 Found 57 hits of Enzyme Inhibition Constant Data
Found 57 hits of Enzyme Inhibition Constant Data Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50149902
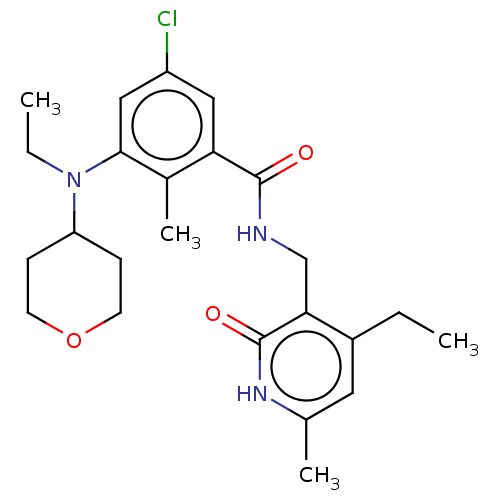
(CHEMBL3771372)Show SMILES CCN(C1CCOCC1)c1cc(Cl)cc(C(=O)NCc2c(CC)cc(C)[nH]c2=O)c1C Show InChI InChI=1S/C24H32ClN3O3/c1-5-17-11-15(3)27-24(30)21(17)14-26-23(29)20-12-18(25)13-22(16(20)4)28(6-2)19-7-9-31-10-8-19/h11-13,19H,5-10,14H2,1-4H3,(H,26,29)(H,27,30) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme
Curated by ChEMBL
| Assay Description
Inhibition of methyltransferase activity of human EZH2 using chicken oligonucleotide as substrate by pull down assay |
J Med Chem 59: 1556-64 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01501
BindingDB Entry DOI: 10.7270/Q2B56MM7 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50149889

(CHEMBL3769571)Show SMILES CCN(C1CCOCC1)c1cc(Cl)cc(C(=O)NCc2c(C)cc(C)[nH]c2=O)c1C Show InChI InChI=1S/C23H30ClN3O3/c1-5-27(18-6-8-30-9-7-18)21-12-17(24)11-19(16(21)4)22(28)25-13-20-14(2)10-15(3)26-23(20)29/h10-12,18H,5-9,13H2,1-4H3,(H,25,28)(H,26,29) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme
Curated by ChEMBL
| Assay Description
Inhibition of methyltransferase activity of human EZH2 using chicken oligonucleotide as substrate by pull down assay |
J Med Chem 59: 1556-64 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01501
BindingDB Entry DOI: 10.7270/Q2B56MM7 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50149895

(CHEMBL3770000)Show SMILES CCN(C1CCOCC1)c1cc(Br)cc(C(=O)NCc2c(C)cc(C)[nH]c2=O)c1C Show InChI InChI=1S/C23H30BrN3O3/c1-5-27(18-6-8-30-9-7-18)21-12-17(24)11-19(16(21)4)22(28)25-13-20-14(2)10-15(3)26-23(20)29/h10-12,18H,5-9,13H2,1-4H3,(H,25,28)(H,26,29) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme
Curated by ChEMBL
| Assay Description
Inhibition of methyltransferase activity of human EZH2 using chicken oligonucleotide as substrate by pull down assay |
J Med Chem 59: 1556-64 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01501
BindingDB Entry DOI: 10.7270/Q2B56MM7 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM190198
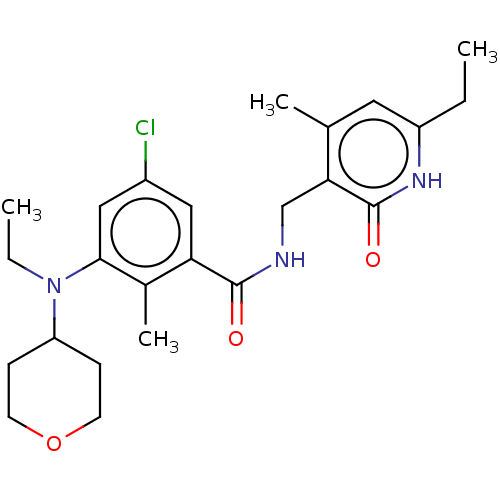
(EPZ008279 | US9175331, 27)Show SMILES CCN(C1CCOCC1)c1cc(Cl)cc(C(=O)NCc2c(C)cc(CC)[nH]c2=O)c1C Show InChI InChI=1S/C24H32ClN3O3/c1-5-18-11-15(3)21(24(30)27-18)14-26-23(29)20-12-17(25)13-22(16(20)4)28(6-2)19-7-9-31-10-8-19/h11-13,19H,5-10,14H2,1-4H3,(H,26,29)(H,27,30) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme
Curated by ChEMBL
| Assay Description
Inhibition of methyltransferase activity of human EZH2 using chicken oligonucleotide as substrate by pull down assay |
J Med Chem 59: 1556-64 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01501
BindingDB Entry DOI: 10.7270/Q2B56MM7 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50149884
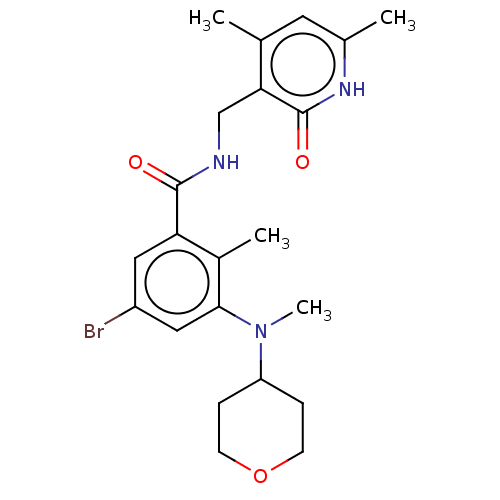
(CHEMBL3770973)Show SMILES CN(C1CCOCC1)c1cc(Br)cc(C(=O)NCc2c(C)cc(C)[nH]c2=O)c1C Show InChI InChI=1S/C22H28BrN3O3/c1-13-9-14(2)25-22(28)19(13)12-24-21(27)18-10-16(23)11-20(15(18)3)26(4)17-5-7-29-8-6-17/h9-11,17H,5-8,12H2,1-4H3,(H,24,27)(H,25,28) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme
Curated by ChEMBL
| Assay Description
Inhibition of methyltransferase activity of human EZH2 using chicken oligonucleotide as substrate by pull down assay |
J Med Chem 59: 1556-64 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01501
BindingDB Entry DOI: 10.7270/Q2B56MM7 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50149882
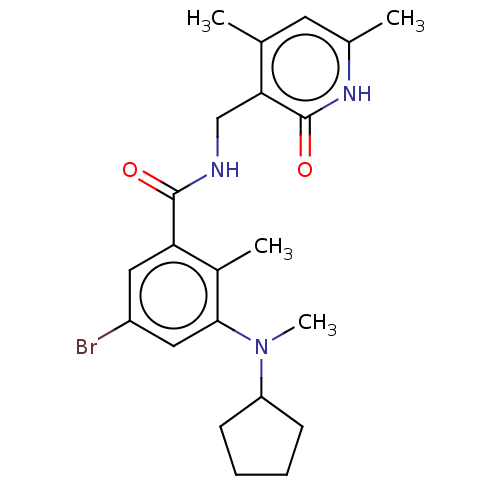
(CHEMBL3771083)Show SMILES CN(C1CCCC1)c1cc(Br)cc(C(=O)NCc2c(C)cc(C)[nH]c2=O)c1C Show InChI InChI=1S/C22H28BrN3O2/c1-13-9-14(2)25-22(28)19(13)12-24-21(27)18-10-16(23)11-20(15(18)3)26(4)17-7-5-6-8-17/h9-11,17H,5-8,12H2,1-4H3,(H,24,27)(H,25,28) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme
Curated by ChEMBL
| Assay Description
Inhibition of methyltransferase activity of human EZH2 using chicken oligonucleotide as substrate by pull down assay |
J Med Chem 59: 1556-64 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01501
BindingDB Entry DOI: 10.7270/Q2B56MM7 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50149896

