 Found 41 hits of Enzyme Inhibition Constant Data
Found 41 hits of Enzyme Inhibition Constant Data Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM50107278
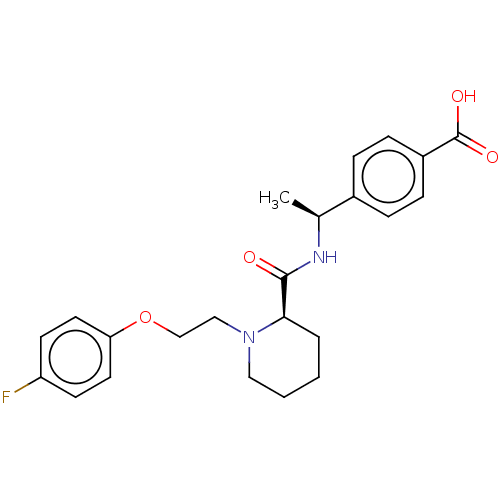
(CHEMBL3600884)Show SMILES Cl.C[C@H](NC(=O)[C@H]1CCCCN1CCOc1ccc(F)cc1)c1ccc(cc1)C(O)=O |r| Show InChI InChI=1S/C23H27FN2O4/c1-16(17-5-7-18(8-6-17)23(28)29)25-22(27)21-4-2-3-13-26(21)14-15-30-20-11-9-19(24)10-12-20/h5-12,16,21H,2-4,13-15H2,1H3,(H,25,27)(H,28,29)/t16-,21+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]-PGE2 from recombinant human EP4 receptor expressed in HEK293 cell membranes after 90 mins by liquid scintillation counting analy... |
Bioorg Med Chem Lett 25: 3176-8 (2015)
Article DOI: 10.1016/j.bmcl.2015.05.091
BindingDB Entry DOI: 10.7270/Q2FX7C79 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM50446847

(CHEMBL3115074)Show SMILES C[C@H](NC(=O)[C@H]1CCCCN1CCOc1ccccc1)c1ccc(cc1)C(O)=O |r| Show InChI InChI=1S/C23H28N2O4/c1-17(18-10-12-19(13-11-18)23(27)28)24-22(26)21-9-5-6-14-25(21)15-16-29-20-7-3-2-4-8-20/h2-4,7-8,10-13,17,21H,5-6,9,14-16H2,1H3,(H,24,26)(H,27,28)/t17-,21+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 41 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]-PGE2 from recombinant human EP4 receptor expressed in HEK293 cell membranes after 90 mins by liquid scintillation counting analy... |
Bioorg Med Chem Lett 25: 3176-8 (2015)
Article DOI: 10.1016/j.bmcl.2015.05.091
BindingDB Entry DOI: 10.7270/Q2FX7C79 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM50107280
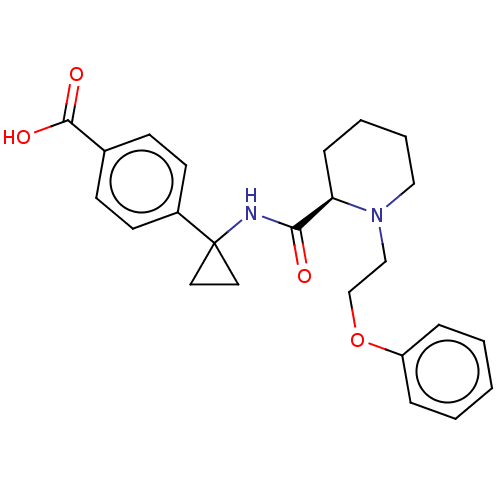
(CHEMBL3600788)Show SMILES Cl.OC(=O)c1ccc(cc1)C1(CC1)NC(=O)[C@H]1CCCCN1CCOc1ccccc1 |r| Show InChI InChI=1S/C24H28N2O4/c27-22(25-24(13-14-24)19-11-9-18(10-12-19)23(28)29)21-8-4-5-15-26(21)16-17-30-20-6-2-1-3-7-20/h1-3,6-7,9-12,21H,4-5,8,13-17H2,(H,25,27)(H,28,29)/t21-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 45 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]-PGE2 from recombinant human EP4 receptor expressed in HEK293 cell membranes after 90 mins by liquid scintillation counting analy... |
Bioorg Med Chem Lett 25: 3176-8 (2015)
Article DOI: 10.1016/j.bmcl.2015.05.091
BindingDB Entry DOI: 10.7270/Q2FX7C79 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM50107282
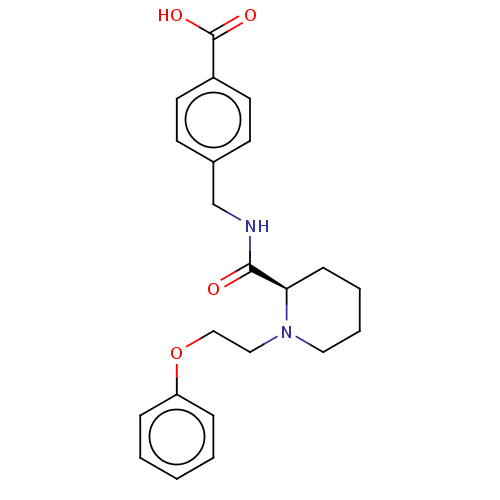
(CHEMBL3600786)Show SMILES Cl.OC(=O)c1ccc(CNC(=O)[C@H]2CCCCN2CCOc2ccccc2)cc1 |r| Show InChI InChI=1S/C22H26N2O4/c25-21(23-16-17-9-11-18(12-10-17)22(26)27)20-8-4-5-13-24(20)14-15-28-19-6-2-1-3-7-19/h1-3,6-7,9-12,20H,4-5,8,13-16H2,(H,23,25)(H,26,27)/t20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 126 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]-PGE2 from recombinant human EP4 receptor expressed in HEK293 cell membranes after 90 mins by liquid scintillation counting analy... |
Bioorg Med Chem Lett 25: 3176-8 (2015)
Article DOI: 10.1016/j.bmcl.2015.05.091
BindingDB Entry DOI: 10.7270/Q2FX7C79 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM50107281
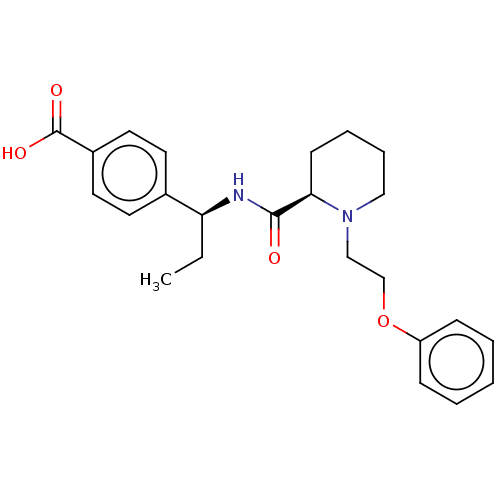
(CHEMBL3600787)Show SMILES Cl.CC[C@H](NC(=O)[C@H]1CCCCN1CCOc1ccccc1)c1ccc(cc1)C(O)=O |r| Show InChI InChI=1S/C24H30N2O4/c1-2-21(18-11-13-19(14-12-18)24(28)29)25-23(27)22-10-6-7-15-26(22)16-17-30-20-8-4-3-5-9-20/h3-5,8-9,11-14,21-22H,2,6-7,10,15-17H2,1H3,(H,25,27)(H,28,29)/t21-,22+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 129 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]-PGE2 from recombinant human EP4 receptor expressed in HEK293 cell membranes after 90 mins by liquid scintillation counting analy... |
Bioorg Med Chem Lett 25: 3176-8 (2015)
Article DOI: 10.1016/j.bmcl.2015.05.091
BindingDB Entry DOI: 10.7270/Q2FX7C79 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM50107279

