

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Serine/threonine-protein kinase B-raf (Homo sapiens (Human)) | BDBM50139157 (CHEMBL3764588) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceuticals International Co. 40 Landsdowne Street, Cambridge, MA 02139, United States. Curated by ChEMBL | Assay Description Inhibition of CDK2/Cyclin E (unknown origin) expressed in baculoviral infected insect Sf9 cells using histone H1 as substrate in presence of [gamma-3... | Bioorg Med Chem Lett 26: 1156-60 (2016) Article DOI: 10.1016/j.bmcl.2016.01.049 BindingDB Entry DOI: 10.7270/Q29P33HD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase B-raf (Homo sapiens (Human)) | BDBM50139150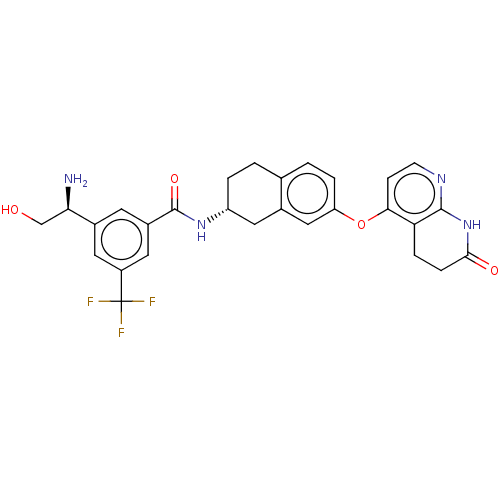 (CHEMBL3763999) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceuticals International Co. 40 Landsdowne Street, Cambridge, MA 02139, United States. Curated by ChEMBL | Assay Description Inhibition of CDK2/Cyclin E (unknown origin) expressed in baculoviral infected insect Sf9 cells using histone H1 as substrate in presence of [gamma-3... | Bioorg Med Chem Lett 26: 1156-60 (2016) Article DOI: 10.1016/j.bmcl.2016.01.049 BindingDB Entry DOI: 10.7270/Q29P33HD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase B-raf (Homo sapiens (Human)) | BDBM50139149 (CHEMBL3765012) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceuticals International Co. 40 Landsdowne Street, Cambridge, MA 02139, United States. Curated by ChEMBL | Assay Description Inhibition of CDK2/Cyclin E (unknown origin) expressed in baculoviral infected insect Sf9 cells using histone H1 as substrate in presence of [gamma-3... | Bioorg Med Chem Lett 26: 1156-60 (2016) Article DOI: 10.1016/j.bmcl.2016.01.049 BindingDB Entry DOI: 10.7270/Q29P33HD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase B-raf (Homo sapiens (Human)) | BDBM50139147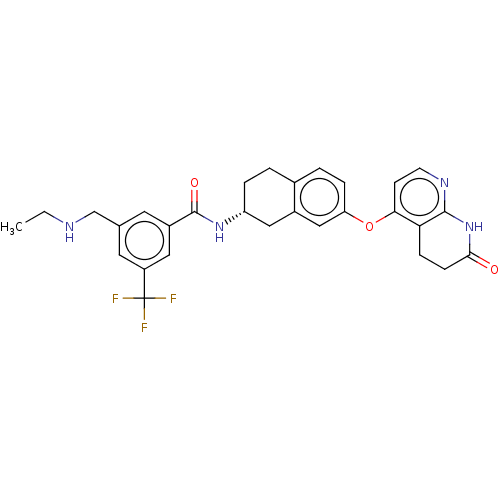 (CHEMBL3765509) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceuticals International Co. 40 Landsdowne Street, Cambridge, MA 02139, United States. Curated by ChEMBL | Assay Description Inhibition of CDK2/Cyclin E (unknown origin) expressed in baculoviral infected