 Found 58 hits of Enzyme Inhibition Constant Data
Found 58 hits of Enzyme Inhibition Constant Data Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM31088
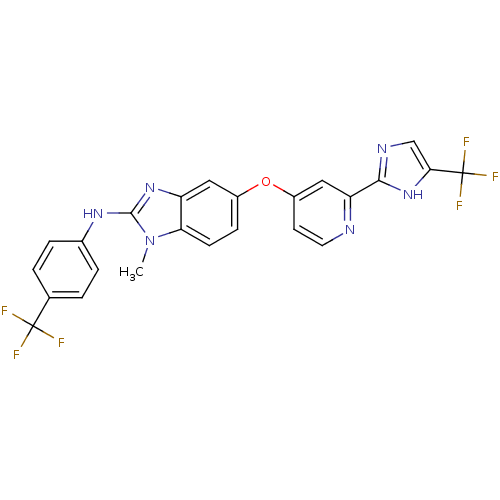
(1-methyl-5-[2-[5-(trifluoromethyl)-1H-imidazol-2-y...)Show SMILES Cn1c(Nc2ccc(cc2)C(F)(F)F)nc2cc(Oc3ccnc(c3)-c3ncc([nH]3)C(F)(F)F)ccc12 Show InChI InChI=1S/C24H16F6N6O/c1-36-19-7-6-15(10-17(19)34-22(36)33-14-4-2-13(3-5-14)23(25,26)27)37-16-8-9-31-18(11-16)21-32-12-20(35-21)24(28,29)30/h2-12H,1H3,(H,32,35)(H,33,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity towards Coagulation factor X was determined using chromogenic substrate, MeO-COD-CHG-Gly-Arg-pNA |
ACS Med Chem Lett 6: 961-5 (2015)
Article DOI: 10.1021/ml500526p
BindingDB Entry DOI: 10.7270/Q28W3G4S |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Platelet-derived growth factor receptor beta
(Homo sapiens (Human)) | BDBM50026679
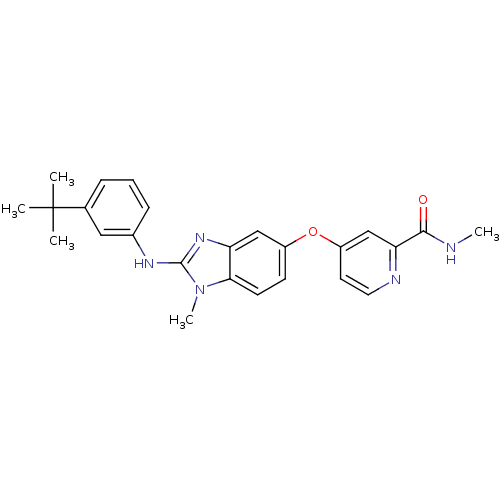
(CHEMBL3335371)Show SMILES CC(=O)Nc1cc(Oc2ccc3n(C)c(Nc4cccc(c4)C(C)(C)C)nc3c2)ccn1 Show InChI InChI=1S/C25H27N5O2/c1-16(31)27-23-15-20(11-12-26-23)32-19-9-10-22-21(14-19)29-24(30(22)5)28-18-8-6-7-17(13-18)25(2,3)4/h6-15H,1-5H3,(H,28,29)(H,26,27,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of Histamine H1 receptor |
ACS Med Chem Lett 6: 961-5 (2015)
Article DOI: 10.1021/ml500526p
BindingDB Entry DOI: 10.7270/Q28W3G4S |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor beta
(Homo sapiens (Human)) | BDBM50131822
(CHEMBL3632719)Show SMILES Cn1c(Nc2cccc(c2)C(C)(C)C)nc2cc(Oc3ccnc(c3)-c3ncc([nH]3)C(F)(F)F)ccc12 Show InChI InChI=1S/C27H25F3N6O/c1-26(2,3)16-6-5-7-17(12-16)33-25-34-20-13-18(8-9-22(20)36(25)4)37-19-10-11-31-21(14-19)24-32-15-23(35-24)27(28,29)30/h5-15H,1-4H3,(H,32,35)(H,33,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of Histamine H1 receptor |
ACS Med Chem Lett 6: 961-5 (2015)
Article DOI: 10.1021/ml500526p
BindingDB Entry DOI: 10.7270/Q28W3G4S |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Fyn
(Homo sapiens (Human)) | BDBM50026679

(CHEMBL3335371)Show SMILES CC(=O)Nc1cc(Oc2ccc3n(C)c(Nc4cccc(c4)C(C)(C)C)nc3c2)ccn1 Show InChI InChI=1S/C25H27N5O2/c1-16(31)27-23-15-20(11-12-26-23)32-19-9-10-22-21(14-19)29-24(30(22)5)28-18-8-6-7-17(13-18)25(2,3)4/h6-15H,1-5H3,(H,28,29)(H,26,27,31) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-FPP incorporation into biotin-linked K-ras decapeptide (CVIM) by bovine farnesyltransferase |
ACS Med Chem Lett 6: 961-5 (2015)
Article DOI: 10.1021/ml500526p
BindingDB Entry DOI: 10.7270/Q28W3G4S |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50026679

(CHEMBL3335371)Show SMILES CC(=O)Nc1cc(Oc2ccc3n(C)c(Nc4cccc(c4)C(C)(C)C)nc3c2)ccn1 Show InChI InChI=1S/C25H27N5O2/c1-16(31)27-23-15-20(11-12-26-23)32-19-9-10-22-21(14-19)29-24(30(22)5)28-18-8-6-7-17(13-18)25(2,3)4/h6-15H,1-5H3,(H,28,29)(H,26,27,31) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-FPP incorporation into biotin-linked K-ras decapeptide (CVIM) by bovine farnesyltransferase |
ACS Med Chem Lett 6: 961-5 (2015)
Article DOI: 10.1021/ml500526p
BindingDB Entry DOI: 10.7270/Q28W3G4S |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 1/2/3
(Homo sapiens (Human)) | BDBM50026679

(CHEMBL3335371)Show SMILES CC(=O)Nc1cc(Oc2ccc3n(C)c(Nc4cccc(c4)C(C)(C)C)nc3c2)ccn1 Show InChI InChI=1S/C25H27N5O2/c1-16(31)27-23-15-20(11-12-26-23)32-19-9-10-22-21(14-19)29-24(30(22)5)28-18-8-6-7-17(13-18)25(2,3)4/h6-15H,1-5H3,(H,28,29)(H,26,27,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibitory activity towards acetylcholine esterase (AChE) |
ACS Med Chem Lett 6: 961-5 (2015)
Article DOI: 10.1021/ml500526p
BindingDB Entry DOI: 10.7270/Q28W3G4S |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50026679

