(H,27,30)(H,31,32)(H2,28,29,33)/t17?,18-,19?,20+,25?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Infection Research
Curated by ChEMBL
| Assay Description
Inhibition of human activated TAFI incubated for 15 mins by microtiter plate reader based assay |
J Med Chem 58: 4839-44 (2015)
Article DOI: 10.1021/jm501840b
BindingDB Entry DOI: 10.7270/Q24B3314 |
More data for this
Ligand-Target Pair | |
Carboxypeptidase B2
(Homo sapiens (Human)) | BDBM50089756
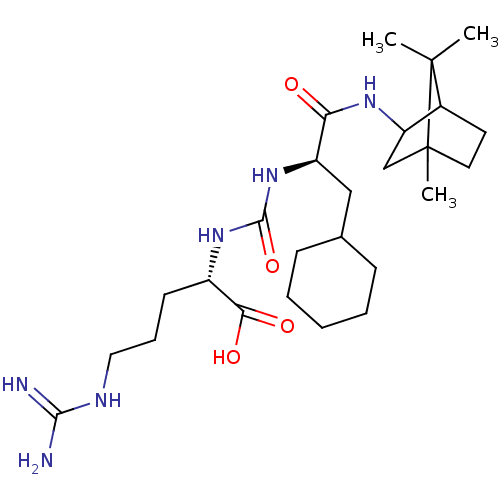
(CHEMBL3577440)Show SMILES CC1(C)C2CCC1(C)CC2NC(=O)[C@@H](CC1CCCCC1)NC(=O)N[C@@H](CCCNC(N)=N)C(O)=O |r,TLB:10:9:1:5.4| Show InChI InChI=1S/C26H46N6O4/c1-25(2)17-11-12-26(25,3)15-20(17)30-21(33)19(14-16-8-5-4-6-9-16)32-24(36)31-18(22(34)35)10-7-13-29-23(27)28/h16-20H,4-15H2,1-3H3,(H,30,33)(H,34,35)(H4,27,28,29)(H2,31,32,36)/t17?,18-,19+,20?,26?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Infection Research
Curated by ChEMBL
| Assay Description
Inhibition of human activated TAFI incubated for 15 mins by microtiter plate reader based assay |
J Med Chem 58: 4839-44 (2015)
Article DOI: 10.1021/jm501840b
BindingDB Entry DOI: 10.7270/Q24B3314 |
More data for this
Ligand-Target Pair | |
Carboxypeptidase B2
(Homo sapiens (Human)) | BDBM50089749
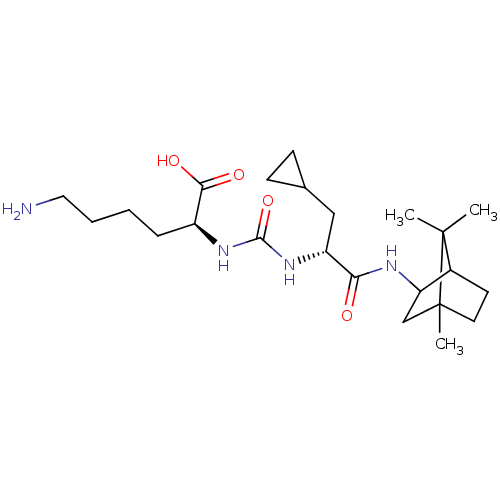
(CHEMBL3577433)Show SMILES CC1(C)C2CCC1(C)CC2NC(=O)[C@@H](CC1CC1)NC(=O)N[C@@H](CCCCN)C(O)=O |r,TLB:10:9:1:5.4| Show InChI InChI=1S/C23H40N4O4/c1-22(2)15-9-10-23(22,3)13-18(15)25-19(28)17(12-14-7-8-14)27-21(31)26-16(20(29)30)6-4-5-11-24/h14-18H,4-13,24H2,1-3H3,(H,25,28)(H,29,30)(H2,26,27,31)/t15?,16-,17+,18?,23?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Infection Research
Curated by ChEMBL
| Assay Description
Inhibition of human activated TAFI incubated for 15 mins by microtiter plate reader based assay |
J Med Chem 58: 4839-44 (2015)
Article DOI: 10.1021/jm501840b
BindingDB Entry DOI: 10.7270/Q24B3314 |
More data for this
Ligand-Target Pair | |
Carboxypeptidase B2
(Homo sapiens (Human)) | BDBM50089691

(CHEMBL3577425)Show SMILES CC1(C)C2CCC1(C)CC2NC(=O)[C@@H](CC1CCCCC1)NC(=O)N[C@@H](CCCCN)C(O)=O |r,TLB:10:9:5.4:1| Show InChI InChI=1S/C26H46N4O4/c1-25(2)18-12-13-26(25,3)16-21(18)28-22(31)20(15-17-9-5-4-6-10-17)30-24(34)29-19(23(32)33)11-7-8-14-27/h17-21H,4-16,27H2,1-3H3,(H,28,31)(H,32,33)(H2,29,30,34)/t18?,19-,20+,21?,26?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Infection Research
Curated by ChEMBL
| Assay Description
Inhibition of human activated TAFI incubated for 15 mins in presence of 1% human serum albumin by microtiter plate reader based assay |
J Med Chem 58: 4839-44 (2015)
Article DOI: 10.1021/jm501840b
BindingDB Entry DOI: 10.7270/Q24B3314 |
More data for this
Ligand-Target Pair | |
Carboxypeptidase B2
(Homo sapiens (Human)) | BDBM50089750
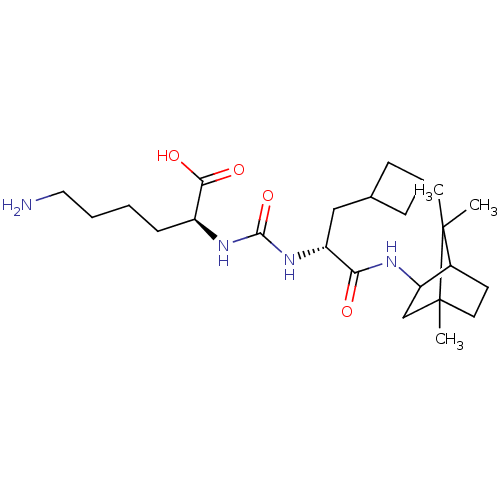
(CHEMBL3577434)Show SMILES CC1(C)C2CCC1(C)CC2NC(=O)[C@@H](CC1CCC1)NC(=O)N[C@@H](CCCCN)C(O)=O |r,TLB:10:9:1:5.4| Show InChI InChI=1S/C24H42N4O4/c1-23(2)16-10-11-24(23,3)14-19(16)26-20(29)18(13-15-7-6-8-15)28-22(32)27-17(21(30)31)9-4-5-12-25/h15-19H,4-14,25H2,1-3H3,(H,26,29)(H,30,31)(H2,27,28,32)/t16?,17-,18+,19?,24?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Infection Research
Curated by ChEMBL
| Assay Description
Inhibition of human activated TAFI incubated for 15 mins by microtiter plate reader based assay |
J Med Chem 58: 4839-44 (2015)
Article DOI: 10.1021/jm501840b
BindingDB Entry DOI: 10.7270/Q24B3314 |
More data for this
Ligand-Target Pair | |
Carboxypeptidase B2
(Homo sapiens (Human)) | BDBM50089748
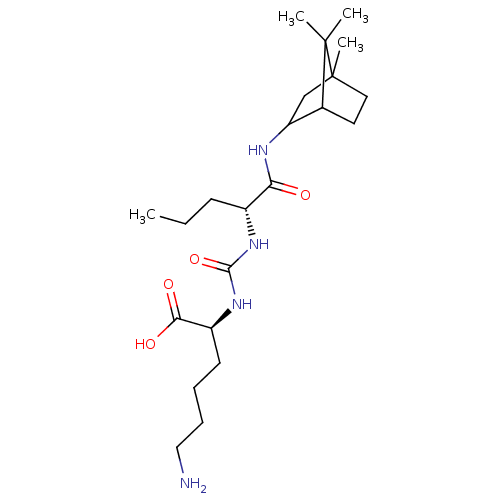
(CHEMBL3577432)Show SMILES CCC[C@@H](NC(=O)N[C@@H](CCCCN)C(O)=O)C(=O)NC1CC2(C)CCC1C2(C)C |r,THB:19:20:27:24.25| Show InChI InChI=1S/C22H40N4O4/c1-5-8-15(25-20(30)26-16(19(28)29)9-6-7-12-23)18(27)24-17-13-22(4)11-10-14(17)21(22,2)3/h14-17H,5-13,23H2,1-4H3,(H,24,27)(H,28,29)(H2,25,26,30)/t14?,15-,16+,17?,22?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Infection Research
Curated by ChEMBL
| Assay Description
Inhibition of human activated TAFI incubated for 15 mins by microtiter plate reader based assay |
J Med Chem 58: 4839-44 (2015)
Article DOI: 10.1021/jm501840b
BindingDB Entry DOI: 10.7270/Q24B3314 |
More data for this
Ligand-Target Pair | |
Carboxypeptidase B2
(Homo sapiens (Human)) | BDBM50089742

(CHEMBL3577426)Show SMILES CC1(C)C2CCC1(C)CC2NC(=O)[C@@H](Cc1ccccc1)NC(=O)N[C@@H](CCCCN)C(O)=O |r,TLB:10:9:1:5.4| Show InChI InChI=1S/C26H40N4O4/c1-25(2)18-12-13-26(25,3)16-21(18)28-22(31)20(15-17-9-5-4-6-10-17)30-24(34)29-19(23(32)33)11-7-8-14-27/h4-6,9-10,18-21H,7-8,11-16,27H2,1-3H3,(H,28,31)(H,32,33)(H2,29,30,34)/t18?,19-,20+,21?,26?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Infection Research
Curated by ChEMBL
| Assay Description
Inhibition of human activated TAFI incubated for 15 mins by microtiter plate reader based assay |
J Med Chem 58: 4839-44 (2015)
Article DOI: 10.1021/jm501840b
BindingDB Entry DOI: 10.7270/Q24B3314 |
More data for this
Ligand-Target Pair | |
Carboxypeptidase B2
(Homo sapiens (Human)) | BDBM50089752

