 Found 153 hits of Enzyme Inhibition Constant Data
Found 153 hits of Enzyme Inhibition Constant Data Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50370572

(CHEMBL85606 | SB-277011)Show SMILES O=C(N[C@H]1CC[C@H](CCN2CCc3cc(ccc3C2)C#N)CC1)c1ccnc2ccccc12 |r,wU:3.2,wD:6.6,(-5.17,2.38,;-5.16,.84,;-3.83,.08,;-2.49,.85,;-2.5,2.39,;-1.16,3.16,;.18,2.39,;1.51,3.16,;2.84,2.39,;4.18,3.16,;4.17,4.69,;5.51,5.46,;6.84,4.68,;8.18,5.44,;9.51,4.67,;9.5,3.12,;8.16,2.36,;6.83,3.14,;5.51,2.38,;10.85,5.43,;12.18,6.19,;.17,.85,;-1.15,.08,;-6.49,.07,;-7.83,.83,;-9.16,.06,;-9.16,-1.48,;-7.83,-2.25,;-7.83,-3.79,;-6.49,-4.56,;-5.15,-3.79,;-5.16,-2.25,;-6.49,-1.48,)| Show InChI InChI=1S/C28H30N4O/c29-18-21-5-8-23-19-32(16-13-22(23)17-21)15-12-20-6-9-24(10-7-20)31-28(33)26-11-14-30-27-4-2-1-3-25(26)27/h1-5,8,11,14,17,20,24H,6-7,9-10,12-13,15-16,19H2,(H,31,33)/t20-,24- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aptuit s.r.l
Curated by ChEMBL
| Assay Description
Antagonist activity at human dopamine D3 receptor expressed in CHO cells after 90 mins by [35S]-GTPgamma S assay |
Bioorg Med Chem Lett 26: 1329-32 (2016)
Article DOI: 10.1016/j.bmcl.2015.12.081
BindingDB Entry DOI: 10.7270/Q2HM5B83 |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Rattus norvegicus (Rat)) | BDBM50139853

(CHEMBL3764143 | US10577361, E14)Show SMILES Cn1c(SCCCN2CCOC(C2)c2ccc(cc2)C(F)(F)F)nnc1-c1ccc(cc1)-c1ncco1 Show InChI InChI=1S/C26H26F3N5O2S/c1-33-23(19-3-5-20(6-4-19)24-30-11-14-36-24)31-32-25(33)37-16-2-12-34-13-15-35-22(17-34)18-7-9-21(10-8-18)26(27,28)29/h3-11,14,22H,2,12-13,15-17H2,1H3 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aptuit s.r.l
Curated by ChEMBL
| Assay Description
Displacement of [3H]-dofetilide from human ERG potassium channel by scintillation proximity assay |
Bioorg Med Chem Lett 26: 1329-32 (2016)
Article DOI: 10.1016/j.bmcl.2015.12.081
BindingDB Entry DOI: 10.7270/Q2HM5B83 |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Rattus norvegicus (Rat)) | BDBM50139853

(CHEMBL3764143 | US10577361, E14)Show SMILES Cn1c(SCCCN2CCOC(C2)c2ccc(cc2)C(F)(F)F)nnc1-c1ccc(cc1)-c1ncco1 Show InChI InChI=1S/C26H26F3N5O2S/c1-33-23(19-3-5-20(6-4-19)24-30-11-14-36-24)31-32-25(33)37-16-2-12-34-13-15-35-22(17-34)18-7-9-21(10-8-18)26(27,28)29/h3-11,14,22H,2,12-13,15-17H2,1H3 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.01 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aptuit s.r.l
Curated by ChEMBL
| Assay Description
Displacement of [3H]-dofetilide from human ERG potassium channel by scintillation proximity assay |
Bioorg Med Chem Lett 26: 1329-32 (2016)
Article DOI: 10.1016/j.bmcl.2015.12.081
BindingDB Entry DOI: 10.7270/Q2HM5B83 |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Rattus norvegicus (Rat)) | BDBM50370572

(CHEMBL85606 | SB-277011)Show SMILES O=C(N[C@H]1CC[C@H](CCN2CCc3cc(ccc3C2)C#N)CC1)c1ccnc2ccccc12 |r,wU:3.2,wD:6.6,(-5.17,2.38,;-5.16,.84,;-3.83,.08,;-2.49,.85,;-2.5,2.39,;-1.16,3.16,;.18,2.39,;1.51,3.16,;2.84,2.39,;4.18,3.16,;4.17,4.69,;5.51,5.46,;6.84,4.68,;8.18,5.44,;9.51,4.67,;9.5,3.12,;8.16,2.36,;6.83,3.14,;5.51,2.38,;10.85,5.43,;12.18,6.19,;.17,.85,;-1.15,.08,;-6.49,.07,;-7.83,.83,;-9.16,.06,;-9.16,-1.48,;-7.83,-2.25,;-7.83,-3.79,;-6.49,-4.56,;-5.15,-3.79,;-5.16,-2.25,;-6.49,-1.48,)| Show InChI InChI=1S/C28H30N4O/c29-18-21-5-8-23-19-32(16-13-22(23)17-21)15-12-20-6-9-24(10-7-20)31-28(33)26-11-14-30-27-4-2-1-3-25(26)27/h1-5,8,11,14,17,20,24H,6-7,9-10,12-13,15-16,19H2,(H,31,33)/t20-,24- | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aptuit s.r.l
Curated by ChEMBL
| Assay Description
Displacement of [125I]-7-OH-PIPAT from rat brain dopamine D3 receptor after 45 mins by microplate scintillation counting analysis |
Bioorg Med Chem Lett 26: 1329-32 (2016)
Article DOI: 10.1016/j.bmcl.2015.12.081
BindingDB Entry DOI: 10.7270/Q2HM5B83 |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50139853

(CHEMBL3764143 | US10577361, E14)Show SMILES Cn1c(SCCCN2CCOC(C2)c2ccc(cc2)C(F)(F)F)nnc1-c1ccc(cc1)-c1ncco1 Show InChI InChI=1S/C26H26F3N5O2S/c1-33-23(19-3-5-20(6-4-19)24-30-11-14-36-24)31-32-25(33)37-16-2-12-34-13-15-35-22(17-34)18-7-9-21(10-8-18)26(27,28)29/h3-11,14,22H,2,12-13,15-17H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aptuit s.r.l
Curated by ChEMBL
| Assay Description
Agonist activity at human dopamine D3 receptor expressed in CHO cells after 90 mins by [35S]-GTPgamma S assay |
Bioorg Med Chem Lett 26: 1329-32 (2016)
Article DOI: 10.1016/j.bmcl.2015.12.081
BindingDB Entry DOI: 10.7270/Q2HM5B83 |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50139853

(CHEMBL3764143 | US10577361, E14)Show SMILES Cn1c(SCCCN2CCOC(C2)c2ccc(cc2)C(F)(F)F)nnc1-c1ccc(cc1)-c1ncco1 Show InChI InChI=1S/C26H26F3N5O2S/c1-33-23(19-3-5-20(6-4-19)24-30-11-14-36-24)31-32-25(33)37-16-2-12-34-13-15-35-22(17-34)18-7-9-21(10-8-18)26(27,28)29/h3-11,14,22H,2,12-13,15-17H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aptuit s.r.l
Curated by ChEMBL
| Assay Description
Agonist activity at human dopamine D3 receptor expressed in CHO cells after 90 mins by [35S]-GTPgamma S assay |
Bioorg Med Chem Lett 26: 1329-32 (2016)
Article DOI: 10.1016/j.bmcl.2015.12.081
BindingDB Entry DOI: 10.7270/Q2HM5B83 |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50139924
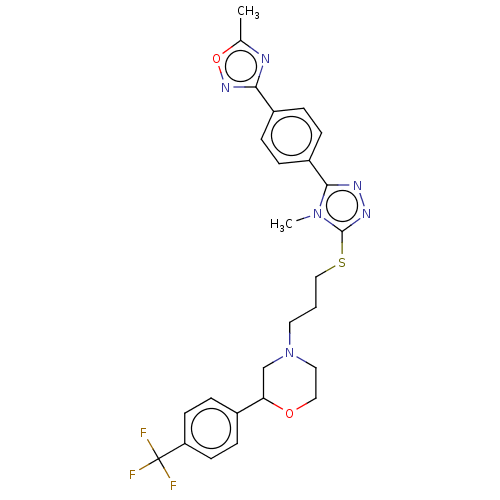
(CHEMBL3765266 | US10577361, E30)Show SMILES Cc1nc(no1)-c1ccc(cc1)-c1nnc(SCCCN2CCOC(C2)c2ccc(cc2)C(F)(F)F)n1C Show InChI InChI=1S/C26H27F3N6O2S/c1-17-30-23(33-37-17)19-4-6-20(7-5-19)24-31-32-25(34(24)2)38-15-3-12-35-13-14-36-22(16-35)18-8-10-21(11-9-18)26(27,28)29/h4-11,22H,3,12-16H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aptuit s.r.l
Curated by ChEMBL
| Assay Description
Antagonist activity at human dopamine D3 receptor expressed in CHO cells after 90 mins by [35S]-GTPgamma S assay |
Bioorg Med Chem Lett 26: 1329-32 (2016)
Article DOI: 10.1016/j.bmcl.2015.12.081
BindingDB Entry DOI: 10.7270/Q2HM5B83 |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50139923
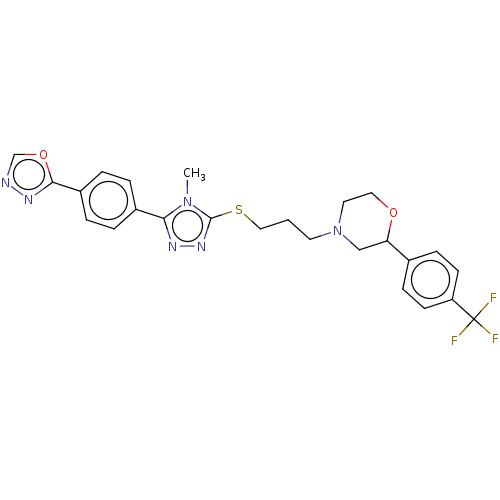
(CHEMBL3765285 | US10577361, E29)Show SMILES Cn1c(SCCCN2CCOC(C2)c2ccc(cc2)C(F)(F)F)nnc1-c1ccc(cc1)-c1nnco1 Show InChI InChI=1S/C25H25F3N6O2S/c1-33-22(18-3-5-19(6-4-18)23-31-29-16-36-23)30-32-24(33)37-14-2-11-34-12-13-35-21(15-34)17-7-9-20(10-8-17)25(26,27)28/h3-10,16,21H,2,11-15H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aptuit s.r.l
Curated by ChEMBL
| Assay Description
Antagonist activity at human dopamine D3 receptor expressed in CHO cells after 90 mins by [35S]-GTPgamma S assay |
Bioorg Med Chem Lett 26: 1329-32 (2016)
Article DOI: 10.1016/j.bmcl.2015.12.081
BindingDB Entry DOI: 10.7270/Q2HM5B83 |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Rattus norvegicus (Rat)) | BDBM50139923

(CHEMBL3765285 | US10577361, E29)Show SMILES Cn1c(SCCCN2CCOC(C2)c2ccc(cc2)C(F)(F)F)nnc1-c1ccc(cc1)-c1nnco1 Show InChI InChI=1S/C25H25F3N6O2S/c1-33-22(18-3-5-19(6-4-18)23-31-29-16-36-23)30-32-24(33)37-14-2-11-34-12-13-35-21(15-34)17-7-9-20(10-8-17)25(26,27)28/h3-10,16,21H,2,11-15H2,1H3 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aptuit s.r.l
Curated by ChEMBL
| Assay Description
Displacement of [125I]-7-OH-PIPAT from rat brain dopamine D3 receptor after 45 mins by microplate scintillation counting analysis |
Bioorg Med Chem Lett 26: 1329-32 (2016)
Article DOI: 10.1016/j.bmcl.2015.12.081
BindingDB Entry DOI: 10.7270/Q2HM5B83 |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50139851

(CHEMBL3764306 | US10577361, E20)Show SMILES Cn1c(SCCCN2CCOC(C2)c2ccc(cc2)C(F)(F)F)nnc1-c1ccc(nc1)C(N)=O Show InChI InChI=1S/C23H25F3N6O2S/c1-31-21(16-5-8-18(20(27)33)28-13-16)29-30-22(31)35-12-2-9-32-10-11-34-19(14-32)15-3-6-17(7-4-15)23(24,25)26/h3-8,13,19H,2,9-12,14H2,1H3,(H2,27,33) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aptuit s.r.l
Curated by ChEMBL
| Assay Description
Antagonist activity at human dopamine D3 receptor expressed in CHO cells after 90 mins by [35S]-GTPgamma S assay |
Bioorg Med Chem Lett 26: 1329-32 (2016)
Article DOI: 10.1016/j.bmcl.2015.12.081
BindingDB Entry DOI: 10.7270/Q2HM5B83 |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50139925
(CHEMBL3764246 | US10577361, E37)Show SMILES Cc1nc(no1)-c1ccc(cc1)-c1nnc(SCCCN2CCOC(C2)c2ccc(C)cc2)n1C Show InChI InChI=1S/C26H30N6O2S/c1-18-5-7-20(8-6-18)23-17-32(14-15-33-23)13-4-16-35-26-29-28-25(31(26)3)22-11-9-21(10-12-22)24-27-19(2)34-30-24/h5-12,23H,4,13-17H2,1-3H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aptuit s.r.l
Curated by ChEMBL
| Assay Description
Antagonist activity at human dopamine D3 receptor expressed in CHO cells after 90 mins by [35S]-GTPgamma S assay |
Bioorg Med Chem Lett 26: 1329-32 (2016)
Article DOI: 10.1016/j.bmcl.2015.12.081
BindingDB Entry DOI: 10.7270/Q2HM5B83 |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50139895
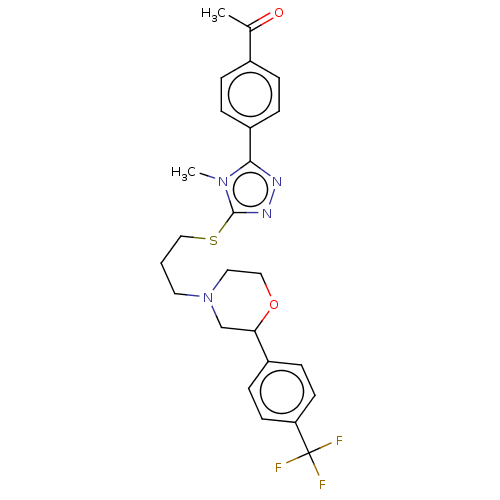
(CHEMBL3765267 | US10577361, E32)Show SMILES CC(=O)c1ccc(cc1)-c1nnc(SCCCN2CCOC(C2)c2ccc(cc2)C(F)(F)F)n1C Show InChI InChI=1S/C25H27F3N4O2S/c1-17(33)18-4-6-20(7-5-18)23-29-30-24(31(23)2)35-15-3-12-32-13-14-34-22(16-32)19-8-10-21(11-9-19)25(26,27)28/h4-11,22H,3,12-16H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aptuit s.r.l
Curated by ChEMBL
| Assay Description
Displacement of [125I]-7-OH-PIPAT from rat brain dopamine D2 receptor after 45 mins by microplate scintillation counting analysis |
Bioorg Med Chem Lett 26: 1329-32 (2016)
Article DOI: 10.1016/j.bmcl.2015.12.081
BindingDB Entry DOI: 10.7270/Q2HM5B83 |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50139852
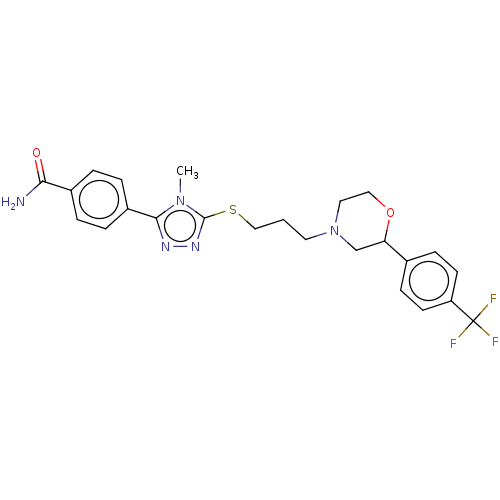
(CHEMBL3765426 | US10577361, E11)Show SMILES Cn1c(SCCCN2CCOC(C2)c2ccc(cc2)C(F)(F)F)nnc1-c1ccc(cc1)C(N)=O Show InChI InChI=1S/C24H26F3N5O2S/c1-31-22(18-5-3-17(4-6-18)21(28)33)29-30-23(31)35-14-2-11-32-12-13-34-20(15-32)16-7-9-19(10-8-16)24(25,26)27/h3-10,20H,2,11-15H2,1H3,(H2,28,33) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aptuit s.r.l
Curated by ChEMBL
| Assay Description
Antagonist activity at human dopamine D3 receptor expressed in CHO cells after 90 mins by [35S]-GTPgamma S assay |
Bioorg Med Chem Lett 26: 1329-32 (2016)
Article DOI: 10.1016/j.bmcl.2015.12.081
BindingDB Entry DOI: 10.7270/Q2HM5B83 |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50139852

(CHEMBL3765426 | US10577361, E11)Show SMILES Cn1c(SCCCN2CCOC(C2)c2ccc(cc2)C(F)(F)F)nnc1-c1ccc(cc1)C(N)=O Show InChI InChI=1S/C24H26F3N5O2S/c1-31-22(18-5-3-17(4-6-18)21(28)33)29-30-23(31)35-14-2-11-32-12-13-34-20(15-32)16-7-9-19(10-8-16)24(25,26)27/h3-10,20H,2,11-15H2,1H3,(H2,28,33) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aptuit s.r.l
Curated by ChEMBL
| Assay Description
Antagonist activity at human dopamine D3 receptor expressed in CHO cells after 90 mins by [35S]-GTPgamma S assay |
Bioorg Med Chem Lett 26: 1329-32 (2016)
Article DOI: 10.1016/j.bmcl.2015.12.081
BindingDB Entry DOI: 10.7270/Q2HM5B83 |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50139891
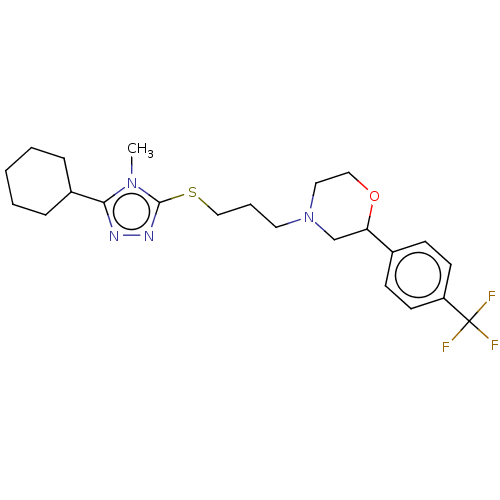
(CHEMBL3764245 | US10577361, E25)Show SMILES Cn1c(SCCCN2CCOC(C2)c2ccc(cc2)C(F)(F)F)nnc1C1CCCCC1 Show InChI InChI=1S/C23H31F3N4OS/c1-29-21(18-6-3-2-4-7-18)27-28-22(29)32-15-5-12-30-13-14-31-20(16-30)17-8-10-19(11-9-17)23(24,25)26/h8-11,18,20H,2-7,12-16H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aptuit s.r.l
Curated by ChEMBL
| Assay Description
Antagonist activity at human dopamine D3 receptor expressed in CHO cells after 90 mins by [35S]-GTPgamma S assay |
Bioorg Med Chem Lett 26: 1329-32 (2016)
Article DOI: 10.1016/j.bmcl.2015.12.081
BindingDB Entry DOI: 10.7270/Q2HM5B83 |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50139850
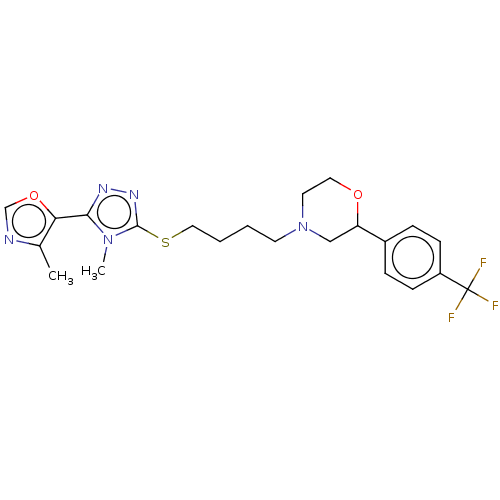
(CHEMBL3763418 | US10577361, E4)Show SMILES Cc1ncoc1-c1nnc(SCCCCN2CCOC(C2)c2ccc(cc2)C(F)(F)F)n1C Show InChI InChI=1S/C22H26F3N5O2S/c1-15-19(32-14-26-15)20-27-28-21(29(20)2)33-12-4-3-9-30-10-11-31-18(13-30)16-5-7-17(8-6-16)22(23,24)25/h5-8,14,18H,3-4,9-13H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aptuit s.r.l
Curated by ChEMBL
| Assay Description
Antagonist activity at human dopamine D3 receptor expressed in CHO cells after 90 mins by [35S]-GTPgamma S assay |
Bioorg Med Chem Lett 26: 1329-32 (2016)
Article DOI: 10.1016/j.bmcl.2015.12.081
BindingDB Entry DOI: 10.7270/Q2HM5B83 |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Rattus norvegicus (Rat)) | BDBM50139924

