

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prothrombin (Homo sapiens (Human)) | BDBM50123490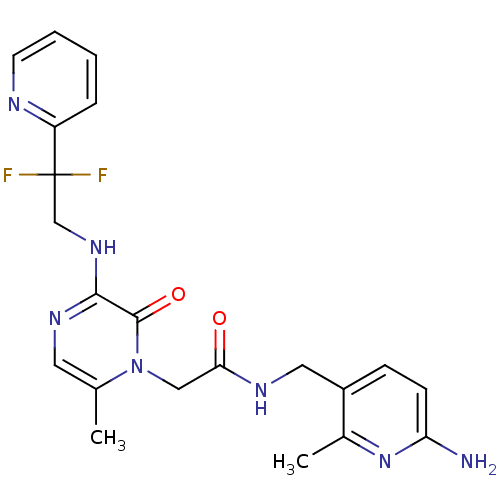 (CHEMBL143418 | N-(6-Amino-2-methyl-pyridin-3-ylmet...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of Thrombin (unknown origin) | J Med Chem 58: 8315-59 (2015) Article DOI: 10.1021/acs.jmedchem.5b00258 BindingDB Entry DOI: 10.7270/Q22J6DPZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50123504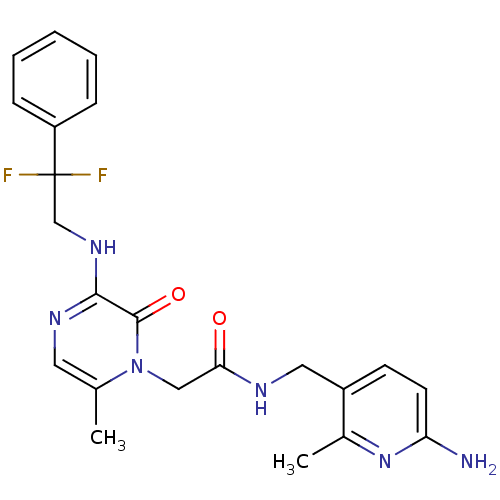 (CHEMBL142546 | N-((6-amino-2-methylpyridin-3-yl)me...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of Thrombin (unknown origin) | J Med Chem 58: 8315-59 (2015) Article DOI: 10.1021/acs.jmedchem.5b00258 BindingDB Entry DOI: 10.7270/Q22J6DPZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50123496 (CHEMBL143138 | N-(6-Amino-2-methyl-pyridin-3-ylmet...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of Thrombin (unknown origin) | J Med Chem 58: 8315-59 (2015) Article DOI: 10.1021/acs.jmedchem.5b00258 BindingDB Entry DOI: 10.7270/Q22J6DPZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50067797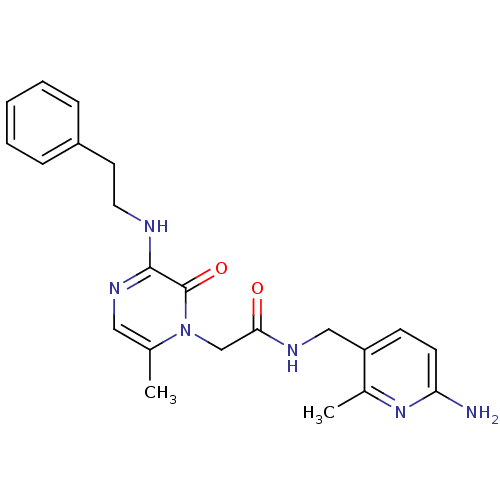 (CHEMBL19080 | L-37378 | N-(6-Amino-2-methyl-pyridi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of Thrombin (unknown origin) | J Med Chem 58: 8315-59 (2015) Article DOI: 10.1021/acs.jmedchem.5b00258 BindingDB Entry DOI: 10.7270/Q22J6DPZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50123500 (CHEMBL143139 | N-(6-Amino-2-methyl-pyridin-3-ylmet...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of Thrombin (unknown origin) | J Med Chem 58: 8315-59 (2015) Article DOI: 10.1021/acs.jmedchem.5b00258 BindingDB Entry DOI: 10.7270/Q22J6DPZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50130216 (CHEMBL3632874) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of human Thrombin using S-2238 as substrate assessed as release of p-nitroaniline preincubated for 240 secs followed by substrate addition... | J Med Chem 58: 8315-59 (2015) Article DOI: 