(CHEMBL3771013)Show SMILES CN(C1CCNCC1)c1cc(Br)cc(C(=O)NCc2c(C)cc(C)[nH]c2=O)c1C Show InChI InChI=1S/C22H29BrN4O2/c1-13-9-14(2)26-22(29)19(13)12-25-21(28)18-10-16(23)11-20(15(18)3)27(4)17-5-7-24-8-6-17/h9-11,17,24H,5-8,12H2,1-4H3,(H,25,28)(H,26,29) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme
Curated by ChEMBL
| Assay Description
Inhibition of methyltransferase activity of human EZH2 using chicken oligonucleotide as substrate by pull down assay |
J Med Chem 59: 1556-64 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01501
BindingDB Entry DOI: 10.7270/Q2B56MM7 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50149901
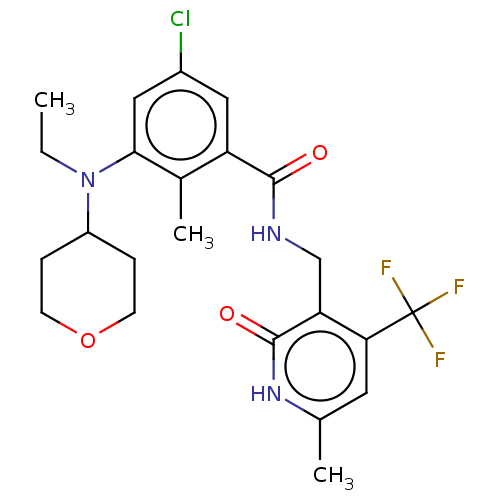
(CHEMBL3770820)Show SMILES CCN(C1CCOCC1)c1cc(Cl)cc(C(=O)NCc2c(cc(C)[nH]c2=O)C(F)(F)F)c1C Show InChI InChI=1S/C23H27ClF3N3O3/c1-4-30(16-5-7-33-8-6-16)20-11-15(24)10-17(14(20)3)21(31)28-12-18-19(23(25,26)27)9-13(2)29-22(18)32/h9-11,16H,4-8,12H2,1-3H3,(H,28,31)(H,29,32) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme
Curated by ChEMBL
| Assay Description
Inhibition of methyltransferase activity of human EZH2 using chicken oligonucleotide as substrate by pull down assay |
J Med Chem 59: 1556-64 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01501
BindingDB Entry DOI: 10.7270/Q2B56MM7 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50149706
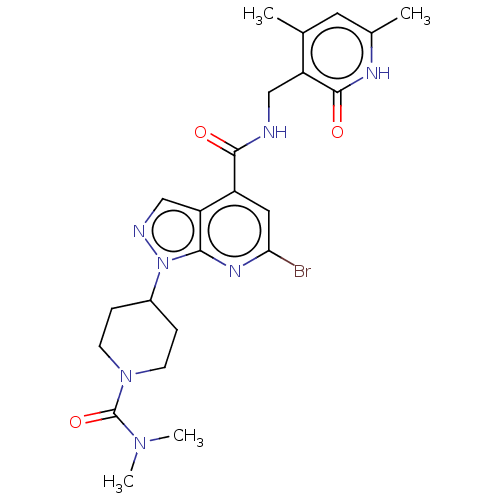
(CHEMBL3769652)Show SMILES CN(C)C(=O)N1CCC(CC1)n1ncc2c(cc(Br)nc12)C(=O)NCc1c(C)cc(C)[nH]c1=O Show InChI InChI=1S/C23H28BrN7O3/c1-13-9-14(2)27-22(33)17(13)11-25-21(32)16-10-19(24)28-20-18(16)12-26-31(20)15-5-7-30(8-6-15)23(34)29(3)4/h9-10,12,15H,5-8,11H2,1-4H3,(H,25,32)(H,27,33) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme
Curated by ChEMBL
| Assay Description
Inhibition of methyltransferase activity of human EZH2 using chicken oligonucleotide as substrate by pull down assay |
J Med Chem 59: 1556-64 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01501
BindingDB Entry DOI: 10.7270/Q2B56MM7 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50149705

(CHEMBL3771343)Show SMILES Cc1cc(C)c(CNC(=O)c2cc(Br)nc3n(ncc23)C2CCN(CC2)C(=O)C(C)(C)C)c(=O)[nH]1 Show InChI InChI=1S/C25H31BrN6O3/c1-14-10-15(2)29-23(34)18(14)12-27-22(33)17-11-20(26)30-21-19(17)13-28-32(21)16-6-8-31(9-7-16)24(35)25(3,4)5/h10-11,13,16H,6-9,12H2,1-5H3,(H,27,33)(H,29,34) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme
Curated by ChEMBL
| Assay Description
Inhibition of methyltransferase activity of human EZH2 using chicken oligonucleotide as substrate by pull down assay |
J Med Chem 59: 1556-64 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01501
BindingDB Entry DOI: 10.7270/Q2B56MM7 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50149703
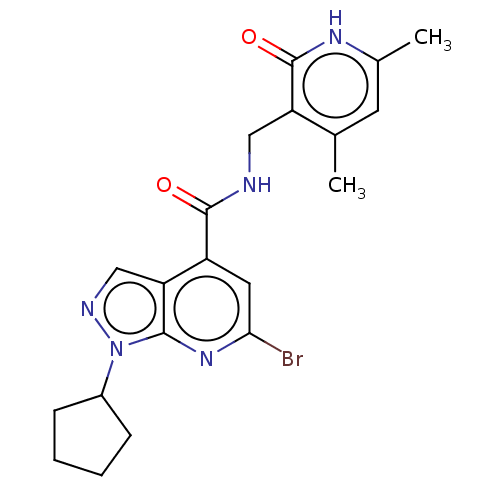
(CHEMBL3770438)Show SMILES Cc1cc(C)c(CNC(=O)c2cc(Br)nc3n(ncc23)C2CCCC2)c(=O)[nH]1 Show InChI InChI=1S/C20H22BrN5O2/c1-11-7-12(2)24-20(28)15(11)9-22-19(27)14-8-17(21)25-18-16(14)10-23-26(18)13-5-3-4-6-13/h7-8,10,13H,3-6,9H2,1-2H3,(H,22,27)(H,24,28) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme
Curated by ChEMBL
| Assay Description
Inhibition of methyltransferase activity of human EZH2 using chicken oligonucleotide as substrate by pull down assay |
J Med Chem 59: 1556-64 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01501
BindingDB Entry DOI: 10.7270/Q2B56MM7 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50149898

(CHEMBL3771269)Show SMILES CCN(C1CCOCC1)c1cc(Cl)cc(C(=O)NCc2c(C)cc[nH]c2=O)c1C Show InChI InChI=1S/C22H28ClN3O3/c1-4-26(17-6-9-29-10-7-17)20-12-16(23)11-18(15(20)3)21(27)25-13-19-14(2)5-8-24-22(19)28/h5,8,11-12,17H,4,6-7,9-10,13H2,1-3H3,(H,24,28)(H,25,27) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme
Curated by ChEMBL
| Assay Description
Inhibition of methyltransferase activity of human EZH2 using chicken oligonucleotide as substrate by pull down assay |
J Med Chem 59: 1556-64 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01501
BindingDB Entry DOI: 10.7270/Q2B56MM7 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50149909

(CHEMBL3770154)Show SMILES CCN(C1CCOCC1)c1cc(Cl)cc(C(=O)NCc2c(C)cc(C)n(C)c2=O)c1C Show InChI InChI=1S/C24H32ClN3O3/c1-6-28(19-7-9-31-10-8-19)22-13-18(25)12-20(17(22)4)23(29)26-14-21-15(2)11-16(3)27(5)24(21)30/h11-13,19H,6-10,14H2,1-5H3,(H,26,29) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme
Curated by ChEMBL
| Assay Description
Inhibition of methyltransferase activity of human EZH2 using chicken oligonucleotide as substrate by pull down assay |
J Med Chem 59: 1556-64 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01501
BindingDB Entry DOI: 10.7270/Q2B56MM7 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50149900
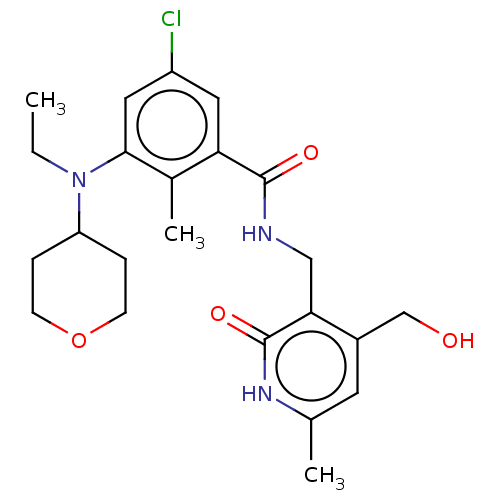
(CHEMBL3769624)Show SMILES CCN(C1CCOCC1)c1cc(Cl)cc(C(=O)NCc2c(CO)cc(C)[nH]c2=O)c1C Show InChI InChI=1S/C23H30ClN3O4/c1-4-27(18-5-7-31-8-6-18)21-11-17(24)10-19(15(21)3)22(29)25-12-20-16(13-28)9-14(2)26-23(20)30/h9-11,18,28H,4-8,12-13H2,1-3H3,(H,25,29)(H,26,30) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme
Curated by ChEMBL
| Assay Description
Inhibition of methyltransferase activity of human EZH2 using chicken oligonucleotide as substrate by pull down assay |
J Med Chem 59: 1556-64 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01501
BindingDB Entry DOI: 10.7270/Q2B56MM7 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50149704