(CHEMBL3335371)Show SMILES CC(=O)Nc1cc(Oc2ccc3n(C)c(Nc4cccc(c4)C(C)(C)C)nc3c2)ccn1 Show InChI InChI=1S/C25H27N5O2/c1-16(31)27-23-15-20(11-12-26-23)32-19-9-10-22-21(14-19)29-24(30(22)5)28-18-8-6-7-17(13-18)25(2,3)4/h6-15H,1-5H3,(H,28,29)(H,26,27,31) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Binding affinity towards Kappa opioid receptor |
ACS Med Chem Lett 6: 961-5 (2015)
Article DOI: 10.1021/ml500526p
BindingDB Entry DOI: 10.7270/Q28W3G4S |
More data for this
Ligand-Target Pair | |
Mast/stem cell growth factor receptor Kit
(Homo sapiens (Human)) | BDBM50026679

(CHEMBL3335371)Show SMILES CC(=O)Nc1cc(Oc2ccc3n(C)c(Nc4cccc(c4)C(C)(C)C)nc3c2)ccn1 Show InChI InChI=1S/C25H27N5O2/c1-16(31)27-23-15-20(11-12-26-23)32-19-9-10-22-21(14-19)29-24(30(22)5)28-18-8-6-7-17(13-18)25(2,3)4/h6-15H,1-5H3,(H,28,29)(H,26,27,31) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibitory activity towards acetylcholine esterase (AChE) |
ACS Med Chem Lett 6: 961-5 (2015)
Article DOI: 10.1021/ml500526p
BindingDB Entry DOI: 10.7270/Q28W3G4S |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50026679

(CHEMBL3335371)Show SMILES CC(=O)Nc1cc(Oc2ccc3n(C)c(Nc4cccc(c4)C(C)(C)C)nc3c2)ccn1 Show InChI InChI=1S/C25H27N5O2/c1-16(31)27-23-15-20(11-12-26-23)32-19-9-10-22-21(14-19)29-24(30(22)5)28-18-8-6-7-17(13-18)25(2,3)4/h6-15H,1-5H3,(H,28,29)(H,26,27,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Reduced farnesylation of H-ras transformed NIH3T3 cells |
ACS Med Chem Lett 6: 961-5 (2015)
Article DOI: 10.1021/ml500526p
BindingDB Entry DOI: 10.7270/Q28W3G4S |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Fyn
(Homo sapiens (Human)) | BDBM26028
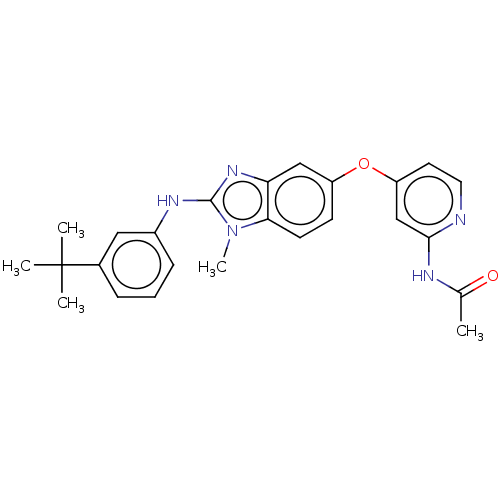
(4-({2-[(3-tert-butylphenyl)(methyl)amino]-1H-1,3-b...)Show SMILES CNC(=O)c1cc(Oc2ccc3n(C)c(Nc4cccc(c4)C(C)(C)C)nc3c2)ccn1 Show InChI InChI=1S/C25H27N5O2/c1-25(2,3)16-7-6-8-17(13-16)28-24-29-20-14-18(9-10-22(20)30(24)5)32-19-11-12-27-21(15-19)23(31)26-4/h6-15H,1-5H3,(H,26,31)(H,28,29) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-FPP incorporation into biotin-linked K-ras decapeptide (CVIM) by bovine farnesyltransferase |
ACS Med Chem Lett 6: 961-5 (2015)
Article DOI: 10.1021/ml500526p
BindingDB Entry DOI: 10.7270/Q28W3G4S |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 1/2/3
(Homo sapiens (Human)) | BDBM26028

(4-({2-[(3-tert-butylphenyl)(methyl)amino]-1H-1,3-b...)Show SMILES CNC(=O)c1cc(Oc2ccc3n(C)c(Nc4cccc(c4)C(C)(C)C)nc3c2)ccn1 Show InChI InChI=1S/C25H27N5O2/c1-25(2,3)16-7-6-8-17(13-16)28-24-29-20-14-18(9-10-22(20)30(24)5)32-19-11-12-27-21(15-19)23(31)26-4/h6-15H,1-5H3,(H,26,31)(H,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibitory activity towards acetylcholine esterase (AChE) |
ACS Med Chem Lett 6: 961-5 (2015)
Article DOI: 10.1021/ml500526p
BindingDB Entry DOI: 10.7270/Q28W3G4S |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor beta
(Homo sapiens (Human)) | BDBM31088

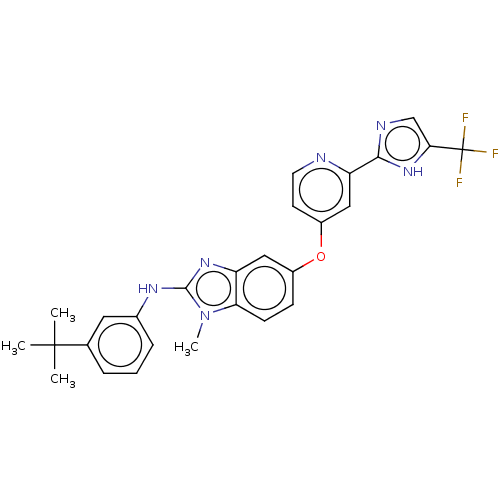
(1-methyl-5-[2-[5-(trifluoromethyl)-1H-imidazol-2-y...)Show SMILES Cn1c(Nc2ccc(cc2)C(F)(F)F)nc2cc(Oc3ccnc(c3)-c3ncc([nH]3)C(F)(F)F)ccc12 Show InChI InChI=1S/C24H16F6N6O/c1-36-19-7-6-15(10-17(19)34-22(36)33-14-4-2-13(3-5-14)23(25,26)27)37-16-8-9-31-18(11-16)21-32-12-20(35-21)24(28,29)30/h2-12H,1H3,(H,32,35)(H,33,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Binding affinity towards Kappa opioid receptor |
ACS Med Chem Lett 6: 961-5 (2015)
Article DOI: 10.1021/ml500526p
BindingDB Entry DOI: 10.7270/Q28W3G4S |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor beta
(Homo sapiens (Human)) | BDBM26028