(CHEMBL3577436)Show SMILES CC1(C)C2CCC1(C)CC2NC(=O)[C@@H](CC1CCCCC1)NC(=O)N[C@@H](CCCN)C(O)=O |r,TLB:10:9:1:5.4| Show InChI InChI=1S/C25H44N4O4/c1-24(2)17-11-12-25(24,3)15-20(17)27-21(30)19(14-16-8-5-4-6-9-16)29-23(33)28-18(22(31)32)10-7-13-26/h16-20H,4-15,26H2,1-3H3,(H,27,30)(H,31,32)(H2,28,29,33)/t17?,18-,19+,20?,25?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Infection Research
Curated by ChEMBL
| Assay Description
Inhibition of human activated TAFI incubated for 15 mins by microtiter plate reader based assay |
J Med Chem 58: 4839-44 (2015)
Article DOI: 10.1021/jm501840b
BindingDB Entry DOI: 10.7270/Q24B3314 |
More data for this
Ligand-Target Pair | |
Carboxypeptidase B2
(Homo sapiens (Human)) | BDBM50089707

(CHEMBL3577336)Show SMILES NCCCC[C@H](NC(=O)N[C@H](CC1CCCCC1)C(=O)NC1C2CC3CC(C2)CC1C3)C(O)=O |r,wD:10.10,5.4,TLB:27:22:30:26.25.28,27:26:30:23.22.21,THB:21:22:25:30.29.28,21:29:23.22.27:25,20:21:30:26.25.28,(.32,12.37,;.25,11.14,;1.54,10.3,;1.46,8.76,;2.74,7.92,;2.66,6.38,;3.95,5.53,;3.86,3.99,;2.76,3.44,;5.15,3.15,;5.06,1.61,;6.35,.77,;7.73,1.47,;7.81,3,;9.18,3.7,;10.47,2.86,;10.39,1.33,;9.02,.63,;3.69,.91,;3.62,-.32,;2.4,1.75,;1.01,1.05,;-.28,1.69,;-1.04,.44,;-1.04,-1.32,;-2.25,-2.11,;-1.85,-.7,;-2.02,.73,;-.4,-.88,;1.11,-.36,;.37,-1.59,;1.28,5.69,;1.21,4.46,;.25,6.36,)| Show InChI InChI=1S/C26H44N4O4/c27-9-5-4-8-21(25(32)33)28-26(34)29-22(15-16-6-2-1-3-7-16)24(31)30-23-19-11-17-10-18(13-19)14-20(23)12-17/h16-23H,1-15,27H2,(H,30,31)(H,32,33)(H2,28,29,34)/t17?,18?,19?,20?,21-,22+,23?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Infection Research
Curated by ChEMBL
| Assay Description
Inhibition of human activated TAFI incubated for 15 mins by microtiter plate reader based assay |
J Med Chem 58: 4839-44 (2015)
Article DOI: 10.1021/jm501840b
BindingDB Entry DOI: 10.7270/Q24B3314 |
More data for this
Ligand-Target Pair | |
Carboxypeptidase B2
(Homo sapiens (Human)) | BDBM50089702

(CHEMBL3577335)Show SMILES NCCCC[C@H](NC(=O)N[C@H](CC1CCCCC1)C(=O)NC12CC3CC(CC(C3)C1)C2)C(O)=O |r,TLB:28:23:30:27.26.29,28:27:30:24.23.22,THB:22:23:26:30.21.29,22:21:24.23.28:26,20:21:24.23.28:26| Show InChI InChI=1S/C26H44N4O4/c27-9-5-4-8-21(24(32)33)28-25(34)29-22(13-17-6-2-1-3-7-17)23(31)30-26-14-18-10-19(15-26)12-20(11-18)16-26/h17-22H,1-16,27H2,(H,30,31)(H,32,33)(H2,28,29,34)/t18?,19?,20?,21-,22+,26?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Infection Research
Curated by ChEMBL
| Assay Description
Inhibition of human activated TAFI incubated for 15 mins by microtiter plate reader based assay |
J Med Chem 58: 4839-44 (2015)
Article DOI: 10.1021/jm501840b
BindingDB Entry DOI: 10.7270/Q24B3314 |
More data for this
Ligand-Target Pair | |
Carboxypeptidase B2
(Homo sapiens (Human)) | BDBM50089754

(CHEMBL3577438)Show SMILES CC1(C)C2CCC1(C)CC2NC(=O)[C@@H](CC1CCCCC1)NC(=O)N[C@@H](CS(=O)(=O)CCN)C(O)=O |r,TLB:10:9:1:5.4| Show InChI InChI=1S/C25H44N4O6S/c1-24(2)17-9-10-25(24,3)14-19(17)27-21(30)18(13-16-7-5-4-6-8-16)28-23(33)29-20(22(31)32)15-36(34,35)12-11-26/h16-20H,4-15,26H2,1-3H3,(H,27,30)(H,31,32)(H2,28,29,33)/t17?,18-,19?,20+,25?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Infection Research
Curated by ChEMBL
| Assay Description
Inhibition of human activated TAFI incubated for 15 mins by microtiter plate reader based assay |
J Med Chem 58: 4839-44 (2015)
Article DOI: 10.1021/jm501840b
BindingDB Entry DOI: 10.7270/Q24B3314 |
More data for this
Ligand-Target Pair | |
Carboxypeptidase B2
(Homo sapiens (Human)) | BDBM50089741
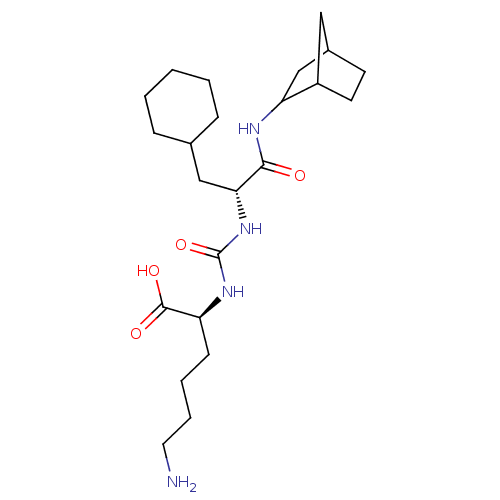
(CHEMBL3577424)Show SMILES NCCCC[C@H](NC(=O)N[C@H](CC1CCCCC1)C(=O)NC1CC2CCC1C2)C(O)=O |r| Show InChI InChI=1S/C23H40N4O4/c24-11-5-4-8-18(22(29)30)26-23(31)27-20(13-15-6-2-1-3-7-15)21(28)25-19-14-16-9-10-17(19)12-16/h15-20H,1-14,24H2,(H,25,28)(H,29,30)(H2,26,27,31)/t16?,17?,18-,19?,20+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Infection Research
Curated by ChEMBL
| Assay Description
Inhibition of human activated TAFI incubated for 15 mins by microtiter plate reader based assay |
J Med Chem 58: 4839-44 (2015)
Article DOI: 10.1021/jm501840b
BindingDB Entry DOI: 10.7270/Q24B3314 |
More data for this
Ligand-Target Pair | |
Carboxypeptidase B2
(Homo sapiens (Human)) | BDBM50089743