(CHEMBL3765266 | US10577361, E30)Show SMILES Cc1nc(no1)-c1ccc(cc1)-c1nnc(SCCCN2CCOC(C2)c2ccc(cc2)C(F)(F)F)n1C Show InChI InChI=1S/C26H27F3N6O2S/c1-17-30-23(33-37-17)19-4-6-20(7-5-19)24-31-32-25(34(24)2)38-15-3-12-35-13-14-36-22(16-35)18-8-10-21(11-9-18)26(27,28)29/h4-11,22H,3,12-16H2,1-2H3 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aptuit s.r.l
Curated by ChEMBL
| Assay Description
Displacement of [125I]-7-OH-PIPAT from rat brain dopamine D3 receptor after 45 mins by microplate scintillation counting analysis |
Bioorg Med Chem Lett 26: 1329-32 (2016)
Article DOI: 10.1016/j.bmcl.2015.12.081
BindingDB Entry DOI: 10.7270/Q2HM5B83 |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50139861

(CHEMBL3764557 | US10577361, E7)Show SMILES Cc1ncoc1-c1nnc(SCCCN2CCOC(C2)c2ccc(cc2)C(F)(F)F)n1C Show InChI InChI=1S/C21H24F3N5O2S/c1-14-18(31-13-25-14)19-26-27-20(28(19)2)32-11-3-8-29-9-10-30-17(12-29)15-4-6-16(7-5-15)21(22,23)24/h4-7,13,17H,3,8-12H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aptuit s.r.l
Curated by ChEMBL
| Assay Description
Antagonist activity at human dopamine D3 receptor expressed in CHO cells after 90 mins by [35S]-GTPgamma S assay |
Bioorg Med Chem Lett 26: 1329-32 (2016)
Article DOI: 10.1016/j.bmcl.2015.12.081
BindingDB Entry DOI: 10.7270/Q2HM5B83 |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50139850

(CHEMBL3763418 | US10577361, E4)Show SMILES Cc1ncoc1-c1nnc(SCCCCN2CCOC(C2)c2ccc(cc2)C(F)(F)F)n1C Show InChI InChI=1S/C22H26F3N5O2S/c1-15-19(32-14-26-15)20-27-28-21(29(20)2)33-12-4-3-9-30-10-11-31-18(13-30)16-5-7-17(8-6-16)22(23,24)25/h5-8,14,18H,3-4,9-13H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 25.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aptuit s.r.l
Curated by ChEMBL
| Assay Description
Displacement of [125I]-7-OH-PIPAT from rat brain dopamine D2 receptor after 45 mins by microplate scintillation counting analysis |
Bioorg Med Chem Lett 26: 1329-32 (2016)
Article DOI: 10.1016/j.bmcl.2015.12.081
BindingDB Entry DOI: 10.7270/Q2HM5B83 |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Rattus norvegicus (Rat)) | BDBM50139852

(CHEMBL3765426 | US10577361, E11)Show SMILES Cn1c(SCCCN2CCOC(C2)c2ccc(cc2)C(F)(F)F)nnc1-c1ccc(cc1)C(N)=O Show InChI InChI=1S/C24H26F3N5O2S/c1-31-22(18-5-3-17(4-6-18)21(28)33)29-30-23(31)35-14-2-11-32-12-13-34-20(15-32)16-7-9-19(10-8-16)24(25,26)27/h3-10,20H,2,11-15H2,1H3,(H2,28,33) | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 31.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aptuit s.r.l
Curated by ChEMBL
| Assay Description
Displacement of [125I]-7-OH-PIPAT from rat brain dopamine D3 receptor after 45 mins by microplate scintillation counting analysis |
Bioorg Med Chem Lett 26: 1329-32 (2016)
Article DOI: 10.1016/j.bmcl.2015.12.081
BindingDB Entry DOI: 10.7270/Q2HM5B83 |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Rattus norvegicus (Rat)) | BDBM50139851

(CHEMBL3764306 | US10577361, E20)Show SMILES Cn1c(SCCCN2CCOC(C2)c2ccc(cc2)C(F)(F)F)nnc1-c1ccc(nc1)C(N)=O Show InChI InChI=1S/C23H25F3N6O2S/c1-31-21(16-5-8-18(20(27)33)28-13-16)29-30-22(31)35-12-2-9-32-10-11-34-19(14-32)15-3-6-17(7-4-15)23(24,25)26/h3-8,13,19H,2,9-12,14H2,1H3,(H2,27,33) | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aptuit s.r.l
Curated by ChEMBL
| Assay Description
Displacement of [125I]-7-OH-PIPAT from rat brain dopamine D3 receptor after 45 mins by microplate scintillation counting analysis |
Bioorg Med Chem Lett 26: 1329-32 (2016)
Article DOI: 10.1016/j.bmcl.2015.12.081
BindingDB Entry DOI: 10.7270/Q2HM5B83 |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Rattus norvegicus (Rat)) | BDBM50139852

(CHEMBL3765426 | US10577361, E11)Show SMILES Cn1c(SCCCN2CCOC(C2)c2ccc(cc2)C(F)(F)F)nnc1-c1ccc(cc1)C(N)=O Show InChI InChI=1S/C24H26F3N5O2S/c1-31-22(18-5-3-17(4-6-18)21(28)33)29-30-23(31)35-14-2-11-32-12-13-34-20(15-32)16-7-9-19(10-8-16)24(25,26)27/h3-10,20H,2,11-15H2,1H3,(H2,28,33) | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aptuit s.r.l
Curated by ChEMBL
| Assay Description
Agonist activity at human dopamine D3 receptor expressed in CHO cells after 90 mins by [35S]-GTPgamma S assay |
Bioorg Med Chem Lett 26: 1329-32 (2016)
Article DOI: 10.1016/j.bmcl.2015.12.081
BindingDB Entry DOI: 10.7270/Q2HM5B83 |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Rattus norvegicus (Rat)) | BDBM50139891

(CHEMBL3764245 | US10577361, E25)Show SMILES Cn1c(SCCCN2CCOC(C2)c2ccc(cc2)C(F)(F)F)nnc1C1CCCCC1 Show InChI InChI=1S/C23H31F3N4OS/c1-29-21(18-6-3-2-4-7-18)27-28-22(29)32-15-5-12-30-13-14-31-20(16-30)17-8-10-19(11-9-17)23(24,25)26/h8-11,18,20H,2-7,12-16H2,1H3 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aptuit s.r.l
Curated by ChEMBL
| Assay Description
Displacement of [125I]-7-OH-PIPAT from rat brain dopamine D3 receptor after 45 mins by microplate scintillation counting analysis |
Bioorg Med Chem Lett 26: 1329-32 (2016)
Article DOI: 10.1016/j.bmcl.2015.12.081
BindingDB Entry DOI: 10.7270/Q2HM5B83 |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50139921
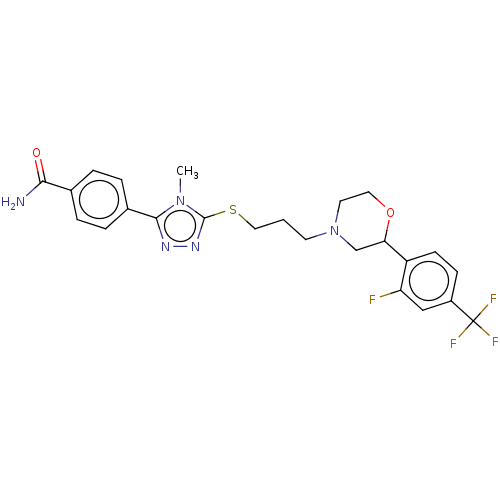
(CHEMBL3764597 | US10577361, E35)Show SMILES Cn1c(SCCCN2CCOC(C2)c2ccc(cc2F)C(F)(F)F)nnc1-c1ccc(cc1)C(N)=O Show InChI InChI=1S/C24H25F4N5O2S/c1-32-22(16-5-3-15(4-6-16)21(29)34)30-31-23(32)36-12-2-9-33-10-11-35-20(14-33)18-8-7-17(13-19(18)25)24(26,27)28/h3-8,13,20H,2,9-12,14H2,1H3,(H2,29,34) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aptuit s.r.l
Curated by ChEMBL
| Assay Description
Agonist activity at human dopamine D3 receptor expressed in CHO cells after 90 mins by [35S]-GTPgamma S assay |
Bioorg Med Chem Lett 26: 1329-32 (2016)
Article DOI: 10.1016/j.bmcl.2015.12.081
BindingDB Entry DOI: 10.7270/Q2HM5B83 |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Rattus norvegicus (Rat)) | BDBM50139921

(CHEMBL3764597 | US10577361, E35)Show SMILES Cn1c(SCCCN2CCOC(C2)c2ccc(cc2F)C(F)(F)F)nnc1-c1ccc(cc1)C(N)=O Show InChI InChI=1S/C24H25F4N5O2S/c1-32-22(16-5-3-15(4-6-16)21(29)34)30-31-23(32)36-12-2-9-33-10-11-35-20(14-33)18-8-7-17(13-19(18)25)24(26,27)28/h3-8,13,20H,2,9-12,14H2,1H3,(H2,29,34) | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 63 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aptuit s.r.l
Curated by ChEMBL
| Assay Description
Displacement of [125I]-7-OH-PIPAT from rat brain dopamine D3 receptor after 45 mins by microplate scintillation counting analysis |
Bioorg Med Chem Lett 26: 1329-32 (2016)
Article DOI: 10.1016/j.bmcl.2015.12.081
BindingDB Entry DOI: 10.7270/Q2HM5B83 |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Rattus norvegicus (Rat)) | BDBM50139895

(CHEMBL3765267 | US10577361, E32)Show SMILES CC(=O)c1ccc(cc1)-c1nnc(SCCCN2CCOC(C2)c2ccc(cc2)C(F)(F)F)n1C Show InChI InChI=1S/C25H27F3N4O2S/c1-17(33)18-4-6-20(7-5-18)23-29-30-24(31(23)2)35-15-3-12-32-13-14-34-22(16-32)19-8-10-21(11-9-19)25(26,27)28/h4-11,22H,3,12-16H2,1-2H3 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 63 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aptuit s.r.l
Curated by ChEMBL
| Assay Description
Displacement of [125I]-7-OH-PIPAT from rat brain dopamine D3 receptor after 45 mins by microplate scintillation counting analysis |
Bioorg Med Chem Lett 26: 1329-32 (2016)
Article DOI: 10.1016/j.bmcl.2015.12.081
BindingDB Entry DOI: 10.7270/Q2HM5B83 |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Rattus norvegicus (Rat)) | BDBM50139850

(CHEMBL3763418 | US10577361, E4)Show SMILES Cc1ncoc1-c1nnc(SCCCCN2CCOC(C2)c2ccc(cc2)C(F)(F)F)n1C Show InChI InChI=1S/C22H26F3N5O2S/c1-15-19(32-14-26-15)20-27-28-21(29(20)2)33-12-4-3-9-30-10-11-31-18(13-30)16-5-7-17(8-6-16)22(23,24)25/h5-8,14,18H,3-4,9-13H2,1-2H3 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 79 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aptuit s.r.l
Curated by ChEMBL
| Assay Description
Agonist activity at human dopamine D3 receptor expressed in CHO cells after 90 mins by [35S]-GTPgamma S assay |
Bioorg Med Chem Lett 26: 1329-32 (2016)
Article DOI: 10.1016/j.bmcl.2015.12.081
BindingDB Entry DOI: 10.7270/Q2HM5B83 |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Rattus norvegicus (Rat)) | BDBM50139925

(CHEMBL3764246 | US10577361, E37)Show SMILES Cc1nc(no1)-c1ccc(cc1)-c1nnc(SCCCN2CCOC(C2)c2ccc(C)cc2)n1C Show InChI InChI=1S/C26H30N6O2S/c1-18-5-7-20(8-6-18)23-17-32(14-15-33-23)13-4-16-35-26-29-28-25(31(26)3)22-11-9-21(10-12-22)24-27-19(2)34-30-24/h5-12,23H,4,13-17H2,1-3H3 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 79 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aptuit s.r.l
Curated by ChEMBL
| Assay Description
Displacement of [125I]-7-OH-PIPAT from rat brain dopamine D3 receptor after 45 mins by microplate scintillation counting analysis |
Bioorg Med Chem Lett 26: 1329-32 (2016)
Article DOI: 10.1016/j.bmcl.2015.12.081
BindingDB Entry DOI: 10.7270/Q2HM5B83 |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Rattus norvegicus (Rat)) | BDBM50139850

(CHEMBL3763418 | US10577361, E4)Show SMILES Cc1ncoc1-c1nnc(SCCCCN2CCOC(C2)c2ccc(cc2)C(F)(F)F)n1C Show InChI InChI=1S/C22H26F3N5O2S/c1-15-19(32-14-26-15)20-27-28-21(29(20)2)33-12-4-3-9-30-10-11-31-18(13-30)16-5-7-17(8-6-16)22(23,24)25/h5-8,14,18H,3-4,9-13H2,1-2H3 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 79 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aptuit s.r.l
Curated by ChEMBL
| Assay Description
Displacement of [125I]-7-OH-PIPAT from rat brain dopamine D3 receptor after 45 mins by microplate scintillation counting analysis |
Bioorg Med Chem Lett 26: 1329-32 (2016)
Article DOI: 10.1016/j.bmcl.2015.12.081
BindingDB Entry DOI: 10.7270/Q2HM5B83 |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Rattus norvegicus (Rat)) | BDBM50139866
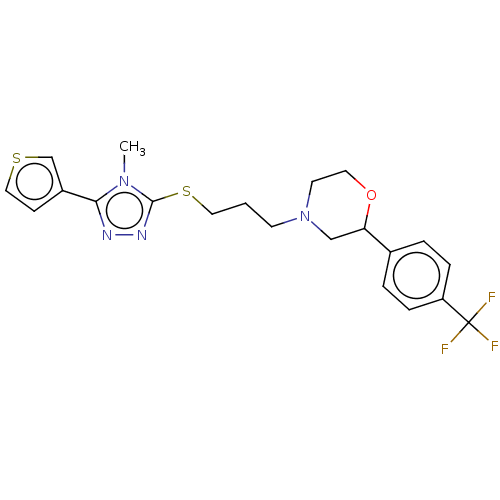
(CHEMBL3764191 | US10577361, E28)Show SMILES Cn1c(SCCCN2CCOC(C2)c2ccc(cc2)C(F)(F)F)nnc1-c1ccsc1 Show InChI InChI=1S/C21H23F3N4OS2/c1-27-19(16-7-12-30-14-16)25-26-20(27)31-11-2-8-28-9-10-29-18(13-28)15-3-5-17(6-4-15)21(22,23)24/h3-7,12,14,18H,2,8-11,13H2,1H3 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aptuit s.r.l
Curated by ChEMBL
| Assay Description
Displacement of [125I]-7-OH-PIPAT from rat brain dopamine D3 receptor after 45 mins by microplate scintillation counting analysis |
Bioorg Med Chem Lett 26: 1329-32 (2016)
Article DOI: 10.1016/j.bmcl.2015.12.081
BindingDB Entry DOI: 10.7270/Q2HM5B83 |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Rattus norvegicus (Rat)) | BDBM50139898
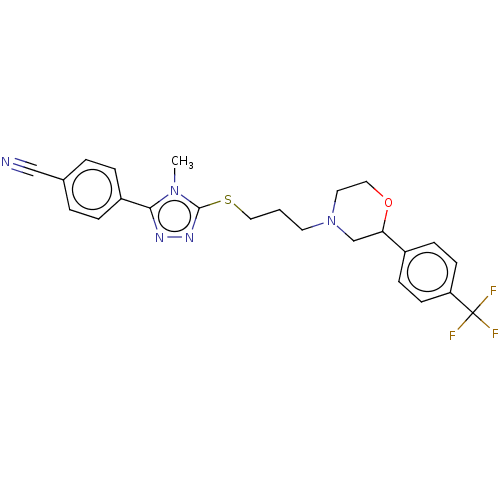
(CHEMBL3764639 | US10577361, E31)Show SMILES Cn1c(SCCCN2CCOC(C2)c2ccc(cc2)C(F)(F)F)nnc1-c1ccc(cc1)C#N Show InChI InChI=1S/C24H24F3N5OS/c1-31-22(19-5-3-17(15-28)4-6-19)29-30-23(31)34-14-2-11-32-12-13-33-21(16-32)18-7-9-20(10-8-18)24(25,26)27/h3-10,21H,2,11-14,16H2,1H3 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aptuit s.r.l
Curated by ChEMBL
| Assay Description
Displacement of [125I]-7-OH-PIPAT from rat brain dopamine D3 receptor after 45 mins by microplate scintillation counting analysis |
Bioorg Med Chem Lett 26: 1329-32 (2016)
Article DOI: 10.1016/j.bmcl.2015.12.081
BindingDB Entry DOI: 10.7270/Q2HM5B83 |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Rattus norvegicus (Rat)) | BDBM50139854
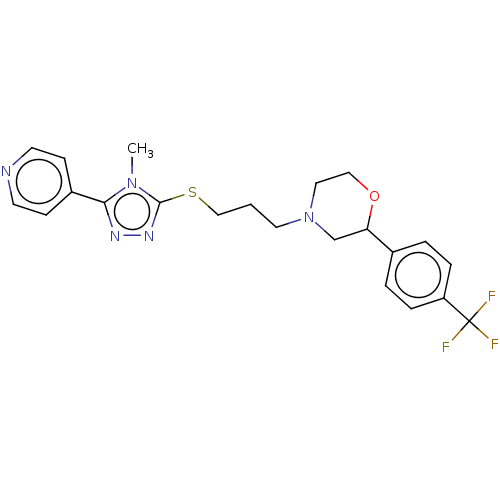
(CHEMBL3763200 | US10577361, E17)Show SMILES Cn1c(SCCCN2CCOC(C2)c2ccc(cc2)C(F)(F)F)nnc1-c1ccncc1 Show InChI InChI=1S/C22H24F3N5OS/c1-29-20(17-7-9-26-10-8-17)27-28-21(29)32-14-2-11-30-12-13-31-19(15-30)16-3-5-18(6-4-16)22(23,24)25/h3-10,19H,2,11-15H2,1H3 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aptuit s.r.l
Curated by ChEMBL
| Assay Description
Displacement of [125I]-7-OH-PIPAT from rat brain dopamine D3 receptor after 45 mins by microplate scintillation counting analysis |
Bioorg Med Chem Lett 26: 1329-32 (2016)
Article DOI: 10.1016/j.bmcl.2015.12.081
BindingDB Entry DOI: 10.7270/Q2HM5B83 |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Rattus norvegicus (Rat)) | BDBM50139857
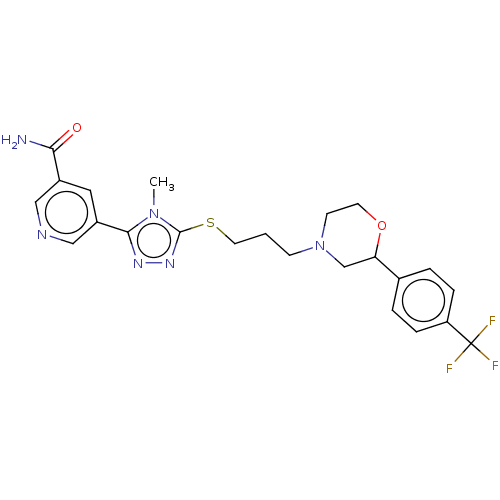
(CHEMBL3765422)Show SMILES Cn1c(SCCCN2CCOC(C2)c2ccc(cc2)C(F)(F)F)nnc1-c1cncc(c1)C(N)=O Show InChI InChI=1S/C23H25F3N6O2S/c1-31-21(17-11-16(20(27)33)12-28-13-17)29-30-22(31)35-10-2-7-32-8-9-34-19(14-32)15-3-5-18(6-4-15)23(24,25)26/h3-6,11-13,19H,2,7-10,14H2,1H3,(H2,27,33) | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aptuit s.r.l
Curated by ChEMBL
| Assay Description
Displacement of [125I]-7-OH-PIPAT from rat brain dopamine D3 receptor after 45 mins by microplate scintillation counting analysis |
Bioorg Med Chem Lett 26: 1329-32 (2016)
Article DOI: 10.1016/j.bmcl.2015.12.081
BindingDB Entry DOI: 10.7270/Q2HM5B83 |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Rattus norvegicus (Rat)) | BDBM50139861

