 Found 24 hits of Enzyme Inhibition Constant Data
Found 24 hits of Enzyme Inhibition Constant Data Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50149477
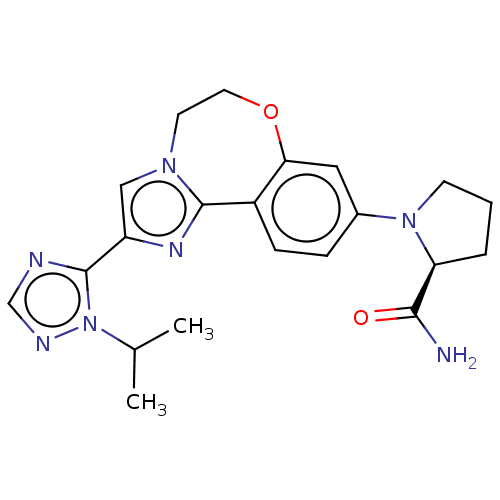
(CHEMBL3770993 | US10851091, U.S. Pat. No. 8,242,10...)Show SMILES CC(C)n1ncnc1-c1cn2CCOc3cc(ccc3-c2n1)N1CCC[C@H]1C(N)=O |r| Show InChI InChI=1S/C21H25N7O2/c1-13(2)28-21(23-12-24-28)16-11-26-8-9-30-18-10-14(5-6-15(18)20(26)25-16)27-7-3-4-17(27)19(22)29/h5-6,10-13,17H,3-4,7-9H2,1-2H3,(H2,22,29)/t17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of recombinant PI3K alpha (unknown origin) using PIP2 as substrate by fluorescence polarization assay |
J Med Chem 59: 985-1002 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01483
BindingDB Entry DOI: 10.7270/Q2QF8VRV |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50149553

(CHEMBL3770306)Show SMILES CC(C)n1ncnc1-c1cn2CCOc3cc(ccc3-c2n1)N1CC[C@H]1C(N)=O |r| Show InChI InChI=1S/C20H23N7O2/c1-12(2)27-20(22-11-23-27)15-10-25-7-8-29-17-9-13(3-4-14(17)19(25)24-15)26-6-5-16(26)18(21)28/h3-4,9-12,16H,5-8H2,1-2H3,(H2,21,28)/t16-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of recombinant PI3K alpha (unknown origin) using PIP2 as substrate by fluorescence polarization assay |
J Med Chem 59: 985-1002 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01483
BindingDB Entry DOI: 10.7270/Q2QF8VRV |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50149482
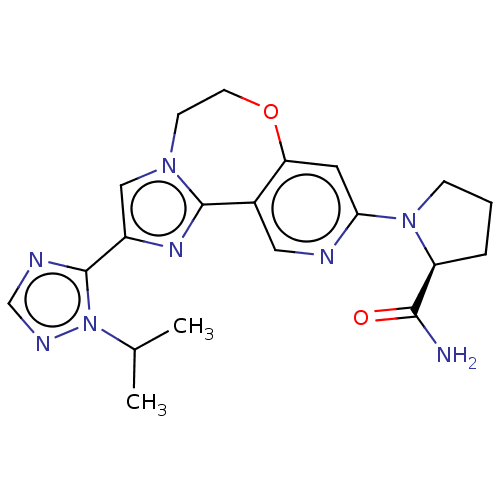
(CHEMBL3770709)Show SMILES CC(C)n1ncnc1-c1cn2CCOc3cc(ncc3-c2n1)N1CCC[C@H]1C(N)=O |r| Show InChI InChI=1S/C20H24N8O2/c1-12(2)28-20(23-11-24-28)14-10-26-6-7-30-16-8-17(22-9-13(16)19(26)25-14)27-5-3-4-15(27)18(21)29/h8-12,15H,3-7H2,1-2H3,(H2,21,29)/t15-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of recombinant PI3K alpha (unknown origin) using PIP2 as substrate by fluorescence polarization assay |
J Med Chem 59: 985-1002 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01483
BindingDB Entry DOI: 10.7270/Q2QF8VRV |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50149548