insect Sf9 cells using histone H1 as substrate in presence of [gamma-3... | Bioorg Med Chem Lett 26: 1156-60 (2016) Article DOI: 10.1016/j.bmcl.2016.01.049 BindingDB Entry DOI: 10.7270/Q29P33HD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase B-raf (Homo sapiens (Human)) | BDBM50139153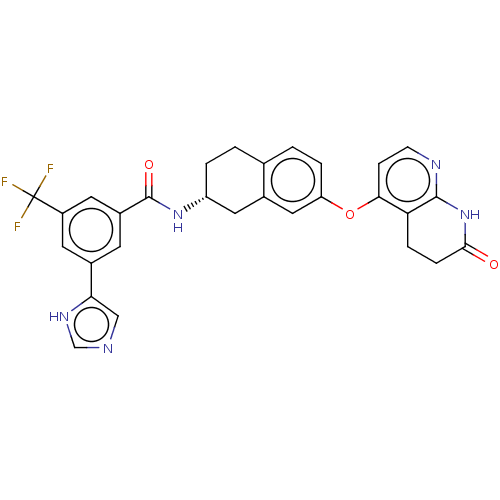 (CHEMBL3765355) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceuticals International Co. 40 Landsdowne Street, Cambridge, MA 02139, United States. Curated by ChEMBL | Assay Description Inhibition of CDK2/Cyclin E (unknown origin) expressed in baculoviral infected insect Sf9 cells using histone H1 as substrate in presence of [gamma-3... | Bioorg Med Chem Lett 26: 1156-60 (2016) Article DOI: 10.1016/j.bmcl.2016.01.049 BindingDB Entry DOI: 10.7270/Q29P33HD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase B-raf (Homo sapiens (Human)) | BDBM50139158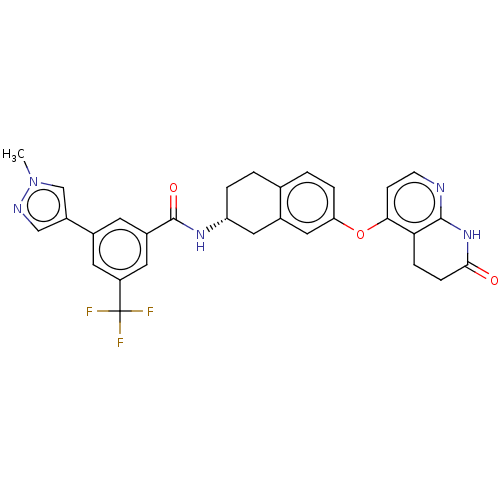 (CHEMBL3763646) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceuticals International Co. 40 Landsdowne Street, Cambridge, MA 02139, United States. Curated by ChEMBL | Assay Description Inhibition of CDK2/Cyclin E (unknown origin) expressed in baculoviral infected insect Sf9 cells using histone H1 as substrate in presence of [gamma-3... | Bioorg Med Chem Lett 26: 1156-60 (2016) Article DOI: 10.1016/j.bmcl.2016.01.049 BindingDB Entry DOI: 10.7270/Q29P33HD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase B-raf (Homo sapiens (Human)) | BDBM50139156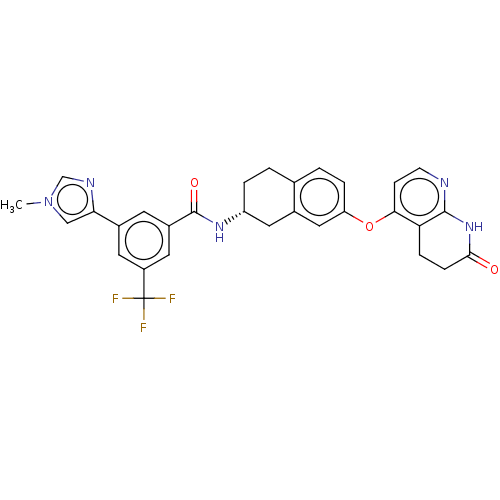 (CHEMBL3763694) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceuticals International Co. 40 Landsdowne Street, Cambridge, MA 02139, United States. Curated by ChEMBL | Assay Description Inhibition of CDK2/Cyclin E (unknown origin) expressed in baculoviral infected insect Sf9 cells using histone H1 as substrate in presence of [gamma-3... | Bioorg Med Chem Lett 26: 1156-60 (2016) Article DOI: 10.1016/j.bmcl.2016.01.049 BindingDB Entry DOI: 10.7270/Q29P33HD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase B-raf (Homo sapiens (Human)) | BDBM50339608 (3-(1-Amino-1-methylethyl)-N-{(2R)-7-[(7-oxo-5,6,7,...