(CHEMBL3577427)Show SMILES CC(C)C[C@@H](NC(=O)N[C@@H](CCCCN)C(O)=O)C(=O)NC1CC2(C)CCC1C2(C)C |r,THB:20:21:28:25.26| Show InChI InChI=1S/C23H42N4O4/c1-14(2)12-17(27-21(31)26-16(20(29)30)8-6-7-11-24)19(28)25-18-13-23(5)10-9-15(18)22(23,3)4/h14-18H,6-13,24H2,1-5H3,(H,25,28)(H,29,30)(H2,26,27,31)/t15?,16-,17+,18?,23?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 57 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Infection Research
Curated by ChEMBL
| Assay Description
Inhibition of human activated TAFI incubated for 15 mins by microtiter plate reader based assay |
J Med Chem 58: 4839-44 (2015)
Article DOI: 10.1021/jm501840b
BindingDB Entry DOI: 10.7270/Q24B3314 |
More data for this
Ligand-Target Pair | |
Carboxypeptidase B2
(Homo sapiens (Human)) | BDBM50089757
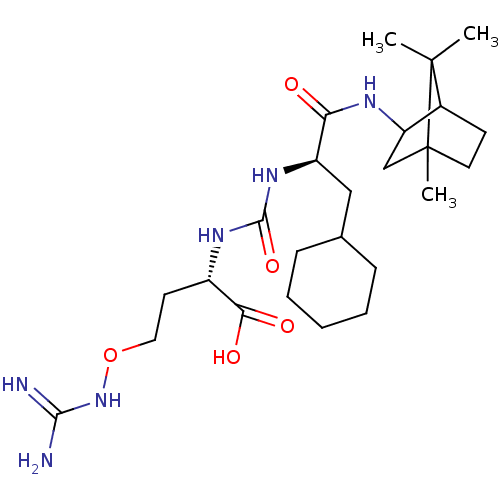
(CHEMBL3577441)Show SMILES CC1(C)C2CCC1(C)CC2NC(=O)[C@@H](CC1CCCCC1)NC(=O)N[C@@H](CCONC(N)=N)C(O)=O |r,TLB:10:9:1:5.4| Show InChI InChI=1S/C25H44N6O5/c1-24(2)16-9-11-25(24,3)14-19(16)28-20(32)18(13-15-7-5-4-6-8-15)30-23(35)29-17(21(33)34)10-12-36-31-22(26)27/h15-19H,4-14H2,1-3H3,(H,28,32)(H,33,34)(H4,26,27,31)(H2,29,30,35)/t16?,17-,18+,19?,25?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Infection Research
Curated by ChEMBL
| Assay Description
Inhibition of human activated TAFI incubated for 15 mins by microtiter plate reader based assay |
J Med Chem 58: 4839-44 (2015)
Article DOI: 10.1021/jm501840b
BindingDB Entry DOI: 10.7270/Q24B3314 |
More data for this
Ligand-Target Pair | |
Carboxypeptidase B2
(Homo sapiens (Human)) | BDBM50089740
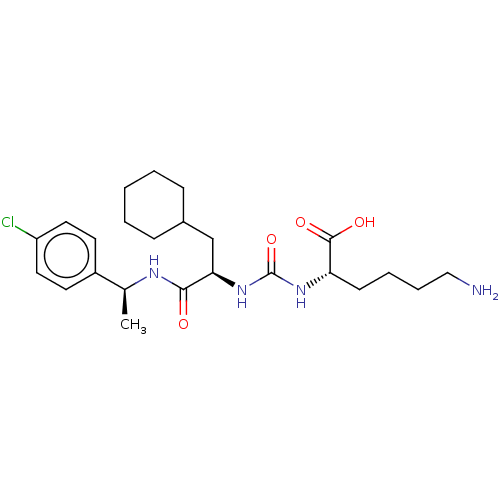
(CHEMBL3577423)Show SMILES C[C@H](NC(=O)[C@@H](CC1CCCCC1)NC(=O)N[C@@H](CCCCN)C(O)=O)c1ccc(Cl)cc1 |r| Show InChI InChI=1S/C24H37ClN4O4/c1-16(18-10-12-19(25)13-11-18)27-22(30)21(15-17-7-3-2-4-8-17)29-24(33)28-20(23(31)32)9-5-6-14-26/h10-13,16-17,20-21H,2-9,14-15,26H2,1H3,(H,27,30)(H,31,32)(H2,28,29,33)/t16-,20-,21+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Infection Research
Curated by ChEMBL
| Assay Description
Inhibition of human activated TAFI incubated for 15 mins by microtiter plate reader based assay |
J Med Chem 58: 4839-44 (2015)
Article DOI: 10.1021/jm501840b
BindingDB Entry DOI: 10.7270/Q24B3314 |
More data for this
Ligand-Target Pair | |
Carboxypeptidase B2
(Homo sapiens (Human)) | BDBM50089753
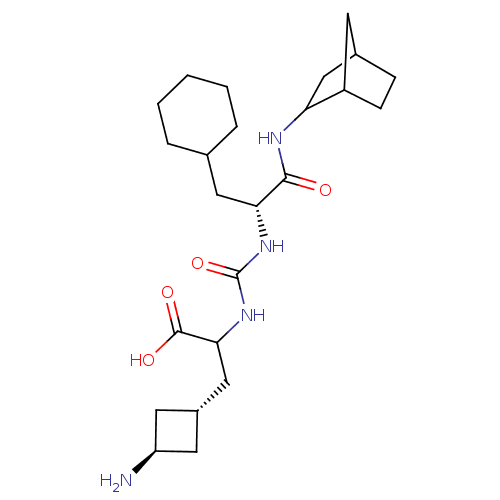
(CHEMBL3577437)Show SMILES N[C@H]1C[C@H](CC(NC(=O)N[C@H](CC2CCCCC2)C(=O)NC2CC3CCC2C3)C(O)=O)C1 |r,wU:1.0,wD:3.3,10.10,(8.03,9.24,;6.97,8.62,;6.58,7.13,;5.14,7.54,;3.81,6.76,;3.82,5.22,;2.49,4.44,;2.51,2.9,;3.58,2.29,;1.18,2.11,;1.19,.57,;2.53,-.18,;2.55,-1.72,;1.22,-2.51,;1.24,-4.05,;2.58,-4.8,;3.91,-4.02,;3.89,-2.48,;-.14,-.21,;-1.21,.4,;-.12,-1.75,;-1.45,-2.53,;-2.78,-1.78,;-4.04,-2.58,;-4.01,-4.04,;-2.7,-4.79,;-1.45,-4.01,;-2.31,-2.53,;5.16,4.46,;6.22,5.08,;5.17,3.23,;5.48,9.01,)| Show InChI InChI=1S/C24H40N4O4/c25-18-9-16(10-18)13-21(23(30)31)28-24(32)27-20(11-14-4-2-1-3-5-14)22(29)26-19-12-15-6-7-17(19)8-15/h14-21H,1-13,25H2,(H,26,29)(H,30,31)(H2,27,28,32)/t15?,16-,17?,18-,19?,20-,21?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Infection Research
Curated by ChEMBL
| Assay Description
Inhibition of human activated TAFI incubated for 15 mins by microtiter plate reader based assay |
J Med Chem 58: 4839-44 (2015)
Article DOI: 10.1021/jm501840b
BindingDB Entry DOI: 10.7270/Q24B3314 |
More data for this
Ligand-Target Pair | |
Carboxypeptidase B2
(Homo sapiens (Human)) | BDBM50089737

(CHEMBL3577337)Show SMILES NCCCC[C@H](NC(=O)N[C@H](CC1CCCCC1)C(=O)NCC(c1ccccc1)c1ccccc1)C(O)=O |r| Show InChI InChI=1S/C30H42N4O4/c31-19-11-10-18-26(29(36)37)33-30(38)34-27(20-22-12-4-1-5-13-22)28(35)32-21-25(23-14-6-2-7-15-23)24-16-8-3-9-17-24/h2-3,6-9,14-17,22,25-27H,1,4-5,10-13,18-21,31H2,(H,32,35)(H,36,37)(H2,33,34,38)/t26-,27+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Infection Research
Curated by ChEMBL
| Assay Description
Inhibition of human activated TAFI incubated for 15 mins by microtiter plate reader based assay |
J Med Chem 58: 4839-44 (2015)
Article DOI: 10.1021/jm501840b
BindingDB Entry DOI: 10.7270/Q24B3314 |
More data for this
Ligand-Target Pair | |
Carboxypeptidase B2
(Homo sapiens (Human)) | BDBM50089700

(CHEMBL3577325)Show SMILES C[C@H](NC(=O)[C@@H](CC1CCCCC1)NC(=O)N[C@@H](CCCCN)C(O)=O)C1CCCCC1 |r| Show InChI InChI=1S/C24H44N4O4/c1-17(19-12-6-3-7-13-19)26-22(29)21(16-18-10-4-2-5-11-18)28-24(32)27-20(23(30)31)14-8-9-15-25/h17-21H,2-16,25H2,1H3,(H,26,29)(H,30,31)(H2,27,28,32)/t17-,20-,21+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Infection Research
Curated by ChEMBL
| Assay Description
Inhibition of human activated TAFI incubated for 15 mins by microtiter plate reader based assay |
J Med Chem 58: 4839-44 (2015)
Article DOI: 10.1021/jm501840b
BindingDB Entry DOI: 10.7270/Q24B3314 |
More data for this
Ligand-Target Pair | |
Carboxypeptidase B2
(Homo sapiens (Human)) | BDBM50089694
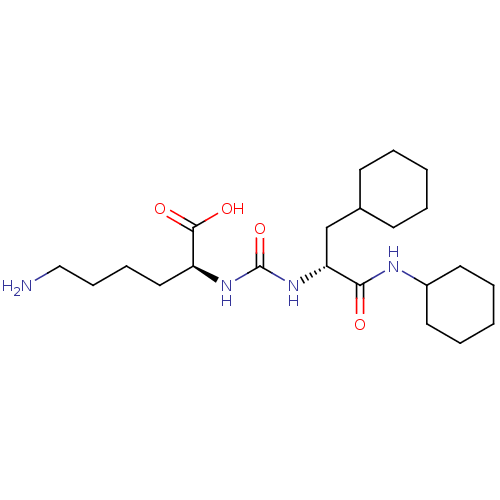
(CHEMBL3577330)Show SMILES NCCCC[C@H](NC(=O)N[C@H](CC1CCCCC1)C(=O)NC1CCCCC1)C(O)=O |r| Show InChI InChI=1S/C22H40N4O4/c23-14-8-7-13-18(21(28)29)25-22(30)26-19(15-16-9-3-1-4-10-16)20(27)24-17-11-5-2-6-12-17/h16-19H,1-15,23H2,(H,24,27)(H,28,29)(H2,25,26,30)/t18-,19+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Infection Research
Curated by ChEMBL
| Assay Description
Inhibition of human activated TAFI incubated for 15 mins by microtiter plate reader based assay |
J Med Chem 58: 4839-44 (2015)
Article DOI: 10.1021/jm501840b
BindingDB Entry DOI: 10.7270/Q24B3314 |
More data for this
Ligand-Target Pair | |
Carboxypeptidase B
(Homo sapiens (Human)) | BDBM50089685
(CHEMBL3577333)Show SMILES CC(C)CCNC(=O)[C@@H](CCCCNC(=O)OCc1ccccc1)NC(=O)N[C@@H](CCCCN)C(O)=O |r| Show InChI InChI=1S/C26H43N5O6/c1-19(2)14-17-28-23(32)21(30-25(35)31-22(24(33)34)13-6-8-15-27)12-7-9-16-29-26(36)37-18-20-10-4-3-5-11-20/h3-5,10-11,19,21-22H,6-9,12-18,27H2,1-2H3,(H,28,32)(H,29,36)(H,33,34)(H2,30,31,35)/t21-,22+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Infection Research
Curated by ChEMBL
| Assay Description
Inhibition of CBP (unknown origin) |
J Med Chem 58: 4839-44 (2015)
Article DOI: 10.1021/jm501840b
BindingDB Entry DOI: 10.7270/Q24B3314 |
More data for this
Ligand-Target Pair | |
Carboxypeptidase B2
(Homo sapiens (Human)) | BDBM50089695