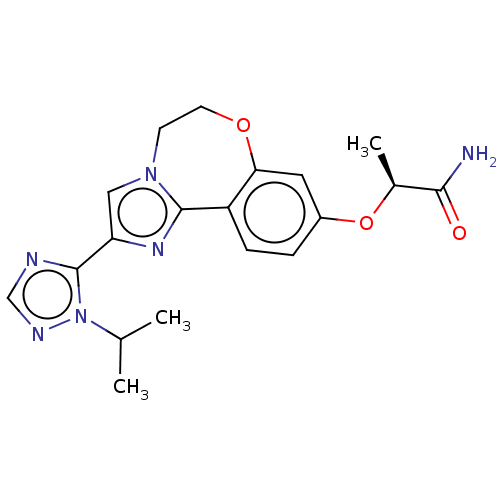
(CHEMBL3771364 | US10851091, U.S. Pat. No. 8,242,10...)Show SMILES CC(C)n1ncnc1-c1cn2CCOc3cc(O[C@@H](C)C(N)=O)ccc3-c2n1 |r| Show InChI InChI=1S/C19H22N6O3/c1-11(2)25-19(21-10-22-25)15-9-24-6-7-27-16-8-13(28-12(3)17(20)26)4-5-14(16)18(24)23-15/h4-5,8-12H,6-7H2,1-3H3,(H2,20,26)/t12-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of recombinant PI3K alpha (unknown origin) using PIP2 as substrate by fluorescence polarization assay |
J Med Chem 59: 985-1002 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01483
BindingDB Entry DOI: 10.7270/Q2QF8VRV |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50149483
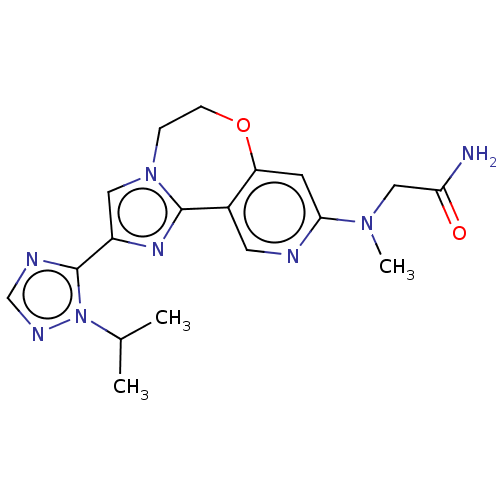
(CHEMBL3770325)Show SMILES CC(C)n1ncnc1-c1cn2CCOc3cc(ncc3-c2n1)N(C)CC(N)=O Show InChI InChI=1S/C18H22N8O2/c1-11(2)26-18(21-10-22-26)13-8-25-4-5-28-14-6-16(24(3)9-15(19)27)20-7-12(14)17(25)23-13/h6-8,10-11H,4-5,9H2,1-3H3,(H2,19,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of recombinant PI3K alpha (unknown origin) using PIP2 as substrate by fluorescence polarization assay |
J Med Chem 59: 985-1002 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01483
BindingDB Entry DOI: 10.7270/Q2QF8VRV |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50434806

(2-(4-(2-(1-isopropyl-3-methyl-1H-1,2,4-triazol-5-y...)Show SMILES CC(C)n1nc(C)nc1-c1cn2CCOc3cc(ccc3-c2n1)-c1cnn(c1)C(C)(C)C(N)=O Show InChI InChI=1S/C24H28N8O2/c1-14(2)32-22(27-15(3)29-32)19-13-30-8-9-34-20-10-16(6-7-18(20)21(30)28-19)17-11-26-31(12-17)24(4,5)23(25)33/h6-7,10-14H,8-9H2,1-5H3,(H2,25,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of recombinant PI3K alpha (unknown origin) using PIP2 as substrate by fluorescence polarization assay |
J Med Chem 59: 985-1002 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01483
BindingDB Entry DOI: 10.7270/Q2QF8VRV |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50149476
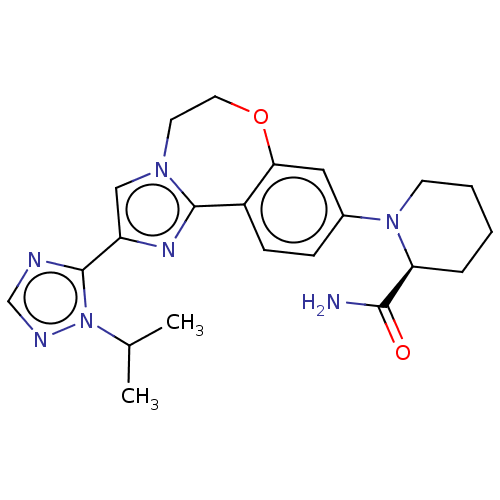