(4-({2-[(3-tert-butylphenyl)(methyl)amino]-1H-1,3-b...)Show SMILES CNC(=O)c1cc(Oc2ccc3n(C)c(Nc4cccc(c4)C(C)(C)C)nc3c2)ccn1 Show InChI InChI=1S/C25H27N5O2/c1-25(2,3)16-7-6-8-17(13-16)28-24-29-20-14-18(9-10-22(20)30(24)5)32-19-11-12-27-21(15-19)23(31)26-4/h6-15H,1-5H3,(H,26,31)(H,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of Histamine H1 receptor |
ACS Med Chem Lett 6: 961-5 (2015)
Article DOI: 10.1021/ml500526p
BindingDB Entry DOI: 10.7270/Q28W3G4S |
More data for this
Ligand-Target Pair | |
Mast/stem cell growth factor receptor Kit
(Homo sapiens (Human)) | BDBM26028

(4-({2-[(3-tert-butylphenyl)(methyl)amino]-1H-1,3-b...)Show SMILES CNC(=O)c1cc(Oc2ccc3n(C)c(Nc4cccc(c4)C(C)(C)C)nc3c2)ccn1 Show InChI InChI=1S/C25H27N5O2/c1-25(2,3)16-7-6-8-17(13-16)28-24-29-20-14-18(9-10-22(20)30(24)5)32-19-11-12-27-21(15-19)23(31)26-4/h6-15H,1-5H3,(H,26,31)(H,28,29) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibitory activity towards acetylcholine esterase (AChE) |
ACS Med Chem Lett 6: 961-5 (2015)
Article DOI: 10.1021/ml500526p
BindingDB Entry DOI: 10.7270/Q28W3G4S |
More data for this
Ligand-Target Pair | |
RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM31088

(1-methyl-5-[2-[5-(trifluoromethyl)-1H-imidazol-2-y...)Show SMILES Cn1c(Nc2ccc(cc2)C(F)(F)F)nc2cc(Oc3ccnc(c3)-c3ncc([nH]3)C(F)(F)F)ccc12 Show InChI InChI=1S/C24H16F6N6O/c1-36-19-7-6-15(10-17(19)34-22(36)33-14-4-2-13(3-5-14)23(25,26)27)37-16-8-9-31-18(11-16)21-32-12-20(35-21)24(28,29)30/h2-12H,1H3,(H,32,35)(H,33,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Reduced farnesylation of H-ras transformed NIH3T3 cells |
ACS Med Chem Lett 6: 961-5 (2015)
Article DOI: 10.1021/ml500526p
BindingDB Entry DOI: 10.7270/Q28W3G4S |
More data for this
Ligand-Target Pair | |
Mast/stem cell growth factor receptor Kit
(Homo sapiens (Human)) | BDBM31088

(1-methyl-5-[2-[5-(trifluoromethyl)-1H-imidazol-2-y...)Show SMILES Cn1c(Nc2ccc(cc2)C(F)(F)F)nc2cc(Oc3ccnc(c3)-c3ncc([nH]3)C(F)(F)F)ccc12 Show InChI InChI=1S/C24H16F6N6O/c1-36-19-7-6-15(10-17(19)34-22(36)33-14-4-2-13(3-5-14)23(25,26)27)37-16-8-9-31-18(11-16)21-32-12-20(35-21)24(28,29)30/h2-12H,1H3,(H,32,35)(H,33,34) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of kappa opioid receptor |
ACS Med Chem Lett 6: 961-5 (2015)
Article DOI: 10.1021/ml500526p
BindingDB Entry DOI: 10.7270/Q28W3G4S |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 1/2/3
(Homo sapiens (Human)) | BDBM31088

(1-methyl-5-[2-[5-(trifluoromethyl)-1H-imidazol-2-y...)Show SMILES Cn1c(Nc2ccc(cc2)C(F)(F)F)nc2cc(Oc3ccnc(c3)-c3ncc([nH]3)C(F)(F)F)ccc12 Show InChI InChI=1S/C24H16F6N6O/c1-36-19-7-6-15(10-17(19)34-22(36)33-14-4-2-13(3-5-14)23(25,26)27)37-16-8-9-31-18(11-16)21-32-12-20(35-21)24(28,29)30/h2-12H,1H3,(H,32,35)(H,33,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibitory activity towards acetylcholine esterase (AChE) |
ACS Med Chem Lett 6: 961-5 (2015)
Article DOI: 10.1021/ml500526p
BindingDB Entry DOI: 10.7270/Q28W3G4S |
More data for this
Ligand-Target Pair | |
Mast/stem cell growth factor receptor Kit
(Homo sapiens (Human)) | BDBM50131822

(CHEMBL3632719)Show SMILES Cn1c(Nc2cccc(c2)C(C)(C)C)nc2cc(Oc3ccnc(c3)-c3ncc([nH]3)C(F)(F)F)ccc12 Show InChI InChI=1S/C27H25F3N6O/c1-26(2,3)16-6-5-7-17(12-16)33-25-34-20-13-18(8-9-22(20)36(25)4)37-19-10-11-31-21(14-19)24-32-15-23(35-24)27(28,29)30/h5-15H,1-4H3,(H,32,35)(H,33,34) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition against rat hydroxytryptamine 3 receptor |
ACS Med Chem Lett 6: 961-5 (2015)
Article DOI: 10.1021/ml500526p
BindingDB Entry DOI: 10.7270/Q28W3G4S |
More data for this
Ligand-Target Pair | |
Mast/stem cell growth factor receptor Kit
(Homo sapiens (Human)) | BDBM50127687
(CHEMBL3632717)Show SMILES Cn1c(Nc2cccc(c2)C(C)(C)C)nc2cc(Oc3ccnc(c3)-c3ncc[nH]3)ccc12 Show InChI InChI=1S/C16H15FN4O/c1-2-22-11-21-19-15(12-3-5-14(17)6-4-12)16(20-21)13-7-9-18-10-8-13/h3-10H,2,11H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of histamine H1 receptor |
ACS Med Chem Lett 6: 961-5 (2015)
Article DOI: 10.1021/ml500526p
BindingDB Entry DOI: 10.7270/Q28W3G4S |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 1/2/3
(Homo sapiens (Human)) | BDBM50127687

(CHEMBL3632717)Show SMILES Cn1c(Nc2cccc(c2)C(C)(C)C)nc2cc(Oc3ccnc(c3)-c3ncc[nH]3)ccc12 Show InChI InChI=1S/C16H15FN4O/c1-2-22-11-21-19-15(12-3-5-14(17)6-4-12)16(20-21)13-7-9-18-10-8-13/h3-10H,2,11H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibitory activity towards acetylcholine esterase (AChE) |
ACS Med Chem Lett 6: 961-5 (2015)
Article DOI: 10.1021/ml500526p
BindingDB Entry DOI: 10.7270/Q28W3G4S |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Fyn
(Homo sapiens (Human)) | BDBM50127687