10.1021/acs.jmedchem.5b00258 BindingDB Entry DOI: 10.7270/Q22J6DPZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50130256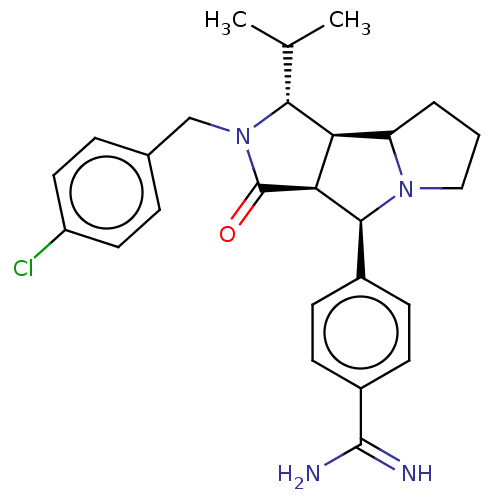 (CHEMBL3632875) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of human Thrombin using S-2238 as substrate assessed as release of p-nitroaniline preincubated for 240 secs followed by substrate addition... | J Med Chem 58: 8315-59 (2015) Article DOI: 10.1021/acs.jmedchem.5b00258 BindingDB Entry DOI: 10.7270/Q22J6DPZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Mus musculus (Mouse)) | BDBM50130307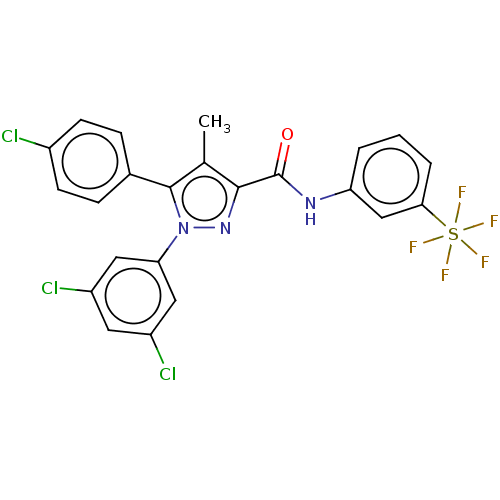 (CHEMBL3633035) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from mouse brain membrane CB1 receptor after 60 mins by liquid scintillation counting analysis | J Med Chem 58: 8315-59 (2015) Article DOI: 10.1021/acs.jmedchem.5b00258 BindingDB Entry DOI: 10.7270/Q22J6DPZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50130296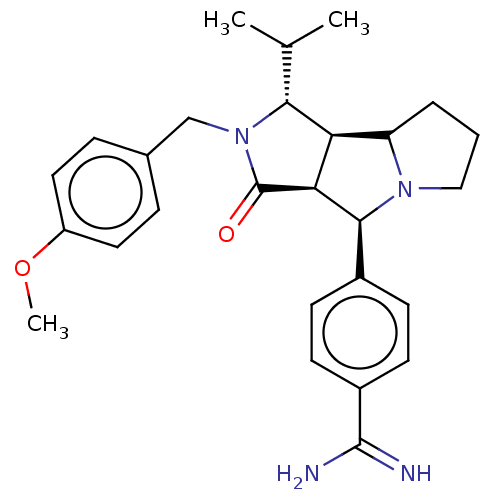 (CHEMBL3632876) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of human Thrombin using S-2238 as substrate assessed as release of p-nitroaniline preincubated for 240 secs followed by substrate addition... | J Med Chem 58: 8315-59 (2015) Article DOI: 10.1021/acs.jmedchem.5b00258 BindingDB Entry DOI: 10.7270/Q22J6DPZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase FKBP1A (Homo sapiens (Human)) | BDBM50400862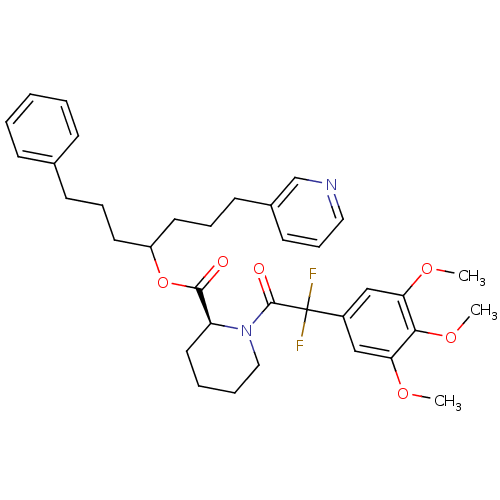 (CHEMBL2205015) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of FKBP12 (unknown origin)-mediated rotamase activity by spectrophotometric analysis | J Med Chem 58: 8315-59 (2015) Article DOI: 10.1021/acs.jmedchem.5b00258 BindingDB Entry DOI: 10.7270/Q22J6DPZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Mus musculus (Mouse)) | BDBM50130306 (CHEMBL3633034) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from mouse brain membrane CB1 receptor after 60 mins by liquid scintillation counting analysis | J Med Chem 58: 8315-59 (2015) Article DOI: 10.1021/acs.jmedchem.5b00258 