(CHEMBL3600883)Show SMILES Cl.C[C@H](NC(=O)[C@H]1CCCCN1CCOc1ccc(cc1)C#N)c1ccc(cc1)C(O)=O |r| Show InChI InChI=1S/C24H27N3O4/c1-17(19-7-9-20(10-8-19)24(29)30)26-23(28)22-4-2-3-13-27(22)14-15-31-21-11-5-18(16-25)6-12-21/h5-12,17,22H,2-4,13-15H2,1H3,(H,26,28)(H,29,30)/t17-,22+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 372 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]-PGE2 from recombinant human EP4 receptor expressed in HEK293 cell membranes after 90 mins by liquid scintillation counting analy... |
Bioorg Med Chem Lett 25: 3176-8 (2015)
Article DOI: 10.1016/j.bmcl.2015.05.091
BindingDB Entry DOI: 10.7270/Q2FX7C79 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM50107283
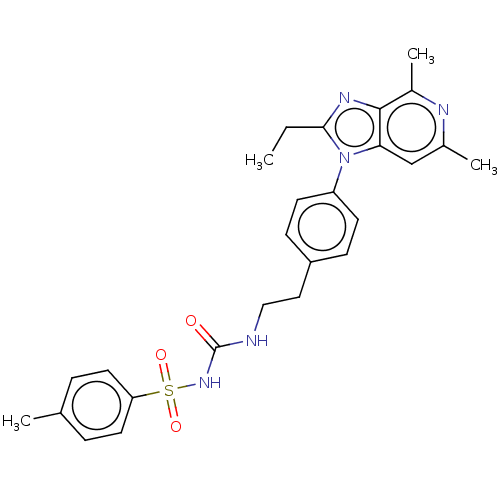
(AAT-007 | CJ-023 | Grapiprant | MR-10A7 | RQ-00000...)Show SMILES CCc1nc2c(C)nc(C)cc2n1-c1ccc(CCNC(=O)NS(=O)(=O)c2ccc(C)cc2)cc1 Show InChI InChI=1S/C26H29N5O3S/c1-5-24-29-25-19(4)28-18(3)16-23(25)31(24)21-10-8-20(9-11-21)14-15-27-26(32)30-35(33,34)22-12-6-17(2)7-13-22/h6-13,16H,5,14-15H2,1-4H3,(H2,27,30,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 449 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]-PGE2 from recombinant human EP4 receptor expressed in HEK293 cell membranes after 90 mins by liquid scintillation counting analy... |
Bioorg Med Chem Lett 25: 3176-8 (2015)
Article DOI: 10.1016/j.bmcl.2015.05.091
BindingDB Entry DOI: 10.7270/Q2FX7C79 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP2 subtype
(Homo sapiens (Human)) | BDBM50446847