(CHEMBL3770717)Show SMILES CC(C)n1ncnc1-c1cn2CCOc3cc(ccc3-c2n1)N1CCCC[C@H]1C(N)=O |r| Show InChI InChI=1S/C22H27N7O2/c1-14(2)29-22(24-13-25-29)17-12-27-9-10-31-19-11-15(6-7-16(19)21(27)26-17)28-8-4-3-5-18(28)20(23)30/h6-7,11-14,18H,3-5,8-10H2,1-2H3,(H2,23,30)/t18-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of recombinant PI3K alpha (unknown origin) using PIP2 as substrate by fluorescence polarization assay |
J Med Chem 59: 985-1002 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01483
BindingDB Entry DOI: 10.7270/Q2QF8VRV |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50149554
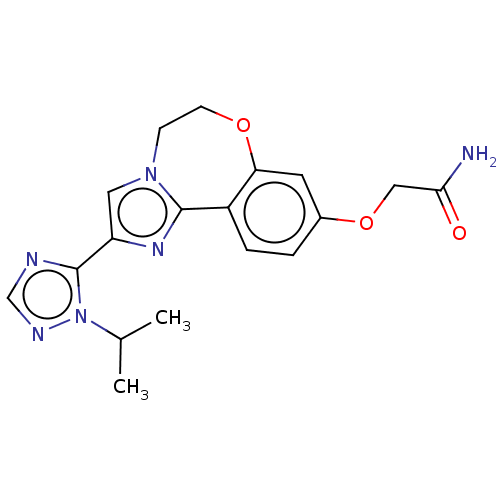
(CHEMBL3770140)Show SMILES CC(C)n1ncnc1-c1cn2CCOc3cc(OCC(N)=O)ccc3-c2n1 Show InChI InChI=1S/C18H20N6O3/c1-11(2)24-18(20-10-21-24)14-8-23-5-6-26-15-7-12(27-9-16(19)25)3-4-13(15)17(23)22-14/h3-4,7-8,10-11H,5-6,9H2,1-2H3,(H2,19,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of recombinant PI3K alpha (unknown origin) using PIP2 as substrate by fluorescence polarization assay |
J Med Chem 59: 985-1002 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01483
BindingDB Entry DOI: 10.7270/Q2QF8VRV |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50149465
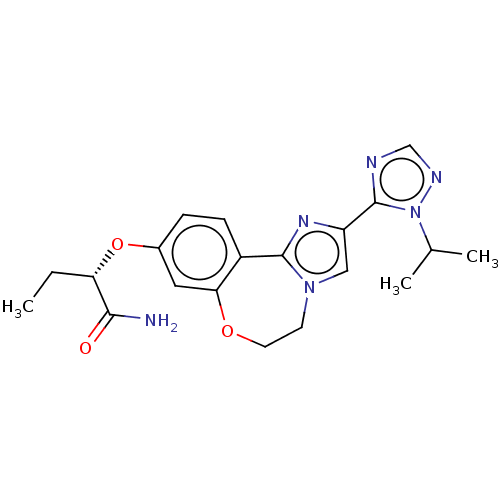
(CHEMBL3769854)Show SMILES CC[C@H](Oc1ccc2-c3nc(cn3CCOc2c1)-c1ncnn1C(C)C)C(N)=O |r| Show InChI InChI=1S/C20H24N6O3/c1-4-16(18(21)27)29-13-5-6-14-17(9-13)28-8-7-25-10-15(24-19(14)25)20-22-11-23-26(20)12(2)3/h5-6,9-12,16H,4,7-8H2,1-3H3,(H2,21,27)/t16-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of recombinant PI3K alpha (unknown origin) using PIP2 as substrate by fluorescence polarization assay |
J Med Chem 59: 985-1002 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01483
BindingDB Entry DOI: 10.7270/Q2QF8VRV |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50149546
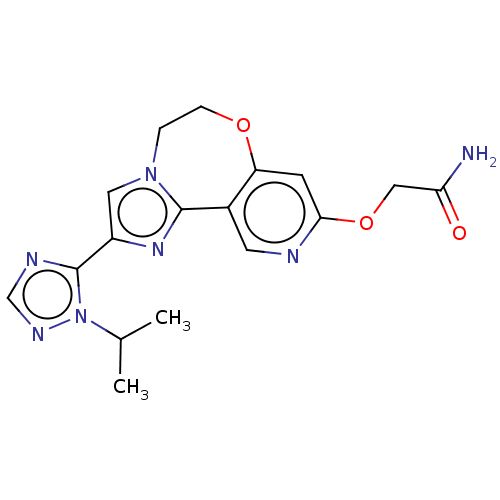
(CHEMBL3769966)Show SMILES CC(C)n1ncnc1-c1cn2CCOc3cc(OCC(N)=O)ncc3-c2n1 Show InChI InChI=1S/C17H19N7O3/c1-10(2)24-17(20-9-21-24)12-7-23-3-4-26-13-5-15(27-8-14(18)25)19-6-11(13)16(23)22-12/h5-7,9-10H,3-4,8H2,1-2H3,(H2,18,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of recombinant PI3K alpha (unknown origin) using PIP2 as substrate by fluorescence polarization assay |