BindingDB Entry DOI: 10.7270/Q22J6DPZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50130213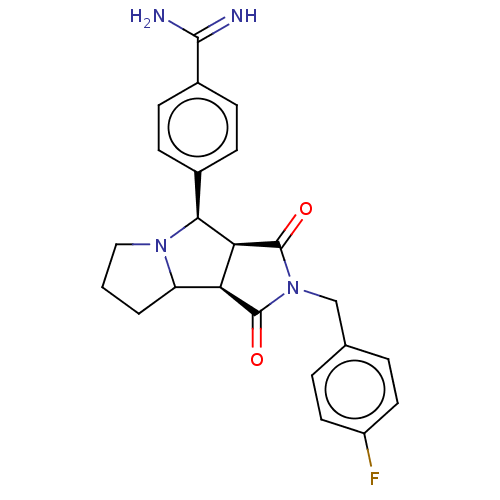 (CHEMBL3632871) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 57 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of human Thrombin using H-D-Phe-Pip-Arg-paranitroanilide/methylsulfonyl-D-Leu-Gly-Arg-paranitroanilide as substrate by spectrophotometric ... | J Med Chem 58: 8315-59 (2015) Article DOI: 10.1021/acs.jmedchem.5b00258 BindingDB Entry DOI: 10.7270/Q22J6DPZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50130215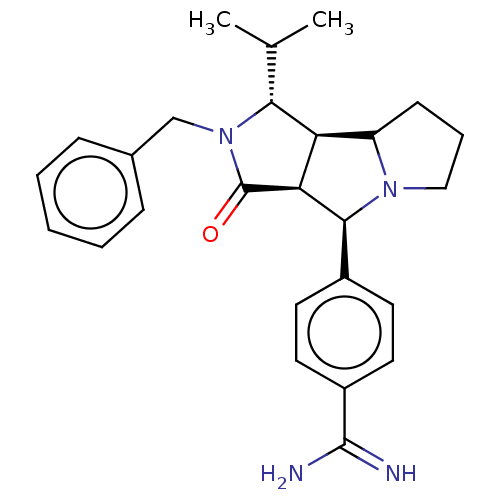 (CHEMBL3632873) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 65 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of human Thrombin using S-2238 as substrate assessed as release of p-nitroaniline preincubated for 240 secs followed by substrate addition... | J Med Chem 58: 8315-59 (2015) Article DOI: 10.1021/acs.jmedchem.5b00258 BindingDB Entry DOI: 10.7270/Q22J6DPZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50130211 (CHEMBL3632869) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of human Thrombin using H-D-Phe-Pip-Arg-paranitroanilide/methylsulfonyl-D-Leu-Gly-Arg-paranitroanilide as substrate by spectrophotometric ... | J Med Chem 58: 8315-59 (2015) Article DOI: 10.1021/acs.jmedchem.5b00258 BindingDB Entry DOI: 10.7270/Q22J6DPZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50130212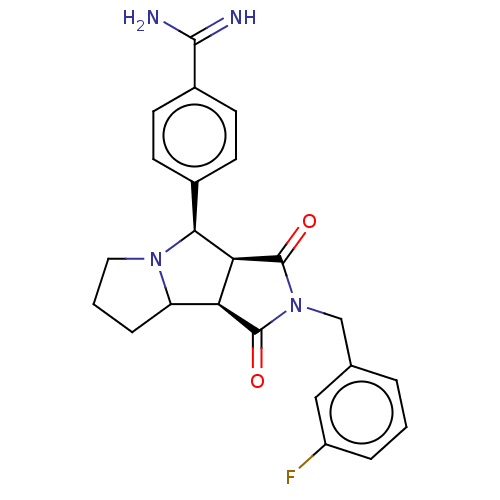 (CHEMBL3632870) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of human Thrombin using H-D-Phe-Pip-Arg-paranitroanilide/methylsulfonyl-D-Leu-Gly-Arg-paranitroanilide as substrate by spectrophotometric ... | J Med Chem 58: 8315-59 (2015) Article DOI: 10.1021/acs.jmedchem.5b00258 BindingDB Entry DOI: 10.7270/Q22J6DPZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50130214 (CHEMBL3632872) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 590 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of human Thrombin using