(CHEMBL3764557 | US10577361, E7)Show SMILES Cc1ncoc1-c1nnc(SCCCN2CCOC(C2)c2ccc(cc2)C(F)(F)F)n1C Show InChI InChI=1S/C21H24F3N5O2S/c1-14-18(31-13-25-14)19-26-27-20(28(19)2)32-11-3-8-29-9-10-30-17(12-29)15-4-6-16(7-5-15)21(22,23)24/h4-7,13,17H,3,8-12H2,1-2H3 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 125 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aptuit s.r.l
Curated by ChEMBL
| Assay Description
Displacement of [125I]-7-OH-PIPAT from rat brain dopamine D3 receptor after 45 mins by microplate scintillation counting analysis |
Bioorg Med Chem Lett 26: 1329-32 (2016)
Article DOI: 10.1016/j.bmcl.2015.12.081
BindingDB Entry DOI: 10.7270/Q2HM5B83 |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Rattus norvegicus (Rat)) | BDBM50139888
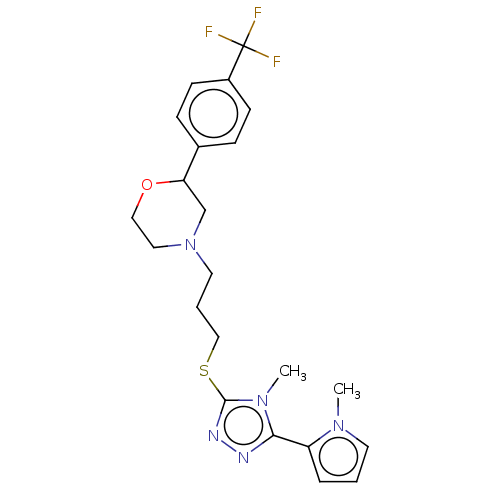
(CHEMBL3764739 | US10577361, E24)Show SMILES Cn1cccc1-c1nnc(SCCCN2CCOC(C2)c2ccc(cc2)C(F)(F)F)n1C Show InChI InChI=1S/C22H26F3N5OS/c1-28-10-3-5-18(28)20-26-27-21(29(20)2)32-14-4-11-30-12-13-31-19(15-30)16-6-8-17(9-7-16)22(23,24)25/h3,5-10,19H,4,11-15H2,1-2H3 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 126 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aptuit s.r.l
Curated by ChEMBL
| Assay Description
Displacement of [125I]-7-OH-PIPAT from rat brain dopamine D3 receptor after 45 mins by microplate scintillation counting analysis |
Bioorg Med Chem Lett 26: 1329-32 (2016)
Article DOI: 10.1016/j.bmcl.2015.12.081
BindingDB Entry DOI: 10.7270/Q2HM5B83 |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Rattus norvegicus (Rat)) | BDBM50139897

(CHEMBL3764830 | US10577361, E33)Show SMILES Cn1c(SCCCN2CCOC(C2)c2ccc(cc2)C(F)(F)F)nnc1-c1ccc(cc1)S(N)(=O)=O Show InChI InChI=1S/C23H26F3N5O3S2/c1-30-21(17-5-9-19(10-6-17)36(27,32)33)28-29-22(30)35-14-2-11-31-12-13-34-20(15-31)16-3-7-18(8-4-16)23(24,25)26/h3-10,20H,2,11-15H2,1H3,(H2,27,32,33) | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 126 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aptuit s.r.l
Curated by ChEMBL
| Assay Description
Displacement of [125I]-7-OH-PIPAT from rat brain dopamine D3 receptor after 45 mins by microplate scintillation counting analysis |
Bioorg Med Chem Lett 26: 1329-32 (2016)
Article DOI: 10.1016/j.bmcl.2015.12.081
BindingDB Entry DOI: 10.7270/Q2HM5B83 |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Rattus norvegicus (Rat)) | BDBM50139861

(CHEMBL3764557 | US10577361, E7)Show SMILES Cc1ncoc1-c1nnc(SCCCN2CCOC(C2)c2ccc(cc2)C(F)(F)F)n1C Show InChI InChI=1S/C21H24F3N5O2S/c1-14-18(31-13-25-14)19-26-27-20(28(19)2)32-11-3-8-29-9-10-30-17(12-29)15-4-6-16(7-5-15)21(22,23)24/h4-7,13,17H,3,8-12H2,1-2H3 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 126 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aptuit s.r.l
Curated by ChEMBL
| Assay Description
Agonist activity at human dopamine D3 receptor expressed in CHO cells after 90 mins by [35S]-GTPgamma S assay |
Bioorg Med Chem Lett 26: 1329-32 (2016)
Article DOI: 10.1016/j.bmcl.2015.12.081
BindingDB Entry DOI: 10.7270/Q2HM5B83 |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Rattus norvegicus (Rat)) | BDBM50139922
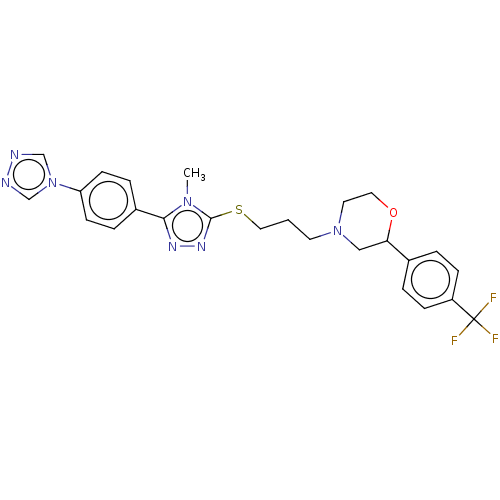
(CHEMBL3765072 | US10577361, E21)Show SMILES Cn1c(SCCCN2CCOC(C2)c2ccc(cc2)C(F)(F)F)nnc1-c1ccc(cc1)-n1cnnc1 Show InChI InChI=1S/C25H26F3N7OS/c1-33-23(19-5-9-21(10-6-19)35-16-29-30-17-35)31-32-24(33)37-14-2-11-34-12-13-36-22(15-34)18-3-7-20(8-4-18)25(26,27)28/h3-10,16-17,22H,2,11-15H2,1H3 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 126 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aptuit s.r.l
Curated by ChEMBL
| Assay Description
Displacement of [125I]-7-OH-PIPAT from rat brain dopamine D3 receptor after 45 mins by microplate scintillation counting analysis |
Bioorg Med Chem Lett 26: 1329-32 (2016)
Article DOI: 10.1016/j.bmcl.2015.12.081
BindingDB Entry DOI: 10.7270/Q2HM5B83 |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Rattus norvegicus (Rat)) | BDBM50139856

(CHEMBL3765169 | US10577361, E19)Show SMILES Cn1c(SCCCN2CCOC(C2)c2ccc(cc2)C(F)(F)F)nnc1-c1cnccn1 Show InChI InChI=1S/C21H23F3N6OS/c1-29-19(17-13-25-7-8-26-17)27-28-20(29)32-12-2-9-30-10-11-31-18(14-30)15-3-5-16(6-4-15)21(22,23)24/h3-8,13,18H,2,9-12,14H2,1H3 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 158 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aptuit s.r.l
Curated by ChEMBL
| Assay Description
Displacement of [125I]-7-OH-PIPAT from rat brain dopamine D3 receptor after 45 mins by microplate scintillation counting analysis |
Bioorg Med Chem Lett 26: 1329-32 (2016)
Article DOI: 10.1016/j.bmcl.2015.12.081
BindingDB Entry DOI: 10.7270/Q2HM5B83 |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Rattus norvegicus (Rat)) | BDBM50139863
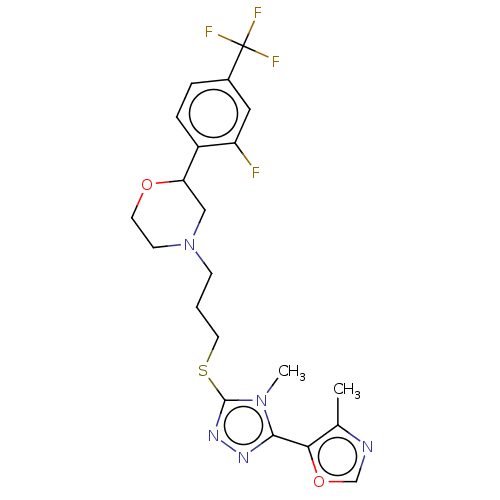
(CHEMBL3764586 | US10577361, E34)Show SMILES Cc1ncoc1-c1nnc(SCCCN2CCOC(C2)c2ccc(cc2F)C(F)(F)F)n1C Show InChI InChI=1S/C21H23F4N5O2S/c1-13-18(32-12-26-13)19-27-28-20(29(19)2)33-9-3-6-30-7-8-31-17(11-30)15-5-4-14(10-16(15)22)21(23,24)25/h4-5,10,12,17H,3,6-9,11H2,1-2H3 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aptuit s.r.l
Curated by ChEMBL
| Assay Description
Displacement of [125I]-7-OH-PIPAT from rat brain dopamine D3 receptor after 45 mins by microplate scintillation counting analysis |
Bioorg Med Chem Lett 26: 1329-32 (2016)
Article DOI: 10.1016/j.bmcl.2015.12.081
BindingDB Entry DOI: 10.7270/Q2HM5B83 |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Rattus norvegicus (Rat)) | BDBM50139865
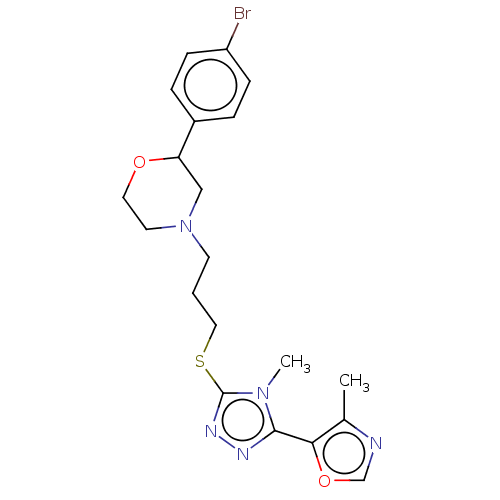
(CHEMBL3764833 | US10577361, E38)Show SMILES Cc1ncoc1-c1nnc(SCCCN2CCOC(C2)c2ccc(Br)cc2)n1C Show InChI InChI=1S/C20H24BrN5O2S/c1-14-18(28-13-22-14)19-23-24-20(25(19)2)29-11-3-8-26-9-10-27-17(12-26)15-4-6-16(21)7-5-15/h4-7,13,17H,3,8-12H2,1-2H3 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aptuit s.r.l
Curated by ChEMBL
| Assay Description
Displacement of [125I]-7-OH-PIPAT from rat brain dopamine D3 receptor after 45 mins by microplate scintillation counting analysis |
Bioorg Med Chem Lett 26: 1329-32 (2016)
Article DOI: 10.1016/j.bmcl.2015.12.081
BindingDB Entry DOI: 10.7270/Q2HM5B83 |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Rattus norvegicus (Rat)) | BDBM50139867
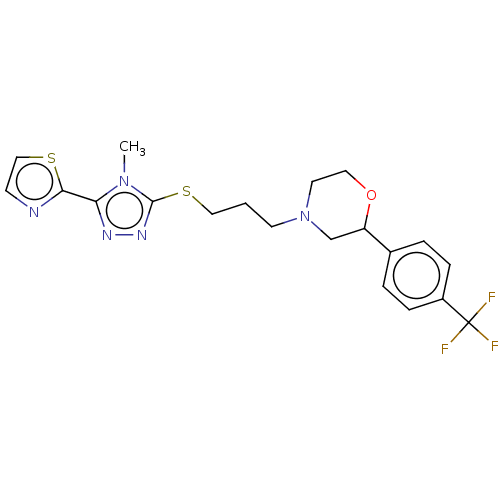
(CHEMBL3764884 | US10577361, E26)Show SMILES Cn1c(SCCCN2CCOC(C2)c2ccc(cc2)C(F)(F)F)nnc1-c1nccs1 Show InChI InChI=1S/C20H22F3N5OS2/c1-27-17(18-24-7-12-30-18)25-26-19(27)31-11-2-8-28-9-10-29-16(13-28)14-3-5-15(6-4-14)20(21,22)23/h3-7,12,16H,2,8-11,13H2,1H3 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 251 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aptuit s.r.l
Curated by ChEMBL
| Assay Description
Displacement of [125I]-7-OH-PIPAT from rat brain dopamine D3 receptor after 45 mins by microplate scintillation counting analysis |
Bioorg Med Chem Lett 26: 1329-32 (2016)
Article DOI: 10.1016/j.bmcl.2015.12.081
BindingDB Entry DOI: 10.7270/Q2HM5B83 |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Rattus norvegicus (Rat)) | BDBM50139855
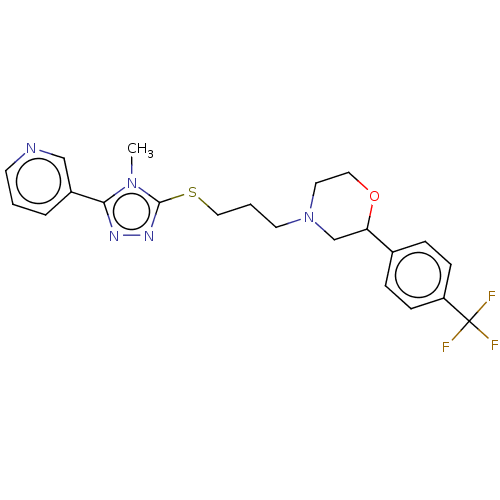
(CHEMBL3764612 | US10577361, E18)Show SMILES Cn1c(SCCCN2CCOC(C2)c2ccc(cc2)C(F)(F)F)nnc1-c1cccnc1 Show InChI InChI=1S/C22H24F3N5OS/c1-29-20(17-4-2-9-26-14-17)27-28-21(29)32-13-3-10-30-11-12-31-19(15-30)16-5-7-18(8-6-16)22(23,24)25/h2,4-9,14,19H,3,10-13,15H2,1H3 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 251 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aptuit s.r.l
Curated by ChEMBL
| Assay Description
Displacement of [125I]-7-OH-PIPAT from rat brain dopamine D3 receptor after 45 mins by microplate scintillation counting analysis |
Bioorg Med Chem Lett 26: 1329-32 (2016)
Article DOI: 10.1016/j.bmcl.2015.12.081
BindingDB Entry DOI: 10.7270/Q2HM5B83 |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Rattus norvegicus (Rat)) | BDBM50139859
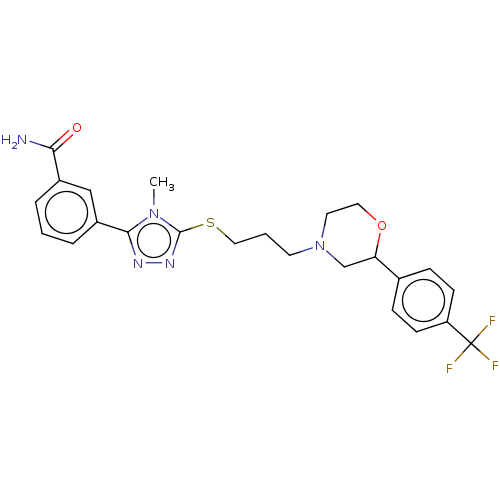
(CHEMBL3764212 | US10577361, E23)Show SMILES Cn1c(SCCCN2CCOC(C2)c2ccc(cc2)C(F)(F)F)nnc1-c1cccc(c1)C(N)=O Show InChI InChI=1S/C24H26F3N5O2S/c1-31-22(18-5-2-4-17(14-18)21(28)33)29-30-23(31)35-13-3-10-32-11-12-34-20(15-32)16-6-8-19(9-7-16)24(25,26)27/h2,4-9,14,20H,3,10-13,15H2,1H3,(H2,28,33) | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aptuit s.r.l
Curated by ChEMBL
| Assay Description
Displacement of [125I]-7-OH-PIPAT from rat brain dopamine D3 receptor after 45 mins by microplate scintillation counting analysis |
Bioorg Med Chem Lett 26: 1329-32 (2016)
Article DOI: 10.1016/j.bmcl.2015.12.081
BindingDB Entry DOI: 10.7270/Q2HM5B83 |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Rattus norvegicus (Rat)) | BDBM50139860
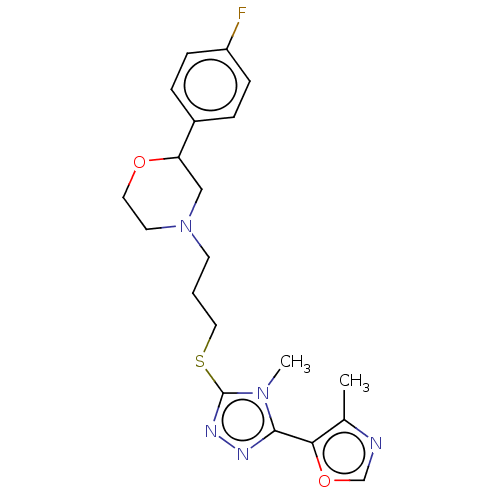
(CHEMBL3765754 | US10577361, E1)Show SMILES Cc1ncoc1-c1nnc(SCCCN2CCOC(C2)c2ccc(F)cc2)n1C Show InChI InChI=1S/C20H24FN5O2S/c1-14-18(28-13-22-14)19-23-24-20(25(19)2)29-11-3-8-26-9-10-27-17(12-26)15-4-6-16(21)7-5-15/h4-7,13,17H,3,8-12H2,1-2H3 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 398 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aptuit s.r.l
Curated by ChEMBL
| Assay Description
Agonist activity at human dopamine D3 receptor expressed in CHO cells after 90 mins by [35S]-GTPgamma S assay |
Bioorg Med Chem Lett 26: 1329-32 (2016)
Article DOI: 10.1016/j.bmcl.2015.12.081
BindingDB Entry DOI: 10.7270/Q2HM5B83 |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Rattus norvegicus (Rat)) | BDBM50139860