J Med Chem 59: 985-1002 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01483
BindingDB Entry DOI: 10.7270/Q2QF8VRV |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50149550
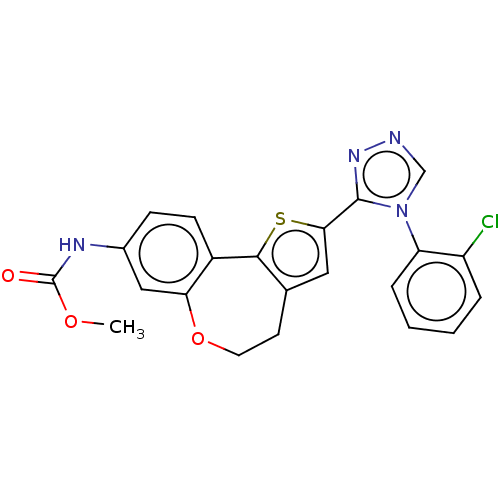
(CHEMBL3770332)Show SMILES COC(=O)Nc1ccc2-c3sc(cc3CCOc2c1)-c1nncn1-c1ccccc1Cl Show InChI InChI=1S/C22H17ClN4O3S/c1-29-22(28)25-14-6-7-15-18(11-14)30-9-8-13-10-19(31-20(13)15)21-26-24-12-27(21)17-5-3-2-4-16(17)23/h2-7,10-12H,8-9H2,1H3,(H,25,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of recombinant PI3K alpha (unknown origin) using PIP2 as substrate by fluorescence polarization assay |
J Med Chem 59: 985-1002 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01483
BindingDB Entry DOI: 10.7270/Q2QF8VRV |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50149545

(CHEMBL3770824)Show SMILES CC(C)n1ncnc1-c1cn2CCOc3cc(O[C@@H](C)C(N)=O)ncc3-c2n1 |r| Show InChI InChI=1S/C18H21N7O3/c1-10(2)25-18(21-9-22-25)13-8-24-4-5-27-14-6-15(28-11(3)16(19)26)20-7-12(14)17(24)23-13/h6-11H,4-5H2,1-3H3,(H2,19,26)/t11-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of recombinant PI3K alpha (unknown origin) using PIP2 as substrate by fluorescence polarization assay |
J Med Chem 59: 985-1002 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01483
BindingDB Entry DOI: 10.7270/Q2QF8VRV |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50149543
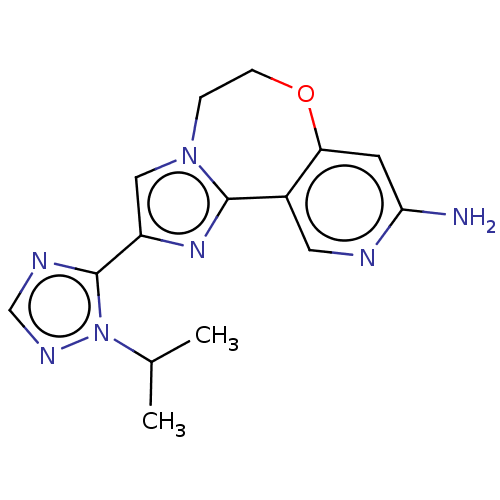
(CHEMBL3770630)Show InChI InChI=1S/C15H17N7O/c1-9(2)22-15(18-8-19-22)11-7-21-3-4-23-12-5-13(16)17-6-10(12)14(21)20-11/h5-9H,3-4H2,1-2H3,(H2,16,17) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of recombinant PI3K alpha (unknown origin) using PIP2 as substrate by fluorescence polarization assay |
J Med Chem 59: 985-1002 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01483
BindingDB Entry DOI: 10.7270/Q2QF8VRV |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50149479
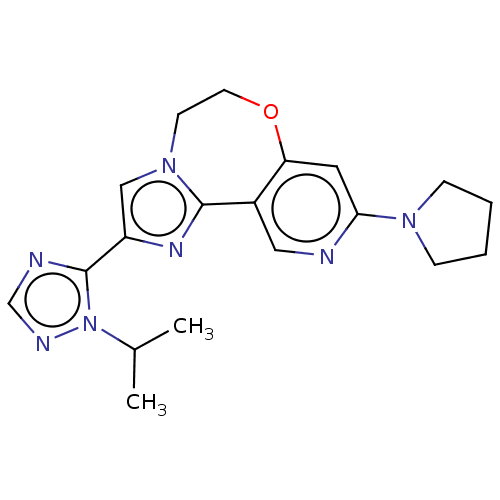
