

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50005711 (CHEBI:46024 | GNF-Pf-1011 | TRICHOSTATIN | Trichos...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Ronzoni Institute for Chemical and Biochem. Research Curated by ChEMBL | Assay Description Inhibition of HDAC1 (unknown origin) incubated for 1 hr by fluor de lys substrate based fluorescence method | Bioorg Med Chem 23: 6785-93 (2015) Article DOI: 10.1016/j.bmc.2015.10.004 BindingDB Entry DOI: 10.7270/Q21N82XF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50354086 (FK-228 | Istodax | ROMIDEPSIN) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Ronzoni Institute for Chemical and Biochem. Research Curated by ChEMBL | Assay Description Inhibition of HDAC1 (unknown origin) incubated for 1 hr by fluor de lys substrate based fluorescence method | Bioorg Med Chem 23: 6785-93 (2015) Article DOI: 10.1016/j.bmc.2015.10.004 BindingDB Entry DOI: 10.7270/Q21N82XF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 8 (Homo sapiens (Human)) | BDBM50354086 (FK-228 | Istodax | ROMIDEPSIN) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Ronzoni Institute for Chemical and Biochem. Research Curated by ChEMBL | Assay Description Inhibition of HDAC8 (unknown origin) incubated for 1 hr by fluor de lys substrate based fluorescence method | Bioorg Med Chem 23: 6785-93 (2015) Article DOI: 10.1016/j.bmc.2015.10.004 BindingDB Entry DOI: 10.7270/Q21N82XF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50005711 (CHEBI:46024 | GNF-Pf-1011 | TRICHOSTATIN | Trichos...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 65 | n/a | n/a | n/a | n/a | n/a | n/a |
Ronzoni Institute for Chemical and Biochem. Research Curated by ChEMBL | Assay Description Inhibition of HDAC6 (unknown origin) incubated for 1 hr by fluor de lys substrate based fluorescence method | Bioorg Med Chem 23: 6785-93 (2015) Article DOI: 10.1016/j.bmc.2015.10.004 BindingDB Entry DOI: 10.7270/Q21N82XF | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histone deacetylase 8 (Homo sapiens (Human)) | BDBM50005711 (CHEBI:46024 | GNF-Pf-1011 | TRICHOSTATIN | Trichos...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
Ronzoni Institute for Chemical and Biochem. Research Curated by ChEMBL | Assay Description Inhibition of HDAC8 (unknown origin) incubated for 1 hr by fluor de lys substrate based fluorescence method | Bioorg Med Chem 23: 6785-93 (2015) Article DOI: 10.1016/j.bmc.2015.10.004 BindingDB Entry DOI: 10.7270/Q21N82XF | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50354086 (FK-228 | Istodax | ROMIDEPSIN) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 281 | n/a | n/a | n/a | n/a | n/a | n/a |
Ronzoni Institute for Chemical and Biochem. Research Curated by ChEMBL | Assay Description Inhibition of HDAC6 (unknown origin) incubated for 1 hr by fluor de lys substrate based fluorescence method | Bioorg Med Chem 23: 6785-93 (2015) Article DOI: 10.1016/j.bmc.2015.10.004 BindingDB Entry DOI: 10.7270/Q21N82XF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50129133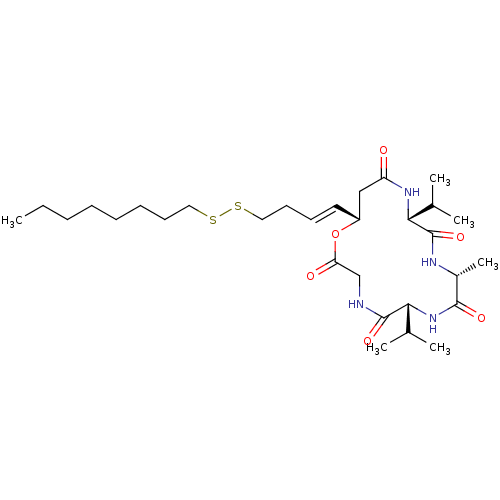 (CHEMBL3628320) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 820 | n/a | n/a | n/a | n/a | n/a | n/a |