(CHEMBL3577329)Show SMILES CCC(C)C(C)NC(=O)[C@@H](CC1CCCCC1)NC(=O)N[C@@H](CCCCN)C(O)=O |r| Show InChI InChI=1S/C22H42N4O4/c1-4-15(2)16(3)24-20(27)19(14-17-10-6-5-7-11-17)26-22(30)25-18(21(28)29)12-8-9-13-23/h15-19H,4-14,23H2,1-3H3,(H,24,27)(H,28,29)(H2,25,26,30)/t15?,16?,18-,19+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Infection Research
Curated by ChEMBL
| Assay Description
Inhibition of human activated TAFI incubated for 15 mins by microtiter plate reader based assay |
J Med Chem 58: 4839-44 (2015)
Article DOI: 10.1021/jm501840b
BindingDB Entry DOI: 10.7270/Q24B3314 |
More data for this
Ligand-Target Pair | |
Carboxypeptidase B2
(Homo sapiens (Human)) | BDBM50089778
(CHEMBL3577443)Show SMILES CC12CCC(C(C1)NC(=O)[C@@H](CC1CCCCC1)NC(=O)N[C@H](Cc1ccc(N)nc1)C(O)=O)C2(C)C |r,THB:7:5:34:2.3| Show InChI InChI=1S/C28H43N5O4/c1-27(2)19-11-12-28(27,3)15-22(19)31-24(34)20(13-17-7-5-4-6-8-17)32-26(37)33-21(25(35)36)14-18-9-10-23(29)30-16-18/h9-10,16-17,19-22H,4-8,11-15H2,1-3H3,(H2,29,30)(H,31,34)(H,35,36)(H2,32,33,37)/t19?,20-,21-,22?,28?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Infection Research
Curated by ChEMBL
| Assay Description
Inhibition of human activated TAFI incubated for 15 mins by microtiter plate reader based assay |
J Med Chem 58: 4839-44 (2015)
Article DOI: 10.1021/jm501840b
BindingDB Entry DOI: 10.7270/Q24B3314 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50089687

(Anabaenopeptin F)Show SMILES [H][C@]1(NC(=O)[C@@H](CCCCNC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](C)N(C)C(=O)[C@H](CCc2ccc(O)cc2)NC1=O)NC(=O)N[C@@H](CCCNC(N)=N)C(O)=O)[C@@H](C)CC |r| Show InChI InChI=1S/C42H62N10O9/c1-5-25(2)34-38(57)47-31(21-18-27-16-19-29(53)20-17-27)39(58)52(4)26(3)35(54)48-33(24-28-12-7-6-8-13-28)36(55)45-22-10-9-14-30(37(56)51-34)49-42(61)50-32(40(59)60)15-11-23-46-41(43)44/h6-8,12-13,16-17,19-20,25-26,30-34,53H,5,9-11,14-15,18,21-24H2,1-4H3,(H,45,55)(H,47,57)(H,48,54)(H,51,56)(H,59,60)(H4,43,44,46)(H2,49,50,61)/t25-,26-,30+,31-,32-,33-,34-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 350 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Infection Research
Curated by ChEMBL
| Assay Description
Inhibition of factor2a (unknown origin) |
J Med Chem 58: 4839-44 (2015)
Article DOI: 10.1021/jm501840b
BindingDB Entry DOI: 10.7270/Q24B3314 |
More data for this
Ligand-Target Pair | |
Carboxypeptidase B2
(Homo sapiens (Human)) | BDBM50089699

(CHEMBL3577326)Show SMILES CC(C)[C@@H](C)NC(=O)[C@@H](CC1CCCCC1)NC(=O)N[C@@H](CCCCN)C(O)=O |r| Show InChI InChI=1S/C21H40N4O4/c1-14(2)15(3)23-19(26)18(13-16-9-5-4-6-10-16)25-21(29)24-17(20(27)28)11-7-8-12-22/h14-18H,4-13,22H2,1-3H3,(H,23,26)(H,27,28)(H2,24,25,29)/t15-,17+,18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 390 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Infection Research
Curated by ChEMBL
| Assay Description
Inhibition of human activated TAFI incubated for 15 mins by microtiter plate reader based assay |
J Med Chem 58: 4839-44 (2015)
Article DOI: 10.1021/jm501840b
BindingDB Entry DOI: 10.7270/Q24B3314 |
More data for this
Ligand-Target Pair | |
Carboxypeptidase B2
(Homo sapiens (Human)) | BDBM50089689

(Oscillamide Y)Show SMILES [H][C@]1(NC(=O)[C@@H](CCCCNC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](C)N(C)C(=O)[C@H](CCc2ccc(O)cc2)NC1=O)NC(=O)N[C@@H](Cc1ccc(O)cc1)C(O)=O)[C@H](C)CC |r| Show InChI InChI=1S/C45H59N7O10/c1-5-27(2)38-42(58)47-35(23-18-29-14-19-32(53)20-15-29)43(59)52(4)28(3)39(55)48-36(25-30-11-7-6-8-12-30)40(56)46-24-10-9-13-34(41(57)51-38)49-45(62)50-37(44(60)61)26-31-16-21-33(54)22-17-31/h6-8,11-12,14-17,19-22,27-28,34-38,53-54H,5,9-10,13,18,23-26H2,1-4H3,(H,46,56)(H,47,58)(H,48,55)(H,51,57)(H,60,61)(H2,49,50,62)/t27-,28+,34-,35+,36+,37+,38+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Infection Research
Curated by ChEMBL
| Assay Description
Inhibition of human activated TAFI incubated for 15 mins by microtiter plate reader based assay |
J Med Chem 58: 4839-44 (2015)
Article DOI: 10.1021/jm501840b
BindingDB Entry DOI: 10.7270/Q24B3314 |
More data for this
Ligand-Target Pair | |
Carboxypeptidase B2
(Homo sapiens (Human)) | BDBM50089744

(CHEMBL3577428)Show SMILES CC(C)[C@@H](NC(=O)N[C@@H](CCCCN)C(O)=O)C(=O)NC1CC2(C)CCC1C2(C)C |r,THB:19:20:27:24.25| Show InChI InChI=1S/C22H40N4O4/c1-13(2)17(26-20(30)25-15(19(28)29)8-6-7-11-23)18(27)24-16-12-22(5)10-9-14(16)21(22,3)4/h13-17H,6-12,23H2,1-5H3,(H,24,27)(H,28,29)(H2,25,26,30)/t14?,15-,16?,17+,22?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 460 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Infection Research
Curated by ChEMBL
| Assay Description
Inhibition of human activated TAFI incubated for 15 mins by microtiter plate reader based assay |
J Med Chem 58: 4839-44 (2015)
Article DOI: 10.1021/jm501840b
BindingDB Entry DOI: 10.7270/Q24B3314 |
More data for this
Ligand-Target Pair | |
Carboxypeptidase B2
(Homo sapiens (Human)) | BDBM50089781
(CHEMBL3577445)Show SMILES CC12CCC(C(C1)NC(=O)[C@@H](CC1CCCCC1)NC(=O)N[C@@H](Cc1ccc(N)nc1)C(N)=O)C2(C)C |r,THB:7:5:34:2.3| Show InChI InChI=1S/C28H44N6O3/c1-27(2)19-11-12-28(27,3)15-22(19)32-25(36)21(13-17-7-5-4-6-8-17)34-26(37)33-20(24(30)35)14-18-9-10-23(29)31-16-18/h9-10,16-17,19-22H,4-8,11-15H2,1-3H3,(H2,29,31)(H2,30,35)(H,32,36)(H2,33,34,37)/t19?,20-,21+,22?,28?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 570 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Infection Research
Curated by ChEMBL
| Assay Description
Inhibition of human activated TAFI incubated for 15 mins by microtiter plate reader based assay |
J Med Chem 58: 4839-44 (2015)
Article DOI: 10.1021/jm501840b
BindingDB Entry DOI: 10.7270/Q24B3314 |
More data for this
Ligand-Target Pair | |
Carboxypeptidase B2
(Homo sapiens (Human)) | BDBM50089697
(CHEMBL3577327)Show SMILES CC(C)(C)CNC(=O)[C@@H](CC1CCCCC1)NC(=O)N[C@@H](CCCCN)C(O)=O |r| Show InChI InChI=1S/C21H40N4O4/c1-21(2,3)14-23-18(26)17(13-15-9-5-4-6-10-15)25-20(29)24-16(19(27)28)11-7-8-12-22/h15-17H,4-14,22H2,1-3H3,(H,23,26)(H,27,28)(H2,24,25,29)/t16-,17+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 690 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Infection Research
Curated by ChEMBL
| Assay Description
Inhibition of human activated TAFI incubated for 15 mins by microtiter plate reader based assay |
J Med Chem 58: 4839-44 (2015)
Article DOI: 10.1021/jm501840b
BindingDB Entry DOI: 10.7270/Q24B3314 |
More data for this
Ligand-Target Pair | |
Carboxypeptidase B2
(Homo sapiens (Human)) | BDBM50089780
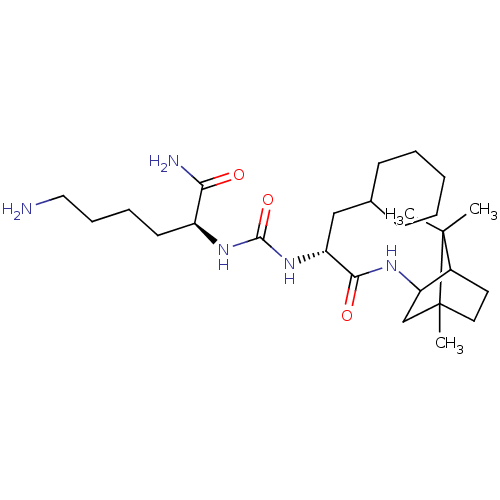
(CHEMBL3577444)Show SMILES CC1(C)C2CCC1(C)CC2NC(=O)[C@@H](CC1CCCCC1)NC(=O)N[C@@H](CCCCN)C(N)=O |r,TLB:10:9:1:5.4| Show InChI InChI=1S/C26H47N5O3/c1-25(2)18-12-13-26(25,3)16-21(18)29-23(33)20(15-17-9-5-4-6-10-17)31-24(34)30-19(22(28)32)11-7-8-14-27/h17-21H,4-16,27H2,1-3H3,(H2,28,32)(H,29,33)(H2,30,31,34)/t18?,19-,20+,21?,26?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 750 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Infection Research
Curated by ChEMBL
| Assay Description
Inhibition of human activated TAFI incubated for 15 mins by microtiter plate reader based assay |
J Med Chem 58: 4839-44 (2015)
Article DOI: 10.1021/jm501840b
BindingDB Entry DOI: 10.7270/Q24B3314 |
More data for this
Ligand-Target Pair | |
Carboxypeptidase B2
(Homo sapiens (Human)) | BDBM50089696