H-D-Phe-Pip-Arg-paranitroanilide/methylsulfonyl-D-Leu-Gly-Arg-paranitroanilide as substrate by spectrophotometric ... | J Med Chem 58: 8315-59 (2015) Article DOI: 10.1021/acs.jmedchem.5b00258 BindingDB Entry DOI: 10.7270/Q22J6DPZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma cruzi) | BDBM50130309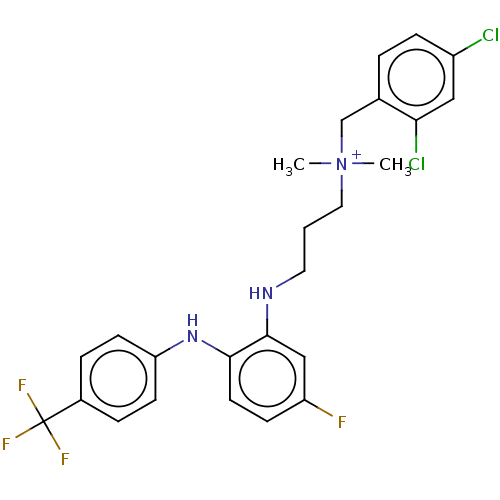 (CHEMBL3633037) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 2.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Competitive inhibition of recombinant Trypanosoma cruzi Trypanothione reductase by photometric assay | J Med Chem 58: 8315-59 (2015) Article DOI: 10.1021/acs.jmedchem.5b00258 BindingDB Entry DOI: 10.7270/Q22J6DPZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma cruzi) | BDBM50130310 (CHEMBL3633038) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Competitive inhibition of recombinant Trypanosoma cruzi Trypanothione reductase by photometric assay | J Med Chem 58: 8315-59 (2015) Article DOI: 10.1021/acs.jmedchem.5b00258 BindingDB Entry DOI: 10.7270/Q22J6DPZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma cruzi) | BDBM50130308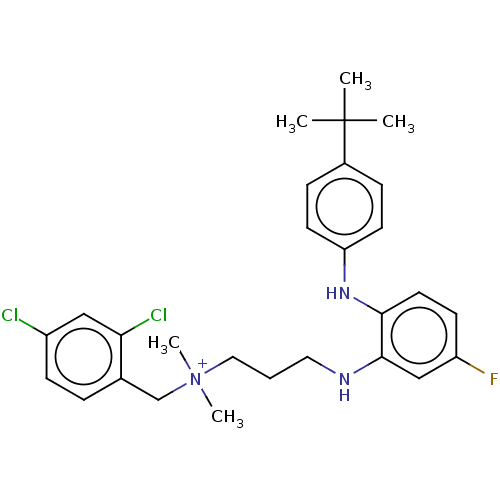 (CHEMBL3633036) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Competitive inhibition of recombinant Trypanosoma cruzi Trypanothione reductase by photometric assay | J Med Chem 58: 8315-59 (2015) Article DOI: 10.1021/acs.jmedchem.5b00258 BindingDB Entry DOI: 10.7270/Q22J6DPZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 4-aminobutyrate aminotransferase, mitochondrial (Sus scrofa) | BDBM50082127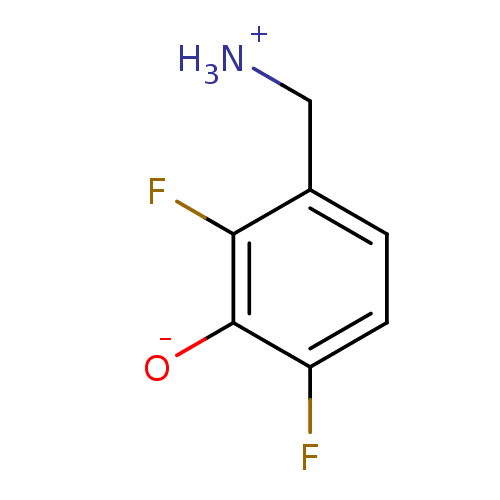 (3-(ammoniomethyl)-2,6-difluorobenzenolate | 3-Amin...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 6.30E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Competitive inhibition of pig brain GABA aminotransferase by Dixon/Cornish-Bowden plot analysis in presence of GABA | J Med Chem 58: 8315-59 (2015) Article DOI: 10.1021/acs.jmedchem.5b00258 BindingDB Entry DOI: 10.7270/Q22J6DPZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 4-aminobutyrate aminotransferase, mitochondrial (Sus scrofa) | BDBM50073151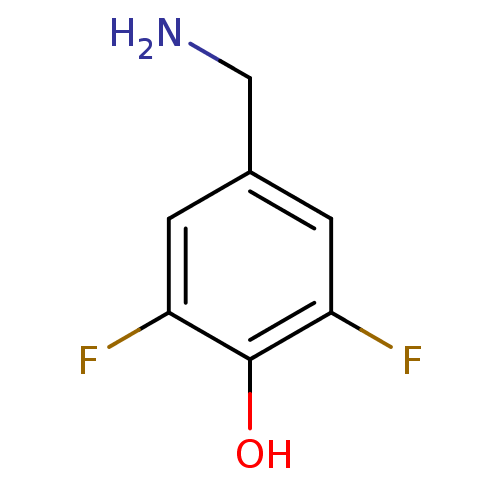 (4-(ammoniomethyl)-2,6-difluorobenzenolate | 4-Amin...