

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50149592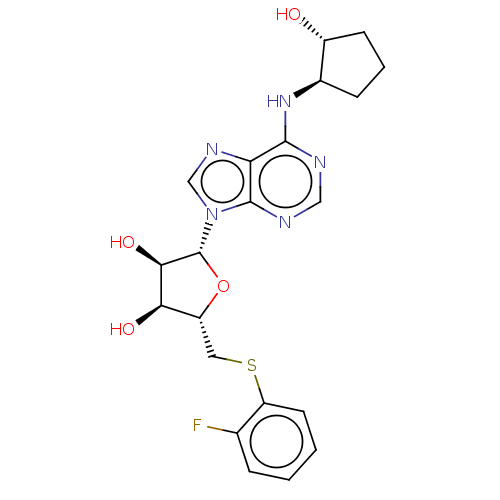 (CVT-3619 | GS-9667) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 113 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Warwick Curated by ChEMBL | Assay Description Displacement of [3H]CPX from human Adenosine A1 receptor expressed in DDT1MF-2 cells | J Med Chem 59: 947-64 (2016) Article DOI: 10.1021/acs.jmedchem.5b01402 BindingDB Entry DOI: 10.7270/Q2FX7CBN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50149592 (CVT-3619 | GS-9667) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Warwick Curated by ChEMBL | Assay Description Displacement of [3H]CPX from human Adenosine A1 receptor expressed in CHO cells | J Med Chem 59: 947-64 (2016) Article DOI: 10.1021/acs.jmedchem.5b01402 BindingDB Entry DOI: 10.7270/Q2FX7CBN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50149598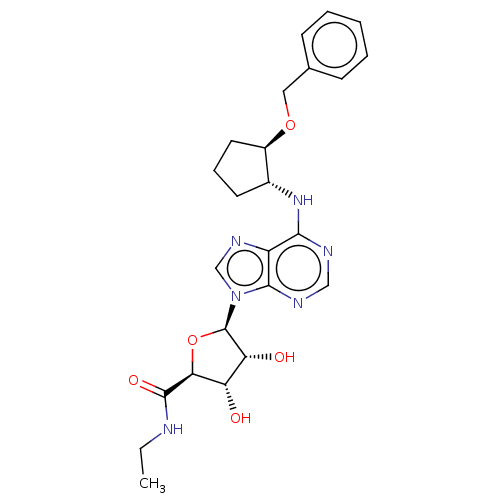 (CHEMBL3770679) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.0295 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Warwick Curated by ChEMBL | Assay Description Agonist activity at human Adenosine A1 receptor transfected in CHO-K1 cells assessed as inhibition of forskolin-stimulated cAMP production after 30 m... | J Med Chem 59: 947-64 (2016) Article DOI: 10.1021/acs.jmedchem.5b01402 BindingDB Entry DOI: 10.7270/Q2FX7CBN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM21220 ((2S,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-N-ethyl-3,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 0.209 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Warwick Curated by ChEMBL | Assay Description Agonist activity at human Adenosine A1 receptor transfected in CHO-K1 cells assessed as inhibition of forskolin-stimulated cAMP production after 30 m... | J Med Chem 59: 947-64 (2016) Article DOI: 10.1021/acs.jmedchem.5b01402 BindingDB Entry DOI: 10.7270/Q2FX7CBN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50149597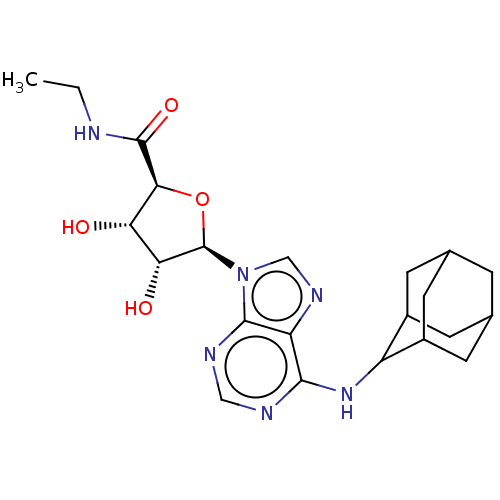 (CHEMBL3771184) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.398 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Warwick Curated by ChEMBL | Assay Description Agonist activity at human Adenosine A1 receptor transfected in CHO-K1 cells assessed as inhibition of forskolin-stimulated cAMP production after 30 m... | J Med Chem 59: 947-64 (2016) Article DOI: 10.1021/acs.jmedchem.5b01402 BindingDB Entry DOI: 10.7270/Q2FX7CBN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM21220 ((2S,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-N-ethyl-3,...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 0.490 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Warwick Curated by ChEMBL | Assay Description Agonist activity at human Adenosine A3 receptor transfected in CHO-K1 cells assessed as inhibition of forskolin-stimulated cAMP production after 30 m... | J Med Chem 59: 947-64 (2016) Article DOI: 10.1021/acs.jmedchem.5b01402 BindingDB Entry DOI: 10.7270/Q2FX7CBN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50085658 ((2R,3R,4S,5R)-2-(2-Chloro-6-cyclopentylamino-purin...