(CHEMBL3577328)Show SMILES CC(C)CNC(=O)[C@@H](CC1CCCCC1)NC(=O)N[C@@H](CCCCN)C(O)=O |r| Show InChI InChI=1S/C20H38N4O4/c1-14(2)13-22-18(25)17(12-15-8-4-3-5-9-15)24-20(28)23-16(19(26)27)10-6-7-11-21/h14-17H,3-13,21H2,1-2H3,(H,22,25)(H,26,27)(H2,23,24,28)/t16-,17+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 850 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Infection Research
Curated by ChEMBL
| Assay Description
Inhibition of human activated TAFI incubated for 15 mins by microtiter plate reader based assay |
J Med Chem 58: 4839-44 (2015)
Article DOI: 10.1021/jm501840b
BindingDB Entry DOI: 10.7270/Q24B3314 |
More data for this
Ligand-Target Pair | |
Carboxypeptidase B2
(Homo sapiens (Human)) | BDBM50089738
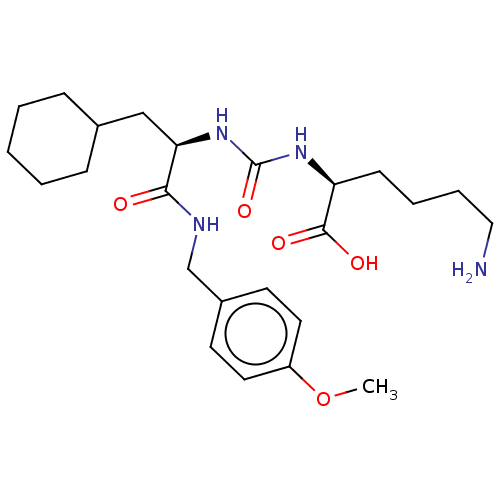
(CHEMBL3577338)Show SMILES COc1ccc(CNC(=O)[C@@H](CC2CCCCC2)NC(=O)N[C@@H](CCCCN)C(O)=O)cc1 |r| Show InChI InChI=1S/C24H38N4O5/c1-33-19-12-10-18(11-13-19)16-26-22(29)21(15-17-7-3-2-4-8-17)28-24(32)27-20(23(30)31)9-5-6-14-25/h10-13,17,20-21H,2-9,14-16,25H2,1H3,(H,26,29)(H,30,31)(H2,27,28,32)/t20-,21+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Infection Research
Curated by ChEMBL
| Assay Description
Inhibition of human activated TAFI incubated for 15 mins by microtiter plate reader based assay |
J Med Chem 58: 4839-44 (2015)
Article DOI: 10.1021/jm501840b
BindingDB Entry DOI: 10.7270/Q24B3314 |
More data for this
Ligand-Target Pair | |
Carboxypeptidase B2
(Homo sapiens (Human)) | BDBM50089745
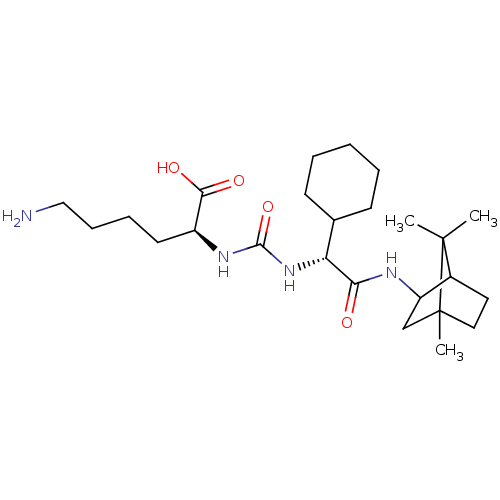
(CHEMBL3577429)Show SMILES CC1(C)C2CCC1(C)CC2NC(=O)[C@H](NC(=O)N[C@@H](CCCCN)C(O)=O)C1CCCCC1 |r,TLB:10:9:1:5.4| Show InChI InChI=1S/C25H44N4O4/c1-24(2)17-12-13-25(24,3)15-19(17)27-21(30)20(16-9-5-4-6-10-16)29-23(33)28-18(22(31)32)11-7-8-14-26/h16-20H,4-15,26H2,1-3H3,(H,27,30)(H,31,32)(H2,28,29,33)/t17?,18-,19?,20+,25?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Infection Research
Curated by ChEMBL
| Assay Description
Inhibition of human activated TAFI incubated for 15 mins by microtiter plate reader based assay |
J Med Chem 58: 4839-44 (2015)
Article DOI: 10.1021/jm501840b
BindingDB Entry DOI: 10.7270/Q24B3314 |
More data for this
Ligand-Target Pair | |
Carboxypeptidase A1
(Homo sapiens (Human)) | BDBM50089687

(Anabaenopeptin F)Show SMILES [H][C@]1(NC(=O)[C@@H](CCCCNC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](C)N(C)C(=O)[C@H](CCc2ccc(O)cc2)NC1=O)NC(=O)N[C@@H](CCCNC(N)=N)C(O)=O)[C@@H](C)CC |r| Show InChI InChI=1S/C42H62N10O9/c1-5-25(2)34-38(57)47-31(21-18-27-16-19-29(53)20-17-27)39(58)52(4)26(3)35(54)48-33(24-28-12-7-6-8-13-28)36(55)45-22-10-9-14-30(37(56)51-34)49-42(61)50-32(40(59)60)15-11-23-46-41(43)44/h6-8,12-13,16-17,19-20,25-26,30-34,53H,5,9-11,14-15,18,21-24H2,1-4H3,(H,45,55)(H,47,57)(H,48,54)(H,51,56)(H,59,60)(H4,43,44,46)(H2,49,50,61)/t25-,26-,30+,31-,32-,33-,34-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Infection Research
Curated by ChEMBL
| Assay Description
Inhibition of CPA (unknown origin) |
J Med Chem 58: 4839-44 (2015)
Article DOI: 10.1021/jm501840b
BindingDB Entry DOI: 10.7270/Q24B3314 |
More data for this
Ligand-Target Pair | |
Carboxypeptidase B2
(Homo sapiens (Human)) | BDBM50089739
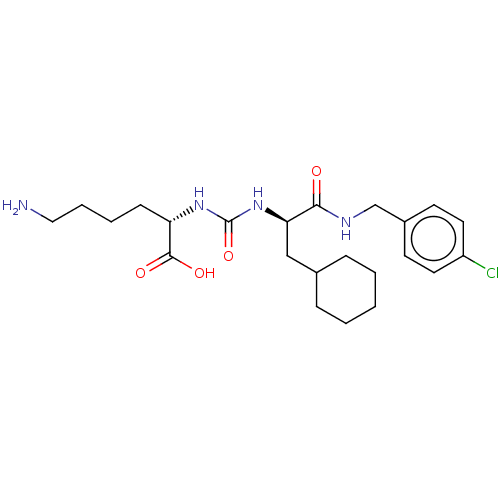
(CHEMBL3577422)Show SMILES NCCCC[C@H](NC(=O)N[C@H](CC1CCCCC1)C(=O)NCc1ccc(Cl)cc1)C(O)=O |r| Show InChI InChI=1S/C23H35ClN4O4/c24-18-11-9-17(10-12-18)15-26-21(29)20(14-16-6-2-1-3-7-16)28-23(32)27-19(22(30)31)8-4-5-13-25/h9-12,16,19-20H,1-8,13-15,25H2,(H,26,29)(H,30,31)(H2,27,28,32)/t19-,20+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Infection Research
Curated by ChEMBL
| Assay Description
Inhibition of human activated TAFI incubated for 15 mins by microtiter plate reader based assay |
J Med Chem 58: 4839-44 (2015)
Article DOI: 10.1021/jm501840b
BindingDB Entry DOI: 10.7270/Q24B3314 |
More data for this
Ligand-Target Pair | |
Carboxypeptidase B2
(Homo sapiens (Human)) | BDBM50089685

(CHEMBL3577333)Show SMILES CC(C)CCNC(=O)[C@@H](CCCCNC(=O)OCc1ccccc1)NC(=O)N[C@@H](CCCCN)C(O)=O |r| Show InChI InChI=1S/C26H43N5O6/c1-19(2)14-17-28-23(32)21(30-25(35)31-22(24(33)34)13-6-8-15-27)12-7-9-16-29-26(36)37-18-20-10-4-3-5-11-20/h3-5,10-11,19,21-22H,6-9,12-18,27H2,1-2H3,(H,28,32)(H,29,36)(H,33,34)(H2,30,31,35)/t21-,22+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Infection Research
Curated by ChEMBL
| Assay Description
Inhibition of human activated TAFI incubated for 15 mins by microtiter plate reader based assay |
J Med Chem 58: 4839-44 (2015)
Article DOI: 10.1021/jm501840b
BindingDB Entry DOI: 10.7270/Q24B3314 |
More data for this
Ligand-Target Pair | |
Carboxypeptidase B2
(Homo sapiens (Human)) | BDBM50089692