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.10E+7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Competitive inhibition of pig brain GABA aminotransferase by Dixon/Cornish-Bowden plot analysis in presence of GABA | J Med Chem 58: 8315-59 (2015) Article DOI: 10.1021/acs.jmedchem.5b00258 BindingDB Entry DOI: 10.7270/Q22J6DPZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (Homo sapiens (Human)) | BDBM50060440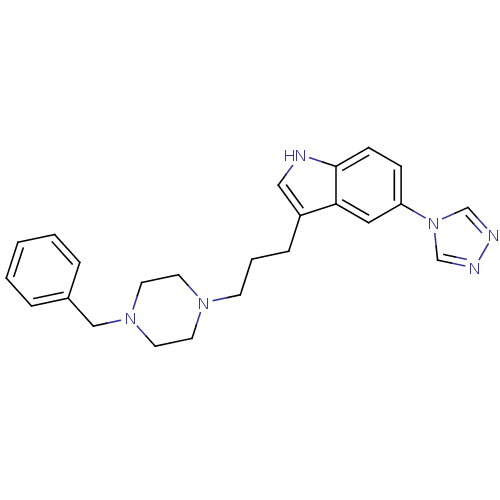 (3-[3-(4-Benzyl-piperazin-1-yl)-propyl]-5-[1,2,4]tr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]-5-HT from human 5HT-1D receptor expressed in CHO cells | J Med Chem 58: 8315-59 (2015) Article DOI: 10.1021/acs.jmedchem.5b00258 BindingDB Entry DOI: 10.7270/Q22J6DPZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (Homo sapiens (Human)) | BDBM50065419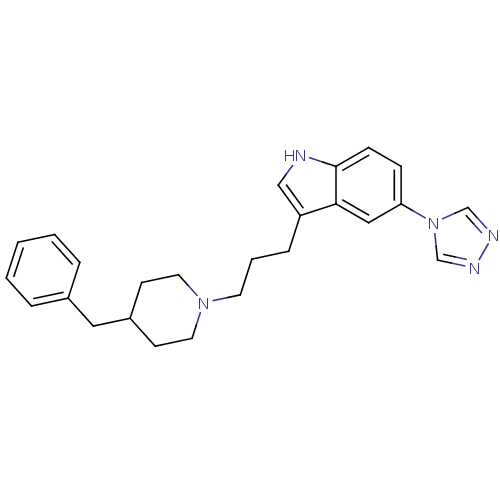 (3-[3-(4-Benzyl-piperidin-1-yl)-propyl]-5-[1,2,4]tr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]-5-HT from human 5HT-1D receptor expressed in CHO cells | J Med Chem 58: 8315-59 (2015) Article DOI: 10.1021/acs.jmedchem.5b00258 BindingDB Entry DOI: 10.7270/Q22J6DPZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50130304 (CHEMBL3632865) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of human plasma DPP-4 using Gly-Pro-4-methylcoumaryl-7-amide as substrate assessed as formation of 7-amino-4-methylcoumarin after 2 hrs by... | J Med Chem 58: 8315-59 (2015) Article DOI: 10.1021/acs.jmedchem.5b00258 BindingDB Entry DOI: 10.7270/Q22J6DPZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50130302 (CHEMBL3632867) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of human plasma DPP-4 using Gly-Pro-4-methylcoumaryl-7-amide as substrate assessed as formation of 7-amino-4-methylcoumarin after 2 hrs by... | J Med Chem 58: 8315-59 (2015) Article DOI: 