

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM50444549 (CHEMBL3099695) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Pharma Research and Early Development (pRED) Curated by ChEMBL | Assay Description Inhibition of human CYP11B2 expressed in renal leiomyoblastoma cells | J Med Chem 58: 8054-65 (2015) Article DOI: 10.1021/acs.jmedchem.5b00851 BindingDB Entry DOI: 10.7270/Q2K0763C | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM8611 (4-{5H,6H,7H,8H-imidazo[1,5-a]pyridin-5-yl}benzonit...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Pharma Research and Early Development (pRED) Curated by ChEMBL | Assay Description Inhibition of human CYP11B2 expressed in renal leiomyoblastoma cells | J Med Chem 58: 8054-65 (2015) Article DOI: 10.1021/acs.jmedchem.5b00851 BindingDB Entry DOI: 10.7270/Q2K0763C | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM50125049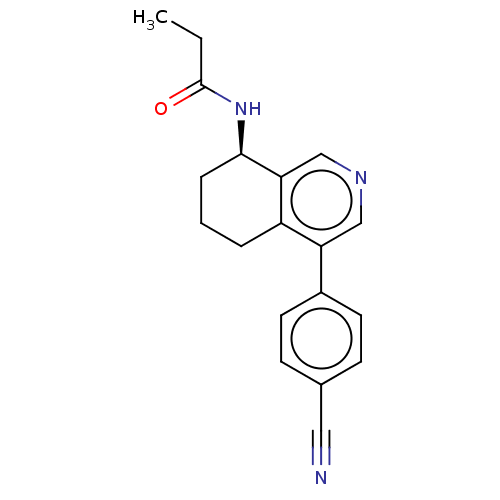 (CHEMBL3623830) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Pharma Research and Early Development (pRED) Curated by ChEMBL | Assay Description Inhibition of human CYP11B2 expressed in human renal leiomyoblastoma cells using 11-deoxycorticosterone as substrate assessed as formation of aldoste... | J Med Chem 58: 8054-65 (2015) Article DOI: 10.1021/acs.jmedchem.5b00851 BindingDB Entry DOI: 10.7270/Q2K0763C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM191903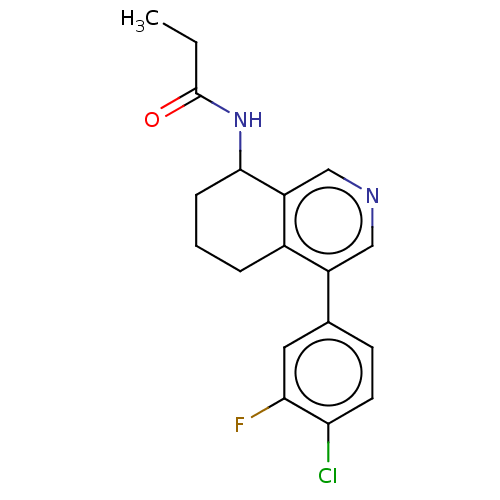 (US9187429, 36 | US9187429, 37 | US9187429, 38) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Pharma Research and Early Development (pRED) Curated by ChEMBL | Assay Description Inhibition of human CYP11B2 expressed in human renal leiomyoblastoma cells using 11-deoxycorticosterone as substrate assessed as formation of aldoste... | J Med Chem 58: 8054-65 (2015) Article DOI: 10.1021/acs.jmedchem.5b00851 BindingDB Entry DOI: 10.7270/Q2K0763C | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM191882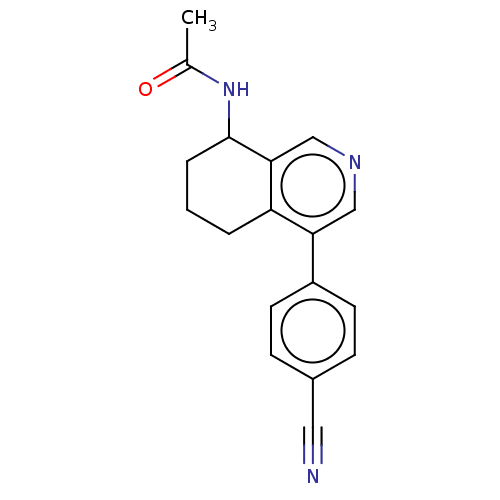 (US9187429, 8) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Pharma Research and Early Development (pRED) Curated by ChEMBL | Assay Description Inhibition of human CYP11B2 expressed in human renal leiomyoblastoma cells using 11-deoxycorticosterone as substrate assessed as formation of aldoste... | J Med Chem 58: 8054-65 (2015) Article DOI: 10.1021/acs.jmedchem.5b00851 BindingDB Entry DOI: 10.7270/Q2K0763C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM191880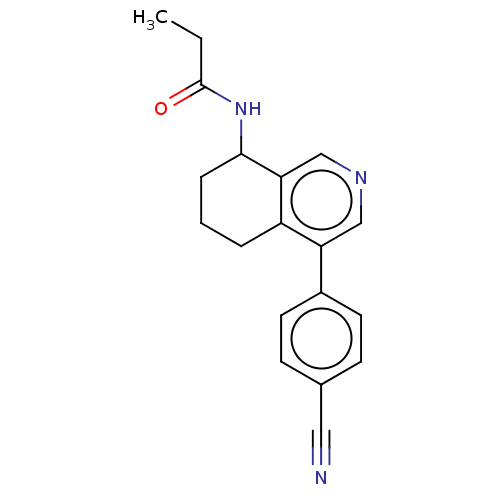 (US9187429, 5 | US9187429, 6 | US9187429, 7) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Pharma Research and Early Development (pRED) Curated by ChEMBL | Assay Description Inhibition of human CYP11B2 expressed in human renal leiomyoblastoma cells using 11-deoxycorticosterone as substrate assessed as formation