(CHEMBL3771135)Show SMILES Cc1cc(C)c(CNC(=O)c2cc(Br)nc3n(ncc23)C2CCN(CC2)C(=O)OC(C)(C)C)c(=O)[nH]1 Show InChI InChI=1S/C25H31BrN6O4/c1-14-10-15(2)29-23(34)18(14)12-27-22(33)17-11-20(26)30-21-19(17)13-28-32(21)16-6-8-31(9-7-16)24(35)36-25(3,4)5/h10-11,13,16H,6-9,12H2,1-5H3,(H,27,33)(H,29,34) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme
Curated by ChEMBL
| Assay Description
Inhibition of methyltransferase activity of human EZH2 using chicken oligonucleotide as substrate by pull down assay |
J Med Chem 59: 1556-64 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01501
BindingDB Entry DOI: 10.7270/Q2B56MM7 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50149899

(CHEMBL3770788)Show SMILES CCN(C1CCOCC1)c1cc(Cl)cc(C(=O)NCc2ccc(C)[nH]c2=O)c1C Show InChI InChI=1S/C22H28ClN3O3/c1-4-26(18-7-9-29-10-8-18)20-12-17(23)11-19(15(20)3)22(28)24-13-16-6-5-14(2)25-21(16)27/h5-6,11-12,18H,4,7-10,13H2,1-3H3,(H,24,28)(H,25,27) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme
Curated by ChEMBL
| Assay Description
Inhibition of methyltransferase activity of human EZH2 using chicken oligonucleotide as substrate by pull down assay |
J Med Chem 59: 1556-64 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01501
BindingDB Entry DOI: 10.7270/Q2B56MM7 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50149907

(CHEMBL3770109)Show SMILES CCN(C1CCOCC1)c1ccc(Cl)c(C(=O)N(C)Cc2c(C)cc(C)[nH]c2=O)c1C Show InChI InChI=1S/C24H32ClN3O3/c1-6-28(18-9-11-31-12-10-18)21-8-7-20(25)22(17(21)4)24(30)27(5)14-19-15(2)13-16(3)26-23(19)29/h7-8,13,18H,6,9-12,14H2,1-5H3,(H,26,29) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme
Curated by ChEMBL
| Assay Description
Inhibition of methyltransferase activity of human EZH2 using chicken oligonucleotide as substrate by pull down assay |
J Med Chem 59: 1556-64 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01501
BindingDB Entry DOI: 10.7270/Q2B56MM7 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50149602
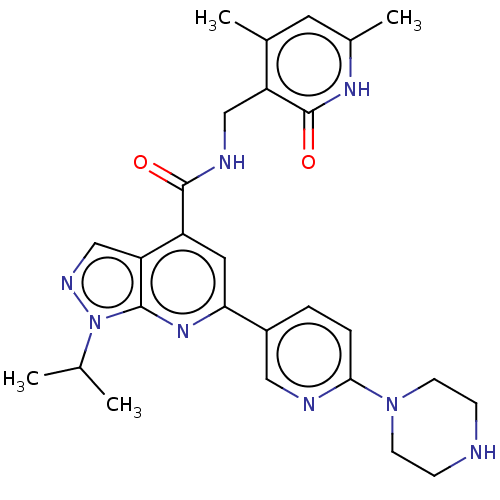
(CHEMBL3770239 | US10273223, Compound A-10 | US9637...)Show SMILES CC(C)n1ncc2c(cc(nc12)-c1ccc(nc1)N1CCNCC1)C(=O)NCc1c(C)cc(C)[nH]c1=O Show InChI InChI=1S/C27H32N8O2/c1-16(2)35-25-22(15-31-35)20(26(36)30-14-21-17(3)11-18(4)32-27(21)37)12-23(33-25)19-5-6-24(29-13-19)34-9-7-28-8-10-34/h5-6,11-13,15-16,28H,7-10,14H2,1-4H3,(H,30,36)(H,32,37) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme
Curated by ChEMBL
| Assay Description
Inhibition of methyltransferase activity of human EZH2 using chicken oligonucleotide as substrate by pull down assay |
J Med Chem 59: 1556-64 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01501
BindingDB Entry DOI: 10.7270/Q2B56MM7 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50149616
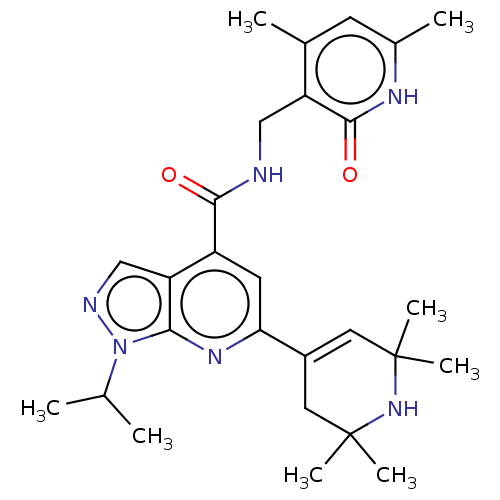
(CHEMBL3770922 | US10273223, Compound B-36 | US9637...)Show SMILES CC(C)n1ncc2c(cc(nc12)C1=CC(C)(C)NC(C)(C)C1)C(=O)NCc1c(C)cc(C)[nH]c1=O |t:14| Show InChI InChI=1S/C27H36N6O2/c1-15(2)33-23-21(14-29-33)19(24(34)28-13-20-16(3)9-17(4)30-25(20)35)10-22(31-23)18-11-26(5,6)32-27(7,8)12-18/h9-11,14-15,32H,12-13H2,1-8H3,(H,28,34)(H,30,35) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme
Curated by ChEMBL
| Assay Description
Inhibition of methyltransferase activity of human EZH2 using chicken oligonucleotide as substrate by pull down assay |
J Med Chem 59: 1556-64 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01501
BindingDB Entry DOI: 10.7270/Q2B56MM7 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM190179

(EPZ005030 | US10273223, Compound A-2 | US9175331, ...)Show SMILES CC(C)n1ncc2c(cc(nc12)-c1ccc(CN2CCOCC2)cc1)C(=O)NCc1c(C)cc(C)[nH]c1=O Show InChI InChI=1S/C29H34N6O3/c1-18(2)35-27-25(16-31-35)23(28(36)30-15-24-19(3)13-20(4)32-29(24)37)14-26(33-27)22-7-5-21(6-8-22)17-34-9-11-38-12-10-34/h5-8,13-14,16,18H,9-12,15,17H2,1-4H3,(H,30,36)(H,32,37) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme
Curated by ChEMBL
| Assay Description
Inhibition of methyltransferase activity of human EZH2 using chicken oligonucleotide as substrate by pull down assay |
J Med Chem 59: 1556-64 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01501
BindingDB Entry DOI: 10.7270/Q2B56MM7 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50149883
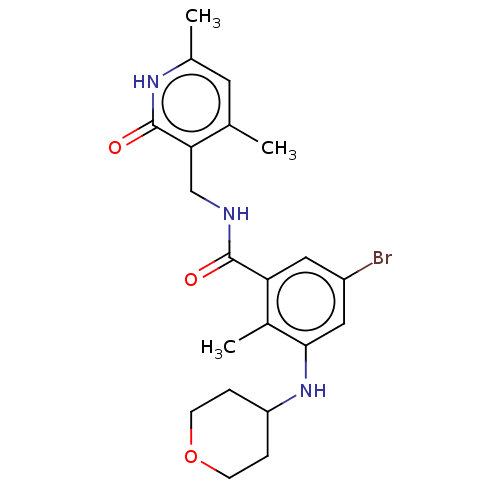
(CHEMBL3770619)Show SMILES Cc1cc(C)c(CNC(=O)c2cc(Br)cc(NC3CCOCC3)c2C)c(=O)[nH]1 Show InChI InChI=1S/C21H26BrN3O3/c1-12-8-13(2)24-21(27)18(12)11-23-20(26)17-9-15(22)10-19(14(17)3)25-16-4-6-28-7-5-16/h8-10,16,25H,4-7,11H2,1-3H3,(H,23,26)(H,24,27) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme
Curated by ChEMBL
| Assay Description
Inhibition of methyltransferase activity of human EZH2 using chicken oligonucleotide as substrate by pull down assay |
J Med Chem 59: 1556-64 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01501
BindingDB Entry DOI: 10.7270/Q2B56MM7 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50075053
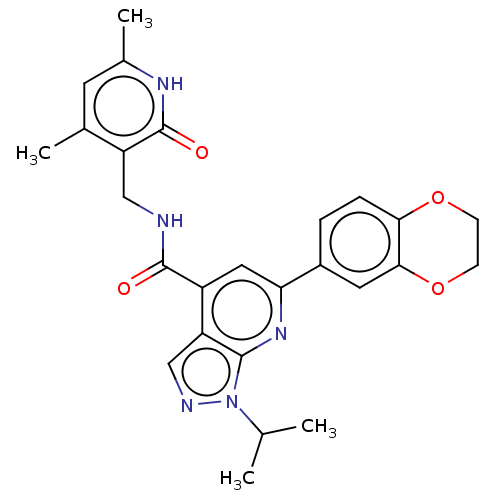
(CHEMBL3414618)Show SMILES CC(C)n1ncc2c(cc(nc12)-c1ccc2OCCOc2c1)C(=O)NCc1c(C)cc(C)[nH]c1=O Show InChI InChI=1S/C26H27N5O4/c1-14(2)31-24-20(13-28-31)18(25(32)27-12-19-15(3)9-16(4)29-26(19)33)11-21(30-24)17-5-6-22-23(10-17)35-8-7-34-22/h5-6,9-11,13-14H,7-8,12H2,1-4H3,(H,27,32)(H,29,33) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme
Curated by ChEMBL
| Assay Description
Inhibition of methyltransferase activity of human EZH2 using chicken oligonucleotide as substrate by pull down assay |
J Med Chem 59: 1556-64 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01501
BindingDB Entry DOI: 10.7270/Q2B56MM7 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50149879