(CHEMBL3632717)Show SMILES Cn1c(Nc2cccc(c2)C(C)(C)C)nc2cc(Oc3ccnc(c3)-c3ncc[nH]3)ccc12 Show InChI InChI=1S/C16H15FN4O/c1-2-22-11-21-19-15(12-3-5-14(17)6-4-12)16(20-21)13-7-9-18-10-8-13/h3-10H,2,11H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
inhibitory concentration needed to to reduce the bovine GGTase-catalyzed incorporation of [3H]-FPP into a biotin-linked K-ras (B) decapeptide |
ACS Med Chem Lett 6: 961-5 (2015)
Article DOI: 10.1021/ml500526p
BindingDB Entry DOI: 10.7270/Q28W3G4S |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM31088

(1-methyl-5-[2-[5-(trifluoromethyl)-1H-imidazol-2-y...)Show SMILES Cn1c(Nc2ccc(cc2)C(F)(F)F)nc2cc(Oc3ccnc(c3)-c3ncc([nH]3)C(F)(F)F)ccc12 Show InChI InChI=1S/C24H16F6N6O/c1-36-19-7-6-15(10-17(19)34-22(36)33-14-4-2-13(3-5-14)23(25,26)27)37-16-8-9-31-18(11-16)21-32-12-20(35-21)24(28,29)30/h2-12H,1H3,(H,32,35)(H,33,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-FPP incorporation into biotin-linked K-ras decapeptide (CVIM) by bovine farnesyltransferase |
ACS Med Chem Lett 6: 961-5 (2015)
Article DOI: 10.1021/ml500526p
BindingDB Entry DOI: 10.7270/Q28W3G4S |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM31088

(1-methyl-5-[2-[5-(trifluoromethyl)-1H-imidazol-2-y...)Show SMILES Cn1c(Nc2ccc(cc2)C(F)(F)F)nc2cc(Oc3ccnc(c3)-c3ncc([nH]3)C(F)(F)F)ccc12 Show InChI InChI=1S/C24H16F6N6O/c1-36-19-7-6-15(10-17(19)34-22(36)33-14-4-2-13(3-5-14)23(25,26)27)37-16-8-9-31-18(11-16)21-32-12-20(35-21)24(28,29)30/h2-12H,1H3,(H,32,35)(H,33,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-FPP incorporation into biotin-linked K-ras decapeptide (CVIM) by bovine farnesyltransferase |
ACS Med Chem Lett 6: 961-5 (2015)
Article DOI: 10.1021/ml500526p
BindingDB Entry DOI: 10.7270/Q28W3G4S |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM31088

(1-methyl-5-[2-[5-(trifluoromethyl)-1H-imidazol-2-y...)Show SMILES Cn1c(Nc2ccc(cc2)C(F)(F)F)nc2cc(Oc3ccnc(c3)-c3ncc([nH]3)C(F)(F)F)ccc12 Show InChI InChI=1S/C24H16F6N6O/c1-36-19-7-6-15(10-17(19)34-22(36)33-14-4-2-13(3-5-14)23(25,26)27)37-16-8-9-31-18(11-16)21-32-12-20(35-21)24(28,29)30/h2-12H,1H3,(H,32,35)(H,33,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-FPP incorporation into biotin-linked K-ras decapeptide (CVIM) by bovine farnesyltransferase |
ACS Med Chem Lett 6: 961-5 (2015)
Article DOI: 10.1021/ml500526p
BindingDB Entry DOI: 10.7270/Q28W3G4S |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50131827
(CHEMBL3632721)Show SMILES Cn1c(Nc2cc(ccc2F)C(C)(C)C)nc2cc(Oc3ccnc(c3)-c3ncc([nH]3)C(F)(F)F)ccc12 Show InChI InChI=1S/C27H24F4N6O/c1-26(2,3)15-5-7-18(28)19(11-15)34-25-35-20-12-16(6-8-22(20)37(25)4)38-17-9-10-32-21(13-17)24-33-14-23(36-24)27(29,30)31/h5-14H,1-4H3,(H,33,36)(H,34,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity towards Coagulation factor X was determined using chromogenic substrate, MeO-COD-CHG-Gly-Arg-pNA |
ACS Med Chem Lett 6: 961-5 (2015)
Article DOI: 10.1021/ml500526p
BindingDB Entry DOI: 10.7270/Q28W3G4S |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50026679

(CHEMBL3335371)Show SMILES CC(=O)Nc1cc(Oc2ccc3n(C)c(Nc4cccc(c4)C(C)(C)C)nc3c2)ccn1 Show InChI InChI=1S/C25H27N5O2/c1-16(31)27-23-15-20(11-12-26-23)32-19-9-10-22-21(14-19)29-24(30(22)5)28-18-8-6-7-17(13-18)25(2,3)4/h6-15H,1-5H3,(H,28,29)(H,26,27,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibitory activity towards acetylcholine esterase (AChE) |
ACS Med Chem Lett 6: 961-5 (2015)
Article DOI: 10.1021/ml500526p
BindingDB Entry DOI: 10.7270/Q28W3G4S |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM26028

(4-({2-[(3-tert-butylphenyl)(methyl)amino]-1H-1,3-b...)Show SMILES CNC(=O)c1cc(Oc2ccc3n(C)c(Nc4cccc(c4)C(C)(C)C)nc3c2)ccn1 Show InChI InChI=1S/C25H27N5O2/c1-25(2,3)16-7-6-8-17(13-16)28-24-29-20-14-18(9-10-22(20)30(24)5)32-19-11-12-27-21(15-19)23(31)26-4/h6-15H,1-5H3,(H,26,31)(H,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Reduced farnesylation of H-ras transformed NIH3T3 cells |
ACS Med Chem Lett 6: 961-5 (2015)
Article DOI: 10.1021/ml500526p
BindingDB Entry DOI: 10.7270/Q28W3G4S |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50131822

(CHEMBL3632719)Show SMILES Cn1c(Nc2cccc(c2)C(C)(C)C)nc2cc(Oc3ccnc(c3)-c3ncc([nH]3)C(F)(F)F)ccc12 Show InChI InChI=1S/C27H25F3N6O/c1-26(2,3)16-6-5-7-17(12-16)33-25-34-20-13-18(8-9-22(20)36(25)4)37-19-10-11-31-21(14-19)24-32-15-23(35-24)27(28,29)30/h5-15H,1-4H3,(H,32,35)(H,33,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity towards Coagulation factor X was determined using chromogenic substrate, MeO-COD-CHG-Gly-Arg-pNA |
ACS Med Chem Lett 6: 961-5 (2015)
Article DOI: 10.1021/ml500526p
BindingDB Entry DOI: 10.7270/Q28W3G4S |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM26028