of aldoste... | J Med Chem 58: 8054-65 (2015) Article DOI: 10.1021/acs.jmedchem.5b00851 BindingDB Entry DOI: 10.7270/Q2K0763C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM191908 (US9187429, 43) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Pharma Research and Early Development (pRED) Curated by ChEMBL | Assay Description Inhibition of human CYP11B2 expressed in human renal leiomyoblastoma cells using 11-deoxycorticosterone as substrate assessed as formation of aldoste... | J Med Chem 58: 8054-65 (2015) Article DOI: 10.1021/acs.jmedchem.5b00851 BindingDB Entry DOI: 10.7270/Q2K0763C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM191899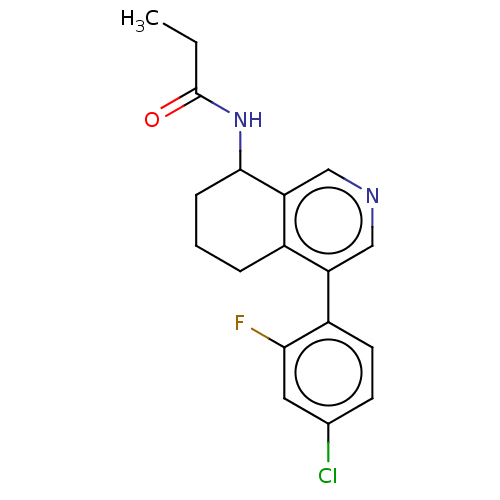 (US9187429, 30 | US9187429, 31 | US9187429, 32) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Pharma Research and Early Development (pRED) Curated by ChEMBL | Assay Description Inhibition of human CYP11B2 expressed in human renal leiomyoblastoma cells using 11-deoxycorticosterone as substrate assessed as formation of aldoste... | J Med Chem 58: 8054-65 (2015) Article DOI: 10.1021/acs.jmedchem.5b00851 BindingDB Entry DOI: 10.7270/Q2K0763C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM50125051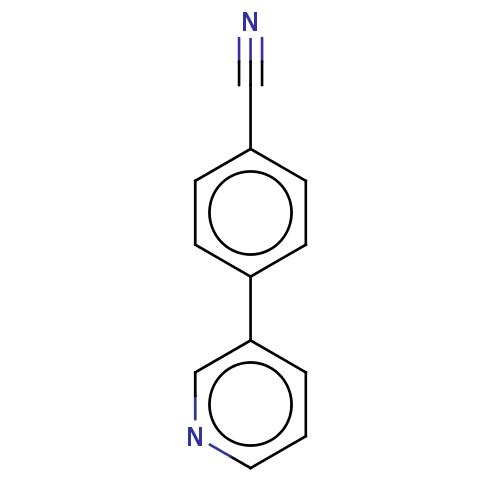 (CHEMBL3623821) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Pharma Research and Early Development (pRED) Curated by ChEMBL | Assay Description Inhibition of human CYP11B2 expressed in human renal leiomyoblastoma cells using 11-deoxycorticosterone as substrate assessed as formation of aldoste... | J Med Chem 58: 8054-65 (2015) Article DOI: 10.1021/acs.jmedchem.5b00851 BindingDB Entry DOI: 10.7270/Q2K0763C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM191876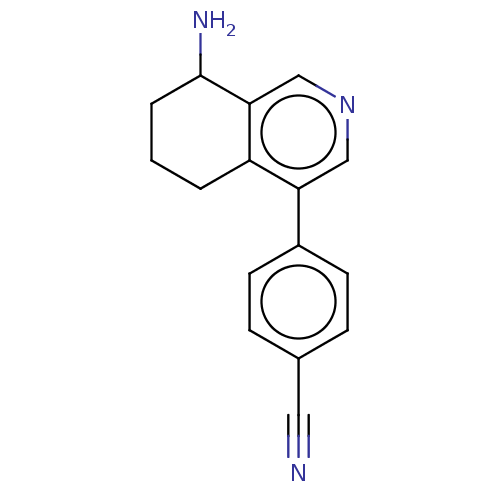 (US9187429, 1) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Pharma Research and Early Development (pRED) Curated by ChEMBL | Assay Description Inhibition of human CYP11B2 expressed in human renal leiomyoblastoma cells using 11-deoxycorticosterone as substrate assessed as formation of aldoste... | J Med Chem 58: 8054-65 (2015) Article DOI: 10.1021/acs.jmedchem.5b00851 BindingDB Entry DOI: 10.7270/Q2K0763C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM191909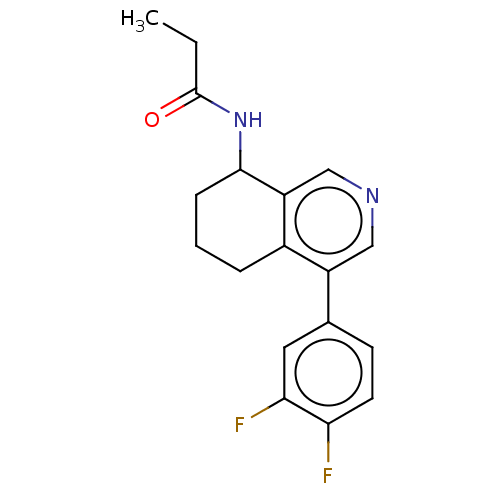 (US9187429, 44) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Pharma Research and Early Development (pRED) Curated by ChEMBL | Assay Description Inhibition of human CYP11B2 expressed in human renal leiomyoblastoma cells using 11-deoxycorticosterone as substrate assessed as formation of aldoste... | J Med Chem 58: 8054-65 (2015) Article DOI: 10.1021/acs.jmedchem.5b00851 BindingDB Entry DOI: 10.7270/Q2K0763C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM191883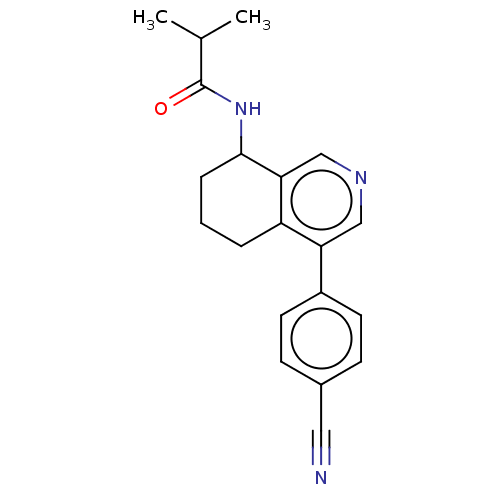 (US9187429, 9) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 123 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Pharma Research and Early Development (pRED) Curated by ChEMBL | Assay Description Inhibition of human CYP11B2 expressed in human renal leiomyoblastoma cells using 11-deoxycorticosterone as substrate assessed as formation of aldoste... | J Med Chem 58: 8054-65 (2015) Article DOI: 10.1021/acs.jmedchem.5b00851 BindingDB Entry DOI: 10.7270/Q2K0763C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Mus musculus) | BDBM8611 (4-{5H,6H,7H,8H-imidazo[1,5-a]pyridin-5-yl}benzonit...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Pharma Research and Early Development (pRED) Curated by ChEMBL | Assay Description Inhibition of mouse CYP11B2 expressed in renal leiomyoblastoma cells | J Med Chem 58: 8054-65 (2015) Article DOI: 10.1021/acs.jmedchem.5b00851 BindingDB Entry DOI: 10.7270/Q2K0763C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Mus musculus) | BDBM50444549 (CHEMBL3099695) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Pharma Research and Early Development (pRED) Curated by ChEMBL | Assay Description Inhibition of mouse CYP11B2 expressed in renal leiomyoblastoma cells | J Med Chem 58: 8054-65 (2015) Article DOI: 10.1021/acs.jmedchem.5b00851 BindingDB Entry DOI: 10.7270/Q2K0763C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Mus musculus) | BDBM50125049 (CHEMBL3623830) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 530 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Pharma Research and Early Development (pRED) Curated by ChEMBL | Assay Description Inhibition of mouse CYP11B2 expressed in human renal leiomyoblastoma cells using 11-deoxycorticosterone as substrate assessed as formation of aldoste... | J Med Chem 58: 8054-65 (2015) Article DOI: 10.1021/acs.jmedchem.5b00851 BindingDB Entry DOI: 10.7270/Q2K0763C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM50125050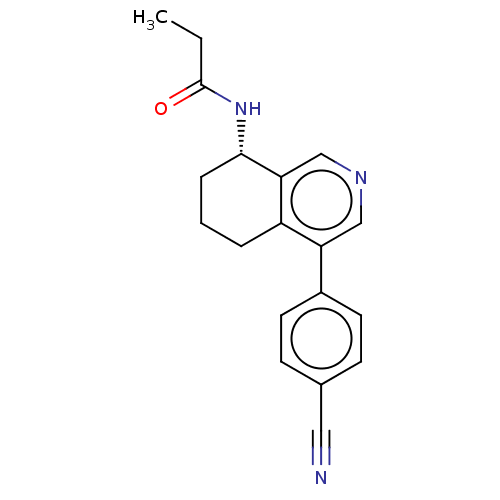 (CHEMBL3623831) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 942 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Pharma Research and Early Development (pRED) Curated by ChEMBL | Assay Description Inhibition of human CYP11B2 expressed in human renal leiomyoblastoma cells using 11-deoxycorticosterone as substrate assessed as formation of aldoste... | J Med Chem 58: 8054-65 (2015) Article DOI: 10.1021/acs.jmedchem.5b00851 BindingDB Entry DOI: 10.7270/Q2K0763C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Mus musculus) | BDBM50125050 (CHEMBL3623831) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Pharma Research and Early Development (pRED) Curated by ChEMBL | Assay Description Inhibition of mouse CYP11B2 expressed in human renal leiomyoblastoma cells using 11-deoxycorticosterone as substrate assessed as formation of aldoste... | J Med