(CHEMBL3770626)Show SMILES Cc1cc(C)c(CNC(=O)c2cc(Br)nc3n(ncc23)C2CCOCC2)c(=O)[nH]1 Show InChI InChI=1S/C20H22BrN5O3/c1-11-7-12(2)24-20(28)15(11)9-22-19(27)14-8-17(21)25-18-16(14)10-23-26(18)13-3-5-29-6-4-13/h7-8,10,13H,3-6,9H2,1-2H3,(H,22,27)(H,24,28) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme
Curated by ChEMBL
| Assay Description
Inhibition of methyltransferase activity of human EZH2 using chicken oligonucleotide as substrate by pull down assay |
J Med Chem 59: 1556-64 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01501
BindingDB Entry DOI: 10.7270/Q2B56MM7 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50149638
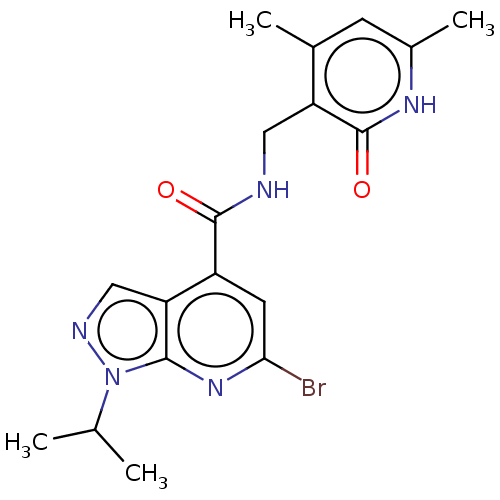
(CHEMBL3770418 | US10273223, Compound B-88 | US9637...)Show SMILES CC(C)n1ncc2c(cc(Br)nc12)C(=O)NCc1c(C)cc(C)[nH]c1=O Show InChI InChI=1S/C18H20BrN5O2/c1-9(2)24-16-14(8-21-24)12(6-15(19)23-16)17(25)20-7-13-10(3)5-11(4)22-18(13)26/h5-6,8-9H,7H2,1-4H3,(H,20,25)(H,22,26) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme
Curated by ChEMBL
| Assay Description
Inhibition of methyltransferase activity of human EZH2 using chicken oligonucleotide as substrate by pull down assay |
J Med Chem 59: 1556-64 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01501
BindingDB Entry DOI: 10.7270/Q2B56MM7 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50149903

(CHEMBL3769599)Show SMILES CCN(C1CCOCC1)c1cc(Cl)cc(C(=O)NCc2c(CN(C)C)cc(C)[nH]c2=O)c1C Show InChI InChI=1S/C25H35ClN4O3/c1-6-30(20-7-9-33-10-8-20)23-13-19(26)12-21(17(23)3)24(31)27-14-22-18(15-29(4)5)11-16(2)28-25(22)32/h11-13,20H,6-10,14-15H2,1-5H3,(H,27,31)(H,28,32) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme
Curated by ChEMBL
| Assay Description
Inhibition of methyltransferase activity of human EZH2 using chicken oligonucleotide as substrate by pull down assay |
J Med Chem 59: 1556-64 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01501
BindingDB Entry DOI: 10.7270/Q2B56MM7 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50149647
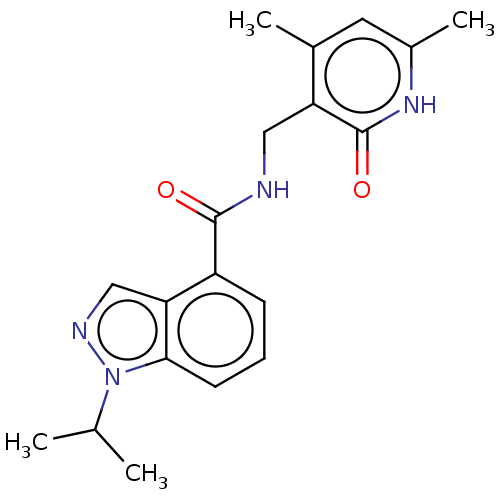
(CHEMBL3771291 | US10273223, Compound D-71 | US9637...)Show SMILES CC(C)n1ncc2c(cccc12)C(=O)NCc1c(C)cc(C)[nH]c1=O Show InChI InChI=1S/C19H22N4O2/c1-11(2)23-17-7-5-6-14(16(17)10-21-23)18(24)20-9-15-12(3)8-13(4)22-19(15)25/h5-8,10-11H,9H2,1-4H3,(H,20,24)(H,22,25) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme
Curated by ChEMBL
| Assay Description
Inhibition of methyltransferase activity of human EZH2 using chicken oligonucleotide as substrate by pull down assay |
J Med Chem 59: 1556-64 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01501
BindingDB Entry DOI: 10.7270/Q2B56MM7 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50149624
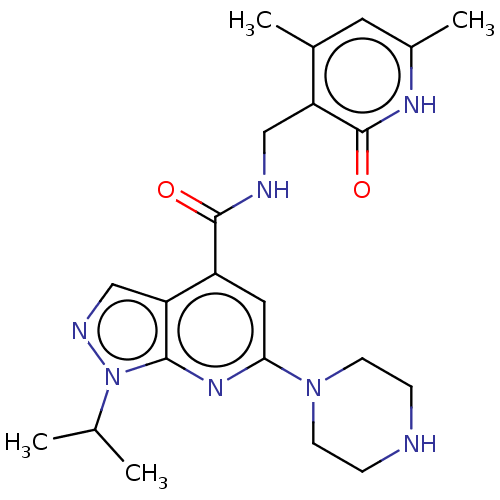
(CHEMBL3769631 | US10273223, Compound B-92 | US9637...)Show SMILES CC(C)n1ncc2c(cc(nc12)N1CCNCC1)C(=O)NCc1c(C)cc(C)[nH]c1=O Show InChI InChI=1S/C22H29N7O2/c1-13(2)29-20-18(12-25-29)16(10-19(27-20)28-7-5-23-6-8-28)21(30)24-11-17-14(3)9-15(4)26-22(17)31/h9-10,12-13,23H,5-8,11H2,1-4H3,(H,24,30)(H,26,31) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme
Curated by ChEMBL
| Assay Description
Inhibition of methyltransferase activity of human EZH2 using chicken oligonucleotide as substrate by pull down assay |
J Med Chem 59: 1556-64 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01501
BindingDB Entry DOI: 10.7270/Q2B56MM7 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50149887
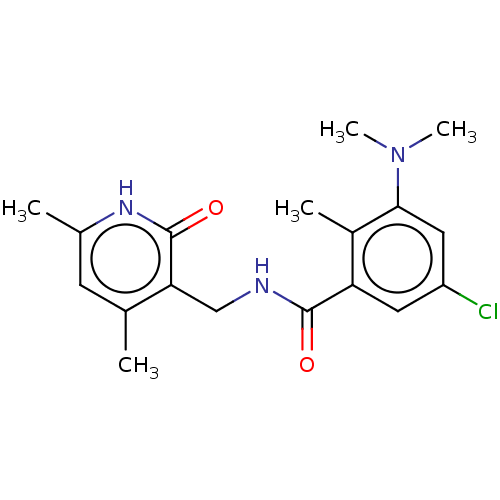
(CHEMBL3771140)Show SMILES CN(C)c1cc(Cl)cc(C(=O)NCc2c(C)cc(C)[nH]c2=O)c1C Show InChI InChI=1S/C18H22ClN3O2/c1-10-6-11(2)21-18(24)15(10)9-20-17(23)14-7-13(19)8-16(12(14)3)22(4)5/h6-8H,9H2,1-5H3,(H,20,23)(H,21,24) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme
Curated by ChEMBL
| Assay Description
Inhibition of methyltransferase activity of human EZH2 using chicken oligonucleotide as substrate by pull down assay |
J Med Chem 59: 1556-64 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01501
BindingDB Entry DOI: 10.7270/Q2B56MM7 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50149878