(CHEMBL3765754 | US10577361, E1)Show SMILES Cc1ncoc1-c1nnc(SCCCN2CCOC(C2)c2ccc(F)cc2)n1C Show InChI InChI=1S/C20H24FN5O2S/c1-14-18(28-13-22-14)19-23-24-20(25(19)2)29-11-3-8-26-9-10-27-17(12-26)15-4-6-16(21)7-5-15/h4-7,13,17H,3,8-12H2,1-2H3 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 398 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aptuit s.r.l
Curated by ChEMBL
| Assay Description
Displacement of [125I]-7-OH-PIPAT from rat brain dopamine D3 receptor after 45 mins by microplate scintillation counting analysis |
Bioorg Med Chem Lett 26: 1329-32 (2016)
Article DOI: 10.1016/j.bmcl.2015.12.081
BindingDB Entry DOI: 10.7270/Q2HM5B83 |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Rattus norvegicus (Rat)) | BDBM50139853

(CHEMBL3764143 | US10577361, E14)Show SMILES Cn1c(SCCCN2CCOC(C2)c2ccc(cc2)C(F)(F)F)nnc1-c1ccc(cc1)-c1ncco1 Show InChI InChI=1S/C26H26F3N5O2S/c1-33-23(19-3-5-20(6-4-19)24-30-11-14-36-24)31-32-25(33)37-16-2-12-34-13-15-35-22(17-34)18-7-9-21(10-8-18)26(27,28)29/h3-11,14,22H,2,12-13,15-17H2,1H3 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 398 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aptuit s.r.l
Curated by ChEMBL
| Assay Description
Displacement of [125I]-7-OH-PIPAT from rat brain dopamine D3 receptor after 45 mins by microplate scintillation counting analysis |
Bioorg Med Chem Lett 26: 1329-32 (2016)
Article DOI: 10.1016/j.bmcl.2015.12.081
BindingDB Entry DOI: 10.7270/Q2HM5B83 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50370572

(CHEMBL85606 | SB-277011)Show SMILES O=C(N[C@H]1CC[C@H](CCN2CCc3cc(ccc3C2)C#N)CC1)c1ccnc2ccccc12 |r,wU:3.2,wD:6.6,(-5.17,2.38,;-5.16,.84,;-3.83,.08,;-2.49,.85,;-2.5,2.39,;-1.16,3.16,;.18,2.39,;1.51,3.16,;2.84,2.39,;4.18,3.16,;4.17,4.69,;5.51,5.46,;6.84,4.68,;8.18,5.44,;9.51,4.67,;9.5,3.12,;8.16,2.36,;6.83,3.14,;5.51,2.38,;10.85,5.43,;12.18,6.19,;.17,.85,;-1.15,.08,;-6.49,.07,;-7.83,.83,;-9.16,.06,;-9.16,-1.48,;-7.83,-2.25,;-7.83,-3.79,;-6.49,-4.56,;-5.15,-3.79,;-5.16,-2.25,;-6.49,-1.48,)| Show InChI InChI=1S/C28H30N4O/c29-18-21-5-8-23-19-32(16-13-22(23)17-21)15-12-20-6-9-24(10-7-20)31-28(33)26-11-14-30-27-4-2-1-3-25(26)27/h1-5,8,11,14,17,20,24H,6-7,9-10,12-13,15-16,19H2,(H,31,33)/t20-,24- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aptuit s.r.l
Curated by ChEMBL
| Assay Description
Displacement of [125I]-7-OH-PIPAT from rat brain dopamine D2 receptor after 45 mins by microplate scintillation counting analysis |
Bioorg Med Chem Lett 26: 1329-32 (2016)
Article DOI: 10.1016/j.bmcl.2015.12.081
BindingDB Entry DOI: 10.7270/Q2HM5B83 |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Rattus norvegicus (Rat)) | BDBM50139864
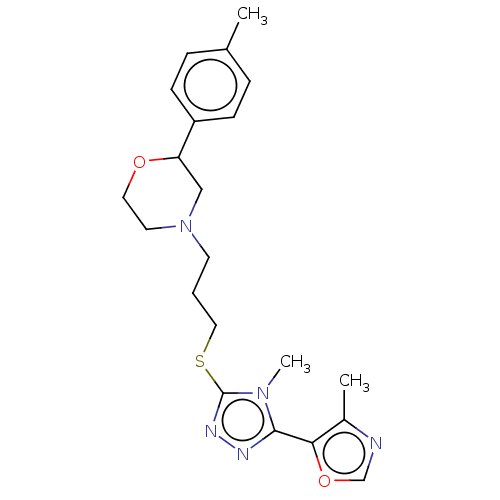
(CHEMBL3765402 | US10577361, E36)Show SMILES Cc1ncoc1-c1nnc(SCCCN2CCOC(C2)c2ccc(C)cc2)n1C Show InChI InChI=1S/C21H27N5O2S/c1-15-5-7-17(8-6-15)18-13-26(10-11-27-18)9-4-12-29-21-24-23-20(25(21)3)19-16(2)22-14-28-19/h5-8,14,18H,4,9-13H2,1-3H3 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aptuit s.r.l
Curated by ChEMBL
| Assay Description
Displacement of [125I]-7-OH-PIPAT from rat brain dopamine D3 receptor after 45 mins by microplate scintillation counting analysis |
Bioorg Med Chem Lett 26: 1329-32 (2016)
Article DOI: 10.1016/j.bmcl.2015.12.081
BindingDB Entry DOI: 10.7270/Q2HM5B83 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50370572

(CHEMBL85606 | SB-277011)Show SMILES O=C(N[C@H]1CC[C@H](CCN2CCc3cc(ccc3C2)C#N)CC1)c1ccnc2ccccc12 |r,wU:3.2,wD:6.6,(-5.17,2.38,;-5.16,.84,;-3.83,.08,;-2.49,.85,;-2.5,2.39,;-1.16,3.16,;.18,2.39,;1.51,3.16,;2.84,2.39,;4.18,3.16,;4.17,4.69,;5.51,5.46,;6.84,4.68,;8.18,5.44,;9.51,4.67,;9.5,3.12,;8.16,2.36,;6.83,3.14,;5.51,2.38,;10.85,5.43,;12.18,6.19,;.17,.85,;-1.15,.08,;-6.49,.07,;-7.83,.83,;-9.16,.06,;-9.16,-1.48,;-7.83,-2.25,;-7.83,-3.79,;-6.49,-4.56,;-5.15,-3.79,;-5.16,-2.25,;-6.49,-1.48,)| Show InChI InChI=1S/C28H30N4O/c29-18-21-5-8-23-19-32(16-13-22(23)17-21)15-12-20-6-9-24(10-7-20)31-28(33)26-11-14-30-27-4-2-1-3-25(26)27/h1-5,8,11,14,17,20,24H,6-7,9-10,12-13,15-16,19H2,(H,31,33)/t20-,24- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aptuit s.r.l
Curated by ChEMBL
| Assay Description
Displacement of [125I]-7-OH-PIPAT from rat brain dopamine D3 receptor after 45 mins by microplate scintillation counting analysis |
Bioorg Med Chem Lett 26: 1329-32 (2016)
Article DOI: 10.1016/j.bmcl.2015.12.081
BindingDB Entry DOI: 10.7270/Q2HM5B83 |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Rattus norvegicus (Rat)) | BDBM50139882
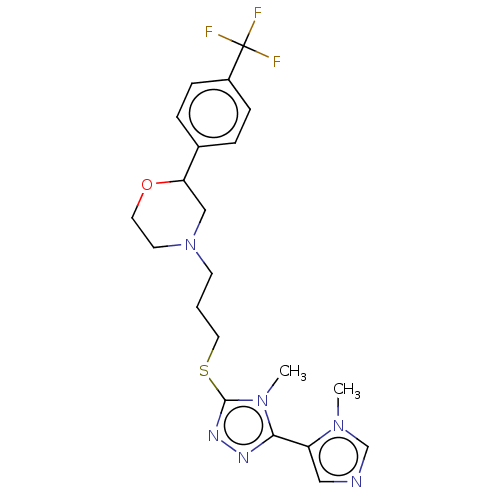
(CHEMBL3764251)Show SMILES Cn1cncc1-c1nnc(SCCCN2CCOC(C2)c2ccc(cc2)C(F)(F)F)n1C Show InChI InChI=1S/C21H25F3N6OS/c1-28-14-25-12-17(28)19-26-27-20(29(19)2)32-11-3-8-30-9-10-31-18(13-30)15-4-6-16(7-5-15)21(22,23)24/h4-7,12,14,18H,3,8-11,13H2,1-2H3 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aptuit s.r.l
Curated by ChEMBL
| Assay Description
Displacement of [125I]-7-OH-PIPAT from rat brain dopamine D3 receptor after 45 mins by microplate scintillation counting analysis |
Bioorg Med Chem Lett 26: 1329-32 (2016)
Article DOI: 10.1016/j.bmcl.2015.12.081
BindingDB Entry DOI: 10.7270/Q2HM5B83 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50139853

(CHEMBL3764143 | US10577361, E14)Show SMILES Cn1c(SCCCN2CCOC(C2)c2ccc(cc2)C(F)(F)F)nnc1-c1ccc(cc1)-c1ncco1 Show InChI InChI=1S/C26H26F3N5O2S/c1-33-23(19-3-5-20(6-4-19)24-30-11-14-36-24)31-32-25(33)37-16-2-12-34-13-15-35-22(17-34)18-7-9-21(10-8-18)26(27,28)29/h3-11,14,22H,2,12-13,15-17H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 999 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aptuit s.r.l
Curated by ChEMBL
| Assay Description
Displacement of [125I]-7-OH-PIPAT from rat brain dopamine D2 receptor after 45 mins by microplate scintillation counting analysis |
Bioorg Med Chem Lett 26: 1329-32 (2016)
Article DOI: 10.1016/j.bmcl.2015.12.081
BindingDB Entry DOI: 10.7270/Q2HM5B83 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50139853

(CHEMBL3764143 | US10577361, E14)Show SMILES Cn1c(SCCCN2CCOC(C2)c2ccc(cc2)C(F)(F)F)nnc1-c1ccc(cc1)-c1ncco1 Show InChI InChI=1S/C26H26F3N5O2S/c1-33-23(19-3-5-20(6-4-19)24-30-11-14-36-24)31-32-25(33)37-16-2-12-34-13-15-35-22(17-34)18-7-9-21(10-8-18)26(27,28)29/h3-11,14,22H,2,12-13,15-17H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aptuit s.r.l
Curated by ChEMBL
| Assay Description
Displacement of [3H]-dofetilide from human ERG potassium channel by scintillation proximity assay |
Bioorg Med Chem Lett 26: 1329-32 (2016)
Article DOI: 10.1016/j.bmcl.2015.12.081
BindingDB Entry DOI: 10.7270/Q2HM5B83 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50139861

(CHEMBL3764557 | US10577361, E7)Show SMILES Cc1ncoc1-c1nnc(SCCCN2CCOC(C2)c2ccc(cc2)C(F)(F)F)n1C Show InChI InChI=1S/C21H24F3N5O2S/c1-14-18(31-13-25-14)19-26-27-20(28(19)2)32-11-3-8-29-9-10-30-17(12-29)15-4-6-16(7-5-15)21(22,23)24/h4-7,13,17H,3,8-12H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aptuit s.r.l
Curated by ChEMBL
| Assay Description
Binding affinity to muscarinic M3 receptor (unknown origin) |
Bioorg Med Chem Lett 26: 1329-32 (2016)
Article DOI: 10.1016/j.bmcl.2015.12.081
BindingDB Entry DOI: 10.7270/Q2HM5B83 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(Homo sapiens (Human)) | BDBM50139861

(CHEMBL3764557 | US10577361, E7)Show SMILES Cc1ncoc1-c1nnc(SCCCN2CCOC(C2)c2ccc(cc2)C(F)(F)F)n1C Show InChI InChI=1S/C21H24F3N5O2S/c1-14-18(31-13-25-14)19-26-27-20(28(19)2)32-11-3-8-29-9-10-30-17(12-29)15-4-6-16(7-5-15)21(22,23)24/h4-7,13,17H,3,8-12H2,1-2H3 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aptuit s.r.l
Curated by ChEMBL
| Assay Description
Antagonist activity at muscarinic M1 receptor (unknown origin) |
Bioorg Med Chem Lett 26: 1329-32 (2016)
Article DOI: 10.1016/j.bmcl.2015.12.081
BindingDB Entry DOI: 10.7270/Q2HM5B83 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50139925

(CHEMBL3764246 | US10577361, E37)Show SMILES Cc1nc(no1)-c1ccc(cc1)-c1nnc(SCCCN2CCOC(C2)c2ccc(C)cc2)n1C Show InChI InChI=1S/C26H30N6O2S/c1-18-5-7-20(8-6-18)23-17-32(14-15-33-23)13-4-16-35-26-29-28-25(31(26)3)22-11-9-21(10-12-22)24-27-19(2)34-30-24/h5-12,23H,4,13-17H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aptuit s.r.l
Curated by ChEMBL
| Assay Description
Agonist activity at human dopamine D3 receptor expressed in CHO cells after 90 mins by [35S]-GTPgamma S assay |
Bioorg Med Chem Lett 26: 1329-32 (2016)
Article DOI: 10.1016/j.bmcl.2015.12.081
BindingDB Entry DOI: 10.7270/Q2HM5B83 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(Homo sapiens (Human)) | BDBM50139852

(CHEMBL3765426 | US10577361, E11)Show SMILES Cn1c(SCCCN2CCOC(C2)c2ccc(cc2)C(F)(F)F)nnc1-c1ccc(cc1)C(N)=O Show InChI InChI=1S/C24H26F3N5O2S/c1-31-22(18-5-3-17(4-6-18)21(28)33)29-30-23(31)35-14-2-11-32-12-13-34-20(15-32)16-7-9-19(10-8-16)24(25,26)27/h3-10,20H,2,11-15H2,1H3,(H2,28,33) | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aptuit s.r.l
Curated by ChEMBL
| Assay Description
Antagonist activity at muscarinic M1 receptor (unknown origin) |
Bioorg Med Chem Lett 26: 1329-32 (2016)
Article DOI: 10.1016/j.bmcl.2015.12.081
BindingDB Entry DOI: 10.7270/Q2HM5B83 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50139853

(CHEMBL3764143 | US10577361, E14)Show SMILES Cn1c(SCCCN2CCOC(C2)c2ccc(cc2)C(F)(F)F)nnc1-c1ccc(cc1)-c1ncco1 Show InChI InChI=1S/C26H26F3N5O2S/c1-33-23(19-3-5-20(6-4-19)24-30-11-14-36-24)31-32-25(33)37-16-2-12-34-13-15-35-22(17-34)18-7-9-21(10-8-18)26(27,28)29/h3-11,14,22H,2,12-13,15-17H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.58E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aptuit s.r.l
Curated by ChEMBL
| Assay Description
Agonist activity at human dopamine D3 receptor expressed in CHO cells after 90 mins by [35S]-GTPgamma S assay |
Bioorg Med Chem Lett 26: 1329-32 (2016)
Article DOI: 10.1016/j.bmcl.2015.12.081
BindingDB Entry DOI: 10.7270/Q2HM5B83 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50139853

(CHEMBL3764143 | US10577361, E14)Show SMILES Cn1c(SCCCN2CCOC(C2)c2ccc(cc2)C(F)(F)F)nnc1-c1ccc(cc1)-c1ncco1 Show InChI InChI=1S/C26H26F3N5O2S/c1-33-23(19-3-5-20(6-4-19)24-30-11-14-36-24)31-32-25(33)37-16-2-12-34-13-15-35-22(17-34)18-7-9-21(10-8-18)26(27,28)29/h3-11,14,22H,2,12-13,15-17H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.59E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aptuit s.r.l
Curated by ChEMBL
| Assay Description
Agonist activity at human dopamine D3 receptor expressed in CHO cells after 90 mins by [35S]-GTPgamma S assay |
Bioorg Med Chem Lett 26: 1329-32 (2016)
Article DOI: 10.1016/j.bmcl.2015.12.081
BindingDB Entry DOI: 10.7270/Q2HM5B83 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50139851

(CHEMBL3764306 | US10577361, E20)Show SMILES Cn1c(SCCCN2CCOC(C2)c2ccc(cc2)C(F)(F)F)nnc1-c1ccc(nc1)C(N)=O Show InChI InChI=1S/C23H25F3N6O2S/c1-31-21(16-5-8-18(20(27)33)28-13-16)29-30-22(31)35-12-2-9-32-10-11-34-19(14-32)15-3-6-17(7-4-15)23(24,25)26/h3-8,13,19H,2,9-12,14H2,1H3,(H2,27,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aptuit s.r.l
Curated by ChEMBL
| Assay Description
Displacement of [125I]-7-OH-PIPAT from rat brain dopamine D3 receptor after 45 mins by microplate scintillation counting analysis |
Bioorg Med Chem Lett 26: 1329-32 (2016)
Article DOI: 10.1016/j.bmcl.2015.12.081
BindingDB Entry DOI: 10.7270/Q2HM5B83 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50139850

(CHEMBL3763418 | US10577361, E4)Show SMILES Cc1ncoc1-c1nnc(SCCCCN2CCOC(C2)c2ccc(cc2)C(F)(F)F)n1C Show InChI InChI=1S/C22H26F3N5O2S/c1-15-19(32-14-26-15)20-27-28-21(29(20)2)33-12-4-3-9-30-10-11-31-18(13-30)16-5-7-17(8-6-16)22(23,24)25/h5-8,14,18H,3-4,9-13H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.51E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aptuit s.r.l
Curated by ChEMBL
| Assay Description
Antagonist activity at human dopamine D3 receptor expressed in CHO cells after 90 mins by [35S]-GTPgamma S assay |
Bioorg Med Chem Lett 26: 1329-32 (2016)
Article DOI: 10.1016/j.bmcl.2015.12.081
BindingDB Entry DOI: 10.7270/Q2HM5B83 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50139860

(CHEMBL3765754 | US10577361, E1)Show SMILES Cc1ncoc1-c1nnc(SCCCN2CCOC(C2)c2ccc(F)cc2)n1C Show InChI InChI=1S/C20H24FN5O2S/c1-14-18(28-13-22-14)19-23-24-20(25(19)2)29-11-3-8-26-9-10-27-17(12-26)15-4-6-16(21)7-5-15/h4-7,13,17H,3,8-12H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.16E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aptuit s.r.l
Curated by ChEMBL
| Assay Description
Displacement of [125I]-7-OH-PIPAT from rat brain dopamine D3 receptor after 45 mins by microplate scintillation counting analysis |
Bioorg Med Chem Lett 26: 1329-32 (2016)
Article DOI: 10.1016/j.bmcl.2015.12.081
BindingDB Entry DOI: 10.7270/Q2HM5B83 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50139861

(CHEMBL3764557 | US10577361, E7)Show SMILES Cc1ncoc1-c1nnc(SCCCN2CCOC(C2)c2ccc(cc2)C(F)(F)F)n1C Show InChI InChI=1S/C21H24F3N5O2S/c1-14-18(31-13-25-14)19-26-27-20(28(19)2)32-11-3-8-29-9-10-30-17(12-29)15-4-6-16(7-5-15)21(22,23)24/h4-7,13,17H,3,8-12H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.16E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aptuit s.r.l
Curated by ChEMBL
| Assay Description
Antagonist activity at human dopamine D3 receptor expressed in CHO cells after 90 mins by [35S]-GTPgamma S assay |
Bioorg Med Chem Lett 26: 1329-32 (2016)
Article DOI: 10.1016/j.bmcl.2015.12.081
BindingDB Entry DOI: 10.7270/Q2HM5B83 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(Homo sapiens (Human)) | BDBM50139860