(4-({2-[(3-tert-butylphenyl)(methyl)amino]-1H-1,3-b...)Show SMILES CNC(=O)c1cc(Oc2ccc3n(C)c(Nc4cccc(c4)C(C)(C)C)nc3c2)ccn1 Show InChI InChI=1S/C25H27N5O2/c1-25(2,3)16-7-6-8-17(13-16)28-24-29-20-14-18(9-10-22(20)30(24)5)32-19-11-12-27-21(15-19)23(31)26-4/h6-15H,1-5H3,(H,26,31)(H,28,29) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 65 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Binding affinity towards Kappa opioid receptor |
ACS Med Chem Lett 6: 961-5 (2015)
Article DOI: 10.1021/ml500526p
BindingDB Entry DOI: 10.7270/Q28W3G4S |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM31088

(1-methyl-5-[2-[5-(trifluoromethyl)-1H-imidazol-2-y...)Show SMILES Cn1c(Nc2ccc(cc2)C(F)(F)F)nc2cc(Oc3ccnc(c3)-c3ncc([nH]3)C(F)(F)F)ccc12 Show InChI InChI=1S/C24H16F6N6O/c1-36-19-7-6-15(10-17(19)34-22(36)33-14-4-2-13(3-5-14)23(25,26)27)37-16-8-9-31-18(11-16)21-32-12-20(35-21)24(28,29)30/h2-12H,1H3,(H,32,35)(H,33,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
inhibitory concentration needed to to reduce the bovine GGTase-catalyzed incorporation of [3H]-FPP into a biotin-linked K-ras (B) decapeptide |
ACS Med Chem Lett 6: 961-5 (2015)
Article DOI: 10.1021/ml500526p
BindingDB Entry DOI: 10.7270/Q28W3G4S |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Vascular endothelial growth factor receptor 1/2/3
(Homo sapiens (Human)) | BDBM50131822

(CHEMBL3632719)Show SMILES Cn1c(Nc2cccc(c2)C(C)(C)C)nc2cc(Oc3ccnc(c3)-c3ncc([nH]3)C(F)(F)F)ccc12 Show InChI InChI=1S/C27H25F3N6O/c1-26(2,3)16-6-5-7-17(12-16)33-25-34-20-13-18(8-9-22(20)36(25)4)37-19-10-11-31-21(14-19)24-32-15-23(35-24)27(28,29)30/h5-15H,1-4H3,(H,32,35)(H,33,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibitory activity towards acetylcholine esterase (AChE) |
ACS Med Chem Lett 6: 961-5 (2015)
Article DOI: 10.1021/ml500526p
BindingDB Entry DOI: 10.7270/Q28W3G4S |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50127687

(CHEMBL3632717)Show SMILES Cn1c(Nc2cccc(c2)C(C)(C)C)nc2cc(Oc3ccnc(c3)-c3ncc[nH]3)ccc12 Show InChI InChI=1S/C16H15FN4O/c1-2-22-11-21-19-15(12-3-5-14(17)6-4-12)16(20-21)13-7-9-18-10-8-13/h3-10H,2,11H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
inhibitory concentration needed to to reduce the bovine GGTase-catalyzed incorporation of [3H]-FPP into a biotin-linked K-ras (B) decapeptide |
ACS Med Chem Lett 6: 961-5 (2015)
Article DOI: 10.1021/ml500526p
BindingDB Entry DOI: 10.7270/Q28W3G4S |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50131822

(CHEMBL3632719)Show SMILES Cn1c(Nc2cccc(c2)C(C)(C)C)nc2cc(Oc3ccnc(c3)-c3ncc([nH]3)C(F)(F)F)ccc12 Show InChI InChI=1S/C27H25F3N6O/c1-26(2,3)16-6-5-7-17(12-16)33-25-34-20-13-18(8-9-22(20)36(25)4)37-19-10-11-31-21(14-19)24-32-15-23(35-24)27(28,29)30/h5-15H,1-4H3,(H,32,35)(H,33,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity towards Coagulation factor X was determined using chromogenic substrate, MeO-COD-CHG-Gly-Arg-pNA |
ACS Med Chem Lett 6: 961-5 (2015)
Article DOI: 10.1021/ml500526p
BindingDB Entry DOI: 10.7270/Q28W3G4S |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50131830
(CHEMBL3632720)Show SMILES Cn1c(Nc2ccc(cc2)C(C)(C)C)nc2cc(Oc3ccnc(c3)-c3ncc([nH]3)C(F)(F)F)ccc12 Show InChI InChI=1S/C27H25F3N6O/c1-26(2,3)16-5-7-17(8-6-16)33-25-34-20-13-18(9-10-22(20)36(25)4)37-19-11-12-31-21(14-19)24-32-15-23(35-24)27(28,29)30/h5-15H,1-4H3,(H,32,35)(H,33,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity towards Coagulation factor X was determined using chromogenic substrate, MeO-COD-CHG-Gly-Arg-pNA |
ACS Med Chem Lett 6: 961-5 (2015)
Article DOI: 10.1021/ml500526p
BindingDB Entry DOI: 10.7270/Q28W3G4S |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM31088

(1-methyl-5-[2-[5-(trifluoromethyl)-1H-imidazol-2-y...)Show SMILES Cn1c(Nc2ccc(cc2)C(F)(F)F)nc2cc(Oc3ccnc(c3)-c3ncc([nH]3)C(F)(F)F)ccc12 Show InChI InChI=1S/C24H16F6N6O/c1-36-19-7-6-15(10-17(19)34-22(36)33-14-4-2-13(3-5-14)23(25,26)27)37-16-8-9-31-18(11-16)21-32-12-20(35-21)24(28,29)30/h2-12H,1H3,(H,32,35)(H,33,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity towards Coagulation factor X was determined using chromogenic substrate, MeO-COD-CHG-Gly-Arg-pNA |
ACS Med Chem Lett 6: 961-5 (2015)
Article DOI: 10.1021/ml500526p
BindingDB Entry DOI: 10.7270/Q28W3G4S |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50127687

(CHEMBL3632717)Show SMILES Cn1c(Nc2cccc(c2)C(C)(C)C)nc2cc(Oc3ccnc(c3)-c3ncc[nH]3)ccc12 Show InChI InChI=1S/C16H15FN4O/c1-2-22-11-21-19-15(12-3-5-14(17)6-4-12)16(20-21)13-7-9-18-10-8-13/h3-10H,2,11H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Binding affinity towards Kappa opioid receptor |
ACS Med Chem Lett 6: 961-5 (2015)
Article DOI: 10.1021/ml500526p
BindingDB Entry DOI: 10.7270/Q28W3G4S |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50131825
(CHEMBL3632723)Show SMILES Cn1c(Nc2cc(ccc2F)C(F)(F)F)nc2cc(Oc3ccnc(c3)-c3ncc([nH]3)C(F)(F)F)ccc12 Show InChI InChI=1S/C24H15F7N6O/c1-37-19-5-3-13(38-14-6-7-32-18(10-14)21-33-11-20(36-21)24(29,30)31)9-17(19)35-22(37)34-16-8-12(23(26,27)28)2-4-15(16)25/h2-11H,1H3,(H,33,36)(H,34,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity towards Coagulation factor X was determined using chromogenic substrate, MeO-COD-CHG-Gly-Arg-pNA |
ACS Med Chem Lett 6: 961-5 (2015)
Article DOI: 10.1021/ml500526p
BindingDB Entry DOI: 10.7270/Q28W3G4S |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Fyn
(Homo sapiens (Human)) | BDBM50131822