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.501 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Warwick Curated by ChEMBL | Assay Description Agonist activity at human Adenosine A1 receptor transfected in CHO-K1 cells assessed as inhibition of forskolin-stimulated cAMP production after 30 m... | J Med Chem 59: 947-64 (2016) Article DOI: 10.1021/acs.jmedchem.5b01402 BindingDB Entry DOI: 10.7270/Q2FX7CBN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50149594 (CHEMBL3771208) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.676 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Warwick Curated by ChEMBL | Assay Description Agonist activity at human Adenosine A1 receptor transfected in CHO-K1 cells assessed as inhibition of forskolin-stimulated cAMP production after 30 m... | J Med Chem 59: 947-64 (2016) Article DOI: 10.1021/acs.jmedchem.5b01402 BindingDB Entry DOI: 10.7270/Q2FX7CBN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM14487 ((2R,3R,4S,5R)-2-(6-amino-9H-purin-9-yl)-5-(hydroxy...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Similars | DrugBank Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Warwick Curated by ChEMBL | Assay Description Agonist activity at human Adenosine A3 receptor transfected in CHO-K1 cells assessed as inhibition of forskolin-stimulated cAMP production after 30 m... | J Med Chem 59: 947-64 (2016) Article DOI: 10.1021/acs.jmedchem.5b01402 BindingDB Entry DOI: 10.7270/Q2FX7CBN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50149599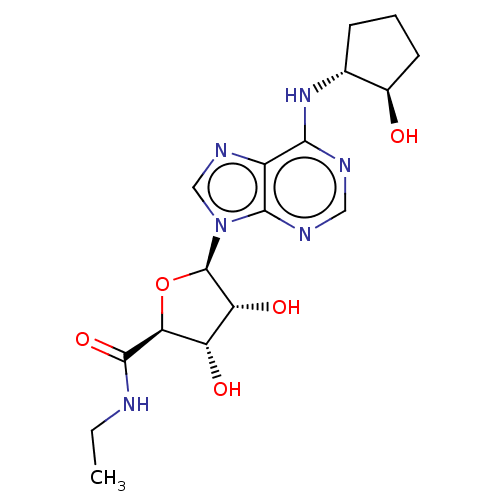 (CHEMBL3770310) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Warwick Curated by ChEMBL | Assay Description Agonist activity at human Adenosine A3 receptor transfected in CHO-K1 cells assessed as inhibition of forskolin-stimulated cAMP production after 30 m... | J Med Chem 59: 947-64 (2016) Article DOI: 10.1021/acs.jmedchem.5b01402 BindingDB Entry DOI: 10.7270/Q2FX7CBN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM14487 ((2R,3R,4S,5R)-2-(6-amino-9H-purin-9-yl)-5-(hydroxy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Similars | DrugBank PDB Article PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Warwick Curated by ChEMBL | Assay Description Agonist activity at human Adenosine A1 receptor transfected in CHO-K1 cells assessed as inhibition of forskolin-stimulated cAMP production after 30 m... | J Med Chem 59: 947-64 (2016) Article DOI: 10.1021/acs.jmedchem.5b01402 BindingDB Entry DOI: 10.7270/Q2FX7CBN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50149595 (CHEMBL3770664) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Warwick Curated by ChEMBL | Assay Description Agonist activity at human Adenosine A1 receptor transfected in CHO-K1 cells assessed as inhibition of forskolin-stimulated cAMP production after 30 m... | J Med Chem 59: 947-64 (2016) Article DOI: 10.1021/acs.jmedchem.5b01402 BindingDB Entry DOI: 10.7270/Q2FX7CBN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50080399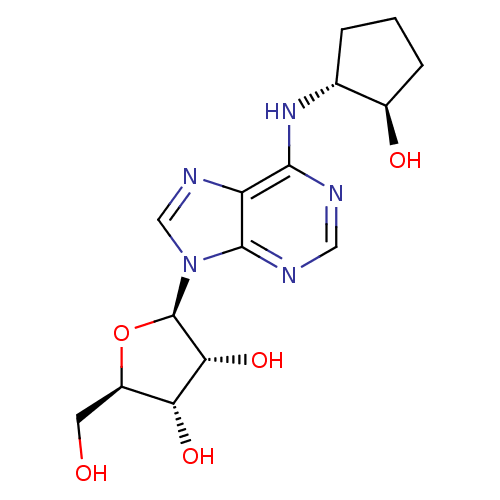 ((2R,3R,4S,5R)-2-[6-((1R,2R)-2-Hydroxy-cyclopentyla...