(CHEMBL3765754 | US10577361, E1)Show SMILES Cc1ncoc1-c1nnc(SCCCN2CCOC(C2)c2ccc(F)cc2)n1C Show InChI InChI=1S/C20H24FN5O2S/c1-14-18(28-13-22-14)19-23-24-20(25(19)2)29-11-3-8-26-9-10-27-17(12-26)15-4-6-16(21)7-5-15/h4-7,13,17H,3,8-12H2,1-2H3 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.16E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aptuit s.r.l
Curated by ChEMBL
| Assay Description
Antagonist activity at muscarinic M1 receptor (unknown origin) |
Bioorg Med Chem Lett 26: 1329-32 (2016)
Article DOI: 10.1016/j.bmcl.2015.12.081
BindingDB Entry DOI: 10.7270/Q2HM5B83 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50139852

(CHEMBL3765426 | US10577361, E11)Show SMILES Cn1c(SCCCN2CCOC(C2)c2ccc(cc2)C(F)(F)F)nnc1-c1ccc(cc1)C(N)=O Show InChI InChI=1S/C24H26F3N5O2S/c1-31-22(18-5-3-17(4-6-18)21(28)33)29-30-23(31)35-14-2-11-32-12-13-34-20(15-32)16-7-9-19(10-8-16)24(25,26)27/h3-10,20H,2,11-15H2,1H3,(H2,28,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.01E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aptuit s.r.l
Curated by ChEMBL
| Assay Description
Antagonist activity at human dopamine D3 receptor expressed in CHO cells after 90 mins by [35S]-GTPgamma S assay |
Bioorg Med Chem Lett 26: 1329-32 (2016)
Article DOI: 10.1016/j.bmcl.2015.12.081
BindingDB Entry DOI: 10.7270/Q2HM5B83 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50139852

(CHEMBL3765426 | US10577361, E11)Show SMILES Cn1c(SCCCN2CCOC(C2)c2ccc(cc2)C(F)(F)F)nnc1-c1ccc(cc1)C(N)=O Show InChI InChI=1S/C24H26F3N5O2S/c1-31-22(18-5-3-17(4-6-18)21(28)33)29-30-23(31)35-14-2-11-32-12-13-34-20(15-32)16-7-9-19(10-8-16)24(25,26)27/h3-10,20H,2,11-15H2,1H3,(H2,28,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.01E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aptuit s.r.l
Curated by ChEMBL
| Assay Description
Antagonist activity at human dopamine D3 receptor expressed in CHO cells after 90 mins by [35S]-GTPgamma S assay |
Bioorg Med Chem Lett 26: 1329-32 (2016)
Article DOI: 10.1016/j.bmcl.2015.12.081
BindingDB Entry DOI: 10.7270/Q2HM5B83 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50139888

(CHEMBL3764739 | US10577361, E24)Show SMILES Cn1cccc1-c1nnc(SCCCN2CCOC(C2)c2ccc(cc2)C(F)(F)F)n1C Show InChI InChI=1S/C22H26F3N5OS/c1-28-10-3-5-18(28)20-26-27-21(29(20)2)32-14-4-11-30-12-13-31-19(15-30)16-6-8-17(9-7-16)22(23,24)25/h3,5-10,19H,4,11-15H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.31E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aptuit s.r.l
Curated by ChEMBL
| Assay Description
Displacement of [125I]-7-OH-PIPAT from rat brain dopamine D2 receptor after 45 mins by microplate scintillation counting analysis |
Bioorg Med Chem Lett 26: 1329-32 (2016)
Article DOI: 10.1016/j.bmcl.2015.12.081
BindingDB Entry DOI: 10.7270/Q2HM5B83 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50139867

(CHEMBL3764884 | US10577361, E26)Show SMILES Cn1c(SCCCN2CCOC(C2)c2ccc(cc2)C(F)(F)F)nnc1-c1nccs1 Show InChI InChI=1S/C20H22F3N5OS2/c1-27-17(18-24-7-12-30-18)25-26-19(27)31-11-2-8-28-9-10-29-16(13-28)14-3-5-15(6-4-14)20(21,22)23/h3-7,12,16H,2,8-11,13H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 7.94E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aptuit s.r.l
Curated by ChEMBL
| Assay Description
Displacement of [125I]-7-OH-PIPAT from rat brain dopamine D2 receptor after 45 mins by microplate scintillation counting analysis |
Bioorg Med Chem Lett 26: 1329-32 (2016)
Article DOI: 10.1016/j.bmcl.2015.12.081
BindingDB Entry DOI: 10.7270/Q2HM5B83 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50139866

(CHEMBL3764191 | US10577361, E28)Show SMILES Cn1c(SCCCN2CCOC(C2)c2ccc(cc2)C(F)(F)F)nnc1-c1ccsc1 Show InChI InChI=1S/C21H23F3N4OS2/c1-27-19(16-7-12-30-14-16)25-26-20(27)31-11-2-8-28-9-10-29-18(13-28)15-3-5-17(6-4-15)21(22,23)24/h3-7,12,14,18H,2,8-11,13H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.94E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aptuit s.r.l
Curated by ChEMBL
| Assay Description
Displacement of [125I]-7-OH-PIPAT from rat brain dopamine D2 receptor after 45 mins by microplate scintillation counting analysis |
Bioorg Med Chem Lett 26: 1329-32 (2016)
Article DOI: 10.1016/j.bmcl.2015.12.081
BindingDB Entry DOI: 10.7270/Q2HM5B83 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50139891

(CHEMBL3764245 | US10577361, E25)Show SMILES Cn1c(SCCCN2CCOC(C2)c2ccc(cc2)C(F)(F)F)nnc1C1CCCCC1 Show InChI InChI=1S/C23H31F3N4OS/c1-29-21(18-6-3-2-4-7-18)27-28-22(29)32-15-5-12-30-13-14-31-20(16-30)17-8-10-19(11-9-17)23(24,25)26/h8-11,18,20H,2-7,12-16H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.94E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aptuit s.r.l
Curated by ChEMBL
| Assay Description
Displacement of [125I]-7-OH-PIPAT from rat brain dopamine D2 receptor after 45 mins by microplate scintillation counting analysis |
Bioorg Med Chem Lett 26: 1329-32 (2016)
Article DOI: 10.1016/j.bmcl.2015.12.081
BindingDB Entry DOI: 10.7270/Q2HM5B83 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50139852

(CHEMBL3765426 | US10577361, E11)Show SMILES Cn1c(SCCCN2CCOC(C2)c2ccc(cc2)C(F)(F)F)nnc1-c1ccc(cc1)C(N)=O Show InChI InChI=1S/C24H26F3N5O2S/c1-31-22(18-5-3-17(4-6-18)21(28)33)29-30-23(31)35-14-2-11-32-12-13-34-20(15-32)16-7-9-19(10-8-16)24(25,26)27/h3-10,20H,2,11-15H2,1H3,(H2,28,33) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9.99E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aptuit s.r.l
Curated by ChEMBL
| Assay Description
Displacement of [125I]-7-OH-PIPAT from rat brain dopamine D2 receptor after 45 mins by microplate scintillation counting analysis |
Bioorg Med Chem Lett 26: 1329-32 (2016)
Article DOI: 10.1016/j.bmcl.2015.12.081
BindingDB Entry DOI: 10.7270/Q2HM5B83 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50139852

(CHEMBL3765426 | US10577361, E11)Show SMILES Cn1c(SCCCN2CCOC(C2)c2ccc(cc2)C(F)(F)F)nnc1-c1ccc(cc1)C(N)=O Show InChI InChI=1S/C24H26F3N5O2S/c1-31-22(18-5-3-17(4-6-18)21(28)33)29-30-23(31)35-14-2-11-32-12-13-34-20(15-32)16-7-9-19(10-8-16)24(25,26)27/h3-10,20H,2,11-15H2,1H3,(H2,28,33) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aptuit s.r.l
Curated by ChEMBL
| Assay Description
Inhibition of human CYP3A4 expressed in bactosome using 7BQ as substrate |
Bioorg Med Chem Lett 26: 1329-32 (2016)
Article DOI: 10.1016/j.bmcl.2015.12.081
BindingDB Entry DOI: 10.7270/Q2HM5B83 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50139924

(CHEMBL3765266 | US10577361, E30)Show SMILES Cc1nc(no1)-c1ccc(cc1)-c1nnc(SCCCN2CCOC(C2)c2ccc(cc2)C(F)(F)F)n1C Show InChI InChI=1S/C26H27F3N6O2S/c1-17-30-23(33-37-17)19-4-6-20(7-5-19)24-31-32-25(34(24)2)38-15-3-12-35-13-14-36-22(16-35)18-8-10-21(11-9-18)26(27,28)29/h4-11,22H,3,12-16H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| <1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aptuit s.r.l
Curated by ChEMBL
| Assay Description
Displacement of [125I]-7-OH-PIPAT from rat brain dopamine D2 receptor after 45 mins by microplate scintillation counting analysis |
Bioorg Med Chem Lett 26: 1329-32 (2016)
Article DOI: 10.1016/j.bmcl.2015.12.081
BindingDB Entry DOI: 10.7270/Q2HM5B83 |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50139853

(CHEMBL3764143 | US10577361, E14)Show SMILES Cn1c(SCCCN2CCOC(C2)c2ccc(cc2)C(F)(F)F)nnc1-c1ccc(cc1)-c1ncco1 Show InChI InChI=1S/C26H26F3N5O2S/c1-33-23(19-3-5-20(6-4-19)24-30-11-14-36-24)31-32-25(33)37-16-2-12-34-13-15-35-22(17-34)18-7-9-21(10-8-18)26(27,28)29/h3-11,14,22H,2,12-13,15-17H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| <1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aptuit s.r.l
Curated by ChEMBL
| Assay Description
Agonist activity at human dopamine D3 receptor expressed in CHO cells after 90 mins by [35S]-GTPgamma S assay |
Bioorg Med Chem Lett 26: 1329-32 (2016)
Article DOI: 10.1016/j.bmcl.2015.12.081
BindingDB Entry DOI: 10.7270/Q2HM5B83 |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50139924

(CHEMBL3765266 | US10577361, E30)Show SMILES Cc1nc(no1)-c1ccc(cc1)-c1nnc(SCCCN2CCOC(C2)c2ccc(cc2)C(F)(F)F)n1C Show InChI InChI=1S/C26H27F3N6O2S/c1-17-30-23(33-37-17)19-4-6-20(7-5-19)24-31-32-25(34(24)2)38-15-3-12-35-13-14-36-22(16-35)18-8-10-21(11-9-18)26(27,28)29/h4-11,22H,3,12-16H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| <1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aptuit s.r.l
Curated by ChEMBL
| Assay Description
Inhibition of human CYP3A4 expressed in bactosome using 7BQ as substrate |
Bioorg Med Chem Lett 26: 1329-32 (2016)
Article DOI: 10.1016/j.bmcl.2015.12.081
BindingDB Entry DOI: 10.7270/Q2HM5B83 |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50139898

(CHEMBL3764639 | US10577361, E31)Show SMILES Cn1c(SCCCN2CCOC(C2)c2ccc(cc2)C(F)(F)F)nnc1-c1ccc(cc1)C#N Show InChI InChI=1S/C24H24F3N5OS/c1-31-22(19-5-3-17(15-28)4-6-19)29-30-23(31)34-14-2-11-32-12-13-33-21(16-32)18-7-9-20(10-8-18)24(25,26)27/h3-10,21H,2,11-14,16H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| <1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aptuit s.r.l
Curated by ChEMBL
| Assay Description
Agonist activity at human dopamine D3 receptor expressed in CHO cells after 90 mins by [35S]-GTPgamma S assay |
Bioorg Med Chem Lett 26: 1329-32 (2016)
Article DOI: 10.1016/j.bmcl.2015.12.081
BindingDB Entry DOI: 10.7270/Q2HM5B83 |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50139888

(CHEMBL3764739 | US10577361, E24)Show SMILES Cn1cccc1-c1nnc(SCCCN2CCOC(C2)c2ccc(cc2)C(F)(F)F)n1C Show InChI InChI=1S/C22H26F3N5OS/c1-28-10-3-5-18(28)20-26-27-21(29(20)2)32-14-4-11-30-12-13-31-19(15-30)16-6-8-17(9-7-16)22(23,24)25/h3,5-10,19H,4,11-15H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| <1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aptuit s.r.l
Curated by ChEMBL
| Assay Description
Agonist activity at human dopamine D3 receptor expressed in CHO cells after 90 mins by [35S]-GTPgamma S assay |
Bioorg Med Chem Lett 26: 1329-32 (2016)
Article DOI: 10.1016/j.bmcl.2015.12.081
BindingDB Entry DOI: 10.7270/Q2HM5B83 |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50139866

(CHEMBL3764191 | US10577361, E28)Show SMILES Cn1c(SCCCN2CCOC(C2)c2ccc(cc2)C(F)(F)F)nnc1-c1ccsc1 Show InChI InChI=1S/C21H23F3N4OS2/c1-27-19(16-7-12-30-14-16)25-26-20(27)31-11-2-8-28-9-10-29-18(13-28)15-3-5-17(6-4-15)21(22,23)24/h3-7,12,14,18H,2,8-11,13H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| <1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aptuit s.r.l
Curated by ChEMBL
| Assay Description
Agonist activity at human dopamine D3 receptor expressed in CHO cells after 90 mins by [35S]-GTPgamma S assay |
Bioorg Med Chem Lett 26: 1329-32 (2016)
Article DOI: 10.1016/j.bmcl.2015.12.081
BindingDB Entry DOI: 10.7270/Q2HM5B83 |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50139865

(CHEMBL3764833 | US10577361, E38)Show SMILES Cc1ncoc1-c1nnc(SCCCN2CCOC(C2)c2ccc(Br)cc2)n1C Show InChI InChI=1S/C20H24BrN5O2S/c1-14-18(28-13-22-14)19-23-24-20(25(19)2)29-11-3-8-26-9-10-27-17(12-26)15-4-6-16(21)7-5-15/h4-7,13,17H,3,8-12H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| <1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aptuit s.r.l
Curated by ChEMBL
| Assay Description
Agonist activity at human dopamine D3 receptor expressed in CHO cells after 90 mins by [35S]-GTPgamma S assay |
Bioorg Med Chem Lett 26: 1329-32 (2016)
Article DOI: 10.1016/j.bmcl.2015.12.081
BindingDB Entry DOI: 10.7270/Q2HM5B83 |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50139850

(CHEMBL3763418 | US10577361, E4)Show SMILES Cc1ncoc1-c1nnc(SCCCCN2CCOC(C2)c2ccc(cc2)C(F)(F)F)n1C Show InChI InChI=1S/C22H26F3N5O2S/c1-15-19(32-14-26-15)20-27-28-21(29(20)2)33-12-4-3-9-30-10-11-31-18(13-30)16-5-7-17(8-6-16)22(23,24)25/h5-8,14,18H,3-4,9-13H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| <1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aptuit s.r.l
Curated by ChEMBL
| Assay Description
Agonist activity at human dopamine D3 receptor expressed in CHO cells after 90 mins by [35S]-GTPgamma S assay |
Bioorg Med Chem Lett 26: 1329-32 (2016)
Article DOI: 10.1016/j.bmcl.2015.12.081
BindingDB Entry DOI: 10.7270/Q2HM5B83 |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50139925

(CHEMBL3764246 | US10577361, E37)Show SMILES Cc1nc(no1)-c1ccc(cc1)-c1nnc(SCCCN2CCOC(C2)c2ccc(C)cc2)n1C Show InChI InChI=1S/C26H30N6O2S/c1-18-5-7-20(8-6-18)23-17-32(14-15-33-23)13-4-16-35-26-29-28-25(31(26)3)22-11-9-21(10-12-22)24-27-19(2)34-30-24/h5-12,23H,4,13-17H2,1-3H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| <1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aptuit s.r.l
Curated by ChEMBL
| Assay Description
Inhibition of human CYP3A4 expressed in bactosome using 7BQ as substrate |
Bioorg Med Chem Lett 26: 1329-32 (2016)
Article DOI: 10.1016/j.bmcl.2015.12.081
BindingDB Entry DOI: 10.7270/Q2HM5B83 |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50139923

(CHEMBL3765285 | US10577361, E29)Show SMILES Cn1c(SCCCN2CCOC(C2)c2ccc(cc2)C(F)(F)F)nnc1-c1ccc(cc1)-c1nnco1 Show InChI InChI=1S/C25H25F3N6O2S/c1-33-22(18-3-5-19(6-4-18)23-31-29-16-36-23)30-32-24(33)37-14-2-11-34-12-13-35-21(15-34)17-7-9-20(10-8-17)25(26,27)28/h3-10,16,21H,2,11-15H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| <1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aptuit s.r.l
Curated by ChEMBL
| Assay Description
Displacement of [125I]-7-OH-PIPAT from rat brain dopamine D2 receptor after 45 mins by microplate scintillation counting analysis |
Bioorg Med Chem Lett 26: 1329-32 (2016)
Article DOI: 10.1016/j.bmcl.2015.12.081
BindingDB Entry DOI: 10.7270/Q2HM5B83 |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50139853

(CHEMBL3764143 | US10577361, E14)Show SMILES Cn1c(SCCCN2CCOC(C2)c2ccc(cc2)C(F)(F)F)nnc1-c1ccc(cc1)-c1ncco1 Show InChI InChI=1S/C26H26F3N5O2S/c1-33-23(19-3-5-20(6-4-19)24-30-11-14-36-24)31-32-25(33)37-16-2-12-34-13-15-35-22(17-34)18-7-9-21(10-8-18)26(27,28)29/h3-11,14,22H,2,12-13,15-17H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| <1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aptuit s.r.l
Curated by ChEMBL
| Assay Description
Displacement of [125I]-7-OH-PIPAT from rat brain dopamine D3 receptor after 45 mins by microplate scintillation counting analysis |
Bioorg Med Chem Lett 26: 1329-32 (2016)
Article DOI: 10.1016/j.bmcl.2015.12.081
BindingDB Entry DOI: 10.7270/Q2HM5B83 |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50139861

(CHEMBL3764557 | US10577361, E7)Show SMILES Cc1ncoc1-c1nnc(SCCCN2CCOC(C2)c2ccc(cc2)C(F)(F)F)n1C Show InChI InChI=1S/C21H24F3N5O2S/c1-14-18(31-13-25-14)19-26-27-20(28(19)2)32-11-3-8-29-9-10-30-17(12-29)15-4-6-16(7-5-15)21(22,23)24/h4-7,13,17H,3,8-12H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| <1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aptuit s.r.l
Curated by ChEMBL
| Assay Description
Displacement of [3H]-dofetilide from human ERG potassium channel by scintillation proximity assay |
Bioorg Med Chem Lett 26: 1329-32 (2016)
Article DOI: 10.1016/j.bmcl.2015.12.081
BindingDB Entry DOI: 10.7270/Q2HM5B83 |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50139860

(CHEMBL3765754 | US10577361, E1)Show SMILES Cc1ncoc1-c1nnc(SCCCN2CCOC(C2)c2ccc(F)cc2)n1C Show InChI InChI=1S/C20H24FN5O2S/c1-14-18(28-13-22-14)19-23-24-20(25(19)2)29-11-3-8-26-9-10-27-17(12-26)15-4-6-16(21)7-5-15/h4-7,13,17H,3,8-12H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| <1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aptuit s.r.l
Curated by ChEMBL
| Assay Description
Displacement of [3H]-dofetilide from human ERG potassium channel by scintillation proximity assay |
Bioorg Med Chem Lett 26: 1329-32 (2016)
Article DOI: 10.1016/j.bmcl.2015.12.081
BindingDB Entry DOI: 10.7270/Q2HM5B83 |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50139922