(CHEMBL3632719)Show SMILES Cn1c(Nc2cccc(c2)C(C)(C)C)nc2cc(Oc3ccnc(c3)-c3ncc([nH]3)C(F)(F)F)ccc12 Show InChI InChI=1S/C27H25F3N6O/c1-26(2,3)16-6-5-7-17(12-16)33-25-34-20-13-18(8-9-22(20)36(25)4)37-19-10-11-31-21(14-19)24-32-15-23(35-24)27(28,29)30/h5-15H,1-4H3,(H,32,35)(H,33,34) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-FPP incorporation into biotin-linked K-ras decapeptide (CVIM) by bovine farnesyltransferase |
ACS Med Chem Lett 6: 961-5 (2015)
Article DOI: 10.1021/ml500526p
BindingDB Entry DOI: 10.7270/Q28W3G4S |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM26028

(4-({2-[(3-tert-butylphenyl)(methyl)amino]-1H-1,3-b...)Show SMILES CNC(=O)c1cc(Oc2ccc3n(C)c(Nc4cccc(c4)C(C)(C)C)nc3c2)ccn1 Show InChI InChI=1S/C25H27N5O2/c1-25(2,3)16-7-6-8-17(13-16)28-24-29-20-14-18(9-10-22(20)30(24)5)32-19-11-12-27-21(15-19)23(31)26-4/h6-15H,1-5H3,(H,26,31)(H,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 280 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibitory activity towards acetylcholine esterase (AChE) |
ACS Med Chem Lett 6: 961-5 (2015)
Article DOI: 10.1021/ml500526p
BindingDB Entry DOI: 10.7270/Q28W3G4S |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor beta
(Homo sapiens (Human)) | BDBM50127687

(CHEMBL3632717)Show SMILES Cn1c(Nc2cccc(c2)C(C)(C)C)nc2cc(Oc3ccnc(c3)-c3ncc[nH]3)ccc12 Show InChI InChI=1S/C16H15FN4O/c1-2-22-11-21-19-15(12-3-5-14(17)6-4-12)16(20-21)13-7-9-18-10-8-13/h3-10H,2,11H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 310 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of Histamine H1 receptor |
ACS Med Chem Lett 6: 961-5 (2015)
Article DOI: 10.1021/ml500526p
BindingDB Entry DOI: 10.7270/Q28W3G4S |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50127687

(CHEMBL3632717)Show SMILES Cn1c(Nc2cccc(c2)C(C)(C)C)nc2cc(Oc3ccnc(c3)-c3ncc[nH]3)ccc12 Show InChI InChI=1S/C16H15FN4O/c1-2-22-11-21-19-15(12-3-5-14(17)6-4-12)16(20-21)13-7-9-18-10-8-13/h3-10H,2,11H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 390 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibitory activity towards acetylcholine esterase (AChE) |
ACS Med Chem Lett 6: 961-5 (2015)
Article DOI: 10.1021/ml500526p
BindingDB Entry DOI: 10.7270/Q28W3G4S |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50131831
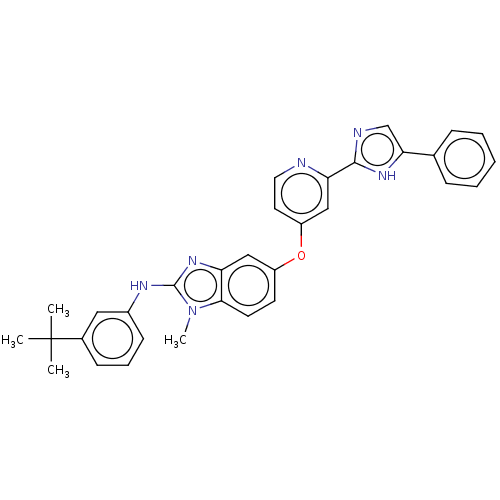
(CHEMBL3632718)Show SMILES Cn1c(Nc2cccc(c2)C(C)(C)C)nc2cc(Oc3ccnc(c3)-c3ncc([nH]3)-c3ccccc3)ccc12 Show InChI InChI=1S/C32H30N6O/c1-32(2,3)22-11-8-12-23(17-22)35-31-37-26-18-24(13-14-29(26)38(31)4)39-25-15-16-33-27(19-25)30-34-20-28(36-30)21-9-6-5-7-10-21/h5-20H,1-4H3,(H,34,36)(H,35,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 480 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity towards Coagulation factor X was determined using chromogenic substrate, MeO-COD-CHG-Gly-Arg-pNA |
ACS Med Chem Lett 6: 961-5 (2015)
Article DOI: 10.1021/ml500526p
BindingDB Entry DOI: 10.7270/Q28W3G4S |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50131824
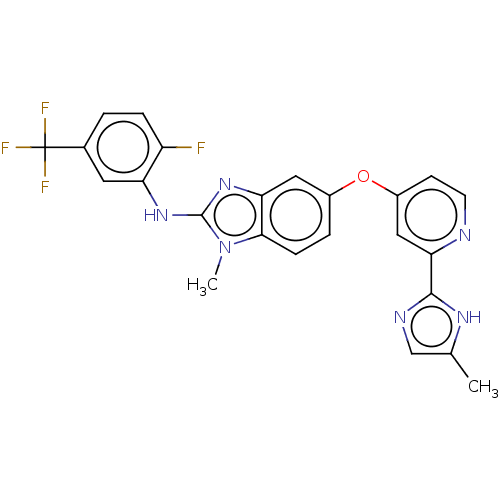
(CHEMBL3632724)Show SMILES Cc1cnc([nH]1)-c1cc(Oc2ccc3n(C)c(Nc4cc(ccc4F)C(F)(F)F)nc3c2)ccn1 Show InChI InChI=1S/C24H18F4N6O/c1-13-12-30-22(31-13)20-11-16(7-8-29-20)35-15-4-6-21-19(10-15)33-23(34(21)2)32-18-9-14(24(26,27)28)3-5-17(18)25/h3-12H,1-2H3,(H,30,31)(H,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 620 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity towards Coagulation factor X was determined using chromogenic substrate, MeO-COD-CHG-Gly-Arg-pNA |
ACS Med Chem Lett 6: 961-5 (2015)
Article DOI: 10.1021/ml500526p
BindingDB Entry DOI: 10.7270/Q28W3G4S |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50131826
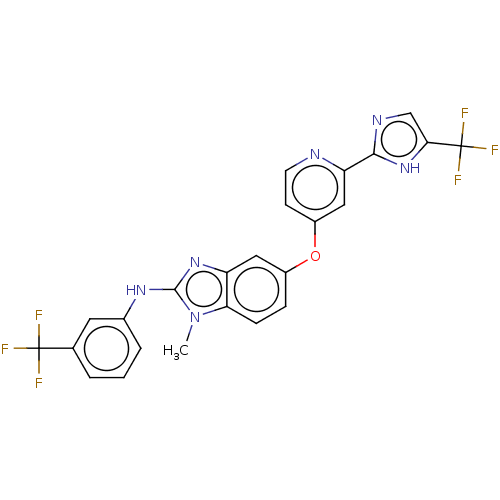
(CHEMBL3632722)Show SMILES Cn1c(Nc2cccc(c2)C(F)(F)F)nc2cc(Oc3ccnc(c3)-c3ncc([nH]3)C(F)(F)F)ccc12 Show InChI InChI=1S/C24H16F6N6O/c1-36-19-6-5-15(10-17(19)34-22(36)33-14-4-2-3-13(9-14)23(25,26)27)37-16-7-8-31-18(11-16)21-32-12-20(35-21)24(28,29)30/h2-12H,1H3,(H,32,35)(H,33,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 780 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity towards Coagulation factor X was determined using chromogenic substrate, MeO-COD-CHG-Gly-Arg-pNA |
ACS Med Chem Lett 6: 961-5 (2015)
Article DOI: 10.1021/ml500526p
BindingDB Entry DOI: 10.7270/Q28W3G4S |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50127687