(CHEMBL3577332)Show SMILES CC(C)CCNC(=O)[C@@H](CC1CCCCC1)NC(=O)N[C@@H](CCCCN)C(O)=O |r| Show InChI InChI=1S/C21H40N4O4/c1-15(2)11-13-23-19(26)18(14-16-8-4-3-5-9-16)25-21(29)24-17(20(27)28)10-6-7-12-22/h15-18H,3-14,22H2,1-2H3,(H,23,26)(H,27,28)(H2,24,25,29)/t17-,18+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Infection Research
Curated by ChEMBL
| Assay Description
Inhibition of human activated TAFI incubated for 15 mins by microtiter plate reader based assay |
J Med Chem 58: 4839-44 (2015)
Article DOI: 10.1021/jm501840b
BindingDB Entry DOI: 10.7270/Q24B3314 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50089688

(ANABAENOPEPTIN B)Show SMILES CC(C)[C@@H]1NC(=O)[C@@H](CCCCNC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](C)N(C)C(=O)[C@H](CCc2ccc(O)cc2)NC1=O)NC(=O)N[C@@H](CCCNC(N)=N)C(O)=O |r| Show InChI InChI=1S/C41H60N10O9/c1-24(2)33-37(56)46-30(20-17-26-15-18-28(52)19-16-26)38(57)51(4)25(3)34(53)47-32(23-27-11-6-5-7-12-27)35(54)44-21-9-8-13-29(36(55)50-33)48-41(60)49-31(39(58)59)14-10-22-45-40(42)43/h5-7,11-12,15-16,18-19,24-25,29-33,52H,8-10,13-14,17,20-23H2,1-4H3,(H,44,54)(H,46,56)(H,47,53)(H,50,55)(H,58,59)(H4,42,43,45)(H2,48,49,60)/t25-,29+,30-,31-,32-,33-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Infection Research
Curated by ChEMBL
| Assay Description
Inhibition of factor2a (unknown origin) |
J Med Chem 58: 4839-44 (2015)
Article DOI: 10.1021/jm501840b
BindingDB Entry DOI: 10.7270/Q24B3314 |
More data for this
Ligand-Target Pair | |
Carboxypeptidase N catalytic chain
(Homo sapiens (Human)) | BDBM50089687

(Anabaenopeptin F)Show SMILES [H][C@]1(NC(=O)[C@@H](CCCCNC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](C)N(C)C(=O)[C@H](CCc2ccc(O)cc2)NC1=O)NC(=O)N[C@@H](CCCNC(N)=N)C(O)=O)[C@@H](C)CC |r| Show InChI InChI=1S/C42H62N10O9/c1-5-25(2)34-38(57)47-31(21-18-27-16-19-29(53)20-17-27)39(58)52(4)26(3)35(54)48-33(24-28-12-7-6-8-13-28)36(55)45-22-10-9-14-30(37(56)51-34)49-42(61)50-32(40(59)60)15-11-23-46-41(43)44/h6-8,12-13,16-17,19-20,25-26,30-34,53H,5,9-11,14-15,18,21-24H2,1-4H3,(H,45,55)(H,47,57)(H,48,54)(H,51,56)(H,59,60)(H4,43,44,46)(H2,49,50,61)/t25-,26-,30+,31-,32-,33-,34-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Infection Research
Curated by ChEMBL
| Assay Description
Inhibition of CPN (unknown origin) |
J Med Chem 58: 4839-44 (2015)
Article DOI: 10.1021/jm501840b
BindingDB Entry DOI: 10.7270/Q24B3314 |
More data for this
Ligand-Target Pair | |
Carboxypeptidase B2
(Homo sapiens (Human)) | BDBM50089746
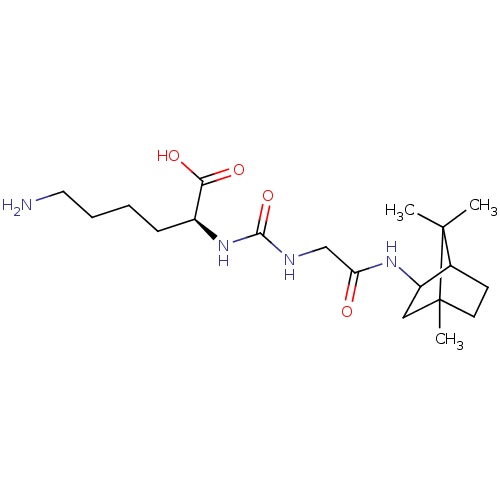
(CHEMBL3577430)Show SMILES CC1(C)C2CCC1(C)CC2NC(=O)CNC(=O)N[C@@H](CCCCN)C(O)=O |r,TLB:10:9:1:5.4| Show InChI InChI=1S/C19H34N4O4/c1-18(2)12-7-8-19(18,3)10-14(12)22-15(24)11-21-17(27)23-13(16(25)26)6-4-5-9-20/h12-14H,4-11,20H2,1-3H3,(H,22,24)(H,25,26)(H2,21,23,27)/t12?,13-,14?,19?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Infection Research
Curated by ChEMBL
| Assay Description
Inhibition of human activated TAFI incubated for 15 mins by microtiter plate reader based assay |
J Med Chem 58: 4839-44 (2015)
Article DOI: 10.1021/jm501840b
BindingDB Entry DOI: 10.7270/Q24B3314 |
More data for this
Ligand-Target Pair | |
Carboxypeptidase A1
(Homo sapiens (Human)) | BDBM50089688

(ANABAENOPEPTIN B)Show SMILES CC(C)[C@@H]1NC(=O)[C@@H](CCCCNC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](C)N(C)C(=O)[C@H](CCc2ccc(O)cc2)NC1=O)NC(=O)N[C@@H](CCCNC(N)=N)C(O)=O |r| Show InChI InChI=1S/C41H60N10O9/c1-24(2)33-37(56)46-30(20-17-26-15-18-28(52)19-16-26)38(57)51(4)25(3)34(53)47-32(23-27-11-6-5-7-12-27)35(54)44-21-9-8-13-29(36(55)50-33)48-41(60)49-31(39(58)59)14-10-22-45-40(42)43/h5-7,11-12,15-16,18-19,24-25,29-33,52H,8-10,13-14,17,20-23H2,1-4H3,(H,44,54)(H,46,56)(H,47,53)(H,50,55)(H,58,59)(H4,42,43,45)(H2,48,49,60)/t25-,29+,30-,31-,32-,33-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Infection Research
Curated by ChEMBL
| Assay Description
Inhibition of CPA (unknown origin) |
J Med Chem 58: 4839-44 (2015)
Article DOI: 10.1021/jm501840b
BindingDB Entry DOI: 10.7270/Q24B3314 |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50089687

(Anabaenopeptin F)Show SMILES [H][C@]1(NC(=O)[C@@H](CCCCNC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](C)N(C)C(=O)[C@H](CCc2ccc(O)cc2)NC1=O)NC(=O)N[C@@H](CCCNC(N)=N)C(O)=O)[C@@H](C)CC |r| Show InChI InChI=1S/C42H62N10O9/c1-5-25(2)34-38(57)47-31(21-18-27-16-19-29(53)20-17-27)39(58)52(4)26(3)35(54)48-33(24-28-12-7-6-8-13-28)36(55)45-22-10-9-14-30(37(56)51-34)49-42(61)50-32(40(59)60)15-11-23-46-41(43)44/h6-8,12-13,16-17,19-20,25-26,30-34,53H,5,9-11,14-15,18,21-24H2,1-4H3,(H,45,55)(H,47,57)(H,48,54)(H,51,56)(H,59,60)(H4,43,44,46)(H2,49,50,61)/t25-,26-,30+,31-,32-,33-,34-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Infection Research
Curated by ChEMBL
| Assay Description
Inhibition of factor11a (unknown origin) |
J Med Chem 58: 4839-44 (2015)
Article DOI: 10.1021/jm501840b
BindingDB Entry DOI: 10.7270/Q24B3314 |
More data for this
Ligand-Target Pair | |
Carboxypeptidase N catalytic chain
(Homo sapiens (Human)) | BDBM50089688

(ANABAENOPEPTIN B)Show SMILES CC(C)[C@@H]1NC(=O)[C@@H](CCCCNC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](C)N(C)C(=O)[C@H](CCc2ccc(O)cc2)NC1=O)NC(=O)N[C@@H](CCCNC(N)=N)C(O)=O |r| Show InChI InChI=1S/C41H60N10O9/c1-24(2)33-37(56)46-30(20-17-26-15-18-28(52)19-16-26)38(57)51(4)25(3)34(53)47-32(23-27-11-6-5-7-12-27)35(54)44-21-9-8-13-29(36(55)50-33)48-41(60)49-31(39(58)59)14-10-22-45-40(42)43/h5-7,11-12,15-16,18-19,24-25,29-33,52H,8-10,13-14,17,20-23H2,1-4H3,(H,44,54)(H,46,56)(H,47,53)(H,50,55)(H,58,59)(H4,42,43,45)(H2,48,49,60)/t25-,29+,30-,31-,32-,33-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Infection Research
Curated by ChEMBL
| Assay Description
Inhibition of CPN (unknown origin) |
J Med Chem 58: 4839-44 (2015)
Article DOI: 10.1021/jm501840b
BindingDB Entry DOI: 10.7270/Q24B3314 |
More data for this
Ligand-Target Pair | |
Carboxypeptidase N catalytic chain
(Homo sapiens (Human)) | BDBM50089686

(CHEMBL3577334)Show SMILES CC(C)[C@@H]1NC(=O)[C@@H](CCCCNC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](C)N(C)C(=O)[C@H](CCc2ccc(O)cc2)NC1=O)NC(=O)N[C@@H](CCCCN)C(O)=O |r| Show InChI InChI=1S/C41H60N8O9/c1-25(2)34-38(54)44-31(21-18-27-16-19-29(50)20-17-27)39(55)49(4)26(3)35(51)45-33(24-28-12-6-5-7-13-28)36(52)43-23-11-9-14-30(37(53)48-34)46-41(58)47-32(40(56)57)15-8-10-22-42/h5-7,12-13,16-17,19-20,25-26,30-34,50H,8-11,14-15,18,21-24,42H2,1-4H3,(H,43,52)(H,44,54)(H,45,51)(H,48,53)(H,56,57)(H2,46,47,58)/t26-,30+,31-,32-,33-,34-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Infection Research
Curated by ChEMBL
| Assay Description
Inhibition of CPN (unknown origin) |
J Med Chem 58: 4839-44 (2015)
Article DOI: 10.1021/jm501840b
BindingDB Entry DOI: 10.7270/Q24B3314 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50089686