(CHEMBL3770059)Show SMILES Cc1cc(C)c(CNC(=O)c2cc(Br)nc3n(ncc23)C2CCNCC2)c(=O)[nH]1 Show InChI InChI=1S/C20H23BrN6O2/c1-11-7-12(2)25-20(29)15(11)9-23-19(28)14-8-17(21)26-18-16(14)10-24-27(18)13-3-5-22-6-4-13/h7-8,10,13,22H,3-6,9H2,1-2H3,(H,23,28)(H,25,29) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme
Curated by ChEMBL
| Assay Description
Inhibition of methyltransferase activity of human EZH2 using chicken oligonucleotide as substrate by pull down assay |
J Med Chem 59: 1556-64 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01501
BindingDB Entry DOI: 10.7270/Q2B56MM7 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50149634

(CHEMBL3771177 | US10273223, Compound B-109)Show SMILES CC(C)c1cc(C(=O)NCc2c(C)cc(C)[nH]c2=O)c2cnn(C(C)C)c2n1 Show InChI InChI=1S/C21H27N5O2/c1-11(2)18-8-15(17-10-23-26(12(3)4)19(17)25-18)20(27)22-9-16-13(5)7-14(6)24-21(16)28/h7-8,10-12H,9H2,1-6H3,(H,22,27)(H,24,28) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme
Curated by ChEMBL
| Assay Description
Inhibition of methyltransferase activity of human EZH2 using chicken oligonucleotide as substrate by pull down assay |
J Med Chem 59: 1556-64 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01501
BindingDB Entry DOI: 10.7270/Q2B56MM7 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50149636
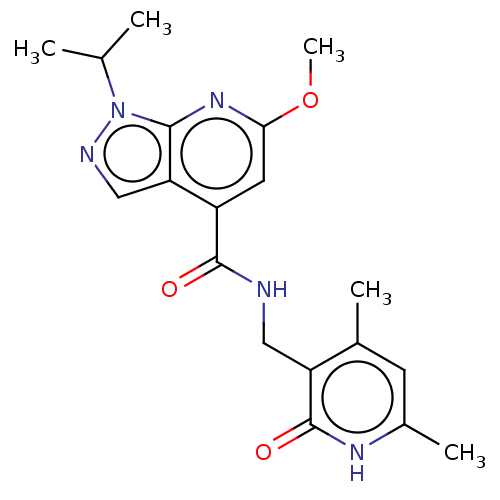
(CHEMBL3770249 | US10273223, Compound B-118 | US963...)Show SMILES COc1cc(C(=O)NCc2c(C)cc(C)[nH]c2=O)c2cnn(C(C)C)c2n1 Show InChI InChI=1S/C19H23N5O3/c1-10(2)24-17-15(9-21-24)13(7-16(23-17)27-5)18(25)20-8-14-11(3)6-12(4)22-19(14)26/h6-7,9-10H,8H2,1-5H3,(H,20,25)(H,22,26) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme
Curated by ChEMBL
| Assay Description
Inhibition of methyltransferase activity of human EZH2 using chicken oligonucleotide as substrate by pull down assay |
J Med Chem 59: 1556-64 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01501
BindingDB Entry DOI: 10.7270/Q2B56MM7 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50149885
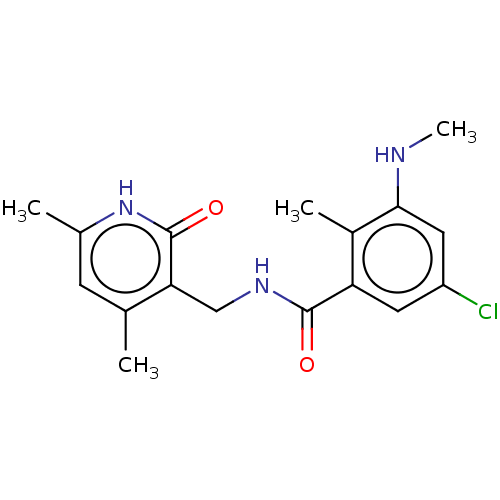
(CHEMBL3770845)Show SMILES CNc1cc(Cl)cc(C(=O)NCc2c(C)cc(C)[nH]c2=O)c1C Show InChI InChI=1S/C17H20ClN3O2/c1-9-5-10(2)21-17(23)14(9)8-20-16(22)13-6-12(18)7-15(19-4)11(13)3/h5-7,19H,8H2,1-4H3,(H,20,22)(H,21,23) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme
Curated by ChEMBL
| Assay Description
Inhibition of methyltransferase activity of human EZH2 using chicken oligonucleotide as substrate by pull down assay |
J Med Chem 59: 1556-64 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01501
BindingDB Entry DOI: 10.7270/Q2B56MM7 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50149882

(CHEMBL3771083)Show SMILES CN(C1CCCC1)c1cc(Br)cc(C(=O)NCc2c(C)cc(C)[nH]c2=O)c1C Show InChI InChI=1S/C22H28BrN3O2/c1-13-9-14(2)25-22(28)19(13)12-24-21(27)18-10-16(23)11-20(15(18)3)26(4)17-7-5-6-8-17/h9-11,17H,5-8,12H2,1-4H3,(H,24,27)(H,25,28) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme
Curated by ChEMBL
| Assay Description
Inhibition of methyltransferase activity of EZH2 in human lymphoma cells assessed as H3K27 methylation |
J Med Chem 59: 1556-64 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01501
BindingDB Entry DOI: 10.7270/Q2B56MM7 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50149908

(CHEMBL3769797)Show SMILES CCN(C1CCOCC1)c1cc(Cl)cc(C(=O)NC(C)c2c(C)cc(C)[nH]c2=O)c1C Show InChI InChI=1S/C24H32ClN3O3/c1-6-28(19-7-9-31-10-8-19)21-13-18(25)12-20(16(21)4)23(29)27-17(5)22-14(2)11-15(3)26-24(22)30/h11-13,17,19H,6-10H2,1-5H3,(H,26,30)(H,27,29) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme
Curated by ChEMBL
| Assay Description
Inhibition of methyltransferase activity of human EZH2 using chicken oligonucleotide as substrate by pull down assay |
J Med Chem 59: 1556-64 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01501
BindingDB Entry DOI: 10.7270/Q2B56MM7 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM190179

(EPZ005030 | US10273223, Compound A-2 | US9175331, ...)Show SMILES CC(C)n1ncc2c(cc(nc12)-c1ccc(CN2CCOCC2)cc1)C(=O)NCc1c(C)cc(C)[nH]c1=O Show InChI InChI=1S/C29H34N6O3/c1-18(2)35-27-25(16-31-35)23(28(36)30-15-24-19(3)13-20(4)32-29(24)37)14-26(33-27)22-7-5-21(6-8-22)17-34-9-11-38-12-10-34/h5-8,13-14,16,18H,9-12,15,17H2,1-4H3,(H,30,36)(H,32,37) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme
Curated by ChEMBL
| Assay Description
Inhibition of methyltransferase activity of EZH2 in human lymphoma cells assessed as H3K27 methylation |
J Med Chem 59: 1556-64 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01501
BindingDB Entry DOI: 10.7270/Q2B56MM7 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50149645
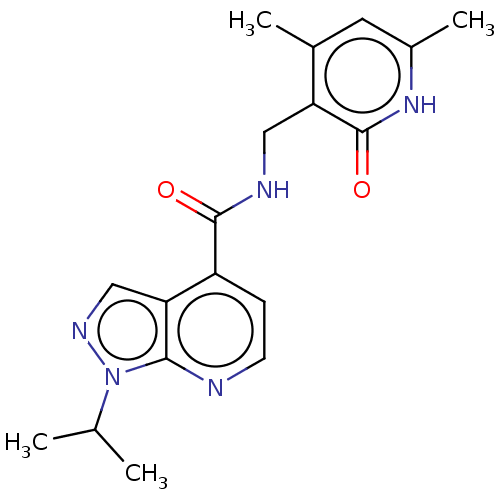
(CHEMBL3771120 | US10273223, Compound B-127 | US963...)Show SMILES CC(C)n1ncc2c(ccnc12)C(=O)NCc1c(C)cc(C)[nH]c1=O Show InChI InChI=1S/C18H21N5O2/c1-10(2)23-16-15(9-21-23)13(5-6-19-16)17(24)20-8-14-11(3)7-12(4)22-18(14)25/h5-7,9-10H,8H2,1-4H3,(H,20,24)(H,22,25) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme
Curated by ChEMBL
| Assay Description
Inhibition of methyltransferase activity of human EZH2 using chicken oligonucleotide as substrate by pull down assay |
J Med Chem 59: 1556-64 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01501
BindingDB Entry DOI: 10.7270/Q2B56MM7 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50149698
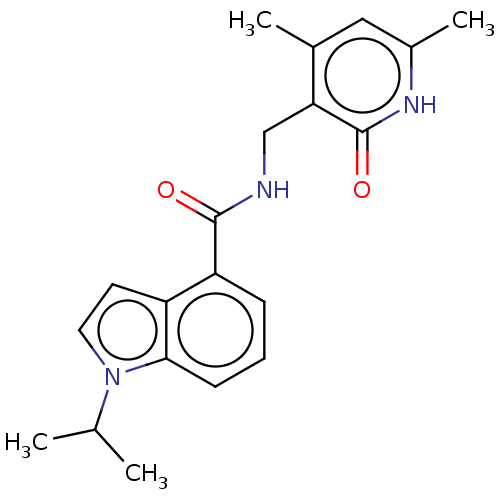
(CHEMBL3771043 | US10273223, Compound E-1 | US96374...)Show SMILES CC(C)n1ccc2c(cccc12)C(=O)NCc1c(C)cc(C)[nH]c1=O Show InChI InChI=1S/C20H23N3O2/c1-12(2)23-9-8-15-16(6-5-7-18(15)23)19(24)21-11-17-13(3)10-14(4)22-20(17)25/h5-10,12H,11H2,1-4H3,(H,21,24)(H,22,25) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme
Curated by ChEMBL
| Assay Description
Inhibition of methyltransferase activity of human EZH2 using chicken oligonucleotide as substrate by pull down assay |
J Med Chem 59: 1556-64 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01501
BindingDB Entry DOI: 10.7270/Q2B56MM7 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50149897