(CHEMBL3632717)Show SMILES Cn1c(Nc2cccc(c2)C(C)(C)C)nc2cc(Oc3ccnc(c3)-c3ncc[nH]3)ccc12 Show InChI InChI=1S/C16H15FN4O/c1-2-22-11-21-19-15(12-3-5-14(17)6-4-12)16(20-21)13-7-9-18-10-8-13/h3-10H,2,11H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity towards Coagulation factor X was determined using chromogenic substrate, MeO-COD-CHG-Gly-Arg-pNA |
ACS Med Chem Lett 6: 961-5 (2015)
Article DOI: 10.1021/ml500526p
BindingDB Entry DOI: 10.7270/Q28W3G4S |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50131822

(CHEMBL3632719)Show SMILES Cn1c(Nc2cccc(c2)C(C)(C)C)nc2cc(Oc3ccnc(c3)-c3ncc([nH]3)C(F)(F)F)ccc12 Show InChI InChI=1S/C27H25F3N6O/c1-26(2,3)16-6-5-7-17(12-16)33-25-34-20-13-18(8-9-22(20)36(25)4)37-19-10-11-31-21(14-19)24-32-15-23(35-24)27(28,29)30/h5-15H,1-4H3,(H,32,35)(H,33,34) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
inhibitory concentration needed to to reduce the bovine GGTase-catalyzed incorporation of [3H]-FPP into a biotin-linked K-ras (B) decapeptide |
ACS Med Chem Lett 6: 961-5 (2015)
Article DOI: 10.1021/ml500526p
BindingDB Entry DOI: 10.7270/Q28W3G4S |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50131827

(CHEMBL3632721)Show SMILES Cn1c(Nc2cc(ccc2F)C(C)(C)C)nc2cc(Oc3ccnc(c3)-c3ncc([nH]3)C(F)(F)F)ccc12 Show InChI InChI=1S/C27H24F4N6O/c1-26(2,3)15-5-7-18(28)19(11-15)34-25-35-20-12-16(6-8-22(20)37(25)4)38-17-9-10-32-21(13-17)24-33-14-23(36-24)27(29,30)31/h5-14H,1-4H3,(H,33,36)(H,34,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibitory activity towards acetylcholine esterase (AChE) |
ACS Med Chem Lett 6: 961-5 (2015)
Article DOI: 10.1021/ml500526p
BindingDB Entry DOI: 10.7270/Q28W3G4S |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50131824

(CHEMBL3632724)Show SMILES Cc1cnc([nH]1)-c1cc(Oc2ccc3n(C)c(Nc4cc(ccc4F)C(F)(F)F)nc3c2)ccn1 Show InChI InChI=1S/C24H18F4N6O/c1-13-12-30-22(31-13)20-11-16(7-8-29-20)35-15-4-6-21-19(10-15)33-23(34(21)2)32-18-9-14(24(26,27)28)3-5-17(18)25/h3-12H,1-2H3,(H,30,31)(H,32,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibitory activity towards acetylcholine esterase (AChE) |
ACS Med Chem Lett 6: 961-5 (2015)
Article DOI: 10.1021/ml500526p
BindingDB Entry DOI: 10.7270/Q28W3G4S |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50131822

(CHEMBL3632719)Show SMILES Cn1c(Nc2cccc(c2)C(C)(C)C)nc2cc(Oc3ccnc(c3)-c3ncc([nH]3)C(F)(F)F)ccc12 Show InChI InChI=1S/C27H25F3N6O/c1-26(2,3)16-6-5-7-17(12-16)33-25-34-20-13-18(8-9-22(20)36(25)4)37-19-10-11-31-21(14-19)24-32-15-23(35-24)27(28,29)30/h5-15H,1-4H3,(H,32,35)(H,33,34) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
inhibitory concentration needed to to reduce the bovine GGTase-catalyzed incorporation of [3H]-FPP into a biotin-linked K-ras (B) decapeptide |
ACS Med Chem Lett 6: 961-5 (2015)
Article DOI: 10.1021/ml500526p
BindingDB Entry DOI: 10.7270/Q28W3G4S |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM31088

(1-methyl-5-[2-[5-(trifluoromethyl)-1H-imidazol-2-y...)Show SMILES Cn1c(Nc2ccc(cc2)C(F)(F)F)nc2cc(Oc3ccnc(c3)-c3ncc([nH]3)C(F)(F)F)ccc12 Show InChI InChI=1S/C24H16F6N6O/c1-36-19-7-6-15(10-17(19)34-22(36)33-14-4-2-13(3-5-14)23(25,26)27)37-16-8-9-31-18(11-16)21-32-12-20(35-21)24(28,29)30/h2-12H,1H3,(H,32,35)(H,33,34) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
inhibitory concentration needed to to reduce the bovine GGTase-catalyzed incorporation of [3H]-FPP into a biotin-linked K-ras (B) decapeptide |
ACS Med Chem Lett 6: 961-5 (2015)
Article DOI: 10.1021/ml500526p
BindingDB Entry DOI: 10.7270/Q28W3G4S |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50131831