10.1021/acs.jmedchem.5b00258 BindingDB Entry DOI: 10.7270/Q22J6DPZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (Homo sapiens (Human)) | BDBM50065417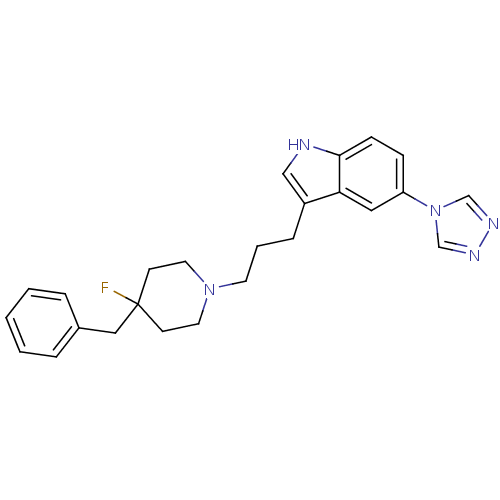 (3-[3-(4-Benzyl-4-fluoro-piperidin-1-yl)-propyl]-5-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]-5-HT from human 5HT-1D receptor expressed in CHO cells | J Med Chem 58: 8315-59 (2015) Article DOI: 10.1021/acs.jmedchem.5b00258 BindingDB Entry DOI: 10.7270/Q22J6DPZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50130305 (CHEMBL3632864) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of human plasma DPP-4 using Gly-Pro-4-methylcoumaryl-7-amide as substrate assessed as formation of 7-amino-4-methylcoumarin after 2 hrs by... | J Med Chem 58: 8315-59 (2015) Article DOI: 10.1021/acs.jmedchem.5b00258 BindingDB Entry DOI: 10.7270/Q22J6DPZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1B (Homo sapiens (Human)) | BDBM50060440 (3-[3-(4-Benzyl-piperazin-1-yl)-propyl]-5-[1,2,4]tr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]-5-HT from human 5HT-1B receptor expressed in CHO cells | J Med Chem 58: 8315-59 (2015) Article DOI: 10.1021/acs.jmedchem.5b00258 BindingDB Entry DOI: 10.7270/Q22J6DPZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1B (Homo sapiens (Human)) | BDBM50065417 (3-[3-(4-Benzyl-4-fluoro-piperidin-1-yl)-propyl]-5-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]-5-HT from human 5HT-1B receptor expressed in CHO cells | J Med Chem 58: 8315-59 (2015) Article DOI: 10.1021/acs.jmedchem.5b00258 BindingDB Entry DOI: 10.7270/Q22J6DPZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11162 ((1R)-3-oxo-3-[3-(trifluoroethyl)-5,6-dihydro[1,2,4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Similars | DrugBank PDB Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of DPP-4 (unknown origin) | J Med Chem 58: 8315-59 (2015) Article DOI: 10.1021/acs.jmedchem.5b00258 BindingDB Entry DOI: 10.7270/Q22J6DPZ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| 5-hydroxytryptamine