(CHEMBL3770092)Show SMILES CC(C)n1ncnc1-c1cn2CCOc3cc(ncc3-c2n1)N1CCCC1 Show InChI InChI=1S/C19H23N7O/c1-13(2)26-19(21-12-22-26)15-11-25-7-8-27-16-9-17(24-5-3-4-6-24)20-10-14(16)18(25)23-15/h9-13H,3-8H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of recombinant PI3K alpha (unknown origin) using PIP2 as substrate by fluorescence polarization assay |
J Med Chem 59: 985-1002 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01483
BindingDB Entry DOI: 10.7270/Q2QF8VRV |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50149544
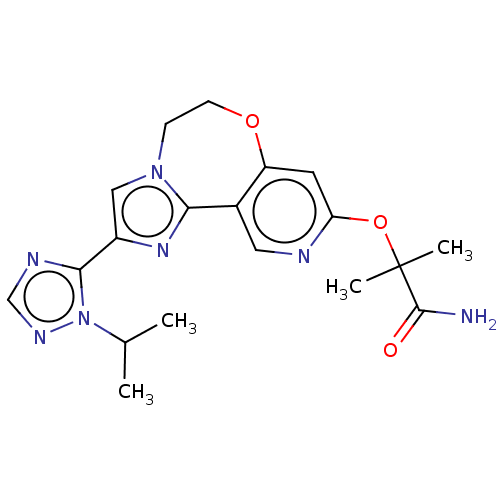
(CHEMBL3769972)Show SMILES CC(C)n1ncnc1-c1cn2CCOc3cc(OC(C)(C)C(N)=O)ncc3-c2n1 Show InChI InChI=1S/C19H23N7O3/c1-11(2)26-17(22-10-23-26)13-9-25-5-6-28-14-7-15(29-19(3,4)18(20)27)21-8-12(14)16(25)24-13/h7-11H,5-6H2,1-4H3,(H2,20,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of recombinant PI3K alpha (unknown origin) using PIP2 as substrate by fluorescence polarization assay |
J Med Chem 59: 985-1002 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01483
BindingDB Entry DOI: 10.7270/Q2QF8VRV |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50149480
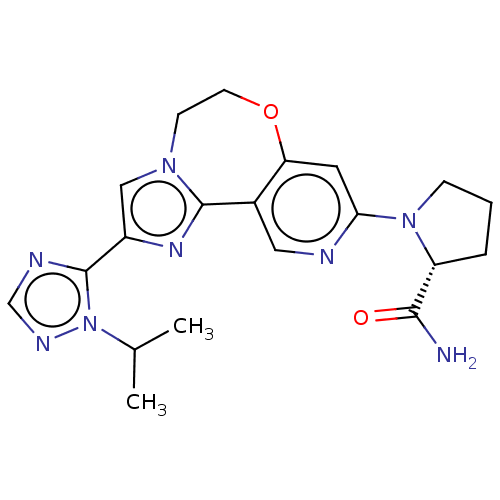
(CHEMBL3769603)Show SMILES CC(C)n1ncnc1-c1cn2CCOc3cc(ncc3-c2n1)N1CCC[C@@H]1C(N)=O |r| Show InChI InChI=1S/C20H24N8O2/c1-12(2)28-20(23-11-24-28)14-10-26-6-7-30-16-8-17(22-9-13(16)19(26)25-14)27-5-3-4-15(27)18(21)29/h8-12,15H,3-7H2,1-2H3,(H2,21,29)/t15-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of recombinant PI3K alpha (unknown origin) using PIP2 as substrate by fluorescence polarization assay |
J Med Chem 59: 985-1002 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01483
BindingDB Entry DOI: 10.7270/Q2QF8VRV |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50149475
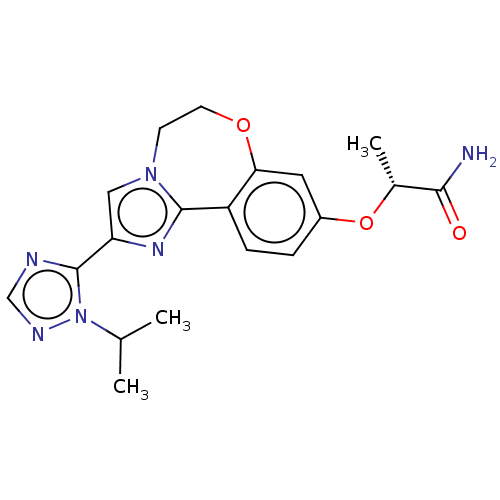
(CHEMBL3770644)Show SMILES CC(C)n1ncnc1-c1cn2CCOc3cc(O[C@H](C)C(N)=O)ccc3-c2n1 |r| Show InChI InChI=1S/C19H22N6O3/c1-11(2)25-19(21-10-22-25)15-9-24-6-7-27-16-8-13(28-12(3)17(20)26)4-5-14(16)18(24)23-15/h4-5,8-12H,6-7H2,1-3H3,(H2,20,26)/t12-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of recombinant PI3K alpha (unknown origin) using PIP2 as substrate by fluorescence polarization assay |