Chem 58: 8054-65 (2015) Article DOI: 10.1021/acs.jmedchem.5b00851 BindingDB Entry DOI: 10.7270/Q2K0763C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50125050 (CHEMBL3623831) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Pharma Research and Early Development (pRED) Curated by ChEMBL | Assay Description Inhibition of CYP2C9 (unknown origin) | J Med Chem 58: 8054-65 (2015) Article DOI: 10.1021/acs.jmedchem.5b00851 BindingDB Entry DOI: 10.7270/Q2K0763C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50125050 (CHEMBL3623831) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Pharma Research and Early Development (pRED) Curated by ChEMBL | Assay Description Inhibition of CYP3A4 (unknown origin) | J Med Chem 58: 8054-65 (2015) Article DOI: 10.1021/acs.jmedchem.5b00851 BindingDB Entry DOI: 10.7270/Q2K0763C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50125050 (CHEMBL3623831) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Pharma Research and Early Development (pRED) Curated by ChEMBL | Assay Description Inhibition of CYP2D6 (unknown origin) | J Med Chem 58: 8054-65 (2015) Article DOI: 10.1021/acs.jmedchem.5b00851 BindingDB Entry DOI: 10.7270/Q2K0763C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM191880 (US9187429, 5 | US9187429, 6 | US9187429, 7) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Pharma Research and Early Development (pRED) Curated by ChEMBL | Assay Description Inhibition of CYP2D6 (unknown origin) | J Med Chem 58: 8054-65 (2015) Article DOI: 10.1021/acs.jmedchem.5b00851 BindingDB Entry DOI: 10.7270/Q2K0763C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM191883 (US9187429, 9) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Pharma Research and Early Development (pRED) Curated by ChEMBL | Assay Description Inhibition of CYP3A4 (unknown origin) | J Med Chem 58: 8054-65 (2015) Article DOI: 10.1021/acs.jmedchem.5b00851 BindingDB Entry DOI: 10.7270/Q2K0763C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM191883 (US9187429, 9) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Pharma Research and Early Development (pRED) Curated by ChEMBL | Assay Description Inhibition of CYP2C9 (unknown origin) | J Med Chem 58: 8054-65 (2015) Article DOI: 10.1021/acs.jmedchem.5b00851 BindingDB Entry DOI: 10.7270/Q2K0763C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM191880 (US9187429, 5 | US9187429, 6 | US9187429, 7) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Pharma Research and Early Development (pRED) Curated by ChEMBL | Assay Description Inhibition of CYP2C9 (unknown origin) | J Med Chem 58: 8054-65 (2015) Article DOI: 10.1021/acs.jmedchem.5b00851 BindingDB Entry DOI: 10.7270/Q2K0763C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM191883 (US9187429, 9) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Pharma Research