(CHEMBL3577334)Show SMILES CC(C)[C@@H]1NC(=O)[C@@H](CCCCNC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](C)N(C)C(=O)[C@H](CCc2ccc(O)cc2)NC1=O)NC(=O)N[C@@H](CCCCN)C(O)=O |r| Show InChI InChI=1S/C41H60N8O9/c1-25(2)34-38(54)44-31(21-18-27-16-19-29(50)20-17-27)39(55)49(4)26(3)35(51)45-33(24-28-12-6-5-7-13-28)36(52)43-23-11-9-14-30(37(53)48-34)46-41(58)47-32(40(56)57)15-8-10-22-42/h5-7,12-13,16-17,19-20,25-26,30-34,50H,8-11,14-15,18,21-24,42H2,1-4H3,(H,43,52)(H,44,54)(H,45,51)(H,48,53)(H,56,57)(H2,46,47,58)/t26-,30+,31-,32-,33-,34-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Infection Research
Curated by ChEMBL
| Assay Description
Inhibition of factor2a (unknown origin) |
J Med Chem 58: 4839-44 (2015)
Article DOI: 10.1021/jm501840b
BindingDB Entry DOI: 10.7270/Q24B3314 |
More data for this
Ligand-Target Pair | |
Carboxypeptidase B2
(Homo sapiens (Human)) | BDBM50089747
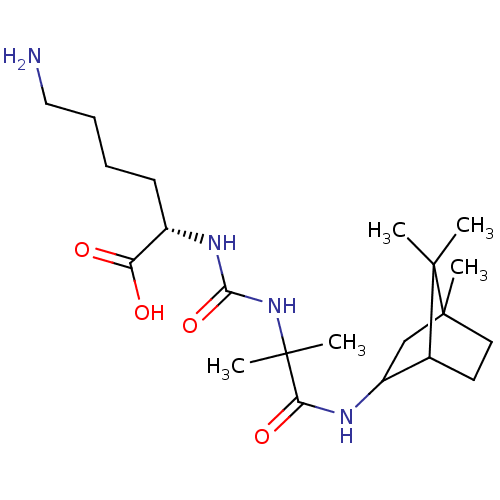
(CHEMBL3577431)Show SMILES CC(C)(NC(=O)N[C@@H](CCCCN)C(O)=O)C(=O)NC1CC2(C)CCC1C2(C)C |r,THB:18:19:26:23.24| Show InChI InChI=1S/C21H38N4O4/c1-19(2)13-9-10-21(19,5)12-15(13)23-17(28)20(3,4)25-18(29)24-14(16(26)27)8-6-7-11-22/h13-15H,6-12,22H2,1-5H3,(H,23,28)(H,26,27)(H2,24,25,29)/t13?,14-,15?,21?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Infection Research
Curated by ChEMBL
| Assay Description
Inhibition of human activated TAFI incubated for 15 mins by microtiter plate reader based assay |
J Med Chem 58: 4839-44 (2015)
Article DOI: 10.1021/jm501840b
BindingDB Entry DOI: 10.7270/Q24B3314 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50089684

(CHEMBL3577447)Show SMILES Cl.CC1(C)C2CCC1(C)CC2NC(=O)[C@@H](CC1CCCCC1)NC(=O)N[C@@H](CCCCN)C(O)=O |r,TLB:11:10:6.5:2| Show InChI InChI=1S/C26H46N4O4/c1-25(2)18-12-13-26(25,3)16-21(18)28-22(31)20(15-17-9-5-4-6-10-17)30-24(34)29-19(23(32)33)11-7-8-14-27/h17-21H,4-16,27H2,1-3H3,(H,28,31)(H,32,33)(H2,29,30,34)/t18?,19-,20+,21?,26?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Infection Research
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 in human liver microsomes using midazolam substrate incubated for 10 mins by LC-MS/MS method |
J Med Chem 58: 4839-44 (2015)
Article DOI: 10.1021/jm501840b
BindingDB Entry DOI: 10.7270/Q24B3314 |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50089686

(CHEMBL3577334)Show SMILES CC(C)[C@@H]1NC(=O)[C@@H](CCCCNC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](C)N(C)C(=O)[C@H](CCc2ccc(O)cc2)NC1=O)NC(=O)N[C@@H](CCCCN)C(O)=O |r| Show InChI InChI=1S/C41H60N8O9/c1-25(2)34-38(54)44-31(21-18-27-16-19-29(50)20-17-27)39(55)49(4)26(3)35(51)45-33(24-28-12-6-5-7-13-28)36(52)43-23-11-9-14-30(37(53)48-34)46-41(58)47-32(40(56)57)15-8-10-22-42/h5-7,12-13,16-17,19-20,25-26,30-34,50H,8-11,14-15,18,21-24,42H2,1-4H3,(H,43,52)(H,44,54)(H,45,51)(H,48,53)(H,56,57)(H2,46,47,58)/t26-,30+,31-,32-,33-,34-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Infection Research
Curated by ChEMBL
| Assay Description
Inhibition of factor11a (unknown origin) |
J Med Chem 58: 4839-44 (2015)
Article DOI: 10.1021/jm501840b
BindingDB Entry DOI: 10.7270/Q24B3314 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50089684

(CHEMBL3577447)Show SMILES Cl.CC1(C)C2CCC1(C)CC2NC(=O)[C@@H](CC1CCCCC1)NC(=O)N[C@@H](CCCCN)C(O)=O |r,TLB:11:10:6.5:2| Show InChI InChI=1S/C26H46N4O4/c1-25(2)18-12-13-26(25,3)16-21(18)28-22(31)20(15-17-9-5-4-6-10-17)30-24(34)29-19(23(32)33)11-7-8-14-27/h17-21H,4-16,27H2,1-3H3,(H,28,31)(H,32,33)(H2,29,30,34)/t18?,19-,20+,21?,26?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Infection Research
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 in human liver microsomes using testosterone substrate incubated for 30 mins by LC-MS/MS method |
J Med Chem 58: 4839-44 (2015)
Article DOI: 10.1021/jm501840b
BindingDB Entry DOI: 10.7270/Q24B3314 |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50089688

(ANABAENOPEPTIN B)Show SMILES CC(C)[C@@H]1NC(=O)[C@@H](CCCCNC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](C)N(C)C(=O)[C@H](CCc2ccc(O)cc2)NC1=O)NC(=O)N[C@@H](CCCNC(N)=N)C(O)=O |r| Show InChI InChI=1S/C41H60N10O9/c1-24(2)33-37(56)46-30(20-17-26-15-18-28(52)19-16-26)38(57)51(4)25(3)34(53)47-32(23-27-11-6-5-7-12-27)35(54)44-21-9-8-13-29(36(55)50-33)48-41(60)49-31(39(58)59)14-10-22-45-40(42)43/h5-7,11-12,15-16,18-19,24-25,29-33,52H,8-10,13-14,17,20-23H2,1-4H3,(H,44,54)(H,46,56)(H,47,53)(H,50,55)(H,58,59)(H4,42,43,45)(H2,48,49,60)/t25-,29+,30-,31-,32-,33-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Infection Research
Curated by ChEMBL
| Assay Description
Inhibition of factor11a (unknown origin) |
J Med Chem 58: 4839-44 (2015)
Article DOI: 10.1021/jm501840b
BindingDB Entry DOI: 10.7270/Q24B3314 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50089684

(CHEMBL3577447)Show SMILES Cl.CC1(C)C2CCC1(C)CC2NC(=O)[C@@H](CC1CCCCC1)NC(=O)N[C@@H](CCCCN)C(O)=O |r,TLB:11:10:6.5:2| Show InChI InChI=1S/C26H46N4O4/c1-25(2)18-12-13-26(25,3)16-21(18)28-22(31)20(15-17-9-5-4-6-10-17)30-24(34)29-19(23(32)33)11-7-8-14-27/h17-21H,4-16,27H2,1-3H3,(H,28,31)(H,32,33)(H2,29,30,34)/t18?,19-,20+,21?,26?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Infection Research
Curated by ChEMBL
| Assay Description
Inhibition of human ERG expressed in CHO cells by whole cell patch clamp assay |
J Med Chem 58: 4839-44 (2015)
Article DOI: 10.1021/jm501840b
BindingDB Entry DOI: 10.7270/Q24B3314 |
More data for this
Ligand-Target Pair | |
Coagulation factor VII
(Homo sapiens (Human)) | BDBM50089687

(Anabaenopeptin F)Show SMILES [H][C@]1(NC(=O)[C@@H](CCCCNC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](C)N(C)C(=O)[C@H](CCc2ccc(O)cc2)NC1=O)NC(=O)N[C@@H](CCCNC(N)=N)C(O)=O)[C@@H](C)CC |r| Show InChI InChI=1S/C42H62N10O9/c1-5-25(2)34-38(57)47-31(21-18-27-16-19-29(53)20-17-27)39(58)52(4)26(3)35(54)48-33(24-28-12-7-6-8-13-28)36(55)45-22-10-9-14-30(37(56)51-34)49-42(61)50-32(40(59)60)15-11-23-46-41(43)44/h6-8,12-13,16-17,19-20,25-26,30-34,53H,5,9-11,14-15,18,21-24H2,1-4H3,(H,45,55)(H,47,57)(H,48,54)(H,51,56)(H,59,60)(H4,43,44,46)(H2,49,50,61)/t25-,26-,30+,31-,32-,33-,34-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Infection Research
Curated by ChEMBL
| Assay Description
Inhibition of factor7a (unknown origin) |
J Med Chem 58: 4839-44 (2015)
Article DOI: 10.1021/jm501840b
BindingDB Entry DOI: 10.7270/Q24B3314 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50089687