(CHEMBL3632718)Show SMILES Cn1c(Nc2cccc(c2)C(C)(C)C)nc2cc(Oc3ccnc(c3)-c3ncc([nH]3)-c3ccccc3)ccc12 Show InChI InChI=1S/C32H30N6O/c1-32(2,3)22-11-8-12-23(17-22)35-31-37-26-18-24(13-14-29(26)38(31)4)39-25-15-16-33-27(19-25)30-34-20-28(36-30)21-9-6-5-7-10-21/h5-20H,1-4H3,(H,34,36)(H,35,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibitory activity towards acetylcholine esterase (AChE) |
ACS Med Chem Lett 6: 961-5 (2015)
Article DOI: 10.1021/ml500526p
BindingDB Entry DOI: 10.7270/Q28W3G4S |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Fyn
(Homo sapiens (Human)) | BDBM31088

(1-methyl-5-[2-[5-(trifluoromethyl)-1H-imidazol-2-y...)Show SMILES Cn1c(Nc2ccc(cc2)C(F)(F)F)nc2cc(Oc3ccnc(c3)-c3ncc([nH]3)C(F)(F)F)ccc12 Show InChI InChI=1S/C24H16F6N6O/c1-36-19-7-6-15(10-17(19)34-22(36)33-14-4-2-13(3-5-14)23(25,26)27)37-16-8-9-31-18(11-16)21-32-12-20(35-21)24(28,29)30/h2-12H,1H3,(H,32,35)(H,33,34) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Reduced farnesylation of H-ras transformed NIH3T3 cells |
ACS Med Chem Lett 6: 961-5 (2015)
Article DOI: 10.1021/ml500526p
BindingDB Entry DOI: 10.7270/Q28W3G4S |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM31088

(1-methyl-5-[2-[5-(trifluoromethyl)-1H-imidazol-2-y...)Show SMILES Cn1c(Nc2ccc(cc2)C(F)(F)F)nc2cc(Oc3ccnc(c3)-c3ncc([nH]3)C(F)(F)F)ccc12 Show InChI InChI=1S/C24H16F6N6O/c1-36-19-7-6-15(10-17(19)34-22(36)33-14-4-2-13(3-5-14)23(25,26)27)37-16-8-9-31-18(11-16)21-32-12-20(35-21)24(28,29)30/h2-12H,1H3,(H,32,35)(H,33,34) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
inhibitory concentration needed to to reduce the bovine GGTase-catalyzed incorporation of [3H]-FPP into a biotin-linked K-ras (B) decapeptide |
ACS Med Chem Lett 6: 961-5 (2015)
Article DOI: 10.1021/ml500526p
BindingDB Entry DOI: 10.7270/Q28W3G4S |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50131822

(CHEMBL3632719)Show SMILES Cn1c(Nc2cccc(c2)C(C)(C)C)nc2cc(Oc3ccnc(c3)-c3ncc([nH]3)C(F)(F)F)ccc12 Show InChI InChI=1S/C27H25F3N6O/c1-26(2,3)16-6-5-7-17(12-16)33-25-34-20-13-18(8-9-22(20)36(25)4)37-19-10-11-31-21(14-19)24-32-15-23(35-24)27(28,29)30/h5-15H,1-4H3,(H,32,35)(H,33,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibitory activity towards acetylcholine esterase (AChE) |
ACS Med Chem Lett 6: 961-5 (2015)
Article DOI: 10.1021/ml500526p
BindingDB Entry DOI: 10.7270/Q28W3G4S |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50131825

(CHEMBL3632723)Show SMILES Cn1c(Nc2cc(ccc2F)C(F)(F)F)nc2cc(Oc3ccnc(c3)-c3ncc([nH]3)C(F)(F)F)ccc12 Show InChI InChI=1S/C24H15F7N6O/c1-37-19-5-3-13(38-14-6-7-32-18(10-14)21-33-11-20(36-21)24(29,30)31)9-17(19)35-22(37)34-16-8-12(23(26,27)28)2-4-15(16)25/h2-11H,1H3,(H,33,36)(H,34,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibitory activity towards acetylcholine esterase (AChE) |
ACS Med Chem Lett 6: 961-5 (2015)
Article DOI: 10.1021/ml500526p
BindingDB Entry DOI: 10.7270/Q28W3G4S |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50131830

(CHEMBL3632720)Show SMILES Cn1c(Nc2ccc(cc2)C(C)(C)C)nc2cc(Oc3ccnc(c3)-c3ncc([nH]3)C(F)(F)F)ccc12 Show InChI InChI=1S/C27H25F3N6O/c1-26(2,3)16-5-7-17(8-6-16)33-25-34-20-13-18(9-10-22(20)36(25)4)37-19-11-12-31-21(14-19)24-32-15-23(35-24)27(28,29)30/h5-15H,1-4H3,(H,32,35)(H,33,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibitory activity towards acetylcholine esterase (AChE) |
ACS Med Chem Lett 6: 961-5 (2015)
Article DOI: 10.1021/ml500526p
BindingDB Entry DOI: 10.7270/Q28W3G4S |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM31088

(1-methyl-5-[2-[5-(trifluoromethyl)-1H-imidazol-2-y...)Show SMILES Cn1c(Nc2ccc(cc2)C(F)(F)F)nc2cc(Oc3ccnc(c3)-c3ncc([nH]3)C(F)(F)F)ccc12 Show InChI InChI=1S/C24H16F6N6O/c1-36-19-7-6-15(10-17(19)34-22(36)33-14-4-2-13(3-5-14)23(25,26)27)37-16-8-9-31-18(11-16)21-32-12-20(35-21)24(28,29)30/h2-12H,1H3,(H,32,35)(H,33,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibitory activity towards acetylcholine esterase (AChE) |
ACS Med Chem Lett 6: 961-5 (2015)
Article DOI: 10.1021/ml500526p
BindingDB Entry DOI: 10.7270/Q28W3G4S |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50131826

(CHEMBL3632722)Show SMILES Cn1c(Nc2cccc(c2)C(F)(F)F)nc2cc(Oc3ccnc(c3)-c3ncc([nH]3)C(F)(F)F)ccc12 Show InChI InChI=1S/C24H16F6N6O/c1-36-19-6-5-15(10-17(19)34-22(36)33-14-4-2-3-13(9-14)23(25,26)27)37-16-7-8-31-18(11-16)21-32-12-20(35-21)24(28,29)30/h2-12H,1H3,(H,32,35)(H,33,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibitory activity towards acetylcholine esterase (AChE) |
ACS Med Chem Lett 6: 961-5 (2015)
Article DOI: 10.1021/ml500526p
BindingDB Entry DOI: 10.7270/Q28W3G4S |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data