(CHEMBL3769574)Show SMILES CCN(C1CCOCC1)c1cc(Cl)cc(C(=O)NCc2ccc[nH]c2=O)c1C Show InChI InChI=1S/C21H26ClN3O3/c1-3-25(17-6-9-28-10-7-17)19-12-16(22)11-18(14(19)2)21(27)24-13-15-5-4-8-23-20(15)26/h4-5,8,11-12,17H,3,6-7,9-10,13H2,1-2H3,(H,23,26)(H,24,27) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme
Curated by ChEMBL
| Assay Description
Inhibition of methyltransferase activity of human EZH2 using chicken oligonucleotide as substrate by pull down assay |
J Med Chem 59: 1556-64 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01501
BindingDB Entry DOI: 10.7270/Q2B56MM7 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50149904

(CHEMBL3769963)Show SMILES Cc1cc(C(=O)NCc2c(C)cc(C)[nH]c2=O)c(C)n1C1CCCCC1 Show InChI InChI=1S/C21H29N3O2/c1-13-10-14(2)23-21(26)19(13)12-22-20(25)18-11-15(3)24(16(18)4)17-8-6-5-7-9-17/h10-11,17H,5-9,12H2,1-4H3,(H,22,25)(H,23,26) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme
Curated by ChEMBL
| Assay Description
Inhibition of methyltransferase activity of human EZH2 using chicken oligonucleotide as substrate by pull down assay |
J Med Chem 59: 1556-64 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01501
BindingDB Entry DOI: 10.7270/Q2B56MM7 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50149600
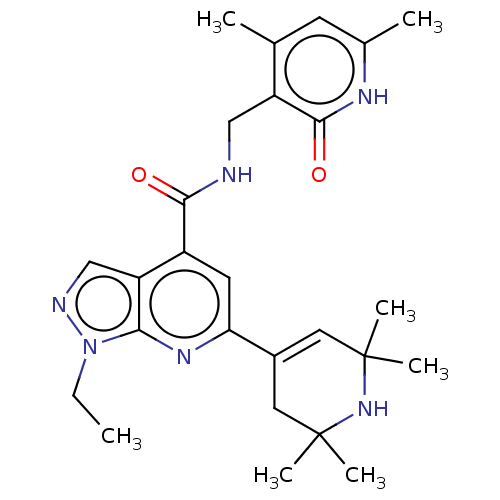
(CHEMBL3770081 | US10273223, Compound B-131 | US963...)Show SMILES CCn1ncc2c(cc(nc12)C1=CC(C)(C)NC(C)(C)C1)C(=O)NCc1c(C)cc(C)[nH]c1=O |t:13| Show InChI InChI=1S/C26H34N6O2/c1-8-32-22-20(14-28-32)18(23(33)27-13-19-15(2)9-16(3)29-24(19)34)10-21(30-22)17-11-25(4,5)31-26(6,7)12-17/h9-11,14,31H,8,12-13H2,1-7H3,(H,27,33)(H,29,34) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme
Curated by ChEMBL
| Assay Description
Inhibition of methyltransferase activity of human EZH2 using chicken oligonucleotide as substrate by pull down assay |
J Med Chem 59: 1556-64 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01501
BindingDB Entry DOI: 10.7270/Q2B56MM7 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50149880
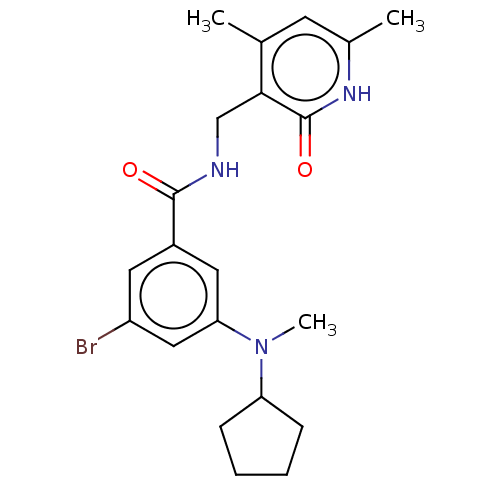
(CHEMBL3769498)Show SMILES CN(C1CCCC1)c1cc(Br)cc(c1)C(=O)NCc1c(C)cc(C)[nH]c1=O Show InChI InChI=1S/C21H26BrN3O2/c1-13-8-14(2)24-21(27)19(13)12-23-20(26)15-9-16(22)11-18(10-15)25(3)17-6-4-5-7-17/h8-11,17H,4-7,12H2,1-3H3,(H,23,26)(H,24,27) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme
Curated by ChEMBL
| Assay Description
Inhibition of methyltransferase activity of human EZH2 using chicken oligonucleotide as substrate by pull down assay |
J Med Chem 59: 1556-64 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01501
BindingDB Entry DOI: 10.7270/Q2B56MM7 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50149906
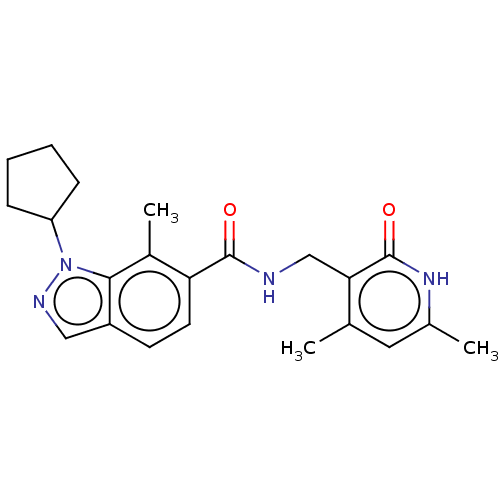
(CHEMBL3770104)Show SMILES Cc1cc(C)c(CNC(=O)c2ccc3cnn(C4CCCC4)c3c2C)c(=O)[nH]1 Show InChI InChI=1S/C22H26N4O2/c1-13-10-14(2)25-22(28)19(13)12-23-21(27)18-9-8-16-11-24-26(20(16)15(18)3)17-6-4-5-7-17/h8-11,17H,4-7,12H2,1-3H3,(H,23,27)(H,25,28) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme
Curated by ChEMBL
| Assay Description
Inhibition of methyltransferase activity of human EZH2 using chicken oligonucleotide as substrate by pull down assay |
J Med Chem 59: 1556-64 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01501
BindingDB Entry DOI: 10.7270/Q2B56MM7 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50149894
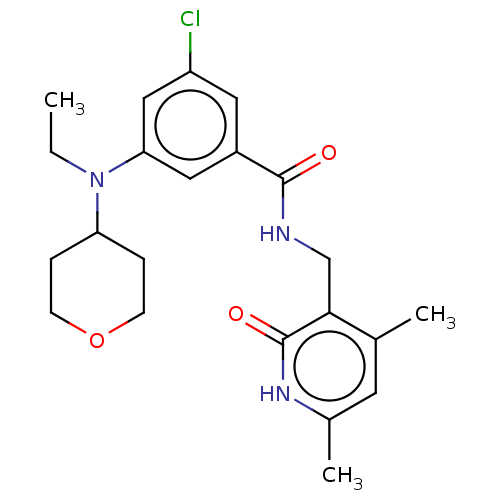
(CHEMBL3769788)Show SMILES CCN(C1CCOCC1)c1cc(Cl)cc(c1)C(=O)NCc1c(C)cc(C)[nH]c1=O Show InChI InChI=1S/C22H28ClN3O3/c1-4-26(18-5-7-29-8-6-18)19-11-16(10-17(23)12-19)21(27)24-13-20-14(2)9-15(3)25-22(20)28/h9-12,18H,4-8,13H2,1-3H3,(H,24,27)(H,25,28) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme
Curated by ChEMBL
| Assay Description
Inhibition of methyltransferase activity of human EZH2 using chicken oligonucleotide as substrate by pull down assay |
J Med Chem 59: 1556-64 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01501
BindingDB Entry DOI: 10.7270/Q2B56MM7 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50149601
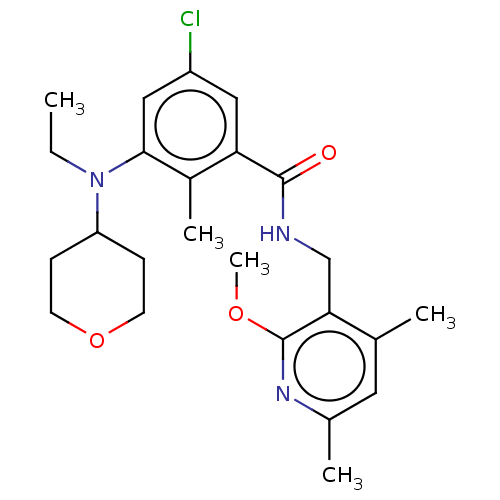
(CHEMBL3770929)Show SMILES CCN(C1CCOCC1)c1cc(Cl)cc(C(=O)NCc2c(C)cc(C)nc2OC)c1C Show InChI InChI=1S/C24H32ClN3O3/c1-6-28(19-7-9-31-10-8-19)22-13-18(25)12-20(17(22)4)23(29)26-14-21-15(2)11-16(3)27-24(21)30-5/h11-13,19H,6-10,14H2,1-5H3,(H,26,29) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme
Curated by ChEMBL
| Assay Description
Inhibition of methyltransferase activity of human EZH2 using chicken oligonucleotide as substrate by pull down assay |
J Med Chem 59: 1556-64 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01501
BindingDB Entry DOI: 10.7270/Q2B56MM7 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50149701
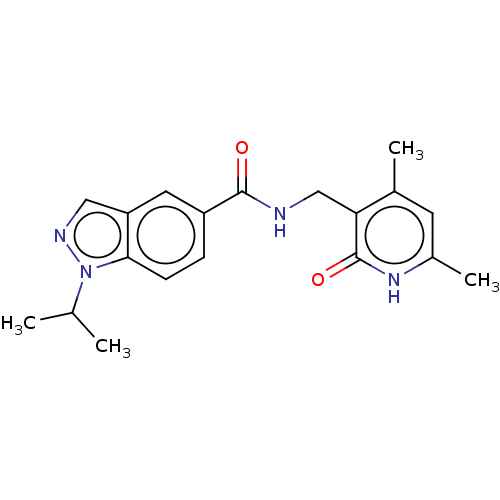
(CHEMBL3769806)Show SMILES CC(C)n1ncc2cc(ccc12)C(=O)NCc1c(C)cc(C)[nH]c1=O Show InChI InChI=1S/C19H22N4O2/c1-11(2)23-17-6-5-14(8-15(17)9-21-23)18(24)20-10-16-12(3)7-13(4)22-19(16)25/h5-9,11H,10H2,1-4H3,(H,20,24)(H,22,25) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme
Curated by ChEMBL
| Assay Description
Inhibition of methyltransferase activity of human EZH2 using chicken oligonucleotide as substrate by pull down assay |
J Med Chem 59: 1556-64 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01501
BindingDB Entry DOI: 10.7270/Q2B56MM7 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50149910