(Anabaenopeptin F)Show SMILES [H][C@]1(NC(=O)[C@@H](CCCCNC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](C)N(C)C(=O)[C@H](CCc2ccc(O)cc2)NC1=O)NC(=O)N[C@@H](CCCNC(N)=N)C(O)=O)[C@@H](C)CC |r| Show InChI InChI=1S/C42H62N10O9/c1-5-25(2)34-38(57)47-31(21-18-27-16-19-29(53)20-17-27)39(58)52(4)26(3)35(54)48-33(24-28-12-7-6-8-13-28)36(55)45-22-10-9-14-30(37(56)51-34)49-42(61)50-32(40(59)60)15-11-23-46-41(43)44/h6-8,12-13,16-17,19-20,25-26,30-34,53H,5,9-11,14-15,18,21-24H2,1-4H3,(H,45,55)(H,47,57)(H,48,54)(H,51,56)(H,59,60)(H4,43,44,46)(H2,49,50,61)/t25-,26-,30+,31-,32-,33-,34-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Infection Research
Curated by ChEMBL
| Assay Description
Inhibition of factor10a (unknown origin) |
J Med Chem 58: 4839-44 (2015)
Article DOI: 10.1021/jm501840b
BindingDB Entry DOI: 10.7270/Q24B3314 |
More data for this
Ligand-Target Pair | |
Carboxypeptidase B2
(Homo sapiens (Human)) | BDBM50089782

(CHEMBL3577446)Show SMILES CC12CCC(C(C1)NC(=O)[C@@H](CC1CCCCC1)NC(=O)N[C@@H](Cc1ccc(N)nc1)C#N)C2(C)C |r,THB:7:5:33:3.2| Show InChI InChI=1S/C28H42N6O2/c1-27(2)21-11-12-28(27,3)15-23(21)33-25(35)22(14-18-7-5-4-6-8-18)34-26(36)32-20(16-29)13-19-9-10-24(30)31-17-19/h9-10,17-18,20-23H,4-8,11-15H2,1-3H3,(H2,30,31)(H,33,35)(H2,32,34,36)/t20-,21?,22+,23?,28?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Infection Research
Curated by ChEMBL
| Assay Description
Inhibition of human activated TAFI incubated for 15 mins by microtiter plate reader based assay |
J Med Chem 58: 4839-44 (2015)
Article DOI: 10.1021/jm501840b
BindingDB Entry DOI: 10.7270/Q24B3314 |
More data for this
Ligand-Target Pair | |
Carboxypeptidase B2
(Homo sapiens (Human)) | BDBM50089693

(CHEMBL3577331)Show SMILES NCCCC[C@H](NC(=O)N[C@H](CC1CCCCC1)C(=O)N1CCCC1)C(O)=O |r| Show InChI InChI=1S/C20H36N4O4/c21-11-5-4-10-16(19(26)27)22-20(28)23-17(14-15-8-2-1-3-9-15)18(25)24-12-6-7-13-24/h15-17H,1-14,21H2,(H,26,27)(H2,22,23,28)/t16-,17+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Infection Research
Curated by ChEMBL
| Assay Description
Inhibition of human activated TAFI incubated for 15 mins by microtiter plate reader based assay |
J Med Chem 58: 4839-44 (2015)
Article DOI: 10.1021/jm501840b
BindingDB Entry DOI: 10.7270/Q24B3314 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50089686

(CHEMBL3577334)Show SMILES CC(C)[C@@H]1NC(=O)[C@@H](CCCCNC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](C)N(C)C(=O)[C@H](CCc2ccc(O)cc2)NC1=O)NC(=O)N[C@@H](CCCCN)C(O)=O |r| Show InChI InChI=1S/C41H60N8O9/c1-25(2)34-38(54)44-31(21-18-27-16-19-29(50)20-17-27)39(55)49(4)26(3)35(51)45-33(24-28-12-6-5-7-13-28)36(52)43-23-11-9-14-30(37(53)48-34)46-41(58)47-32(40(56)57)15-8-10-22-42/h5-7,12-13,16-17,19-20,25-26,30-34,50H,8-11,14-15,18,21-24,42H2,1-4H3,(H,43,52)(H,44,54)(H,45,51)(H,48,53)(H,56,57)(H2,46,47,58)/t26-,30+,31-,32-,33-,34-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Infection Research
Curated by ChEMBL
| Assay Description
Inhibition of factor10a (unknown origin) |
J Med Chem 58: 4839-44 (2015)
Article DOI: 10.1021/jm501840b
BindingDB Entry DOI: 10.7270/Q24B3314 |
More data for this
Ligand-Target Pair | |
Coagulation factor VII
(Homo sapiens (Human)) | BDBM50089686

(CHEMBL3577334)Show SMILES CC(C)[C@@H]1NC(=O)[C@@H](CCCCNC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](C)N(C)C(=O)[C@H](CCc2ccc(O)cc2)NC1=O)NC(=O)N[C@@H](CCCCN)C(O)=O |r| Show InChI InChI=1S/C41H60N8O9/c1-25(2)34-38(54)44-31(21-18-27-16-19-29(50)20-17-27)39(55)49(4)26(3)35(51)45-33(24-28-12-6-5-7-13-28)36(52)43-23-11-9-14-30(37(53)48-34)46-41(58)47-32(40(56)57)15-8-10-22-42/h5-7,12-13,16-17,19-20,25-26,30-34,50H,8-11,14-15,18,21-24,42H2,1-4H3,(H,43,52)(H,44,54)(H,45,51)(H,48,53)(H,56,57)(H2,46,47,58)/t26-,30+,31-,32-,33-,34-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Infection Research
Curated by ChEMBL
| Assay Description
Inhibition of factor7a (unknown origin) |
J Med Chem 58: 4839-44 (2015)
Article DOI: 10.1021/jm501840b
BindingDB Entry DOI: 10.7270/Q24B3314 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50089688

(ANABAENOPEPTIN B)Show SMILES CC(C)[C@@H]1NC(=O)[C@@H](CCCCNC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](C)N(C)C(=O)[C@H](CCc2ccc(O)cc2)NC1=O)NC(=O)N[C@@H](CCCNC(N)=N)C(O)=O |r| Show InChI InChI=1S/C41H60N10O9/c1-24(2)33-37(56)46-30(20-17-26-15-18-28(52)19-16-26)38(57)51(4)25(3)34(53)47-32(23-27-11-6-5-7-12-27)35(54)44-21-9-8-13-29(36(55)50-33)48-41(60)49-31(39(58)59)14-10-22-45-40(42)43/h5-7,11-12,15-16,18-19,24-25,29-33,52H,8-10,13-14,17,20-23H2,1-4H3,(H,44,54)(H,46,56)(H,47,53)(H,50,55)(H,58,59)(H4,42,43,45)(H2,48,49,60)/t25-,29+,30-,31-,32-,33-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Infection Research
Curated by ChEMBL
| Assay Description
Inhibition of factor10a (unknown origin) |
J Med Chem 58: 4839-44 (2015)
Article DOI: 10.1021/jm501840b
BindingDB Entry DOI: 10.7270/Q24B3314 |
More data for this
Ligand-Target Pair | |
Carboxypeptidase A1
(Homo sapiens (Human)) | BDBM50089686

(CHEMBL3577334)Show SMILES CC(C)[C@@H]1NC(=O)[C@@H](CCCCNC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](C)N(C)C(=O)[C@H](CCc2ccc(O)cc2)NC1=O)NC(=O)N[C@@H](CCCCN)C(O)=O |r| Show InChI InChI=1S/C41H60N8O9/c1-25(2)34-38(54)44-31(21-18-27-16-19-29(50)20-17-27)39(55)49(4)26(3)35(51)45-33(24-28-12-6-5-7-13-28)36(52)43-23-11-9-14-30(37(53)48-34)46-41(58)47-32(40(56)57)15-8-10-22-42/h5-7,12-13,16-17,19-20,25-26,30-34,50H,8-11,14-15,18,21-24,42H2,1-4H3,(H,43,52)(H,44,54)(H,45,51)(H,48,53)(H,56,57)(H2,46,47,58)/t26-,30+,31-,32-,33-,34-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Infection Research
Curated by ChEMBL
| Assay Description
Inhibition of CPA (unknown origin) |
J Med Chem 58: 4839-44 (2015)
Article DOI: 10.1021/jm501840b
BindingDB Entry DOI: 10.7270/Q24B3314 |
More data for this
Ligand-Target Pair | |
Coagulation factor VII
(Homo sapiens (Human)) | BDBM50089688

(ANABAENOPEPTIN B)Show SMILES CC(C)[C@@H]1NC(=O)[C@@H](CCCCNC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](C)N(C)C(=O)[C@H](CCc2ccc(O)cc2)NC1=O)NC(=O)N[C@@H](CCCNC(N)=N)C(O)=O |r| Show InChI InChI=1S/C41H60N10O9/c1-24(2)33-37(56)46-30(20-17-26-15-18-28(52)19-16-26)38(57)51(4)25(3)34(53)47-32(23-27-11-6-5-7-12-27)35(54)44-21-9-8-13-29(36(55)50-33)48-41(60)49-31(39(58)59)14-10-22-45-40(42)43/h5-7,11-12,15-16,18-19,24-25,29-33,52H,8-10,13-14,17,20-23H2,1-4H3,(H,44,54)(H,46,56)(H,47,53)(H,50,55)(H,58,59)(H4,42,43,45)(H2,48,49,60)/t25-,29+,30-,31-,32-,33-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Infection Research
Curated by ChEMBL
| Assay Description
Inhibition of factor7a (unknown origin) |
J Med Chem 58: 4839-44 (2015)
Article DOI: 10.1021/jm501840b
BindingDB Entry DOI: 10.7270/Q24B3314 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data