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Warwick Curated by ChEMBL | Assay Description Agonist activity at human Adenosine A1 receptor transfected in CHO-K1 cells assessed as inhibition of forskolin-stimulated cAMP production after 30 m... | J Med Chem 59: 947-64 (2016) Article DOI: 10.1021/acs.jmedchem.5b01402 BindingDB Entry DOI: 10.7270/Q2FX7CBN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50149599 (CHEMBL3770310) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6.20 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Warwick Curated by ChEMBL | Assay Description Agonist activity at human Adenosine A1 receptor transfected in CHO-K1 cells assessed as inhibition of forskolin-stimulated cAMP production after 30 m... | J Med Chem 59: 947-64 (2016) Article DOI: 10.1021/acs.jmedchem.5b01402 BindingDB Entry DOI: 10.7270/Q2FX7CBN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50085658 ((2R,3R,4S,5R)-2-(2-Chloro-6-cyclopentylamino-purin...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Warwick Curated by ChEMBL | Assay Description Agonist activity at human Adenosine A3 receptor transfected in CHO-K1 cells assessed as inhibition of forskolin-stimulated cAMP production after 30 m... | J Med Chem 59: 947-64 (2016) Article DOI: 10.1021/acs.jmedchem.5b01402 BindingDB Entry DOI: 10.7270/Q2FX7CBN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50149593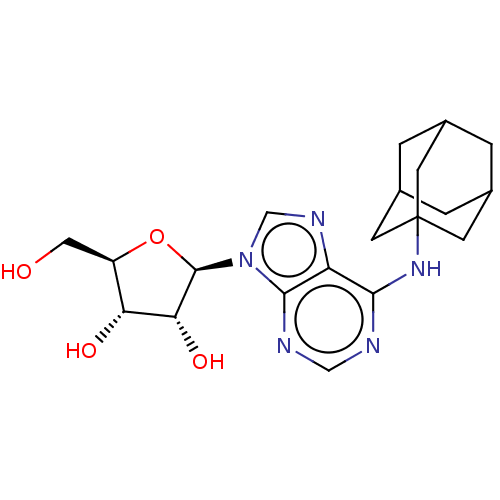 (CHEMBL3771290) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Warwick Curated by ChEMBL | Assay Description Agonist activity at human Adenosine A1 receptor transfected in CHO-K1 cells assessed as inhibition of forskolin-stimulated cAMP production after 30 m... | J Med Chem 59: 947-64 (2016) Article DOI: 10.1021/acs.jmedchem.5b01402 BindingDB Entry DOI: 10.7270/Q2FX7CBN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50080399 ((2R,3R,4S,5R)-2-[6-((1R,2R)-2-Hydroxy-cyclopentyla...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Warwick Curated by ChEMBL | Assay Description Agonist activity at human Adenosine A3 receptor transfected in CHO-K1 cells assessed as inhibition of forskolin-stimulated cAMP production after 30 m... | J Med Chem 59: 947-64 (2016) Article DOI: 10.1021/acs.jmedchem.5b01402 BindingDB Entry DOI: 10.7270/Q2FX7CBN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50149596 (CHEMBL3769641) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Warwick Curated by ChEMBL | Assay Description Agonist activity at human Adenosine A1 receptor transfected in CHO-K1 cells assessed as inhibition of forskolin-stimulated cAMP production after 30 m... | J Med Chem 59: 947-64 (2016) Article DOI: 10.1021/acs.jmedchem.5b01402 BindingDB Entry DOI: 10.7270/Q2FX7CBN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50149596 (CHEMBL3769641) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 79 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Warwick Curated by ChEMBL | Assay Description Agonist activity at human Adenosine A3 receptor transfected in CHO-K1 cells assessed as inhibition of forskolin-stimulated cAMP production after 30 m... | J Med Chem 59: 947-64 (2016) Article DOI: 10.1021/acs.jmedchem.5b01402 BindingDB Entry DOI: 10.7270/Q2FX7CBN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50149598 (CHEMBL3770679) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 269 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Warwick Curated by ChEMBL | Assay Description Agonist activity at human Adenosine A3 receptor transfected in CHO-K1 cells assessed as inhibition of forskolin-stimulated cAMP production after 30 m... | J Med Chem 59: 947-64 (2016) Article DOI: 10.1021/acs.jmedchem.5b01402 BindingDB Entry DOI: 10.7270/Q2FX7CBN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50149599 (CHEMBL3770310) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 5.75E+3 | n/a | n/a | n/a | n/a |