J Med Chem 59: 985-1002 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01483
BindingDB Entry DOI: 10.7270/Q2QF8VRV |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50149551
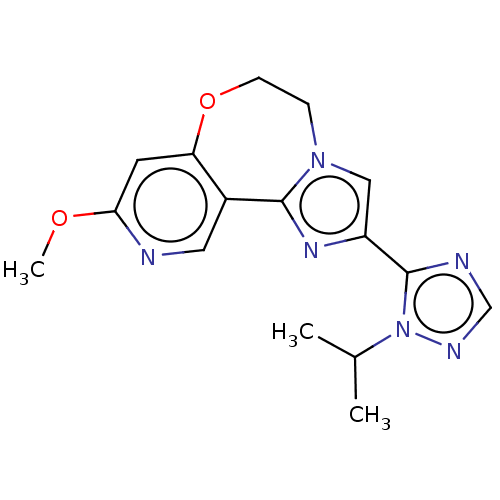
(CHEMBL3770615)Show InChI InChI=1S/C16H18N6O2/c1-10(2)22-16(18-9-19-22)12-8-21-4-5-24-13-6-14(23-3)17-7-11(13)15(21)20-12/h6-10H,4-5H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of recombinant PI3K alpha (unknown origin) using PIP2 as substrate by fluorescence polarization assay |
J Med Chem 59: 985-1002 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01483
BindingDB Entry DOI: 10.7270/Q2QF8VRV |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50149478
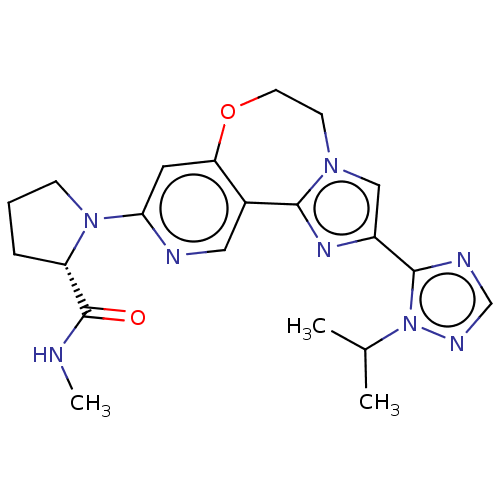
(CHEMBL3769857)Show SMILES CNC(=O)[C@@H]1CCCN1c1cc2OCCn3cc(nc3-c2cn1)-c1ncnn1C(C)C |r| Show InChI InChI=1S/C21H26N8O2/c1-13(2)29-20(24-12-25-29)15-11-27-7-8-31-17-9-18(23-10-14(17)19(27)26-15)28-6-4-5-16(28)21(30)22-3/h9-13,16H,4-8H2,1-3H3,(H,22,30)/t16-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of recombinant PI3K alpha (unknown origin) using PIP2 as substrate by fluorescence polarization assay |
J Med Chem 59: 985-1002 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01483
BindingDB Entry DOI: 10.7270/Q2QF8VRV |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50149548

(CHEMBL3771364 | US10851091, U.S. Pat. No. 8,242,10...)Show SMILES CC(C)n1ncnc1-c1cn2CCOc3cc(O[C@@H](C)C(N)=O)ccc3-c2n1 |r| Show InChI InChI=1S/C19H22N6O3/c1-11(2)25-19(21-10-22-25)15-9-24-6-7-27-16-8-13(28-12(3)17(20)26)4-5-14(16)18(24)23-15/h4-5,8-12H,6-7H2,1-3H3,(H2,20,26)/t12-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
J Med Chem 59: 985-1002 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01483
BindingDB Entry DOI: 10.7270/Q2QF8VRV |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50149548