Ronzoni Institute for Chemical and Biochem. Research Curated by ChEMBL | Assay Description Inhibition of HDAC1 (unknown origin) incubated for 1 hr by fluor de lys substrate based fluorescence method | Bioorg Med Chem 23: 6785-93 (2015) Article DOI: 10.1016/j.bmc.2015.10.004 BindingDB Entry DOI: 10.7270/Q21N82XF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50129135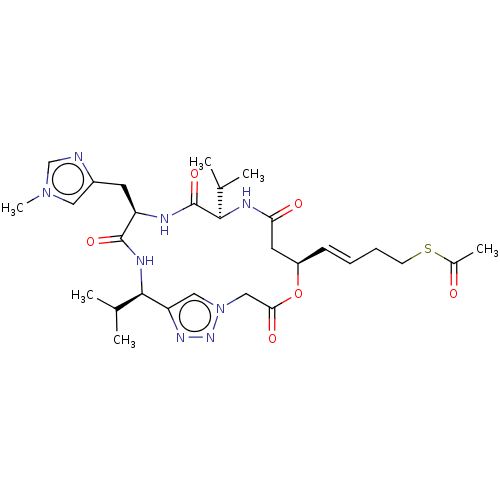 (CHEMBL3628323) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.04E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ronzoni Institute for Chemical and Biochem. Research Curated by ChEMBL | Assay Description Inhibition of HDAC1 (unknown origin) incubated for 1 hr by fluor de lys substrate based fluorescence method | Bioorg Med Chem 23: 6785-93 (2015) Article DOI: 10.1016/j.bmc.2015.10.004 BindingDB Entry DOI: 10.7270/Q21N82XF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50129134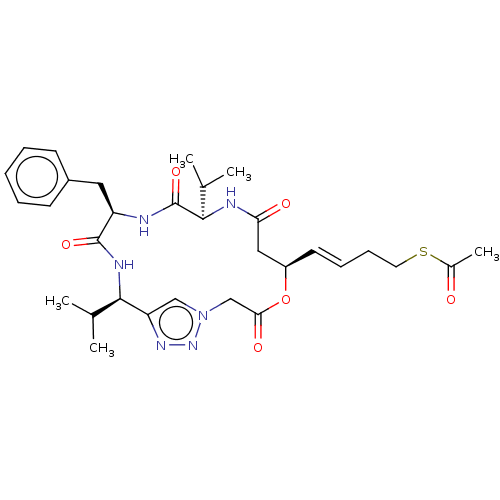 (CHEMBL3628322) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 1.05E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ronzoni Institute for Chemical and Biochem. Research Curated by ChEMBL | Assay Description Inhibition of HDAC1 (unknown origin) incubated for 1 hr by fluor de lys substrate based fluorescence method | Bioorg Med Chem 23: 6785-93 (2015) Article DOI: 10.1016/j.bmc.2015.10.004 BindingDB Entry DOI: 10.7270/Q21N82XF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50129132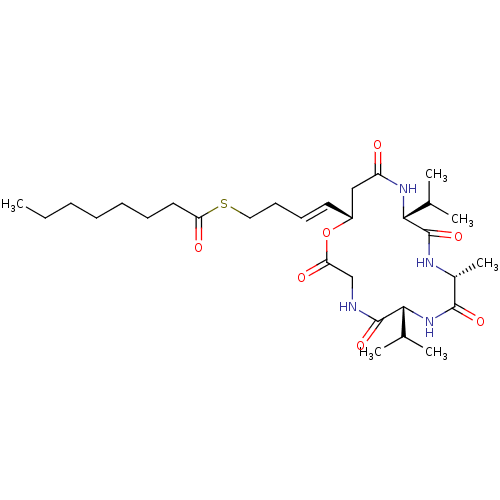 (CHEMBL3628319) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.27E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ronzoni Institute for Chemical and Biochem. Research Curated by ChEMBL | Assay Description Inhibition of HDAC1 (unknown origin) incubated for 1 hr by fluor de lys substrate based fluorescence method | Bioorg Med Chem 23: 6785-93 (2015) Article DOI: 10.1016/j.bmc.2015.10.004 BindingDB Entry DOI: 10.7270/Q21N82XF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 8 (Homo sapiens (Human)) | BDBM50129135 (CHEMBL3628323) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.62E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ronzoni Institute for Chemical and Biochem. Research Curated by ChEMBL | Assay Description Inhibition of HDAC8 (unknown origin) incubated for 1 hr by fluor de lys substrate based fluorescence method | Bioorg Med Chem 23: 6785-93 (2015) Article DOI: 10.1016/j.bmc.2015.10.004 BindingDB Entry DOI: 10.7270/Q21N82XF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 8 (Homo sapiens (Human)) | BDBM50129134 (CHEMBL3628322) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ronzoni Institute for Chemical and Biochem. Research Curated by ChEMBL | Assay Description Inhibition of HDAC8 (unknown origin) incubated for 1 hr by fluor de lys substrate based fluorescence method | Bioorg Med Chem 23: 6785-93 (2015) Article DOI: 10.1016/j.bmc.2015.10.004 BindingDB Entry DOI: 