(CHEMBL3765072 | US10577361, E21)Show SMILES Cn1c(SCCCN2CCOC(C2)c2ccc(cc2)C(F)(F)F)nnc1-c1ccc(cc1)-n1cnnc1 Show InChI InChI=1S/C25H26F3N7OS/c1-33-23(19-5-9-21(10-6-19)35-16-29-30-17-35)31-32-24(33)37-14-2-11-34-12-13-36-22(15-34)18-3-7-20(8-4-18)25(26,27)28/h3-10,16-17,22H,2,11-15H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| <1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aptuit s.r.l
Curated by ChEMBL
| Assay Description
Agonist activity at human dopamine D3 receptor expressed in CHO cells after 90 mins by [35S]-GTPgamma S assay |
Bioorg Med Chem Lett 26: 1329-32 (2016)
Article DOI: 10.1016/j.bmcl.2015.12.081
BindingDB Entry DOI: 10.7270/Q2HM5B83 |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50139856

(CHEMBL3765169 | US10577361, E19)Show SMILES Cn1c(SCCCN2CCOC(C2)c2ccc(cc2)C(F)(F)F)nnc1-c1cnccn1 Show InChI InChI=1S/C21H23F3N6OS/c1-29-19(17-13-25-7-8-26-17)27-28-20(29)32-12-2-9-30-10-11-31-18(14-30)15-3-5-16(6-4-15)21(22,23)24/h3-8,13,18H,2,9-12,14H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| <1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aptuit s.r.l
Curated by ChEMBL
| Assay Description
Agonist activity at human dopamine D3 receptor expressed in CHO cells after 90 mins by [35S]-GTPgamma S assay |
Bioorg Med Chem Lett 26: 1329-32 (2016)
Article DOI: 10.1016/j.bmcl.2015.12.081
BindingDB Entry DOI: 10.7270/Q2HM5B83 |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50139867

(CHEMBL3764884 | US10577361, E26)Show SMILES Cn1c(SCCCN2CCOC(C2)c2ccc(cc2)C(F)(F)F)nnc1-c1nccs1 Show InChI InChI=1S/C20H22F3N5OS2/c1-27-17(18-24-7-12-30-18)25-26-19(27)31-11-2-8-28-9-10-29-16(13-28)14-3-5-15(6-4-14)20(21,22)23/h3-7,12,16H,2,8-11,13H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| <1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aptuit s.r.l
Curated by ChEMBL
| Assay Description
Agonist activity at human dopamine D3 receptor expressed in CHO cells after 90 mins by [35S]-GTPgamma S assay |
Bioorg Med Chem Lett 26: 1329-32 (2016)
Article DOI: 10.1016/j.bmcl.2015.12.081
BindingDB Entry DOI: 10.7270/Q2HM5B83 |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50139864

(CHEMBL3765402 | US10577361, E36)Show SMILES Cc1ncoc1-c1nnc(SCCCN2CCOC(C2)c2ccc(C)cc2)n1C Show InChI InChI=1S/C21H27N5O2S/c1-15-5-7-17(8-6-15)18-13-26(10-11-27-18)9-4-12-29-21-24-23-20(25(21)3)19-16(2)22-14-28-19/h5-8,14,18H,4,9-13H2,1-3H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| <1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aptuit s.r.l
Curated by ChEMBL
| Assay Description
Agonist activity at human dopamine D3 receptor expressed in CHO cells after 90 mins by [35S]-GTPgamma S assay |
Bioorg Med Chem Lett 26: 1329-32 (2016)
Article DOI: 10.1016/j.bmcl.2015.12.081
BindingDB Entry DOI: 10.7270/Q2HM5B83 |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50139860

(CHEMBL3765754 | US10577361, E1)Show SMILES Cc1ncoc1-c1nnc(SCCCN2CCOC(C2)c2ccc(F)cc2)n1C Show InChI InChI=1S/C20H24FN5O2S/c1-14-18(28-13-22-14)19-23-24-20(25(19)2)29-11-3-8-26-9-10-27-17(12-26)15-4-6-16(21)7-5-15/h4-7,13,17H,3,8-12H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| <1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aptuit s.r.l
Curated by ChEMBL
| Assay Description
Agonist activity at human dopamine D3 receptor expressed in CHO cells after 90 mins by [35S]-GTPgamma S assay |
Bioorg Med Chem Lett 26: 1329-32 (2016)
Article DOI: 10.1016/j.bmcl.2015.12.081
BindingDB Entry DOI: 10.7270/Q2HM5B83 |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50139857

(CHEMBL3765422)Show SMILES Cn1c(SCCCN2CCOC(C2)c2ccc(cc2)C(F)(F)F)nnc1-c1cncc(c1)C(N)=O Show InChI InChI=1S/C23H25F3N6O2S/c1-31-21(17-11-16(20(27)33)12-28-13-17)29-30-22(31)35-10-2-7-32-8-9-34-19(14-32)15-3-5-18(6-4-15)23(24,25)26/h3-6,11-13,19H,2,7-10,14H2,1H3,(H2,27,33) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| <1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aptuit s.r.l
Curated by ChEMBL
| Assay Description
Agonist activity at human dopamine D3 receptor expressed in CHO cells after 90 mins by [35S]-GTPgamma S assay |
Bioorg Med Chem Lett 26: 1329-32 (2016)
Article DOI: 10.1016/j.bmcl.2015.12.081
BindingDB Entry DOI: 10.7270/Q2HM5B83 |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50139921

(CHEMBL3764597 | US10577361, E35)Show SMILES Cn1c(SCCCN2CCOC(C2)c2ccc(cc2F)C(F)(F)F)nnc1-c1ccc(cc1)C(N)=O Show InChI InChI=1S/C24H25F4N5O2S/c1-32-22(16-5-3-15(4-6-16)21(29)34)30-31-23(32)36-12-2-9-33-10-11-35-20(14-33)18-8-7-17(13-19(18)25)24(26,27)28/h3-8,13,20H,2,9-12,14H2,1H3,(H2,29,34) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| <1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aptuit s.r.l
Curated by ChEMBL
| Assay Description
Agonist activity at human dopamine D3 receptor expressed in CHO cells after 90 mins by [35S]-GTPgamma S assay |
Bioorg Med Chem Lett 26: 1329-32 (2016)
Article DOI: 10.1016/j.bmcl.2015.12.081
BindingDB Entry DOI: 10.7270/Q2HM5B83 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50139922

(CHEMBL3765072 | US10577361, E21)Show SMILES Cn1c(SCCCN2CCOC(C2)c2ccc(cc2)C(F)(F)F)nnc1-c1ccc(cc1)-n1cnnc1 Show InChI InChI=1S/C25H26F3N7OS/c1-33-23(19-5-9-21(10-6-19)35-16-29-30-17-35)31-32-24(33)37-14-2-11-34-12-13-36-22(15-34)18-3-7-20(8-4-18)25(26,27)28/h3-10,16-17,22H,2,11-15H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aptuit s.r.l
Curated by ChEMBL
| Assay Description
Displacement of [125I]-7-OH-PIPAT from rat brain dopamine D2 receptor after 45 mins by microplate scintillation counting analysis |
Bioorg Med Chem Lett 26: 1329-32 (2016)
Article DOI: 10.1016/j.bmcl.2015.12.081
BindingDB Entry DOI: 10.7270/Q2HM5B83 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50139860

(CHEMBL3765754 | US10577361, E1)Show SMILES Cc1ncoc1-c1nnc(SCCCN2CCOC(C2)c2ccc(F)cc2)n1C Show InChI InChI=1S/C20H24FN5O2S/c1-14-18(28-13-22-14)19-23-24-20(25(19)2)29-11-3-8-26-9-10-27-17(12-26)15-4-6-16(21)7-5-15/h4-7,13,17H,3,8-12H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| <1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aptuit s.r.l
Curated by ChEMBL
| Assay Description
Displacement of [125I]-7-OH-PIPAT from rat brain dopamine D2 receptor after 45 mins by microplate scintillation counting analysis |
Bioorg Med Chem Lett 26: 1329-32 (2016)
Article DOI: 10.1016/j.bmcl.2015.12.081
BindingDB Entry DOI: 10.7270/Q2HM5B83 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50139850

(CHEMBL3763418 | US10577361, E4)Show SMILES Cc1ncoc1-c1nnc(SCCCCN2CCOC(C2)c2ccc(cc2)C(F)(F)F)n1C Show InChI InChI=1S/C22H26F3N5O2S/c1-15-19(32-14-26-15)20-27-28-21(29(20)2)33-12-4-3-9-30-10-11-31-18(13-30)16-5-7-17(8-6-16)22(23,24)25/h5-8,14,18H,3-4,9-13H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| <1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aptuit s.r.l
Curated by ChEMBL
| Assay Description
Displacement of [125I]-7-OH-PIPAT from rat brain dopamine D2 receptor after 45 mins by microplate scintillation counting analysis |
Bioorg Med Chem Lett 26: 1329-32 (2016)
Article DOI: 10.1016/j.bmcl.2015.12.081
BindingDB Entry DOI: 10.7270/Q2HM5B83 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50139863

(CHEMBL3764586 | US10577361, E34)Show SMILES Cc1ncoc1-c1nnc(SCCCN2CCOC(C2)c2ccc(cc2F)C(F)(F)F)n1C Show InChI InChI=1S/C21H23F4N5O2S/c1-13-18(32-12-26-13)19-27-28-20(29(19)2)33-9-3-6-30-7-8-31-17(11-30)15-5-4-14(10-16(15)22)21(23,24)25/h4-5,10,12,17H,3,6-9,11H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aptuit s.r.l
Curated by ChEMBL
| Assay Description
Displacement of [125I]-7-OH-PIPAT from rat brain dopamine D2 receptor after 45 mins by microplate scintillation counting analysis |
Bioorg Med Chem Lett 26: 1329-32 (2016)
Article DOI: 10.1016/j.bmcl.2015.12.081
BindingDB Entry DOI: 10.7270/Q2HM5B83 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50139882

(CHEMBL3764251)Show SMILES Cn1cncc1-c1nnc(SCCCN2CCOC(C2)c2ccc(cc2)C(F)(F)F)n1C Show InChI InChI=1S/C21H25F3N6OS/c1-28-14-25-12-17(28)19-26-27-20(29(19)2)32-11-3-8-30-9-10-31-18(13-30)15-4-6-16(7-5-15)21(22,23)24/h4-7,12,14,18H,3,8-11,13H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| <1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aptuit s.r.l
Curated by ChEMBL
| Assay Description
Displacement of [125I]-7-OH-PIPAT from rat brain dopamine D2 receptor after 45 mins by microplate scintillation counting analysis |
Bioorg Med Chem Lett 26: 1329-32 (2016)
Article DOI: 10.1016/j.bmcl.2015.12.081
BindingDB Entry DOI: 10.7270/Q2HM5B83 |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50139855

(CHEMBL3764612 | US10577361, E18)Show SMILES Cn1c(SCCCN2CCOC(C2)c2ccc(cc2)C(F)(F)F)nnc1-c1cccnc1 Show InChI InChI=1S/C22H24F3N5OS/c1-29-20(17-4-2-9-26-14-17)27-28-21(29)32-13-3-10-30-11-12-31-19(15-30)16-5-7-18(8-6-16)22(23,24)25/h2,4-9,14,19H,3,10-13,15H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| <1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aptuit s.r.l
Curated by ChEMBL
| Assay Description
Agonist activity at human dopamine D3 receptor expressed in CHO cells after 90 mins by [35S]-GTPgamma S assay |
Bioorg Med Chem Lett 26: 1329-32 (2016)
Article DOI: 10.1016/j.bmcl.2015.12.081
BindingDB Entry DOI: 10.7270/Q2HM5B83 |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50139897

(CHEMBL3764830 | US10577361, E33)Show SMILES Cn1c(SCCCN2CCOC(C2)c2ccc(cc2)C(F)(F)F)nnc1-c1ccc(cc1)S(N)(=O)=O Show InChI InChI=1S/C23H26F3N5O3S2/c1-30-21(17-5-9-19(10-6-17)36(27,32)33)28-29-22(30)35-14-2-11-31-12-13-34-20(15-31)16-3-7-18(8-4-16)23(24,25)26/h3-10,20H,2,11-15H2,1H3,(H2,27,32,33) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| <1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aptuit s.r.l
Curated by ChEMBL
| Assay Description
Agonist activity at human dopamine D3 receptor expressed in CHO cells after 90 mins by [35S]-GTPgamma S assay |
Bioorg Med Chem Lett 26: 1329-32 (2016)
Article DOI: 10.1016/j.bmcl.2015.12.081
BindingDB Entry DOI: 10.7270/Q2HM5B83 |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50370572

(CHEMBL85606 | SB-277011)Show SMILES O=C(N[C@H]1CC[C@H](CCN2CCc3cc(ccc3C2)C#N)CC1)c1ccnc2ccccc12 |r,wU:3.2,wD:6.6,(-5.17,2.38,;-5.16,.84,;-3.83,.08,;-2.49,.85,;-2.5,2.39,;-1.16,3.16,;.18,2.39,;1.51,3.16,;2.84,2.39,;4.18,3.16,;4.17,4.69,;5.51,5.46,;6.84,4.68,;8.18,5.44,;9.51,4.67,;9.5,3.12,;8.16,2.36,;6.83,3.14,;5.51,2.38,;10.85,5.43,;12.18,6.19,;.17,.85,;-1.15,.08,;-6.49,.07,;-7.83,.83,;-9.16,.06,;-9.16,-1.48,;-7.83,-2.25,;-7.83,-3.79,;-6.49,-4.56,;-5.15,-3.79,;-5.16,-2.25,;-6.49,-1.48,)| Show InChI InChI=1S/C28H30N4O/c29-18-21-5-8-23-19-32(16-13-22(23)17-21)15-12-20-6-9-24(10-7-20)31-28(33)26-11-14-30-27-4-2-1-3-25(26)27/h1-5,8,11,14,17,20,24H,6-7,9-10,12-13,15-16,19H2,(H,31,33)/t20-,24- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| <1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aptuit s.r.l
Curated by ChEMBL
| Assay Description
Agonist activity at human dopamine D3 receptor expressed in CHO cells after 90 mins by [35S]-GTPgamma S assay |
Bioorg Med Chem Lett 26: 1329-32 (2016)
Article DOI: 10.1016/j.bmcl.2015.12.081
BindingDB Entry DOI: 10.7270/Q2HM5B83 |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50139861

(CHEMBL3764557 | US10577361, E7)Show SMILES Cc1ncoc1-c1nnc(SCCCN2CCOC(C2)c2ccc(cc2)C(F)(F)F)n1C Show InChI InChI=1S/C21H24F3N5O2S/c1-14-18(31-13-25-14)19-26-27-20(28(19)2)32-11-3-8-29-9-10-30-17(12-29)15-4-6-16(7-5-15)21(22,23)24/h4-7,13,17H,3,8-12H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| <1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aptuit s.r.l
Curated by ChEMBL
| Assay Description
Agonist activity at human dopamine D3 receptor expressed in CHO cells after 90 mins by [35S]-GTPgamma S assay |
Bioorg Med Chem Lett 26: 1329-32 (2016)
Article DOI: 10.1016/j.bmcl.2015.12.081
BindingDB Entry DOI: 10.7270/Q2HM5B83 |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50139863

(CHEMBL3764586 | US10577361, E34)Show SMILES Cc1ncoc1-c1nnc(SCCCN2CCOC(C2)c2ccc(cc2F)C(F)(F)F)n1C Show InChI InChI=1S/C21H23F4N5O2S/c1-13-18(32-12-26-13)19-27-28-20(29(19)2)33-9-3-6-30-7-8-31-17(11-30)15-5-4-14(10-16(15)22)21(23,24)25/h4-5,10,12,17H,3,6-9,11H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| <1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aptuit s.r.l
Curated by ChEMBL
| Assay Description
Agonist activity at human dopamine D3 receptor expressed in CHO cells after 90 mins by [35S]-GTPgamma S assay |
Bioorg Med Chem Lett 26: 1329-32 (2016)
Article DOI: 10.1016/j.bmcl.2015.12.081
BindingDB Entry DOI: 10.7270/Q2HM5B83 |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50139882

(CHEMBL3764251)Show SMILES Cn1cncc1-c1nnc(SCCCN2CCOC(C2)c2ccc(cc2)C(F)(F)F)n1C Show InChI InChI=1S/C21H25F3N6OS/c1-28-14-25-12-17(28)19-26-27-20(29(19)2)32-11-3-8-30-9-10-31-18(13-30)15-4-6-16(7-5-15)21(22,23)24/h4-7,12,14,18H,3,8-11,13H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| <1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aptuit s.r.l
Curated by ChEMBL
| Assay Description
Agonist activity at human dopamine D3 receptor expressed in CHO cells after 90 mins by [35S]-GTPgamma S assay |
Bioorg Med Chem Lett 26: 1329-32 (2016)
Article DOI: 10.1016/j.bmcl.2015.12.081
BindingDB Entry DOI: 10.7270/Q2HM5B83 |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50139851

(CHEMBL3764306 | US10577361, E20)Show SMILES Cn1c(SCCCN2CCOC(C2)c2ccc(cc2)C(F)(F)F)nnc1-c1ccc(nc1)C(N)=O Show InChI InChI=1S/C23H25F3N6O2S/c1-31-21(16-5-8-18(20(27)33)28-13-16)29-30-22(31)35-12-2-9-32-10-11-34-19(14-32)15-3-6-17(7-4-15)23(24,25)26/h3-8,13,19H,2,9-12,14H2,1H3,(H2,27,33) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| <1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aptuit s.r.l
Curated by ChEMBL
| Assay Description
Displacement of [3H]-dofetilide from human ERG potassium channel by scintillation proximity assay |
Bioorg Med Chem Lett 26: 1329-32 (2016)
Article DOI: 10.1016/j.bmcl.2015.12.081
BindingDB Entry DOI: 10.7270/Q2HM5B83 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50139898

(CHEMBL3764639 | US10577361, E31)Show SMILES Cn1c(SCCCN2CCOC(C2)c2ccc(cc2)C(F)(F)F)nnc1-c1ccc(cc1)C#N Show InChI InChI=1S/C24H24F3N5OS/c1-31-22(19-5-3-17(15-28)4-6-19)29-30-23(31)34-14-2-11-32-12-13-33-21(16-32)18-7-9-20(10-8-18)24(25,26)27/h3-10,21H,2,11-14,16H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aptuit s.r.l
Curated by ChEMBL
| Assay Description
Displacement of [125I]-7-OH-PIPAT from rat brain dopamine D2 receptor after 45 mins by microplate scintillation counting analysis |
Bioorg Med Chem Lett 26: 1329-32 (2016)
Article DOI: 10.1016/j.bmcl.2015.12.081
BindingDB Entry DOI: 10.7270/Q2HM5B83 |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50139850

(CHEMBL3763418 | US10577361, E4)Show SMILES Cc1ncoc1-c1nnc(SCCCCN2CCOC(C2)c2ccc(cc2)C(F)(F)F)n1C Show InChI InChI=1S/C22H26F3N5O2S/c1-15-19(32-14-26-15)20-27-28-21(29(20)2)33-12-4-3-9-30-10-11-31-18(13-30)16-5-7-17(8-6-16)22(23,24)25/h5-8,14,18H,3-4,9-13H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| <1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aptuit s.r.l
Curated by ChEMBL
| Assay Description
Displacement of [3H]-dofetilide from human ERG potassium channel by scintillation proximity assay |
Bioorg Med Chem Lett 26: 1329-32 (2016)
Article DOI: 10.1016/j.bmcl.2015.12.081
BindingDB Entry DOI: 10.7270/Q2HM5B83 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50139856

(CHEMBL3765169 | US10577361, E19)Show SMILES Cn1c(SCCCN2CCOC(C2)c2ccc(cc2)C(F)(F)F)nnc1-c1cnccn1 Show InChI InChI=1S/C21H23F3N6OS/c1-29-19(17-13-25-7-8-26-17)27-28-20(29)32-12-2-9-30-10-11-31-18(14-30)15-3-5-16(6-4-15)21(22,23)24/h3-8,13,18H,2,9-12,14H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aptuit s.r.l
Curated by ChEMBL
| Assay Description
Displacement of [125I]-7-OH-PIPAT from rat brain dopamine D2 receptor after 45 mins by microplate scintillation counting analysis |
Bioorg Med Chem Lett 26: 1329-32 (2016)
Article DOI: 10.1016/j.bmcl.2015.12.081
BindingDB Entry DOI: 10.7270/Q2HM5B83 |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50139854