(CHEMBL3115074)Show SMILES C[C@H](NC(=O)[C@H]1CCCCN1CCOc1ccccc1)c1ccc(cc1)C(O)=O |r| Show InChI InChI=1S/C23H28N2O4/c1-17(18-10-12-19(13-11-18)23(27)28)24-22(26)21-9-5-6-14-25(21)15-16-29-20-7-3-2-4-8-20/h2-4,7-8,10-13,17,21H,5-6,9,14-16H2,1H3,(H,24,26)(H,27,28)/t17-,21+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 1.21E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]-PGE2 from human EP2 receptor by liquid scintillation counting analysis |
Bioorg Med Chem Lett 25: 3176-8 (2015)
Article DOI: 10.1016/j.bmcl.2015.05.091
BindingDB Entry DOI: 10.7270/Q2FX7C79 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50446847

(CHEMBL3115074)Show SMILES C[C@H](NC(=O)[C@H]1CCCCN1CCOc1ccccc1)c1ccc(cc1)C(O)=O |r| Show InChI InChI=1S/C23H28N2O4/c1-17(18-10-12-19(13-11-18)23(27)28)24-22(26)21-9-5-6-14-25(21)15-16-29-20-7-3-2-4-8-20/h2-4,7-8,10-13,17,21H,5-6,9,14-16H2,1H3,(H,24,26)(H,27,28)/t17-,21+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| >1.27E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]-PGE2 from human EP3 receptor by liquid scintillation counting analysis |
Bioorg Med Chem Lett 25: 3176-8 (2015)
Article DOI: 10.1016/j.bmcl.2015.05.091
BindingDB Entry DOI: 10.7270/Q2FX7C79 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP1 subtype
(Homo sapiens (Human)) | BDBM50446847

(CHEMBL3115074)Show SMILES C[C@H](NC(=O)[C@H]1CCCCN1CCOc1ccccc1)c1ccc(cc1)C(O)=O |r| Show InChI InChI=1S/C23H28N2O4/c1-17(18-10-12-19(13-11-18)23(27)28)24-22(26)21-9-5-6-14-25(21)15-16-29-20-7-3-2-4-8-20/h2-4,7-8,10-13,17,21H,5-6,9,14-16H2,1H3,(H,24,26)(H,27,28)/t17-,21+/m0/s1 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| >1.75E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]-PGE2 from human EP1 receptor by liquid scintillation counting analysis |
Bioorg Med Chem Lett 25: 3176-8 (2015)
Article DOI: 10.1016/j.bmcl.2015.05.091
BindingDB Entry DOI: 10.7270/Q2FX7C79 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM50107278

(CHEMBL3600884)Show SMILES Cl.C[C@H](NC(=O)[C@H]1CCCCN1CCOc1ccc(F)cc1)c1ccc(cc1)C(O)=O |r| Show InChI InChI=1S/C23H27FN2O4/c1-16(17-5-7-18(8-6-17)23(28)29)25-22(27)21-4-2-3-13-26(21)14-15-30-20-11-9-19(24)10-12-20/h5-12,16,21H,2-4,13-15H2,1H3,(H,25,27)(H,28,29)/t16-,21+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity at recombinant human EP4 receptor expressed in HEK293 cells assessed as inhibition of PGE2-stimulated cAMP accumulation by scinti... |
Bioorg Med Chem Lett 25: 3176-8 (2015)
Article DOI: 10.1016/j.bmcl.2015.05.091
BindingDB Entry DOI: 10.7270/Q2FX7C79 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM50107280

(CHEMBL3600788)Show SMILES Cl.OC(=O)c1ccc(cc1)C1(CC1)NC(=O)[C@H]1CCCCN1CCOc1ccccc1 |r| Show InChI InChI=1S/C24H28N2O4/c27-22(25-24(13-14-24)19-11-9-18(10-12-19)23(28)29)21-8-4-5-15-26(21)16-17-30-20-6-2-1-3-7-20/h1-3,6-7,9-12,21H,4-5,8,13-17H2,(H,25,27)(H,28,29)/t21-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity at recombinant human EP4 receptor expressed in HEK293 cells assessed as inhibition of PGE2-stimulated cAMP accumulation by scinti... |
Bioorg Med Chem Lett 25: 3176-8 (2015)
Article DOI: 10.1016/j.bmcl.2015.05.091
BindingDB Entry DOI: 10.7270/Q2FX7C79 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM50446847