10.7270/Q21N82XF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 8 (Homo sapiens (Human)) | BDBM50129133 (CHEMBL3628320) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.01E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ronzoni Institute for Chemical and Biochem. Research Curated by ChEMBL | Assay Description Inhibition of HDAC8 (unknown origin) incubated for 1 hr by fluor de lys substrate based fluorescence method | Bioorg Med Chem 23: 6785-93 (2015) Article DOI: 10.1016/j.bmc.2015.10.004 BindingDB Entry DOI: 10.7270/Q21N82XF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50129134 (CHEMBL3628322) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 8.67E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ronzoni Institute for Chemical and Biochem. Research Curated by ChEMBL | Assay Description Inhibition of HDAC6 (unknown origin) incubated for 1 hr by fluor de lys substrate based fluorescence method | Bioorg Med Chem 23: 6785-93 (2015) Article DOI: 10.1016/j.bmc.2015.10.004 BindingDB Entry DOI: 10.7270/Q21N82XF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50129133 (CHEMBL3628320) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ronzoni Institute for Chemical and Biochem. Research Curated by ChEMBL | Assay Description Inhibition of HDAC6 (unknown origin) incubated for 1 hr by fluor de lys substrate based fluorescence method | Bioorg Med Chem 23: 6785-93 (2015) Article DOI: 10.1016/j.bmc.2015.10.004 BindingDB Entry DOI: 10.7270/Q21N82XF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50129135 (CHEMBL3628323) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9.89E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ronzoni Institute for Chemical and Biochem. Research Curated by ChEMBL | Assay Description Inhibition of HDAC6 (unknown origin) incubated for 1 hr by fluor de lys substrate based fluorescence method | Bioorg Med Chem 23: 6785-93 (2015) Article DOI: 10.1016/j.bmc.2015.10.004 BindingDB Entry DOI: 10.7270/Q21N82XF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 8 (Homo sapiens (Human)) | BDBM50129132 (CHEMBL3628319) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.43E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Ronzoni Institute for Chemical and Biochem. Research Curated by ChEMBL | Assay Description Inhibition of HDAC8 (unknown origin) incubated for 1 hr by fluor de lys substrate based fluorescence method | Bioorg Med Chem 23: 6785-93 (2015) Article DOI: 10.1016/j.bmc.2015.10.004 BindingDB Entry DOI: 10.7270/Q21N82XF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50129132 (CHEMBL3628319) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.83E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Ronzoni Institute for Chemical and Biochem. Research Curated by ChEMBL | Assay Description Inhibition of HDAC6 (unknown origin) incubated for 1 hr by fluor de lys substrate based fluorescence method | Bioorg Med Chem 23: 6785-93 (2015) Article DOI: 10.1016/j.bmc.2015.10.004 BindingDB Entry DOI: 10.7270/Q21N82XF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||