University of Warwick Curated by ChEMBL | Assay Description Agonist activity at human Adenosine A2A receptor expressed in yeast cells coexpressed with chimeric GPA1/Galphas after 16 hrs by beta-galactosidase r... | J Med Chem 59: 947-64 (2016) Article DOI: 10.1021/acs.jmedchem.5b01402 BindingDB Entry DOI: 10.7270/Q2FX7CBN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50080399 ((2R,3R,4S,5R)-2-[6-((1R,2R)-2-Hydroxy-cyclopentyla...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 631 | n/a | n/a | n/a | n/a |
University of Warwick Curated by ChEMBL | Assay Description Agonist activity at human Adenosine A1 receptor expressed in yeast cells coexpressed with chimeric GPA1/Galphai1 after 16 hrs by beta galactosidase r... | J Med Chem 59: 947-64 (2016) Article DOI: 10.1021/acs.jmedchem.5b01402 BindingDB Entry DOI: 10.7270/Q2FX7CBN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50149596 (CHEMBL3769641) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 1.00E+5 | n/a | n/a | n/a | n/a |
University of Warwick Curated by ChEMBL | Assay Description Agonist activity at human Adenosine A1 receptor expressed in yeast cells coexpressed with chimeric GPA1/Galphai1 after 16 hrs by beta galactosidase r... | J Med Chem 59: 947-64 (2016) Article DOI: 10.1021/acs.jmedchem.5b01402 BindingDB Entry DOI: 10.7270/Q2FX7CBN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM14487 ((2R,3R,4S,5R)-2-(6-amino-9H-purin-9-yl)-5-(hydroxy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Similars | DrugBank PDB Article PubMed | n/a | n/a | n/a | n/a | 2.51E+3 | n/a | n/a | n/a | n/a |
University of Warwick Curated by ChEMBL | Assay Description Agonist activity at human Adenosine A1 receptor expressed in yeast cells coexpressed with chimeric GPA1/Galphai1 after 16 hrs by beta galactosidase r... | J Med Chem 59: 947-64 (2016) Article DOI: 10.1021/acs.jmedchem.5b01402 BindingDB Entry DOI: 10.7270/Q2FX7CBN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM14487 ((2R,3R,4S,5R)-2-(6-amino-9H-purin-9-yl)-5-(hydroxy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Similars | DrugBank PDB Article PubMed | n/a | n/a | n/a | n/a | 5.75E+3 | n/a | n/a | n/a | n/a |
University of Warwick Curated by ChEMBL | Assay Description Agonist activity at human Adenosine A2A receptor expressed in yeast cells coexpressed with chimeric GPA1/Galphas after 16 hrs by beta-galactosidase r... | J Med Chem 59: 947-64 (2016) Article DOI: 10.1021/acs.jmedchem.5b01402 BindingDB Entry DOI: 10.7270/Q2FX7CBN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50149599 (CHEMBL3770310) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 316 | n/a | n/a | n/a | n/a |