(CHEMBL3115074)Show SMILES C[C@H](NC(=O)[C@H]1CCCCN1CCOc1ccccc1)c1ccc(cc1)C(O)=O |r| Show InChI InChI=1S/C23H28N2O4/c1-17(18-10-12-19(13-11-18)23(27)28)24-22(26)21-9-5-6-14-25(21)15-16-29-20-7-3-2-4-8-20/h2-4,7-8,10-13,17,21H,5-6,9,14-16H2,1H3,(H,24,26)(H,27,28)/t17-,21+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity at recombinant human EP4 receptor expressed in HEK293 cells assessed as inhibition of PGE2-stimulated cAMP accumulation by scinti... |
Bioorg Med Chem Lett 25: 3176-8 (2015)
Article DOI: 10.1016/j.bmcl.2015.05.091
BindingDB Entry DOI: 10.7270/Q2FX7C79 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM50107283

(AAT-007 | CJ-023 | Grapiprant | MR-10A7 | RQ-00000...)Show SMILES CCc1nc2c(C)nc(C)cc2n1-c1ccc(CCNC(=O)NS(=O)(=O)c2ccc(C)cc2)cc1 Show InChI InChI=1S/C26H29N5O3S/c1-5-24-29-25-19(4)28-18(3)16-23(25)31(24)21-10-8-20(9-11-21)14-15-27-26(32)30-35(33,34)22-12-6-17(2)7-13-22/h6-13,16H,5,14-15H2,1-4H3,(H2,27,30,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity at recombinant human EP4 receptor expressed in HEK293 cells assessed as inhibition of PGE2-stimulated cAMP accumulation by scinti... |
Bioorg Med Chem Lett 25: 3176-8 (2015)
Article DOI: 10.1016/j.bmcl.2015.05.091
BindingDB Entry DOI: 10.7270/Q2FX7C79 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM50107281

(CHEMBL3600787)Show SMILES Cl.CC[C@H](NC(=O)[C@H]1CCCCN1CCOc1ccccc1)c1ccc(cc1)C(O)=O |r| Show InChI InChI=1S/C24H30N2O4/c1-2-21(18-11-13-19(14-12-18)24(28)29)25-23(27)22-10-6-7-15-26(22)16-17-30-20-8-4-3-5-9-20/h3-5,8-9,11-14,21-22H,2,6-7,10,15-17H2,1H3,(H,25,27)(H,28,29)/t21-,22+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity at recombinant human EP4 receptor expressed in HEK293 cells assessed as inhibition of PGE2-stimulated cAMP accumulation by scinti... |
Bioorg Med Chem Lett 25: 3176-8 (2015)
Article DOI: 10.1016/j.bmcl.2015.05.091
BindingDB Entry DOI: 10.7270/Q2FX7C79 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM50107282

(CHEMBL3600786)Show SMILES Cl.OC(=O)c1ccc(CNC(=O)[C@H]2CCCCN2CCOc2ccccc2)cc1 |r| Show InChI InChI=1S/C22H26N2O4/c25-21(23-16-17-9-11-18(12-10-17)22(26)27)20-8-4-5-13-24(20)14-15-28-19-6-2-1-3-7-19/h1-3,6-7,9-12,20H,4-5,8,13-16H2,(H,23,25)(H,26,27)/t20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity at recombinant human EP4 receptor expressed in HEK293 cells assessed as inhibition of PGE2-stimulated cAMP accumulation by scinti... |
Bioorg Med Chem Lett 25: 3176-8 (2015)
Article DOI: 10.1016/j.bmcl.2015.05.091
BindingDB Entry DOI: 10.7270/Q2FX7C79 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM50107279

(CHEMBL3600883)Show SMILES Cl.C[C@H](NC(=O)[C@H]1CCCCN1CCOc1ccc(cc1)C#N)c1ccc(cc1)C(O)=O |r| Show InChI InChI=1S/C24H27N3O4/c1-17(19-7-9-20(10-8-19)24(29)30)26-23(28)22-4-2-3-13-27(22)14-15-31-21-11-5-18(16-25)6-12-21/h5-12,17,22H,2-4,13-15H2,1H3,(H,26,28)(H,29,30)/t17-,22+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity at recombinant human EP4 receptor expressed in HEK293 cells assessed as inhibition of PGE2-stimulated cAMP accumulation by scinti... |
Bioorg Med Chem Lett 25: 3176-8 (2015)
Article DOI: 10.1016/j.bmcl.2015.05.091
BindingDB Entry DOI: 10.7270/Q2FX7C79 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM50446847

(CHEMBL3115074)Show SMILES C[C@H](NC(=O)[C@H]1CCCCN1CCOc1ccccc1)c1ccc(cc1)C(O)=O |r| Show InChI InChI=1S/C23H28N2O4/c1-17(18-10-12-19(13-11-18)23(27)28)24-22(26)21-9-5-6-14-25(21)15-16-29-20-7-3-2-4-8-20/h2-4,7-8,10-13,17,21H,5-6,9,14-16H2,1H3,(H,24,26)(H,27,28)/t17-,21+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 69 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]-PGE2 from recombinant human EP4 receptor expressed in HEK293 cell membranes after 90 mins by liquid scintillation counting analy... |
Bioorg Med Chem Lett 25: 3176-8 (2015)
Article DOI: 10.1016/j.bmcl.2015.05.091
BindingDB Entry DOI: 10.7270/Q2FX7C79 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM50107278

