

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N-lysine methyltransferase SMYD2 (Homo sapiens (Human)) | BDBM50095537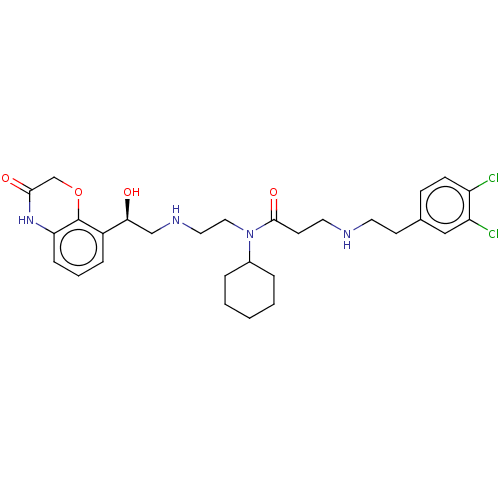 (CHEMBL3590526 | US9598381, 1a (S enantiomer)) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie, Inc. Curated by ChEMBL | Assay Description Inhibition of SMYD2 (unknown origin) using biotinylated GSRAHSSHLKSKKGQSTSRH as substrate assessed as incorporation of tritium labeled methyl group f... | ACS Med Chem Lett 6: 695-700 (2015) Article DOI: 10.1021/acsmedchemlett.5b00124 BindingDB Entry DOI: 10.7270/Q2KP83X5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| N-lysine methyltransferase SMYD2 (Homo sapiens (Human)) | BDBM50095536 (CHEMBL3590527 | US9598381, 1 (racemate)) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie, Inc. Curated by ChEMBL | Assay Description Inhibition of SMYD2 (unknown origin) using biotinylated GSRAHSSHLKSKKGQSTSRH as substrate assessed as incorporation of tritium labeled methyl group f... | ACS Med Chem Lett 6: 695-700 (2015) Article DOI: 10.1021/acsmedchemlett.5b00124 BindingDB Entry DOI: 10.7270/Q2KP83X5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-lysine methyltransferase SMYD2 (Homo sapiens (Human)) | BDBM50075102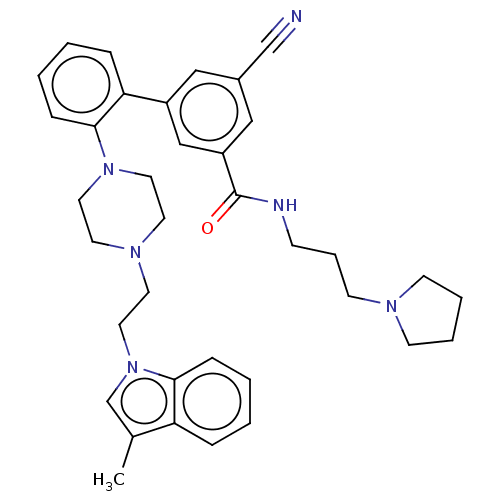 (CHEMBL3414623) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | <15 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie, Inc. Curated by ChEMBL | Assay Description Inhibition of SMYD2 (unknown origin) using p53 (361 to 380) as substrate assessed as incorporation of tritium labeled methyl group from [3H]-SAM to p... | ACS Med Chem Lett 6: 695-700 (2015) Article DOI: 10.1021/acsmedchemlett.5b00124 BindingDB Entry DOI: 10.7270/Q2KP83X5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| N-lysine methyltransferase SMYD2 (Homo sapiens (Human)) | BDBM50396022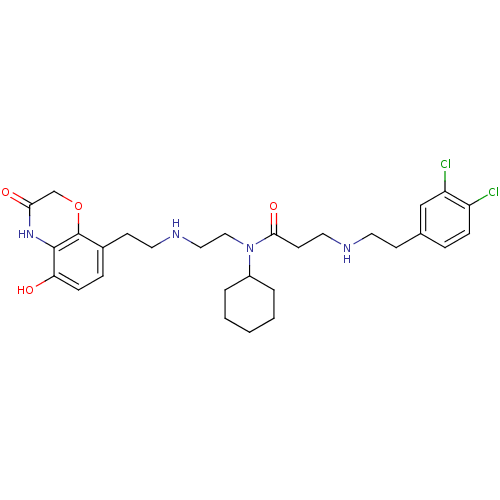 (CHEMBL2169920) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie, Inc. Curated by ChEMBL | Assay Description Inhibition of recombinant full-length human SMYD2 (1 to 433) expressed in Escherichia coli BL21Star(DE3) cells using p53 peptide as substrate after 9... | ACS Med Chem Lett 6: 695-700 (2015) Article DOI: 10.1021/acsmedchemlett.5b00124 BindingDB Entry DOI: 10.7270/Q2KP83X5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| N-lysine methyltransferase SMYD2 (Homo sapiens (Human)) | BDBM50396022 (CHEMBL2169920) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie, Inc. Curated by ChEMBL | Assay Description Inhibition of SMYD2 (unknown origin) using biotinylated GSRAHSSHLKSKKGQSTSRH as substrate assessed as incorporation of tritium labeled methyl group f... | ACS Med Chem Lett 6: 695-700 (2015) Article DOI: 10.1021/acsmedchemlett.5b00124 BindingDB Entry DOI: 10.7270/Q2KP83X5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| N-lysine methyltransferase SMYD2 (Homo sapiens (Human)) | BDBM50095634 (CHEMBL3590519 | US9598381, 11) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 440 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie, Inc. Curated by ChEMBL | Assay Description Inhibition of SMYD2 (unknown origin) using biotinylated GSRAHSSHLKSKKGQSTSRH as substrate assessed as incorporation of tritium labeled methyl group f... | ACS Med Chem Lett 6: 695-700 (2015) Article DOI: 10.1021/acsmedchemlett.5b00124 