(CHEMBL3763200 | US10577361, E17)Show SMILES Cn1c(SCCCN2CCOC(C2)c2ccc(cc2)C(F)(F)F)nnc1-c1ccncc1 Show InChI InChI=1S/C22H24F3N5OS/c1-29-20(17-7-9-26-10-8-17)27-28-21(29)32-14-2-11-30-12-13-31-19(15-30)16-3-5-18(6-4-16)22(23,24)25/h3-10,19H,2,11-15H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| <1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aptuit s.r.l
Curated by ChEMBL
| Assay Description
Agonist activity at human dopamine D3 receptor expressed in CHO cells after 90 mins by [35S]-GTPgamma S assay |
Bioorg Med Chem Lett 26: 1329-32 (2016)
Article DOI: 10.1016/j.bmcl.2015.12.081
BindingDB Entry DOI: 10.7270/Q2HM5B83 |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50139859

(CHEMBL3764212 | US10577361, E23)Show SMILES Cn1c(SCCCN2CCOC(C2)c2ccc(cc2)C(F)(F)F)nnc1-c1cccc(c1)C(N)=O Show InChI InChI=1S/C24H26F3N5O2S/c1-31-22(18-5-2-4-17(14-18)21(28)33)29-30-23(31)35-13-3-10-32-11-12-34-20(15-32)16-6-8-19(9-7-16)24(25,26)27/h2,4-9,14,20H,3,10-13,15H2,1H3,(H2,28,33) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| <1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aptuit s.r.l
Curated by ChEMBL
| Assay Description
Agonist activity at human dopamine D3 receptor expressed in CHO cells after 90 mins by [35S]-GTPgamma S assay |
Bioorg Med Chem Lett 26: 1329-32 (2016)
Article DOI: 10.1016/j.bmcl.2015.12.081
BindingDB Entry DOI: 10.7270/Q2HM5B83 |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50139895

(CHEMBL3765267 | US10577361, E32)Show SMILES CC(=O)c1ccc(cc1)-c1nnc(SCCCN2CCOC(C2)c2ccc(cc2)C(F)(F)F)n1C Show InChI InChI=1S/C25H27F3N4O2S/c1-17(33)18-4-6-20(7-5-18)23-29-30-24(31(23)2)35-15-3-12-32-13-14-34-22(16-32)19-8-10-21(11-9-19)25(26,27)28/h4-11,22H,3,12-16H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| <1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aptuit s.r.l
Curated by ChEMBL
| Assay Description
Agonist activity at human dopamine D3 receptor expressed in CHO cells after 90 mins by [35S]-GTPgamma S assay |
Bioorg Med Chem Lett 26: 1329-32 (2016)
Article DOI: 10.1016/j.bmcl.2015.12.081
BindingDB Entry DOI: 10.7270/Q2HM5B83 |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50139852

(CHEMBL3765426 | US10577361, E11)Show SMILES Cn1c(SCCCN2CCOC(C2)c2ccc(cc2)C(F)(F)F)nnc1-c1ccc(cc1)C(N)=O Show InChI InChI=1S/C24H26F3N5O2S/c1-31-22(18-5-3-17(4-6-18)21(28)33)29-30-23(31)35-14-2-11-32-12-13-34-20(15-32)16-7-9-19(10-8-16)24(25,26)27/h3-10,20H,2,11-15H2,1H3,(H2,28,33) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| <1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aptuit s.r.l
Curated by ChEMBL
| Assay Description
Displacement of [3H]-dofetilide from human ERG potassium channel by scintillation proximity assay |
Bioorg Med Chem Lett 26: 1329-32 (2016)
Article DOI: 10.1016/j.bmcl.2015.12.081
BindingDB Entry DOI: 10.7270/Q2HM5B83 |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50139891

(CHEMBL3764245 | US10577361, E25)Show SMILES Cn1c(SCCCN2CCOC(C2)c2ccc(cc2)C(F)(F)F)nnc1C1CCCCC1 Show InChI InChI=1S/C23H31F3N4OS/c1-29-21(18-6-3-2-4-7-18)27-28-22(29)32-15-5-12-30-13-14-31-20(16-30)17-8-10-19(11-9-17)23(24,25)26/h8-11,18,20H,2-7,12-16H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| <1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aptuit s.r.l
Curated by ChEMBL
| Assay Description
Displacement of [125I]-7-OH-PIPAT from rat brain dopamine D3 receptor after 45 mins by microplate scintillation counting analysis |
Bioorg Med Chem Lett 26: 1329-32 (2016)
Article DOI: 10.1016/j.bmcl.2015.12.081
BindingDB Entry DOI: 10.7270/Q2HM5B83 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50139850

(CHEMBL3763418 | US10577361, E4)Show SMILES Cc1ncoc1-c1nnc(SCCCCN2CCOC(C2)c2ccc(cc2)C(F)(F)F)n1C Show InChI InChI=1S/C22H26F3N5O2S/c1-15-19(32-14-26-15)20-27-28-21(29(20)2)33-12-4-3-9-30-10-11-31-18(13-30)16-5-7-17(8-6-16)22(23,24)25/h5-8,14,18H,3-4,9-13H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| <1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aptuit s.r.l
Curated by ChEMBL
| Assay Description
Antagonist activity at human dopamine D3 receptor expressed in CHO cells after 90 mins by [35S]-GTPgamma S assay |
Bioorg Med Chem Lett 26: 1329-32 (2016)
Article DOI: 10.1016/j.bmcl.2015.12.081
BindingDB Entry DOI: 10.7270/Q2HM5B83 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50139860

(CHEMBL3765754 | US10577361, E1)Show SMILES Cc1ncoc1-c1nnc(SCCCN2CCOC(C2)c2ccc(F)cc2)n1C Show InChI InChI=1S/C20H24FN5O2S/c1-14-18(28-13-22-14)19-23-24-20(25(19)2)29-11-3-8-26-9-10-27-17(12-26)15-4-6-16(21)7-5-15/h4-7,13,17H,3,8-12H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| <1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aptuit s.r.l
Curated by ChEMBL
| Assay Description
Inhibition of human CYP3A4 expressed in bactosome using 7BQ as substrate |
Bioorg Med Chem Lett 26: 1329-32 (2016)
Article DOI: 10.1016/j.bmcl.2015.12.081
BindingDB Entry DOI: 10.7270/Q2HM5B83 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50139923

(CHEMBL3765285 | US10577361, E29)Show SMILES Cn1c(SCCCN2CCOC(C2)c2ccc(cc2)C(F)(F)F)nnc1-c1ccc(cc1)-c1nnco1 Show InChI InChI=1S/C25H25F3N6O2S/c1-33-22(18-3-5-19(6-4-18)23-31-29-16-36-23)30-32-24(33)37-14-2-11-34-12-13-35-21(15-34)17-7-9-20(10-8-17)25(26,27)28/h3-10,16,21H,2,11-15H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.26E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aptuit s.r.l
Curated by ChEMBL
| Assay Description
Displacement of [125I]-7-OH-PIPAT from rat brain dopamine D2 receptor after 45 mins by microplate scintillation counting analysis |
Bioorg Med Chem Lett 26: 1329-32 (2016)
Article DOI: 10.1016/j.bmcl.2015.12.081
BindingDB Entry DOI: 10.7270/Q2HM5B83 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50139854

(CHEMBL3763200 | US10577361, E17)Show SMILES Cn1c(SCCCN2CCOC(C2)c2ccc(cc2)C(F)(F)F)nnc1-c1ccncc1 Show InChI InChI=1S/C22H24F3N5OS/c1-29-20(17-7-9-26-10-8-17)27-28-21(29)32-14-2-11-30-12-13-31-19(15-30)16-3-5-18(6-4-16)22(23,24)25/h3-10,19H,2,11-15H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.26E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aptuit s.r.l
Curated by ChEMBL
| Assay Description
Displacement of [125I]-7-OH-PIPAT from rat brain dopamine D2 receptor after 45 mins by microplate scintillation counting analysis |
Bioorg Med Chem Lett 26: 1329-32 (2016)
Article DOI: 10.1016/j.bmcl.2015.12.081
BindingDB Entry DOI: 10.7270/Q2HM5B83 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50139861

(CHEMBL3764557 | US10577361, E7)Show SMILES Cc1ncoc1-c1nnc(SCCCN2CCOC(C2)c2ccc(cc2)C(F)(F)F)n1C Show InChI InChI=1S/C21H24F3N5O2S/c1-14-18(31-13-25-14)19-26-27-20(28(19)2)32-11-3-8-29-9-10-30-17(12-29)15-4-6-16(7-5-15)21(22,23)24/h4-7,13,17H,3,8-12H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.26E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aptuit s.r.l
Curated by ChEMBL
| Assay Description
Displacement of [125I]-7-OH-PIPAT from rat brain dopamine D2 receptor after 45 mins by microplate scintillation counting analysis |
Bioorg Med Chem Lett 26: 1329-32 (2016)
Article DOI: 10.1016/j.bmcl.2015.12.081
BindingDB Entry DOI: 10.7270/Q2HM5B83 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50139853

(CHEMBL3764143 | US10577361, E14)Show SMILES Cn1c(SCCCN2CCOC(C2)c2ccc(cc2)C(F)(F)F)nnc1-c1ccc(cc1)-c1ncco1 Show InChI InChI=1S/C26H26F3N5O2S/c1-33-23(19-3-5-20(6-4-19)24-30-11-14-36-24)31-32-25(33)37-16-2-12-34-13-15-35-22(17-34)18-7-9-21(10-8-18)26(27,28)29/h3-11,14,22H,2,12-13,15-17H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.58E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aptuit s.r.l
Curated by ChEMBL
| Assay Description
Displacement of [125I]-7-OH-PIPAT from rat brain dopamine D2 receptor after 45 mins by microplate scintillation counting analysis |
Bioorg Med Chem Lett 26: 1329-32 (2016)
Article DOI: 10.1016/j.bmcl.2015.12.081
BindingDB Entry DOI: 10.7270/Q2HM5B83 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50139865

(CHEMBL3764833 | US10577361, E38)Show SMILES Cc1ncoc1-c1nnc(SCCCN2CCOC(C2)c2ccc(Br)cc2)n1C Show InChI InChI=1S/C20H24BrN5O2S/c1-14-18(28-13-22-14)19-23-24-20(25(19)2)29-11-3-8-26-9-10-27-17(12-26)15-4-6-16(21)7-5-15/h4-7,13,17H,3,8-12H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.58E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aptuit s.r.l
Curated by ChEMBL
| Assay Description
Displacement of [125I]-7-OH-PIPAT from rat brain dopamine D2 receptor after 45 mins by microplate scintillation counting analysis |
Bioorg Med Chem Lett 26: 1329-32 (2016)
Article DOI: 10.1016/j.bmcl.2015.12.081
BindingDB Entry DOI: 10.7270/Q2HM5B83 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50139857

(CHEMBL3765422)Show SMILES Cn1c(SCCCN2CCOC(C2)c2ccc(cc2)C(F)(F)F)nnc1-c1cncc(c1)C(N)=O Show InChI InChI=1S/C23H25F3N6O2S/c1-31-21(17-11-16(20(27)33)12-28-13-17)29-30-22(31)35-10-2-7-32-8-9-34-19(14-32)15-3-5-18(6-4-15)23(24,25)26/h3-6,11-13,19H,2,7-10,14H2,1H3,(H2,27,33) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.58E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aptuit s.r.l
Curated by ChEMBL
| Assay Description
Displacement of [125I]-7-OH-PIPAT from rat brain dopamine D2 receptor after 45 mins by microplate scintillation counting analysis |
Bioorg Med Chem Lett 26: 1329-32 (2016)
Article DOI: 10.1016/j.bmcl.2015.12.081
BindingDB Entry DOI: 10.7270/Q2HM5B83 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50139855

(CHEMBL3764612 | US10577361, E18)Show SMILES Cn1c(SCCCN2CCOC(C2)c2ccc(cc2)C(F)(F)F)nnc1-c1cccnc1 Show InChI InChI=1S/C22H24F3N5OS/c1-29-20(17-4-2-9-26-14-17)27-28-21(29)32-13-3-10-30-11-12-31-19(15-30)16-5-7-18(8-6-16)22(23,24)25/h2,4-9,14,19H,3,10-13,15H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.58E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aptuit s.r.l
Curated by ChEMBL
| Assay Description
Displacement of [125I]-7-OH-PIPAT from rat brain dopamine D2 receptor after 45 mins by microplate scintillation counting analysis |
Bioorg Med Chem Lett 26: 1329-32 (2016)
Article DOI: 10.1016/j.bmcl.2015.12.081
BindingDB Entry DOI: 10.7270/Q2HM5B83 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50139921

(CHEMBL3764597 | US10577361, E35)Show SMILES Cn1c(SCCCN2CCOC(C2)c2ccc(cc2F)C(F)(F)F)nnc1-c1ccc(cc1)C(N)=O Show InChI InChI=1S/C24H25F4N5O2S/c1-32-22(16-5-3-15(4-6-16)21(29)34)30-31-23(32)36-12-2-9-33-10-11-35-20(14-33)18-8-7-17(13-19(18)25)24(26,27)28/h3-8,13,20H,2,9-12,14H2,1H3,(H2,29,34) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.58E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aptuit s.r.l
Curated by ChEMBL
| Assay Description
Displacement of [125I]-7-OH-PIPAT from rat brain dopamine D2 receptor after 45 mins by microplate scintillation counting analysis |
Bioorg Med Chem Lett 26: 1329-32 (2016)
Article DOI: 10.1016/j.bmcl.2015.12.081
BindingDB Entry DOI: 10.7270/Q2HM5B83 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50139859

(CHEMBL3764212 | US10577361, E23)Show SMILES Cn1c(SCCCN2CCOC(C2)c2ccc(cc2)C(F)(F)F)nnc1-c1cccc(c1)C(N)=O Show InChI InChI=1S/C24H26F3N5O2S/c1-31-22(18-5-2-4-17(14-18)21(28)33)29-30-23(31)35-13-3-10-32-11-12-34-20(15-32)16-6-8-19(9-7-16)24(25,26)27/h2,4-9,14,20H,3,10-13,15H2,1H3,(H2,28,33) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.58E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aptuit s.r.l
Curated by ChEMBL
| Assay Description
Displacement of [125I]-7-OH-PIPAT from rat brain dopamine D2 receptor after 45 mins by microplate scintillation counting analysis |
Bioorg Med Chem Lett 26: 1329-32 (2016)
Article DOI: 10.1016/j.bmcl.2015.12.081
BindingDB Entry DOI: 10.7270/Q2HM5B83 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50139895

(CHEMBL3765267 | US10577361, E32)Show SMILES CC(=O)c1ccc(cc1)-c1nnc(SCCCN2CCOC(C2)c2ccc(cc2)C(F)(F)F)n1C Show InChI InChI=1S/C25H27F3N4O2S/c1-17(33)18-4-6-20(7-5-18)23-29-30-24(31(23)2)35-15-3-12-32-13-14-34-22(16-32)19-8-10-21(11-9-19)25(26,27)28/h4-11,22H,3,12-16H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aptuit s.r.l
Curated by ChEMBL
| Assay Description
Displacement of [125I]-7-OH-PIPAT from rat brain dopamine D2 receptor after 45 mins by microplate scintillation counting analysis |
Bioorg Med Chem Lett 26: 1329-32 (2016)
Article DOI: 10.1016/j.bmcl.2015.12.081
BindingDB Entry DOI: 10.7270/Q2HM5B83 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50139851

(CHEMBL3764306 | US10577361, E20)Show SMILES Cn1c(SCCCN2CCOC(C2)c2ccc(cc2)C(F)(F)F)nnc1-c1ccc(nc1)C(N)=O Show InChI InChI=1S/C23H25F3N6O2S/c1-31-21(16-5-8-18(20(27)33)28-13-16)29-30-22(31)35-12-2-9-32-10-11-34-19(14-32)15-3-6-17(7-4-15)23(24,25)26/h3-8,13,19H,2,9-12,14H2,1H3,(H2,27,33) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.51E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aptuit s.r.l
Curated by ChEMBL
| Assay Description
Displacement of [125I]-7-OH-PIPAT from rat brain dopamine D2 receptor after 45 mins by microplate scintillation counting analysis |
Bioorg Med Chem Lett 26: 1329-32 (2016)
Article DOI: 10.1016/j.bmcl.2015.12.081
BindingDB Entry DOI: 10.7270/Q2HM5B83 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50139925

(CHEMBL3764246 | US10577361, E37)Show SMILES Cc1nc(no1)-c1ccc(cc1)-c1nnc(SCCCN2CCOC(C2)c2ccc(C)cc2)n1C Show InChI InChI=1S/C26H30N6O2S/c1-18-5-7-20(8-6-18)23-17-32(14-15-33-23)13-4-16-35-26-29-28-25(31(26)3)22-11-9-21(10-12-22)24-27-19(2)34-30-24/h5-12,23H,4,13-17H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.51E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aptuit s.r.l
Curated by ChEMBL
| Assay Description
Displacement of [125I]-7-OH-PIPAT from rat brain dopamine D2 receptor after 45 mins by microplate scintillation counting analysis |
Bioorg Med Chem Lett 26: 1329-32 (2016)
Article DOI: 10.1016/j.bmcl.2015.12.081
BindingDB Entry DOI: 10.7270/Q2HM5B83 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50139897

(CHEMBL3764830 | US10577361, E33)Show SMILES Cn1c(SCCCN2CCOC(C2)c2ccc(cc2)C(F)(F)F)nnc1-c1ccc(cc1)S(N)(=O)=O Show InChI InChI=1S/C23H26F3N5O3S2/c1-30-21(17-5-9-19(10-6-17)36(27,32)33)28-29-22(30)35-14-2-11-31-12-13-34-20(15-31)16-3-7-18(8-4-16)23(24,25)26/h3-10,20H,2,11-15H2,1H3,(H2,27,32,33) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| <3.16E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aptuit s.r.l
Curated by ChEMBL
| Assay Description
Displacement of [125I]-7-OH-PIPAT from rat brain dopamine D2 receptor after 45 mins by microplate scintillation counting analysis |
Bioorg Med Chem Lett 26: 1329-32 (2016)
Article DOI: 10.1016/j.bmcl.2015.12.081
BindingDB Entry DOI: 10.7270/Q2HM5B83 |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM50139861

(CHEMBL3764557 | US10577361, E7)Show SMILES Cc1ncoc1-c1nnc(SCCCN2CCOC(C2)c2ccc(cc2)C(F)(F)F)n1C Show InChI InChI=1S/C21H24F3N5O2S/c1-14-18(31-13-25-14)19-26-27-20(28(19)2)32-11-3-8-29-9-10-30-17(12-29)15-4-6-16(7-5-15)21(22,23)24/h4-7,13,17H,3,8-12H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| <1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aptuit s.r.l
Curated by ChEMBL
| Assay Description
Binding affinity to dopamine D4 receptor (unknown origin) |
Bioorg Med Chem Lett 26: 1329-32 (2016)
Article DOI: 10.1016/j.bmcl.2015.12.081
BindingDB Entry DOI: 10.7270/Q2HM5B83 |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(Homo sapiens (Human)) | BDBM50139861