(CHEMBL3600884)Show SMILES Cl.C[C@H](NC(=O)[C@H]1CCCCN1CCOc1ccc(F)cc1)c1ccc(cc1)C(O)=O |r| Show InChI InChI=1S/C23H27FN2O4/c1-16(17-5-7-18(8-6-17)23(28)29)25-22(27)21-4-2-3-13-26(21)14-15-30-20-11-9-19(24)10-12-20/h5-12,16,21H,2-4,13-15H2,1H3,(H,25,27)(H,28,29)/t16-,21+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 72 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]-PGE2 from recombinant human EP4 receptor expressed in HEK293 cell membranes after 90 mins by liquid scintillation counting analy... |
Bioorg Med Chem Lett 25: 3176-8 (2015)
Article DOI: 10.1016/j.bmcl.2015.05.091
BindingDB Entry DOI: 10.7270/Q2FX7C79 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM50107280

(CHEMBL3600788)Show SMILES Cl.OC(=O)c1ccc(cc1)C1(CC1)NC(=O)[C@H]1CCCCN1CCOc1ccccc1 |r| Show InChI InChI=1S/C24H28N2O4/c27-22(25-24(13-14-24)19-11-9-18(10-12-19)23(28)29)21-8-4-5-15-26(21)16-17-30-20-6-2-1-3-7-20/h1-3,6-7,9-12,21H,4-5,8,13-17H2,(H,25,27)(H,28,29)/t21-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 78 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]-PGE2 from recombinant human EP4 receptor expressed in HEK293 cell membranes after 90 mins by liquid scintillation counting analy... |
Bioorg Med Chem Lett 25: 3176-8 (2015)
Article DOI: 10.1016/j.bmcl.2015.05.091
BindingDB Entry DOI: 10.7270/Q2FX7C79 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM50107278

(CHEMBL3600884)Show SMILES Cl.C[C@H](NC(=O)[C@H]1CCCCN1CCOc1ccc(F)cc1)c1ccc(cc1)C(O)=O |r| Show InChI InChI=1S/C23H27FN2O4/c1-16(17-5-7-18(8-6-17)23(28)29)25-22(27)21-4-2-3-13-26(21)14-15-30-20-11-9-19(24)10-12-20/h5-12,16,21H,2-4,13-15H2,1H3,(H,25,27)(H,28,29)/t16-,21+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 121 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity at EP4 receptor in LPS-stimulated human whole blood assessed as inhibition of PGE2-induced TNF-alpha reduction preincubated for 3... |
Bioorg Med Chem Lett 25: 3176-8 (2015)
Article DOI: 10.1016/j.bmcl.2015.05.091
BindingDB Entry DOI: 10.7270/Q2FX7C79 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM50446847

(CHEMBL3115074)Show SMILES C[C@H](NC(=O)[C@H]1CCCCN1CCOc1ccccc1)c1ccc(cc1)C(O)=O |r| Show InChI InChI=1S/C23H28N2O4/c1-17(18-10-12-19(13-11-18)23(27)28)24-22(26)21-9-5-6-14-25(21)15-16-29-20-7-3-2-4-8-20/h2-4,7-8,10-13,17,21H,5-6,9,14-16H2,1H3,(H,24,26)(H,27,28)/t17-,21+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 126 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity at EP4 receptor in LPS-stimulated human whole blood assessed as inhibition of PGE2-induced TNF-alpha reduction preincubated for 3... |
Bioorg Med Chem Lett 25: 3176-8 (2015)
Article DOI: 10.1016/j.bmcl.2015.05.091
BindingDB Entry DOI: 10.7270/Q2FX7C79 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM50107282

(CHEMBL3600786)Show SMILES Cl.OC(=O)c1ccc(CNC(=O)[C@H]2CCCCN2CCOc2ccccc2)cc1 |r| Show InChI InChI=1S/C22H26N2O4/c25-21(23-16-17-9-11-18(12-10-17)22(26)27)20-8-4-5-13-24(20)14-15-28-19-6-2-1-3-7-19/h1-3,6-7,9-12,20H,4-5,8,13-16H2,(H,23,25)(H,26,27)/t20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 203 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]-PGE2 from recombinant human EP4 receptor expressed in HEK293 cell membranes after 90 mins by liquid scintillation counting analy... |
Bioorg Med Chem Lett 25: 3176-8 (2015)
Article DOI: 10.1016/j.bmcl.2015.05.091
BindingDB Entry DOI: 10.7270/Q2FX7C79 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM50107281