University of Warwick Curated by ChEMBL | Assay Description Agonist activity at human Adenosine A1 receptor expressed in yeast cells coexpressed with chimeric GPA1/Galphai1 after 16 hrs by beta galactosidase r... | J Med Chem 59: 947-64 (2016) Article DOI: 10.1021/acs.jmedchem.5b01402 BindingDB Entry DOI: 10.7270/Q2FX7CBN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM21220 ((2S,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-N-ethyl-3,...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | n/a | n/a | 339 | n/a | n/a | n/a | n/a |
University of Warwick Curated by ChEMBL | Assay Description Agonist activity at human Adenosine A2A receptor expressed in yeast cells coexpressed with chimeric GPA1/Galphas after 16 hrs by beta-galactosidase r... | J Med Chem 59: 947-64 (2016) Article DOI: 10.1021/acs.jmedchem.5b01402 BindingDB Entry DOI: 10.7270/Q2FX7CBN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50149598 (CHEMBL3770679) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a |
University of Warwick Curated by ChEMBL | Assay Description Agonist activity at human Adenosine A1 receptor expressed in yeast cells coexpressed with chimeric GPA1/Galphai1 after 16 hrs by beta galactosidase r... | J Med Chem 59: 947-64 (2016) Article DOI: 10.1021/acs.jmedchem.5b01402 BindingDB Entry DOI: 10.7270/Q2FX7CBN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50149593 (CHEMBL3771290) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.58E+4 | n/a | n/a | n/a | n/a |
University of Warwick Curated by ChEMBL | Assay Description Agonist activity at human Adenosine A1 receptor expressed in yeast cells coexpressed with chimeric GPA1/Galphai1 after 16 hrs by beta galactosidase r... | J Med Chem 59: 947-64 (2016) Article DOI: 10.1021/acs.jmedchem.5b01402 BindingDB Entry DOI: 10.7270/Q2FX7CBN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM35804 ((CGS21680) 3-(4-{2-[6-Amino-9-(5-ethylcarbamoylmet...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 6.31E+5 | n/a | n/a | n/a | n/a |
University of Warwick Curated by ChEMBL | Assay Description Agonist activity at human Adenosine A1 receptor expressed in yeast cells coexpressed with chimeric GPA1/Galphai1 after 16 hrs by beta galactosidase r... | J Med Chem 59: 947-64 (2016) Article DOI: 10.1021/acs.jmedchem.5b01402 BindingDB Entry DOI: 10.7270/Q2FX7CBN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2b (Homo sapiens (Human)) | BDBM50149598 (CHEMBL3770679) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.26E+5 | n/a | n/a | n/a | n/a |
University of Warwick Curated by ChEMBL | Assay Description Agonist activity at human Adenosine A2B receptor expressed in yeast cells coexpressed with chimeric GPA1/Galphas after 16 hrs by beta-galactosidase r... | J Med Chem 59: 947-64 (2016) Article DOI: 10.1021/acs.jmedchem.5b01402 BindingDB Entry DOI: 10.7270/Q2FX7CBN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2b (Homo sapiens (Human)) | BDBM50085658 ((2R,3R,4S,5R)-2-(2-Chloro-6-cyclopentylamino-purin...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 7.24E+4 | n/a | n/a | n/a | n/a |