(CHEMBL3771364 | US10851091, U.S. Pat. No. 8,242,10...)Show SMILES CC(C)n1ncnc1-c1cn2CCOc3cc(O[C@@H](C)C(N)=O)ccc3-c2n1 |r| Show InChI InChI=1S/C19H22N6O3/c1-11(2)25-19(21-10-22-25)15-9-24-6-7-27-16-8-13(28-12(3)17(20)26)4-5-14(16)18(24)23-15/h4-5,8-12H,6-7H2,1-3H3,(H2,20,26)/t12-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C19 (unknown origin) |
J Med Chem 59: 985-1002 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01483
BindingDB Entry DOI: 10.7270/Q2QF8VRV |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50149548

(CHEMBL3771364 | US10851091, U.S. Pat. No. 8,242,10...)Show SMILES CC(C)n1ncnc1-c1cn2CCOc3cc(O[C@@H](C)C(N)=O)ccc3-c2n1 |r| Show InChI InChI=1S/C19H22N6O3/c1-11(2)25-19(21-10-22-25)15-9-24-6-7-27-16-8-13(28-12(3)17(20)26)4-5-14(16)18(24)23-15/h4-5,8-12H,6-7H2,1-3H3,(H2,20,26)/t12-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of CYP1A2 (unknown origin) |
J Med Chem 59: 985-1002 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01483
BindingDB Entry DOI: 10.7270/Q2QF8VRV |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50149548

(CHEMBL3771364 | US10851091, U.S. Pat. No. 8,242,10...)Show SMILES CC(C)n1ncnc1-c1cn2CCOc3cc(O[C@@H](C)C(N)=O)ccc3-c2n1 |r| Show InChI InChI=1S/C19H22N6O3/c1-11(2)25-19(21-10-22-25)15-9-24-6-7-27-16-8-13(28-12(3)17(20)26)4-5-14(16)18(24)23-15/h4-5,8-12H,6-7H2,1-3H3,(H2,20,26)/t12-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 (unknown origin) |
J Med Chem 59: 985-1002 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01483
BindingDB Entry DOI: 10.7270/Q2QF8VRV |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50149548

(CHEMBL3771364 | US10851091, U.S. Pat. No. 8,242,10...)Show SMILES CC(C)n1ncnc1-c1cn2CCOc3cc(O[C@@H](C)C(N)=O)ccc3-c2n1 |r| Show InChI InChI=1S/C19H22N6O3/c1-11(2)25-19(21-10-22-25)15-9-24-6-7-27-16-8-13(28-12(3)17(20)26)4-5-14(16)18(24)23-15/h4-5,8-12H,6-7H2,1-3H3,(H2,20,26)/t12-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 (unknown origin) |
J Med Chem 59: 985-1002 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01483
BindingDB Entry DOI: 10.7270/Q2QF8VRV |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data