(CHEMBL3600787)Show SMILES Cl.CC[C@H](NC(=O)[C@H]1CCCCN1CCOc1ccccc1)c1ccc(cc1)C(O)=O |r| Show InChI InChI=1S/C24H30N2O4/c1-2-21(18-11-13-19(14-12-18)24(28)29)25-23(27)22-10-6-7-15-26(22)16-17-30-20-8-4-3-5-9-20/h3-5,8-9,11-14,21-22H,2,6-7,10,15-17H2,1H3,(H,25,27)(H,28,29)/t21-,22+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 232 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]-PGE2 from recombinant human EP4 receptor expressed in HEK293 cell membranes after 90 mins by liquid scintillation counting analy... |
Bioorg Med Chem Lett 25: 3176-8 (2015)
Article DOI: 10.1016/j.bmcl.2015.05.091
BindingDB Entry DOI: 10.7270/Q2FX7C79 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM50107281

(CHEMBL3600787)Show SMILES Cl.CC[C@H](NC(=O)[C@H]1CCCCN1CCOc1ccccc1)c1ccc(cc1)C(O)=O |r| Show InChI InChI=1S/C24H30N2O4/c1-2-21(18-11-13-19(14-12-18)24(28)29)25-23(27)22-10-6-7-15-26(22)16-17-30-20-8-4-3-5-9-20/h3-5,8-9,11-14,21-22H,2,6-7,10,15-17H2,1H3,(H,25,27)(H,28,29)/t21-,22+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 291 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity at EP4 receptor in LPS-stimulated human whole blood assessed as inhibition of PGE2-induced TNF-alpha reduction preincubated for 3... |
Bioorg Med Chem Lett 25: 3176-8 (2015)
Article DOI: 10.1016/j.bmcl.2015.05.091
BindingDB Entry DOI: 10.7270/Q2FX7C79 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM50107280

(CHEMBL3600788)Show SMILES Cl.OC(=O)c1ccc(cc1)C1(CC1)NC(=O)[C@H]1CCCCN1CCOc1ccccc1 |r| Show InChI InChI=1S/C24H28N2O4/c27-22(25-24(13-14-24)19-11-9-18(10-12-19)23(28)29)21-8-4-5-15-26(21)16-17-30-20-6-2-1-3-7-20/h1-3,6-7,9-12,21H,4-5,8,13-17H2,(H,25,27)(H,28,29)/t21-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 586 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity at EP4 receptor in LPS-stimulated human whole blood assessed as inhibition of PGE2-induced TNF-alpha reduction preincubated for 3... |
Bioorg Med Chem Lett 25: 3176-8 (2015)
Article DOI: 10.1016/j.bmcl.2015.05.091
BindingDB Entry DOI: 10.7270/Q2FX7C79 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM50107279

(CHEMBL3600883)Show SMILES Cl.C[C@H](NC(=O)[C@H]1CCCCN1CCOc1ccc(cc1)C#N)c1ccc(cc1)C(O)=O |r| Show InChI InChI=1S/C24H27N3O4/c1-17(19-7-9-20(10-8-19)24(29)30)26-23(28)22-4-2-3-13-27(22)14-15-31-21-11-5-18(16-25)6-12-21/h5-12,17,22H,2-4,13-15H2,1H3,(H,26,28)(H,29,30)/t17-,22+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 621 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]-PGE2 from recombinant human EP4 receptor expressed in HEK293 cell membranes after 90 mins by liquid scintillation counting analy... |
Bioorg Med Chem Lett 25: 3176-8 (2015)
Article DOI: 10.1016/j.bmcl.2015.05.091
BindingDB Entry DOI: 10.7270/Q2FX7C79 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM50107283

(AAT-007 | CJ-023 | Grapiprant | MR-10A7 | RQ-00000...)Show SMILES CCc1nc2c(C)nc(C)cc2n1-c1ccc(CCNC(=O)NS(=O)(=O)c2ccc(C)cc2)cc1 Show InChI InChI=1S/C26H29N5O3S/c1-5-24-29-25-19(4)28-18(3)16-23(25)31(24)21-10-8-20(9-11-21)14-15-27-26(32)30-35(33,34)22-12-6-17(2)7-13-22/h6-13,16H,5,14-15H2,1-4H3,(H2,27,30,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 689 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]-PGE2 from recombinant human EP4 receptor expressed in HEK293 cell membranes after 90 mins by liquid scintillation counting analy... |
Bioorg Med Chem Lett 25: 3176-8 (2015)
Article DOI: 10.1016/j.bmcl.2015.05.091
BindingDB Entry DOI: 10.7270/Q2FX7C79 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM50107282

(CHEMBL3600786)Show SMILES Cl.OC(=O)c1ccc(CNC(=O)[C@H]2CCCCN2CCOc2ccccc2)cc1 |r| Show InChI InChI=1S/C22H26N2O4/c25-21(23-16-17-9-11-18(12-10-17)22(26)27)20-8-4-5-13-24(20)14-15-28-19-6-2-1-3-7-19/h1-3,6-7,9-12,20H,4-5,8,13-16H2,(H,23,25)(H,26,27)/t20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 899 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity at EP4 receptor in LPS-stimulated human whole blood assessed as inhibition of PGE2-induced TNF-alpha reduction preincubated for 3... |
Bioorg Med Chem Lett 25: 3176-8 (2015)
Article DOI: 10.1016/j.bmcl.2015.05.091
BindingDB Entry DOI: 10.7270/Q2FX7C79 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM50107279