University of Warwick Curated by ChEMBL | Assay Description Agonist activity at human Adenosine A2B receptor expressed in yeast cells coexpressed with chimeric GPA1/Galphas after 16 hrs by beta-galactosidase r... | J Med Chem 59: 947-64 (2016) Article DOI: 10.1021/acs.jmedchem.5b01402 BindingDB Entry DOI: 10.7270/Q2FX7CBN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM35804 ((CGS21680) 3-(4-{2-[6-Amino-9-(5-ethylcarbamoylmet...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a |
University of Warwick Curated by ChEMBL | Assay Description Agonist activity at human Adenosine A2A receptor expressed in yeast cells coexpressed with chimeric GPA1/Galphas after 16 hrs by beta-galactosidase r... | J Med Chem 59: 947-64 (2016) Article DOI: 10.1021/acs.jmedchem.5b01402 BindingDB Entry DOI: 10.7270/Q2FX7CBN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50085658 ((2R,3R,4S,5R)-2-(2-Chloro-6-cyclopentylamino-purin...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a |
University of Warwick Curated by ChEMBL | Assay Description Agonist activity at human Adenosine A2A receptor expressed in yeast cells coexpressed with chimeric GPA1/Galphas after 16 hrs by beta-galactosidase r... | J Med Chem 59: 947-64 (2016) Article DOI: 10.1021/acs.jmedchem.5b01402 BindingDB Entry DOI: 10.7270/Q2FX7CBN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM21220 ((2S,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-N-ethyl-3,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a |
University of Warwick Curated by ChEMBL | Assay Description Agonist activity at human Adenosine A1 receptor expressed in yeast cells coexpressed with chimeric GPA1/Galphai1 after 16 hrs by beta galactosidase r... | J Med Chem 59: 947-64 (2016) Article DOI: 10.1021/acs.jmedchem.5b01402 BindingDB Entry DOI: 10.7270/Q2FX7CBN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2b (Homo sapiens (Human)) | BDBM50149599 (CHEMBL3770310) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 3.31E+5 | n/a | n/a | n/a | n/a |
University of Warwick Curated by ChEMBL | Assay Description Agonist activity at human Adenosine A2B receptor expressed in yeast cells coexpressed with chimeric GPA1/Galphas after 16 hrs by beta-galactosidase r... | J Med Chem 59: 947-64 (2016) Article DOI: 10.1021/acs.jmedchem.5b01402 BindingDB Entry DOI: 10.7270/Q2FX7CBN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2b (Homo sapiens (Human)) | BDBM35804 ((CGS21680) 3-(4-{2-[6-Amino-9-(5-ethylcarbamoylmet...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 6.31E+6 | n/a | n/a | n/a | n/a |
University of Warwick Curated by ChEMBL | Assay Description Agonist activity at human Adenosine A2B receptor expressed in yeast cells coexpressed with chimeric GPA1/Galphas after 16 hrs by beta-galactosidase r... | J Med Chem 59: 947-64 (2016) Article DOI: 10.1021/acs.jmedchem.5b01402 BindingDB Entry DOI: 10.7270/Q2FX7CBN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2b (Homo sapiens (Human)) | BDBM14487 ((2R,3R,4S,5R)-2-(6-amino-9H-purin-9-yl)-5-(hydroxy...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Similars | DrugBank PDB Article PubMed | n/a | n/a | n/a | n/a | 5.13E+4 | n/a | n/a | n/a | n/a |