BindingDB Entry DOI: 10.7270/Q2KP83X5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-lysine methyltransferase SMYD2 (Homo sapiens (Human)) | BDBM50095615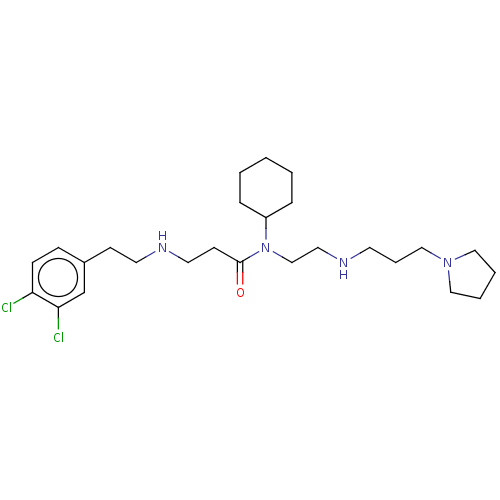 (CHEMBL3590524 | US9598381, 13) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie, Inc. Curated by ChEMBL | Assay Description Inhibition of SMYD2 (unknown origin) using biotinylated GSRAHSSHLKSKKGQSTSRH as substrate assessed as incorporation of tritium labeled methyl group f... | ACS Med Chem Lett 6: 695-700 (2015) Article DOI: 10.1021/acsmedchemlett.5b00124 BindingDB Entry DOI: 10.7270/Q2KP83X5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-lysine methyltransferase SMYD2 (Homo sapiens (Human)) | BDBM50095633 (CHEMBL3590520 | US9598381, 3) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie, Inc. Curated by ChEMBL | Assay Description Inhibition of SMYD2 (unknown origin) using biotinylated GSRAHSSHLKSKKGQSTSRH as substrate assessed as incorporation of tritium labeled methyl group f... | ACS Med Chem Lett 6: 695-700 (2015) Article DOI: 10.1021/acsmedchemlett.5b00124 BindingDB Entry DOI: 10.7270/Q2KP83X5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-lysine methyltransferase SMYD2 (Homo sapiens (Human)) | BDBM50095612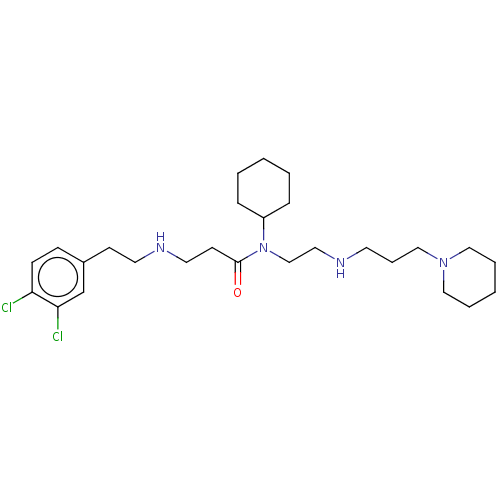 (CHEMBL3590525 | US9598381, 5) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie, Inc. Curated by ChEMBL | Assay Description Inhibition of SMYD2 (unknown origin) using biotinylated GSRAHSSHLKSKKGQSTSRH as substrate assessed as incorporation of tritium labeled methyl group f... | ACS Med Chem Lett 6: 695-700 (2015) Article DOI: 10.1021/acsmedchemlett.5b00124 BindingDB Entry DOI: 10.7270/Q2KP83X5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-lysine methyltransferase SMYD2 (Homo sapiens (Human)) | BDBM50095635 (CHEMBL3590518 | US9598381, 2) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie, Inc. Curated by ChEMBL | Assay Description Inhibition of SMYD2 (unknown origin) using biotinylated GSRAHSSHLKSKKGQSTSRH as substrate assessed as incorporation of tritium labeled methyl group f... | ACS Med Chem Lett 6: 695-700 (2015) Article DOI: 10.1021/acsmedchemlett.5b00124 BindingDB Entry DOI: 10.7270/Q2KP83X5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-lysine methyltransferase SMYD2 (Homo sapiens (Human)) | BDBM50095625 (CHEMBL3590523 | US9598381, 6) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie, Inc. Curated by ChEMBL | Assay Description Inhibition of SMYD2 (unknown origin) using biotinylated GSRAHSSHLKSKKGQSTSRH as substrate assessed as incorporation of tritium labeled methyl group f... | ACS Med Chem Lett 6: 695-700 (2015) Article DOI: 10.1021/acsmedchemlett.5b00124 BindingDB Entry DOI: 10.7270/Q2KP83X5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-lysine methyltransferase SMYD2 (Homo sapiens (Human)) | BDBM50095632 (CHEMBL3590521 | US9598381, 14) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie, Inc. Curated by ChEMBL | Assay Description Inhibition of SMYD2 (unknown origin) using biotinylated GSRAHSSHLKSKKGQSTSRH as substrate assessed as incorporation of tritium labeled methyl group f... | ACS Med Chem Lett 6: 695-700 (2015) Article DOI: 10.1021/acsmedchemlett.5b00124 BindingDB Entry DOI: 10.7270/Q2KP83X5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||