(CHEMBL3769561 | US10273223, Compound B-153 | US963...)Show SMILES Cc1cc(C)c(CNC(=O)c2cc(nc3n(C)ncc23)C2=CC(C)(C)NC(C)(C)C2)c(=O)[nH]1 |t:22| Show InChI InChI=1S/C25H32N6O2/c1-14-8-15(2)28-23(33)18(14)12-26-22(32)17-9-20(29-21-19(17)13-27-31(21)7)16-10-24(3,4)30-25(5,6)11-16/h8-10,13,30H,11-12H2,1-7H3,(H,26,32)(H,28,33) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme
Curated by ChEMBL
| Assay Description
Inhibition of methyltransferase activity of human EZH2 using chicken oligonucleotide as substrate by pull down assay |
J Med Chem 59: 1556-64 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01501
BindingDB Entry DOI: 10.7270/Q2B56MM7 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50149905

(CHEMBL3769731)Show SMILES Cc1cc(C)c(CNC(=O)c2ccc3cnn(C4CCCC4)c3c2)c(=O)[nH]1 Show InChI InChI=1S/C21H24N4O2/c1-13-9-14(2)24-21(27)18(13)12-22-20(26)15-7-8-16-11-23-25(19(16)10-15)17-5-3-4-6-17/h7-11,17H,3-6,12H2,1-2H3,(H,22,26)(H,24,27) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme
Curated by ChEMBL
| Assay Description
Inhibition of methyltransferase activity of human EZH2 using chicken oligonucleotide as substrate by pull down assay |
J Med Chem 59: 1556-64 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01501
BindingDB Entry DOI: 10.7270/Q2B56MM7 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50149702
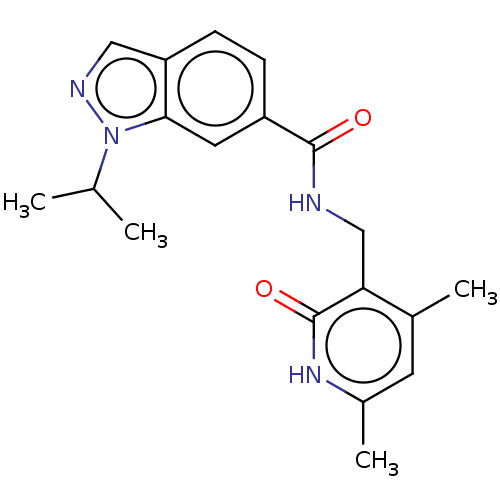
(CHEMBL3770192)Show SMILES CC(C)n1ncc2ccc(cc12)C(=O)NCc1c(C)cc(C)[nH]c1=O Show InChI InChI=1S/C19H22N4O2/c1-11(2)23-17-8-14(5-6-15(17)9-21-23)18(24)20-10-16-12(3)7-13(4)22-19(16)25/h5-9,11H,10H2,1-4H3,(H,20,24)(H,22,25) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme
Curated by ChEMBL
| Assay Description
Inhibition of methyltransferase activity of human EZH2 using chicken oligonucleotide as substrate by pull down assay |
J Med Chem 59: 1556-64 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01501
BindingDB Entry DOI: 10.7270/Q2B56MM7 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50149700

(CHEMBL3769778)Show SMILES CC(C)n1nnc2c(cccc12)C(=O)NCc1c(C)cc(C)[nH]c1=O Show InChI InChI=1S/C18H21N5O2/c1-10(2)23-15-7-5-6-13(16(15)21-22-23)17(24)19-9-14-11(3)8-12(4)20-18(14)25/h5-8,10H,9H2,1-4H3,(H,19,24)(H,20,25) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme
Curated by ChEMBL
| Assay Description
Inhibition of methyltransferase activity of human EZH2 using chicken oligonucleotide as substrate by pull down assay |
J Med Chem 59: 1556-64 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01501
BindingDB Entry DOI: 10.7270/Q2B56MM7 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50149699

(CHEMBL3770275)Show SMILES CC(C)n1cnc2c(cccc12)C(=O)NCc1c(C)cc(C)[nH]c1=O Show InChI InChI=1S/C19H22N4O2/c1-11(2)23-10-21-17-14(6-5-7-16(17)23)18(24)20-9-15-12(3)8-13(4)22-19(15)25/h5-8,10-11H,9H2,1-4H3,(H,20,24)(H,22,25) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme
Curated by ChEMBL
| Assay Description
Inhibition of methyltransferase activity of human EZH2 using chicken oligonucleotide as substrate by pull down assay |
J Med Chem 59: 1556-64 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01501
BindingDB Entry DOI: 10.7270/Q2B56MM7 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50149889