(CHEMBL3764557 | US10577361, E7)Show SMILES Cc1ncoc1-c1nnc(SCCCN2CCOC(C2)c2ccc(cc2)C(F)(F)F)n1C Show InChI InChI=1S/C21H24F3N5O2S/c1-14-18(31-13-25-14)19-26-27-20(28(19)2)32-11-3-8-29-9-10-30-17(12-29)15-4-6-16(7-5-15)21(22,23)24/h4-7,13,17H,3,8-12H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| <1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aptuit s.r.l
Curated by ChEMBL
| Assay Description
Binding affinity to dopamine D1 receptor (unknown origin) |
Bioorg Med Chem Lett 26: 1329-32 (2016)
Article DOI: 10.1016/j.bmcl.2015.12.081
BindingDB Entry DOI: 10.7270/Q2HM5B83 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50139853

(CHEMBL3764143 | US10577361, E14)Show SMILES Cn1c(SCCCN2CCOC(C2)c2ccc(cc2)C(F)(F)F)nnc1-c1ccc(cc1)-c1ncco1 Show InChI InChI=1S/C26H26F3N5O2S/c1-33-23(19-3-5-20(6-4-19)24-30-11-14-36-24)31-32-25(33)37-16-2-12-34-13-15-35-22(17-34)18-7-9-21(10-8-18)26(27,28)29/h3-11,14,22H,2,12-13,15-17H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Aptuit s.r.l
Curated by ChEMBL
| Assay Description
Inhibition of human CYP3A4 expressed in bactosome using DBOMF as substrate |
Bioorg Med Chem Lett 26: 1329-32 (2016)
Article DOI: 10.1016/j.bmcl.2015.12.081
BindingDB Entry DOI: 10.7270/Q2HM5B83 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50139853

(CHEMBL3764143 | US10577361, E14)Show SMILES Cn1c(SCCCN2CCOC(C2)c2ccc(cc2)C(F)(F)F)nnc1-c1ccc(cc1)-c1ncco1 Show InChI InChI=1S/C26H26F3N5O2S/c1-33-23(19-3-5-20(6-4-19)24-30-11-14-36-24)31-32-25(33)37-16-2-12-34-13-15-35-22(17-34)18-7-9-21(10-8-18)26(27,28)29/h3-11,14,22H,2,12-13,15-17H2,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Aptuit s.r.l
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2D6 expressed in bactosome |
Bioorg Med Chem Lett 26: 1329-32 (2016)
Article DOI: 10.1016/j.bmcl.2015.12.081
BindingDB Entry DOI: 10.7270/Q2HM5B83 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50139853

(CHEMBL3764143 | US10577361, E14)Show SMILES Cn1c(SCCCN2CCOC(C2)c2ccc(cc2)C(F)(F)F)nnc1-c1ccc(cc1)-c1ncco1 Show InChI InChI=1S/C26H26F3N5O2S/c1-33-23(19-3-5-20(6-4-19)24-30-11-14-36-24)31-32-25(33)37-16-2-12-34-13-15-35-22(17-34)18-7-9-21(10-8-18)26(27,28)29/h3-11,14,22H,2,12-13,15-17H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Aptuit s.r.l
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2C19 expressed in bactosome |
Bioorg Med Chem Lett 26: 1329-32 (2016)
Article DOI: 10.1016/j.bmcl.2015.12.081
BindingDB Entry DOI: 10.7270/Q2HM5B83 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50139853

(CHEMBL3764143 | US10577361, E14)Show SMILES Cn1c(SCCCN2CCOC(C2)c2ccc(cc2)C(F)(F)F)nnc1-c1ccc(cc1)-c1ncco1 Show InChI InChI=1S/C26H26F3N5O2S/c1-33-23(19-3-5-20(6-4-19)24-30-11-14-36-24)31-32-25(33)37-16-2-12-34-13-15-35-22(17-34)18-7-9-21(10-8-18)26(27,28)29/h3-11,14,22H,2,12-13,15-17H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Aptuit s.r.l
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2C9 expressed in bactosome |
Bioorg Med Chem Lett 26: 1329-32 (2016)
Article DOI: 10.1016/j.bmcl.2015.12.081
BindingDB Entry DOI: 10.7270/Q2HM5B83 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50139853

(CHEMBL3764143 | US10577361, E14)Show SMILES Cn1c(SCCCN2CCOC(C2)c2ccc(cc2)C(F)(F)F)nnc1-c1ccc(cc1)-c1ncco1 Show InChI InChI=1S/C26H26F3N5O2S/c1-33-23(19-3-5-20(6-4-19)24-30-11-14-36-24)31-32-25(33)37-16-2-12-34-13-15-35-22(17-34)18-7-9-21(10-8-18)26(27,28)29/h3-11,14,22H,2,12-13,15-17H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Aptuit s.r.l
Curated by ChEMBL
| Assay Description
Displacement of [125I]-7-OH-PIPAT from rat brain dopamine D3 receptor after 45 mins by microplate scintillation counting analysis |
Bioorg Med Chem Lett 26: 1329-32 (2016)
Article DOI: 10.1016/j.bmcl.2015.12.081
BindingDB Entry DOI: 10.7270/Q2HM5B83 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50139853

(CHEMBL3764143 | US10577361, E14)Show SMILES Cn1c(SCCCN2CCOC(C2)c2ccc(cc2)C(F)(F)F)nnc1-c1ccc(cc1)-c1ncco1 Show InChI InChI=1S/C26H26F3N5O2S/c1-33-23(19-3-5-20(6-4-19)24-30-11-14-36-24)31-32-25(33)37-16-2-12-34-13-15-35-22(17-34)18-7-9-21(10-8-18)26(27,28)29/h3-11,14,22H,2,12-13,15-17H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Aptuit s.r.l
Curated by ChEMBL
| Assay Description
Inhibition of human CYP1A2 expressed in bactosome |
Bioorg Med Chem Lett 26: 1329-32 (2016)
Article DOI: 10.1016/j.bmcl.2015.12.081
BindingDB Entry DOI: 10.7270/Q2HM5B83 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50139861

(CHEMBL3764557 | US10577361, E7)Show SMILES Cc1ncoc1-c1nnc(SCCCN2CCOC(C2)c2ccc(cc2)C(F)(F)F)n1C Show InChI InChI=1S/C21H24F3N5O2S/c1-14-18(31-13-25-14)19-26-27-20(28(19)2)32-11-3-8-29-9-10-30-17(12-29)15-4-6-16(7-5-15)21(22,23)24/h4-7,13,17H,3,8-12H2,1-2H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Aptuit s.r.l
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2D6 expressed in bactosome |
Bioorg Med Chem Lett 26: 1329-32 (2016)
Article DOI: 10.1016/j.bmcl.2015.12.081
BindingDB Entry DOI: 10.7270/Q2HM5B83 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50139861

(CHEMBL3764557 | US10577361, E7)Show SMILES Cc1ncoc1-c1nnc(SCCCN2CCOC(C2)c2ccc(cc2)C(F)(F)F)n1C Show InChI InChI=1S/C21H24F3N5O2S/c1-14-18(31-13-25-14)19-26-27-20(28(19)2)32-11-3-8-29-9-10-30-17(12-29)15-4-6-16(7-5-15)21(22,23)24/h4-7,13,17H,3,8-12H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Aptuit s.r.l
Curated by ChEMBL
| Assay Description
Agonist activity at human dopamine D3 receptor expressed in CHO cells after 90 mins by [35S]-GTPgamma S assay |
Bioorg Med Chem Lett 26: 1329-32 (2016)
Article DOI: 10.1016/j.bmcl.2015.12.081
BindingDB Entry DOI: 10.7270/Q2HM5B83 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50139861

(CHEMBL3764557 | US10577361, E7)Show SMILES Cc1ncoc1-c1nnc(SCCCN2CCOC(C2)c2ccc(cc2)C(F)(F)F)n1C Show InChI InChI=1S/C21H24F3N5O2S/c1-14-18(31-13-25-14)19-26-27-20(28(19)2)32-11-3-8-29-9-10-30-17(12-29)15-4-6-16(7-5-15)21(22,23)24/h4-7,13,17H,3,8-12H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Aptuit s.r.l
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2C9 expressed in bactosome |
Bioorg Med Chem Lett 26: 1329-32 (2016)
Article DOI: 10.1016/j.bmcl.2015.12.081
BindingDB Entry DOI: 10.7270/Q2HM5B83 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50139861

(CHEMBL3764557 | US10577361, E7)Show SMILES Cc1ncoc1-c1nnc(SCCCN2CCOC(C2)c2ccc(cc2)C(F)(F)F)n1C Show InChI InChI=1S/C21H24F3N5O2S/c1-14-18(31-13-25-14)19-26-27-20(28(19)2)32-11-3-8-29-9-10-30-17(12-29)15-4-6-16(7-5-15)21(22,23)24/h4-7,13,17H,3,8-12H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Aptuit s.r.l
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2C19 expressed in bactosome |
Bioorg Med Chem Lett 26: 1329-32 (2016)
Article DOI: 10.1016/j.bmcl.2015.12.081
BindingDB Entry DOI: 10.7270/Q2HM5B83 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50139861

(CHEMBL3764557 | US10577361, E7)Show SMILES Cc1ncoc1-c1nnc(SCCCN2CCOC(C2)c2ccc(cc2)C(F)(F)F)n1C Show InChI InChI=1S/C21H24F3N5O2S/c1-14-18(31-13-25-14)19-26-27-20(28(19)2)32-11-3-8-29-9-10-30-17(12-29)15-4-6-16(7-5-15)21(22,23)24/h4-7,13,17H,3,8-12H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Aptuit s.r.l
Curated by ChEMBL
| Assay Description
Inhibition of human CYP1A2 expressed in bactosome |
Bioorg Med Chem Lett 26: 1329-32 (2016)
Article DOI: 10.1016/j.bmcl.2015.12.081
BindingDB Entry DOI: 10.7270/Q2HM5B83 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50139861

(CHEMBL3764557 | US10577361, E7)Show SMILES Cc1ncoc1-c1nnc(SCCCN2CCOC(C2)c2ccc(cc2)C(F)(F)F)n1C Show InChI InChI=1S/C21H24F3N5O2S/c1-14-18(31-13-25-14)19-26-27-20(28(19)2)32-11-3-8-29-9-10-30-17(12-29)15-4-6-16(7-5-15)21(22,23)24/h4-7,13,17H,3,8-12H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Aptuit s.r.l
Curated by ChEMBL
| Assay Description
Inhibition of human CYP3A4 expressed in bactosome using DBOMF as substrate |
Bioorg Med Chem Lett 26: 1329-32 (2016)
Article DOI: 10.1016/j.bmcl.2015.12.081
BindingDB Entry DOI: 10.7270/Q2HM5B83 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50139852

(CHEMBL3765426 | US10577361, E11)Show SMILES Cn1c(SCCCN2CCOC(C2)c2ccc(cc2)C(F)(F)F)nnc1-c1ccc(cc1)C(N)=O Show InChI InChI=1S/C24H26F3N5O2S/c1-31-22(18-5-3-17(4-6-18)21(28)33)29-30-23(31)35-14-2-11-32-12-13-34-20(15-32)16-7-9-19(10-8-16)24(25,26)27/h3-10,20H,2,11-15H2,1H3,(H2,28,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Aptuit s.r.l
Curated by ChEMBL
| Assay Description
Inhibition of human CYP3A4 expressed in bactosome using DBOMF as substrate |
Bioorg Med Chem Lett 26: 1329-32 (2016)
Article DOI: 10.1016/j.bmcl.2015.12.081
BindingDB Entry DOI: 10.7270/Q2HM5B83 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50139860

(CHEMBL3765754 | US10577361, E1)Show SMILES Cc1ncoc1-c1nnc(SCCCN2CCOC(C2)c2ccc(F)cc2)n1C Show InChI InChI=1S/C20H24FN5O2S/c1-14-18(28-13-22-14)19-23-24-20(25(19)2)29-11-3-8-26-9-10-27-17(12-26)15-4-6-16(21)7-5-15/h4-7,13,17H,3,8-12H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Aptuit s.r.l
Curated by ChEMBL
| Assay Description
Displacement of [125I]-7-OH-PIPAT from rat brain dopamine D2 receptor after 45 mins by microplate scintillation counting analysis |
Bioorg Med Chem Lett 26: 1329-32 (2016)
Article DOI: 10.1016/j.bmcl.2015.12.081
BindingDB Entry DOI: 10.7270/Q2HM5B83 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50139860

(CHEMBL3765754 | US10577361, E1)Show SMILES Cc1ncoc1-c1nnc(SCCCN2CCOC(C2)c2ccc(F)cc2)n1C Show InChI InChI=1S/C20H24FN5O2S/c1-14-18(28-13-22-14)19-23-24-20(25(19)2)29-11-3-8-26-9-10-27-17(12-26)15-4-6-16(21)7-5-15/h4-7,13,17H,3,8-12H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Aptuit s.r.l
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2C19 expressed in bactosome |
Bioorg Med Chem Lett 26: 1329-32 (2016)
Article DOI: 10.1016/j.bmcl.2015.12.081
BindingDB Entry DOI: 10.7270/Q2HM5B83 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50139860

(CHEMBL3765754 | US10577361, E1)Show SMILES Cc1ncoc1-c1nnc(SCCCN2CCOC(C2)c2ccc(F)cc2)n1C Show InChI InChI=1S/C20H24FN5O2S/c1-14-18(28-13-22-14)19-23-24-20(25(19)2)29-11-3-8-26-9-10-27-17(12-26)15-4-6-16(21)7-5-15/h4-7,13,17H,3,8-12H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Aptuit s.r.l
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2C9 expressed in bactosome |
Bioorg Med Chem Lett 26: 1329-32 (2016)
Article DOI: 10.1016/j.bmcl.2015.12.081
BindingDB Entry DOI: 10.7270/Q2HM5B83 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50139852

(CHEMBL3765426 | US10577361, E11)Show SMILES Cn1c(SCCCN2CCOC(C2)c2ccc(cc2)C(F)(F)F)nnc1-c1ccc(cc1)C(N)=O Show InChI InChI=1S/C24H26F3N5O2S/c1-31-22(18-5-3-17(4-6-18)21(28)33)29-30-23(31)35-14-2-11-32-12-13-34-20(15-32)16-7-9-19(10-8-16)24(25,26)27/h3-10,20H,2,11-15H2,1H3,(H2,28,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Aptuit s.r.l
Curated by ChEMBL
| Assay Description
Inhibition of human CYP1A2 expressed in bactosome |
Bioorg Med Chem Lett 26: 1329-32 (2016)
Article DOI: 10.1016/j.bmcl.2015.12.081
BindingDB Entry DOI: 10.7270/Q2HM5B83 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50139852

(CHEMBL3765426 | US10577361, E11)Show SMILES Cn1c(SCCCN2CCOC(C2)c2ccc(cc2)C(F)(F)F)nnc1-c1ccc(cc1)C(N)=O Show InChI InChI=1S/C24H26F3N5O2S/c1-31-22(18-5-3-17(4-6-18)21(28)33)29-30-23(31)35-14-2-11-32-12-13-34-20(15-32)16-7-9-19(10-8-16)24(25,26)27/h3-10,20H,2,11-15H2,1H3,(H2,28,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Aptuit s.r.l
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2C19 expressed in bactosome |
Bioorg Med Chem Lett 26: 1329-32 (2016)
Article DOI: 10.1016/j.bmcl.2015.12.081
BindingDB Entry DOI: 10.7270/Q2HM5B83 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50139852

(CHEMBL3765426 | US10577361, E11)Show SMILES Cn1c(SCCCN2CCOC(C2)c2ccc(cc2)C(F)(F)F)nnc1-c1ccc(cc1)C(N)=O Show InChI InChI=1S/C24H26F3N5O2S/c1-31-22(18-5-3-17(4-6-18)21(28)33)29-30-23(31)35-14-2-11-32-12-13-34-20(15-32)16-7-9-19(10-8-16)24(25,26)27/h3-10,20H,2,11-15H2,1H3,(H2,28,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Aptuit s.r.l
Curated by ChEMBL
| Assay Description
Agonist activity at human dopamine D3 receptor expressed in CHO cells after 90 mins by [35S]-GTPgamma S assay |
Bioorg Med Chem Lett 26: 1329-32 (2016)
Article DOI: 10.1016/j.bmcl.2015.12.081
BindingDB Entry DOI: 10.7270/Q2HM5B83 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50139852

(CHEMBL3765426 | US10577361, E11)Show SMILES Cn1c(SCCCN2CCOC(C2)c2ccc(cc2)C(F)(F)F)nnc1-c1ccc(cc1)C(N)=O Show InChI InChI=1S/C24H26F3N5O2S/c1-31-22(18-5-3-17(4-6-18)21(28)33)29-30-23(31)35-14-2-11-32-12-13-34-20(15-32)16-7-9-19(10-8-16)24(25,26)27/h3-10,20H,2,11-15H2,1H3,(H2,28,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Aptuit s.r.l
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2C9 expressed in bactosome |
Bioorg Med Chem Lett 26: 1329-32 (2016)
Article DOI: 10.1016/j.bmcl.2015.12.081
BindingDB Entry DOI: 10.7270/Q2HM5B83 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50139860

(CHEMBL3765754 | US10577361, E1)Show SMILES Cc1ncoc1-c1nnc(SCCCN2CCOC(C2)c2ccc(F)cc2)n1C Show InChI InChI=1S/C20H24FN5O2S/c1-14-18(28-13-22-14)19-23-24-20(25(19)2)29-11-3-8-26-9-10-27-17(12-26)15-4-6-16(21)7-5-15/h4-7,13,17H,3,8-12H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Aptuit s.r.l
Curated by ChEMBL
| Assay Description
Inhibition of human CYP3A4 expressed in bactosome using DBOMF as substrate |
Bioorg Med Chem Lett 26: 1329-32 (2016)
Article DOI: 10.1016/j.bmcl.2015.12.081
BindingDB Entry DOI: 10.7270/Q2HM5B83 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50139852

(CHEMBL3765426 | US10577361, E11)Show SMILES Cn1c(SCCCN2CCOC(C2)c2ccc(cc2)C(F)(F)F)nnc1-c1ccc(cc1)C(N)=O Show InChI InChI=1S/C24H26F3N5O2S/c1-31-22(18-5-3-17(4-6-18)21(28)33)29-30-23(31)35-14-2-11-32-12-13-34-20(15-32)16-7-9-19(10-8-16)24(25,26)27/h3-10,20H,2,11-15H2,1H3,(H2,28,33) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Aptuit s.r.l
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2D6 expressed in bactosome |
Bioorg Med Chem Lett 26: 1329-32 (2016)
Article DOI: 10.1016/j.bmcl.2015.12.081
BindingDB Entry DOI: 10.7270/Q2HM5B83 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50139860

(CHEMBL3765754 | US10577361, E1)Show SMILES Cc1ncoc1-c1nnc(SCCCN2CCOC(C2)c2ccc(F)cc2)n1C Show InChI InChI=1S/C20H24FN5O2S/c1-14-18(28-13-22-14)19-23-24-20(25(19)2)29-11-3-8-26-9-10-27-17(12-26)15-4-6-16(21)7-5-15/h4-7,13,17H,3,8-12H2,1-2H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Aptuit s.r.l
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2D6 expressed in bactosome |
Bioorg Med Chem Lett 26: 1329-32 (2016)
Article DOI: 10.1016/j.bmcl.2015.12.081
BindingDB Entry DOI: 10.7270/Q2HM5B83 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50139860

(CHEMBL3765754 | US10577361, E1)Show SMILES Cc1ncoc1-c1nnc(SCCCN2CCOC(C2)c2ccc(F)cc2)n1C Show InChI InChI=1S/C20H24FN5O2S/c1-14-18(28-13-22-14)19-23-24-20(25(19)2)29-11-3-8-26-9-10-27-17(12-26)15-4-6-16(21)7-5-15/h4-7,13,17H,3,8-12H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Aptuit s.r.l
Curated by ChEMBL
| Assay Description
Inhibition of human CYP1A2 expressed in bactosome |
Bioorg Med Chem Lett 26: 1329-32 (2016)
Article DOI: 10.1016/j.bmcl.2015.12.081
BindingDB Entry DOI: 10.7270/Q2HM5B83 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data