receptor 1B (Homo sapiens (Human)) | BDBM50065419 (3-[3-(4-Benzyl-piperidin-1-yl)-propyl]-5-[1,2,4]tr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]-5-HT from human 5HT-1B receptor expressed in CHO cells | J Med Chem 58: 8315-59 (2015) Article DOI: 10.1021/acs.jmedchem.5b00258 BindingDB Entry DOI: 10.7270/Q22J6DPZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor VII/Tissue factor (Homo sapiens (Human)) | BDBM14497 (2-Pyridone 14b | N-[(4-carbamimidoylphenyl)methyl]...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 52 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of recombinant human Tissue factor/factor 7a using N-methylsulfonyl-D-phe-gly-arg-p-nitroaniline as substrate assessed as release of p-nit... | J Med Chem 58: 8315-59 (2015) Article DOI: 10.1021/acs.jmedchem.5b00258 BindingDB Entry DOI: 10.7270/Q22J6DPZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50130314 (CHEMBL3633040) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 112 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Antagonist activity at human ETA receptor expressed in CHO cells assessed as inhibition of ET-1-induced Ca2+ efflux from endoplasmic reticulum into c... | J Med Chem 58: 8315-59 (2015) Article DOI: 10.1021/acs.jmedchem.5b00258 BindingDB Entry DOI: 10.7270/Q22J6DPZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50130311 (CHEMBL3633039) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Antagonist activity at human ETA receptor expressed in CHO cells assessed as inhibition of ET-1-induced Ca2+ efflux from endoplasmic reticulum into c... | J Med Chem 58: 8315-59 (2015) Article DOI: 10.1021/acs.jmedchem.5b00258 BindingDB Entry DOI: 10.7270/Q22J6DPZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM50094513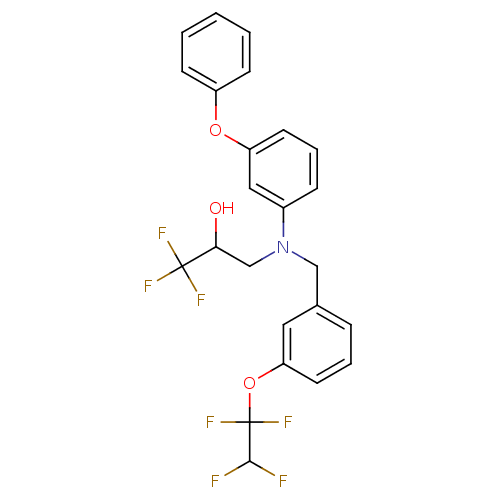 (1,1,1-Trifluoro-3-{(3-phenoxy-phenyl)-[3-(1,1,2,2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of recombinant human CETP in buffer assessed as transfer of [3H]-cholesteryl ester from HDL donor particles to LDL acceptor