(CHEMBL3600883)Show SMILES Cl.C[C@H](NC(=O)[C@H]1CCCCN1CCOc1ccc(cc1)C#N)c1ccc(cc1)C(O)=O |r| Show InChI InChI=1S/C24H27N3O4/c1-17(19-7-9-20(10-8-19)24(29)30)26-23(28)22-4-2-3-13-27(22)14-15-31-21-11-5-18(16-25)6-12-21/h5-12,17,22H,2-4,13-15H2,1H3,(H,26,28)(H,29,30)/t17-,22+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.21E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity at EP4 receptor in LPS-stimulated human whole blood assessed as inhibition of PGE2-induced TNF-alpha reduction preincubated for 3... |
Bioorg Med Chem Lett 25: 3176-8 (2015)
Article DOI: 10.1016/j.bmcl.2015.05.091
BindingDB Entry DOI: 10.7270/Q2FX7C79 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM50107283

(AAT-007 | CJ-023 | Grapiprant | MR-10A7 | RQ-00000...)Show SMILES CCc1nc2c(C)nc(C)cc2n1-c1ccc(CCNC(=O)NS(=O)(=O)c2ccc(C)cc2)cc1 Show InChI InChI=1S/C26H29N5O3S/c1-5-24-29-25-19(4)28-18(3)16-23(25)31(24)21-10-8-20(9-11-21)14-15-27-26(32)30-35(33,34)22-12-6-17(2)7-13-22/h6-13,16H,5,14-15H2,1-4H3,(H2,27,30,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.61E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity at EP4 receptor in LPS-stimulated human whole blood assessed as inhibition of PGE2-induced TNF-alpha reduction preincubated for 3... |
Bioorg Med Chem Lett 25: 3176-8 (2015)
Article DOI: 10.1016/j.bmcl.2015.05.091
BindingDB Entry DOI: 10.7270/Q2FX7C79 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP2 subtype
(Homo sapiens (Human)) | BDBM50446847

(CHEMBL3115074)Show SMILES C[C@H](NC(=O)[C@H]1CCCCN1CCOc1ccccc1)c1ccc(cc1)C(O)=O |r| Show InChI InChI=1S/C23H28N2O4/c1-17(18-10-12-19(13-11-18)23(27)28)24-22(26)21-9-5-6-14-25(21)15-16-29-20-7-3-2-4-8-20/h2-4,7-8,10-13,17,21H,5-6,9,14-16H2,1H3,(H,24,26)(H,27,28)/t17-,21+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.63E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]-PGE2 from human EP2 receptor by liquid scintillation counting analysis |
Bioorg Med Chem Lett 25: 3176-8 (2015)
Article DOI: 10.1016/j.bmcl.2015.05.091
BindingDB Entry DOI: 10.7270/Q2FX7C79 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50446847

(CHEMBL3115074)Show SMILES C[C@H](NC(=O)[C@H]1CCCCN1CCOc1ccccc1)c1ccc(cc1)C(O)=O |r| Show InChI InChI=1S/C23H28N2O4/c1-17(18-10-12-19(13-11-18)23(27)28)24-22(26)21-9-5-6-14-25(21)15-16-29-20-7-3-2-4-8-20/h2-4,7-8,10-13,17,21H,5-6,9,14-16H2,1H3,(H,24,26)(H,27,28)/t17-,21+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C19 (unknown origin) |
Bioorg Med Chem Lett 25: 3176-8 (2015)
Article DOI: 10.1016/j.bmcl.2015.05.091
BindingDB Entry DOI: 10.7270/Q2FX7C79 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C8
(Homo sapiens (Human)) | BDBM50446847

(CHEMBL3115074)Show SMILES C[C@H](NC(=O)[C@H]1CCCCN1CCOc1ccccc1)c1ccc(cc1)C(O)=O |r| Show InChI InChI=1S/C23H28N2O4/c1-17(18-10-12-19(13-11-18)23(27)28)24-22(26)21-9-5-6-14-25(21)15-16-29-20-7-3-2-4-8-20/h2-4,7-8,10-13,17,21H,5-6,9,14-16H2,1H3,(H,24,26)(H,27,28)/t17-,21+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C8 (unknown origin) |
Bioorg Med Chem Lett 25: 3176-8 (2015)
Article DOI: 10.1016/j.bmcl.2015.05.091
BindingDB Entry DOI: 10.7270/Q2FX7C79 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2B6
(Homo sapiens (Human)) | BDBM50446847

(CHEMBL3115074)Show SMILES C[C@H](NC(=O)[C@H]1CCCCN1CCOc1ccccc1)c1ccc(cc1)C(O)=O |r| Show InChI InChI=1S/C23H28N2O4/c1-17(18-10-12-19(13-11-18)23(27)28)24-22(26)21-9-5-6-14-25(21)15-16-29-20-7-3-2-4-8-20/h2-4,7-8,10-13,17,21H,5-6,9,14-16H2,1H3,(H,24,26)(H,27,28)/t17-,21+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of CYP2B6 (unknown origin) |
Bioorg Med Chem Lett 25: 3176-8 (2015)
Article DOI: 10.1016/j.bmcl.2015.05.091
BindingDB Entry DOI: 10.7270/Q2FX7C79 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50446847