University of Warwick Curated by ChEMBL | Assay Description Agonist activity at human Adenosine A2B receptor expressed in yeast cells coexpressed with chimeric GPA1/Galphas after 16 hrs by beta-galactosidase r... | J Med Chem 59: 947-64 (2016) Article DOI: 10.1021/acs.jmedchem.5b01402 BindingDB Entry DOI: 10.7270/Q2FX7CBN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50149597 (CHEMBL3771184) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.58E+4 | n/a | n/a | n/a | n/a |
University of Warwick Curated by ChEMBL | Assay Description Agonist activity at human Adenosine A1 receptor expressed in yeast cells coexpressed with chimeric GPA1/Galphai1 after 16 hrs by beta galactosidase r... | J Med Chem 59: 947-64 (2016) Article DOI: 10.1021/acs.jmedchem.5b01402 BindingDB Entry DOI: 10.7270/Q2FX7CBN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50085658 ((2R,3R,4S,5R)-2-(2-Chloro-6-cyclopentylamino-purin...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 200 | n/a | n/a | n/a | n/a |
University of Warwick Curated by ChEMBL | Assay Description Agonist activity at human Adenosine A1 receptor expressed in yeast cells coexpressed with chimeric GPA1/Galphai1 after 16 hrs by beta galactosidase r... | J Med Chem 59: 947-64 (2016) Article DOI: 10.1021/acs.jmedchem.5b01402 BindingDB Entry DOI: 10.7270/Q2FX7CBN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2b (Homo sapiens (Human)) | BDBM21220 ((2S,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-N-ethyl-3,...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | n/a | n/a | 1.48E+4 | n/a | n/a | n/a | n/a |
University of Warwick Curated by ChEMBL | Assay Description Agonist activity at human Adenosine A2B receptor expressed in yeast cells coexpressed with chimeric GPA1/Galphas after 16 hrs by beta-galactosidase r... | J Med Chem 59: 947-64 (2016) Article DOI: 10.1021/acs.jmedchem.5b01402 BindingDB Entry DOI: 10.7270/Q2FX7CBN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50149598 (CHEMBL3770679) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.58E+4 | n/a | n/a | n/a | n/a |
University of Warwick Curated by ChEMBL | Assay Description Agonist activity at human Adenosine A2A receptor expressed in yeast cells coexpressed with chimeric GPA1/Galphas after 16 hrs by beta-galactosidase r... | J Med Chem 59: 947-64 (2016) Article DOI: 10.1021/acs.jmedchem.5b01402 BindingDB Entry DOI: 10.7270/Q2FX7CBN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50149594 (CHEMBL3771208) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.59E+3 | n/a | n/a | n/a | n/a |
University of Warwick Curated by ChEMBL | Assay Description Agonist activity at human Adenosine A1 receptor expressed in yeast cells coexpressed with chimeric GPA1/Galphai1 after 16 hrs by beta galactosidase r... | J Med Chem 59: 947-64 (2016) Article DOI: 10.1021/acs.jmedchem.5b01402 BindingDB Entry DOI: 10.7270/Q2FX7CBN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50149595 (CHEMBL3770664) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 3.98E+3 | n/a | n/a | n/a | n/a |
University of Warwick Curated by ChEMBL | Assay Description Agonist activity at human Adenosine A1 receptor expressed in yeast cells coexpressed with chimeric GPA1/Galphai1 after 16 hrs by beta galactosidase r... | J Med Chem 59: 947-64 (2016) Article DOI: 10.1021/acs.jmedchem.5b01402 BindingDB Entry DOI: 10.7270/Q2FX7CBN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||