particles | J Med Chem 58: 8315-59 (2015) Article DOI: 10.1021/acs.jmedchem.5b00258 BindingDB Entry DOI: 10.7270/Q22J6DPZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50130303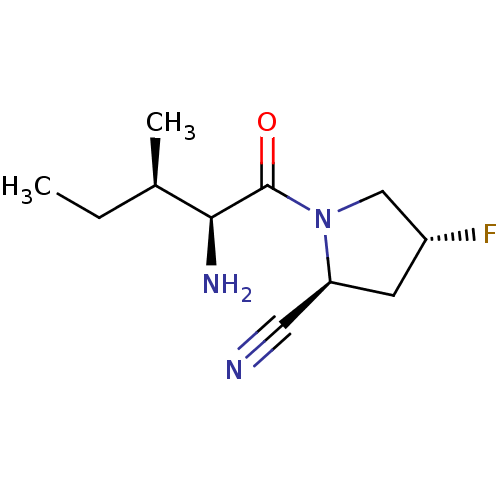 (CHEMBL3632866) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 290 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of human plasma DPP-4 using Gly-Pro-4-methylcoumaryl-7-amide as substrate assessed as formation of 7-amino-4-methylcoumarin after 2 hrs by... | J Med Chem 58: 8315-59 (2015) Article DOI: 10.1021/acs.jmedchem.5b00258 BindingDB Entry DOI: 10.7270/Q22J6DPZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor VII/Tissue factor (Homo sapiens (Human)) | BDBM50130301 (CHEMBL3632877) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 340 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of recombinant human Tissue factor/factor 7a using N-methylsulfonyl-D-phe-gly-arg-p-nitroaniline as substrate assessed as release of p-nit... | J Med Chem 58: 8315-59 (2015) Article DOI: 10.1021/acs.jmedchem.5b00258 BindingDB Entry DOI: 10.7270/Q22J6DPZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM50100997 (1,1,1-Trifluoro-3-[(3-furan-2-yl-benzyl)-(3-phenox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 480 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of recombinant human CETP in buffer assessed as transfer of [3H]-cholesteryl ester from HDL donor particles to LDL acceptor particles | J Med Chem 58: 8315-59 (2015) Article DOI: 10.1021/acs.jmedchem.5b00258 BindingDB Entry DOI: 10.7270/Q22J6DPZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM50101003 (3-((3-ethoxybenzyl)(3-phenoxyphenyl)amino)-1,1,1-t...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of recombinant human CETP in buffer assessed as transfer of [3H]-cholesteryl ester from HDL donor particles to LDL acceptor particles | J Med Chem 58: 8315-59 (2015) Article DOI: 10.1021/acs.jmedchem.5b00258 BindingDB Entry DOI: 10.7270/Q22J6DPZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||