(CHEMBL3115074)Show SMILES C[C@H](NC(=O)[C@H]1CCCCN1CCOc1ccccc1)c1ccc(cc1)C(O)=O |r| Show InChI InChI=1S/C23H28N2O4/c1-17(18-10-12-19(13-11-18)23(27)28)24-22(26)21-9-5-6-14-25(21)15-16-29-20-7-3-2-4-8-20/h2-4,7-8,10-13,17,21H,5-6,9,14-16H2,1H3,(H,24,26)(H,27,28)/t17-,21+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of CYP1A2 (unknown origin) |
Bioorg Med Chem Lett 25: 3176-8 (2015)
Article DOI: 10.1016/j.bmcl.2015.05.091
BindingDB Entry DOI: 10.7270/Q2FX7C79 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50446847

(CHEMBL3115074)Show SMILES C[C@H](NC(=O)[C@H]1CCCCN1CCOc1ccccc1)c1ccc(cc1)C(O)=O |r| Show InChI InChI=1S/C23H28N2O4/c1-17(18-10-12-19(13-11-18)23(27)28)24-22(26)21-9-5-6-14-25(21)15-16-29-20-7-3-2-4-8-20/h2-4,7-8,10-13,17,21H,5-6,9,14-16H2,1H3,(H,24,26)(H,27,28)/t17-,21+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 (unknown origin) |
Bioorg Med Chem Lett 25: 3176-8 (2015)
Article DOI: 10.1016/j.bmcl.2015.05.091
BindingDB Entry DOI: 10.7270/Q2FX7C79 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50446847

(CHEMBL3115074)Show SMILES C[C@H](NC(=O)[C@H]1CCCCN1CCOc1ccccc1)c1ccc(cc1)C(O)=O |r| Show InChI InChI=1S/C23H28N2O4/c1-17(18-10-12-19(13-11-18)23(27)28)24-22(26)21-9-5-6-14-25(21)15-16-29-20-7-3-2-4-8-20/h2-4,7-8,10-13,17,21H,5-6,9,14-16H2,1H3,(H,24,26)(H,27,28)/t17-,21+/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 (unknown origin) |
Bioorg Med Chem Lett 25: 3176-8 (2015)
Article DOI: 10.1016/j.bmcl.2015.05.091
BindingDB Entry DOI: 10.7270/Q2FX7C79 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50446847

(CHEMBL3115074)Show SMILES C[C@H](NC(=O)[C@H]1CCCCN1CCOc1ccccc1)c1ccc(cc1)C(O)=O |r| Show InChI InChI=1S/C23H28N2O4/c1-17(18-10-12-19(13-11-18)23(27)28)24-22(26)21-9-5-6-14-25(21)15-16-29-20-7-3-2-4-8-20/h2-4,7-8,10-13,17,21H,5-6,9,14-16H2,1H3,(H,24,26)(H,27,28)/t17-,21+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
Bioorg Med Chem Lett 25: 3176-8 (2015)
Article DOI: 10.1016/j.bmcl.2015.05.091
BindingDB Entry DOI: 10.7270/Q2FX7C79 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP1 subtype
(Homo sapiens (Human)) | BDBM50446847

(CHEMBL3115074)Show SMILES C[C@H](NC(=O)[C@H]1CCCCN1CCOc1ccccc1)c1ccc(cc1)C(O)=O |r| Show InChI InChI=1S/C23H28N2O4/c1-17(18-10-12-19(13-11-18)23(27)28)24-22(26)21-9-5-6-14-25(21)15-16-29-20-7-3-2-4-8-20/h2-4,7-8,10-13,17,21H,5-6,9,14-16H2,1H3,(H,24,26)(H,27,28)/t17-,21+/m0/s1 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]-PGE2 from human EP1 receptor by liquid scintillation counting analysis |
Bioorg Med Chem Lett 25: 3176-8 (2015)
Article DOI: 10.1016/j.bmcl.2015.05.091
BindingDB Entry DOI: 10.7270/Q2FX7C79 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50446847

(CHEMBL3115074)Show SMILES C[C@H](NC(=O)[C@H]1CCCCN1CCOc1ccccc1)c1ccc(cc1)C(O)=O |r| Show InChI InChI=1S/C23H28N2O4/c1-17(18-10-12-19(13-11-18)23(27)28)24-22(26)21-9-5-6-14-25(21)15-16-29-20-7-3-2-4-8-20/h2-4,7-8,10-13,17,21H,5-6,9,14-16H2,1H3,(H,24,26)(H,27,28)/t17-,21+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]-PGE2 from human EP3 receptor by liquid scintillation counting analysis |
Bioorg Med Chem Lett 25: 3176-8 (2015)
Article DOI: 10.1016/j.bmcl.2015.05.091
BindingDB Entry DOI: 10.7270/Q2FX7C79 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data