(CHEMBL3769571)Show SMILES CCN(C1CCOCC1)c1cc(Cl)cc(C(=O)NCc2c(C)cc(C)[nH]c2=O)c1C Show InChI InChI=1S/C23H30ClN3O3/c1-5-27(18-6-8-30-9-7-18)21-12-17(24)11-19(16(21)4)22(28)25-13-20-14(2)10-15(3)26-23(20)29/h10-12,18H,5-9,13H2,1-4H3,(H,25,28)(H,26,29) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 300 | n/a | n/a | n/a | n/a |
Epizyme
Curated by ChEMBL
| Assay Description
Inhibition of methyltransferase activity of EZH2 in human G401 cells assessed as H3K27 trimethylation after 4 hrs by ELISA |
J Med Chem 59: 1556-64 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01501
BindingDB Entry DOI: 10.7270/Q2B56MM7 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50075051
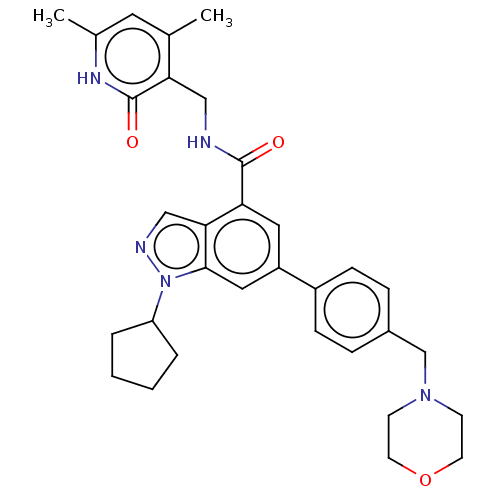
(CHEMBL3360855 | EPZ005687 | US10273223, Compound C...)Show SMILES Cc1cc(C)c(CNC(=O)c2cc(cc3n(ncc23)C2CCCC2)-c2ccc(CN3CCOCC3)cc2)c(=O)[nH]1 Show InChI InChI=1S/C32H37N5O3/c1-21-15-22(2)35-32(39)28(21)18-33-31(38)27-16-25(17-30-29(27)19-34-37(30)26-5-3-4-6-26)24-9-7-23(8-10-24)20-36-11-13-40-14-12-36/h7-10,15-17,19,26H,3-6,11-14,18,20H2,1-2H3,(H,33,38)(H,35,39) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 2.90E+3 | n/a | n/a | n/a | n/a |
Epizyme
Curated by ChEMBL
| Assay Description
Inhibition of methyltransferase activity of EZH2 in human G401 cells assessed as H3K27 trimethylation after 4 hrs by ELISA |
J Med Chem 59: 1556-64 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01501
BindingDB Entry DOI: 10.7270/Q2B56MM7 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM190179

(EPZ005030 | US10273223, Compound A-2 | US9175331, ...)Show SMILES CC(C)n1ncc2c(cc(nc12)-c1ccc(CN2CCOCC2)cc1)C(=O)NCc1c(C)cc(C)[nH]c1=O Show InChI InChI=1S/C29H34N6O3/c1-18(2)35-27-25(16-31-35)23(28(36)30-15-24-19(3)13-20(4)32-29(24)37)14-26(33-27)22-7-5-21(6-8-22)17-34-9-11-38-12-10-34/h5-8,13-14,16,18H,9-12,15,17H2,1-4H3,(H,30,36)(H,32,37) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.05E+4 | n/a | n/a | n/a | n/a |
Epizyme
Curated by ChEMBL
| Assay Description
Inhibition of methyltransferase activity of EZH2 in human G401 cells assessed as H3K27 trimethylation after 4 hrs by ELISA |
J Med Chem 59: 1556-64 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01501
BindingDB Entry DOI: 10.7270/Q2B56MM7 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM172038
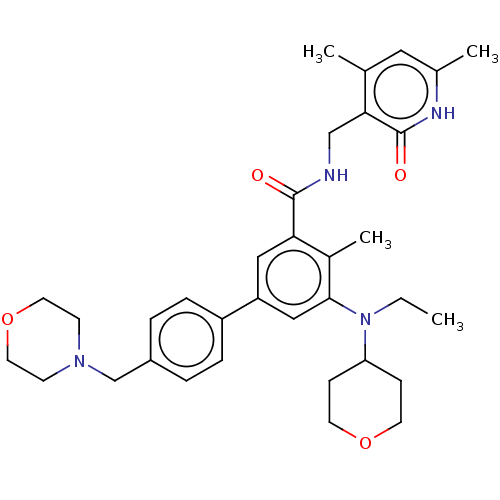
(US10155002, Compound 44 | US10647700, Compound EPZ...)Show SMILES CCN(C1CCOCC1)c1cc(cc(C(=O)NCc2c(C)cc(C)[nH]c2=O)c1C)-c1ccc(CN2CCOCC2)cc1 Show InChI InChI=1S/C34H44N4O4/c1-5-38(29-10-14-41-15-11-29)32-20-28(27-8-6-26(7-9-27)22-37-12-16-42-17-13-37)19-30(25(32)4)33(39)35-21-31-23(2)18-24(3)36-34(31)40/h6-9,18-20,29H,5,10-17,21-22H2,1-4H3,(H,35,39)(H,36,40) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 200 | n/a | n/a | n/a | n/a |
Epizyme
Curated by ChEMBL
| Assay Description
Inhibition of methyltransferase activity of EZH2 in human G401 cells assessed as H3K27 trimethylation after 4 hrs by ELISA |
J Med Chem 59: 1556-64 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01501
BindingDB Entry DOI: 10.7270/Q2B56MM7 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50149882

(CHEMBL3771083)Show SMILES CN(C1CCCC1)c1cc(Br)cc(C(=O)NCc2c(C)cc(C)[nH]c2=O)c1C Show InChI InChI=1S/C22H28BrN3O2/c1-13-9-14(2)25-22(28)19(13)12-24-21(27)18-10-16(23)11-20(15(18)3)26(4)17-7-5-6-8-17/h9-11,17H,5-8,12H2,1-4H3,(H,24,27)(H,25,28) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 500 | n/a | n/a | n/a | n/a |
Epizyme
Curated by ChEMBL
| Assay Description
Inhibition of methyltransferase activity of EZH2 in human G401 cells assessed as H3K27 trimethylation after 4 hrs by ELISA |
J Med Chem 59: 1556-64 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01501
BindingDB Entry DOI: 10.7270/Q2B56MM7 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM171998
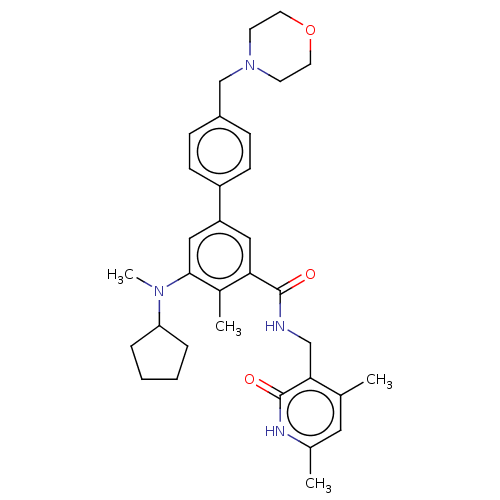
(US10155002, Compound 2 | US9090562, 2)Show SMILES CN(C1CCCC1)c1cc(cc(C(=O)NCc2c(C)cc(C)[nH]c2=O)c1C)-c1ccc(CN2CCOCC2)cc1 Show InChI InChI=1S/C33H42N4O3/c1-22-17-23(2)35-33(39)30(22)20-34-32(38)29-18-27(19-31(24(29)3)36(4)28-7-5-6-8-28)26-11-9-25(10-12-26)21-37-13-15-40-16-14-37/h9-12,17-19,28H,5-8,13-16,20-21H2,1-4H3,(H,34,38)(H,35,39) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 700 | n/a | n/a | n/a | n/a |
Epizyme
Curated by ChEMBL
| Assay Description
Inhibition of methyltransferase activity of EZH2 in human G401 cells assessed as H3K27 trimethylation after 4 hrs by ELISA |
J Med Chem 59: 1556-64 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01501
BindingDB Entry DOI: 10.7270/Q2B56MM7 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50149884

(CHEMBL3770973)Show SMILES CN(C1CCOCC1)c1cc(Br)cc(C(=O)NCc2c(C)cc(C)[nH]c2=O)c1C Show InChI InChI=1S/C22H28BrN3O3/c1-13-9-14(2)25-22(28)19(13)12-24-21(27)18-10-16(23)11-20(15(18)3)26(4)17-5-7-29-8-6-17/h9-11,17H,5-8,12H2,1-4H3,(H,24,27)(H,25,28) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 300 | n/a | n/a | n/a | n/a |
Epizyme
Curated by ChEMBL
| Assay Description
Inhibition of methyltransferase activity of EZH2 in human G401 cells assessed as H3K27 trimethylation after 4 hrs by ELISA |
J Med Chem 59: 1556-64 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01501
BindingDB Entry DOI: 10.7270/Q2B56MM7 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data