and Early Development (pRED) Curated by ChEMBL | Assay Description Inhibition of CYP2D6 (unknown origin) | J Med Chem 58: 8054-65 (2015) Article DOI: 10.1021/acs.jmedchem.5b00851 BindingDB Entry DOI: 10.7270/Q2K0763C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50125049 (CHEMBL3623830) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Pharma Research and Early Development (pRED) Curated by ChEMBL | Assay Description Inhibition of CYP3A4 (unknown origin) | J Med Chem 58: 8054-65 (2015) Article DOI: 10.1021/acs.jmedchem.5b00851 BindingDB Entry DOI: 10.7270/Q2K0763C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM191882 (US9187429, 8) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Pharma Research and Early Development (pRED) Curated by ChEMBL | Assay Description Inhibition of CYP2C9 (unknown origin) | J Med Chem 58: 8054-65 (2015) Article DOI: 10.1021/acs.jmedchem.5b00851 BindingDB Entry DOI: 10.7270/Q2K0763C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM191880 (US9187429, 5 | US9187429, 6 | US9187429, 7) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Pharma Research and Early Development (pRED) Curated by ChEMBL | Assay Description Inhibition of CYP3A4 (unknown origin) | J Med Chem 58: 8054-65 (2015) Article DOI: 10.1021/acs.jmedchem.5b00851 BindingDB Entry DOI: 10.7270/Q2K0763C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM191882 (US9187429, 8) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Pharma Research and Early Development (pRED) Curated by ChEMBL | Assay Description Inhibition of CYP3A4 (unknown origin) | J Med Chem 58: 8054-65 (2015) Article DOI: 10.1021/acs.jmedchem.5b00851 BindingDB Entry DOI: 10.7270/Q2K0763C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50125049 (CHEMBL3623830) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Pharma Research and Early Development (pRED) Curated by ChEMBL | Assay Description Inhibition of CYP2C9 (unknown origin) | J Med Chem 58: 8054-65 (2015) Article DOI: 10.1021/acs.jmedchem.5b00851 BindingDB Entry DOI: 10.7270/Q2K0763C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50125049 (CHEMBL3623830) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Pharma Research and Early Development (pRED) Curated by ChEMBL | Assay Description Inhibition of CYP2D6 (unknown origin) | J Med Chem 58: 8054-65 (2015) Article DOI: 10.1021/acs.jmedchem.5b00851 BindingDB Entry DOI: 10.7270/Q2K0763C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM191882 (US9187429, 8) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Pharma Research and Early Development (pRED) Curated by ChEMBL | Assay Description Inhibition of CYP2D6 (unknown origin) | J Med Chem 58: 8054-65 (2015) Article DOI: 10.1021/acs.jmedchem.5b00851 BindingDB Entry DOI: 10.7270/Q2K0763C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||