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceuticals International Co. 40 Landsdowne Street, Cambridge, MA 02139, United States. Curated by ChEMBL | Assay Description Inhibition of CDK2/Cyclin E (unknown origin) expressed in baculoviral infected insect Sf9 cells using histone H1 as substrate in presence of [gamma-3... | Bioorg Med Chem Lett 26: 1156-60 (2016) Article DOI: 10.1016/j.bmcl.2016.01.049 BindingDB Entry DOI: 10.7270/Q29P33HD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase B-raf (Homo sapiens (Human)) | BDBM50339621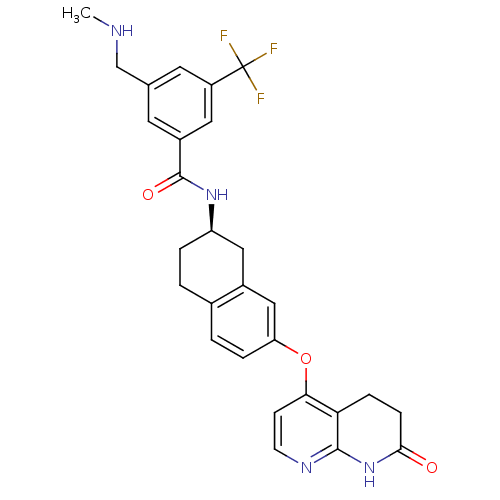 (3-[(methylamino)methyl]-N-{(2R)-7-[(7-oxo-5,6,7,8-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceuticals International Co. 40 Landsdowne Street, Cambridge, MA 02139, United States. Curated by ChEMBL | Assay Description Inhibition of CDK2/Cyclin E (unknown origin) | Bioorg Med Chem Lett 26: 1156-60 (2016) Article DOI: 10.1016/j.bmcl.2016.01.049 BindingDB Entry DOI: 10.7270/Q29P33HD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase B-raf (Homo sapiens (Human)) | BDBM50139151 (CHEMBL3764870) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceuticals International Co. 40 Landsdowne Street, Cambridge, MA 02139, United States. Curated by ChEMBL | Assay Description Inhibition of CDK2/Cyclin E (unknown origin) expressed in baculoviral infected insect Sf9 cells using histone H1 as substrate in presence of [gamma-3... | Bioorg Med Chem Lett 26: 1156-60 (2016) Article DOI: 10.1016/j.bmcl.2016.01.049 BindingDB Entry DOI: 10.7270/Q29P33HD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase B-raf (Homo sapiens (Human)) | BDBM50339609 (3-[(Dimethylamino)methyl]-N-{(2R)-7-[(7-oxo-5,6,7,...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceuticals International Co. 40 Landsdowne Street, Cambridge, MA 02139, United States. Curated by ChEMBL | Assay Description Inhibition of CDK2/Cyclin E (unknown origin) | Bioorg Med Chem Lett 26: 1156-60 (2016) Article DOI: 10.1016/j.bmcl.2016.01.049 BindingDB Entry DOI: 10.7270/Q29P33HD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase B-raf (Homo sapiens (Human)) | BDBM50139155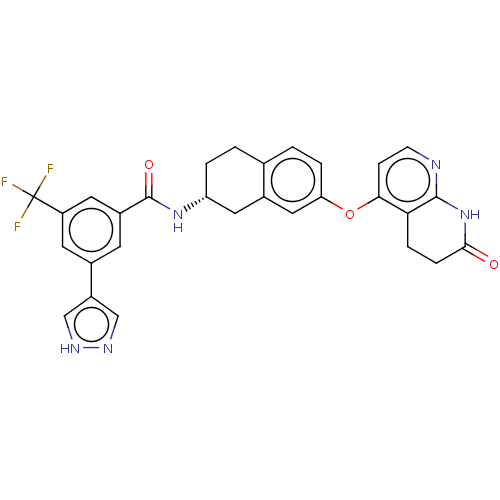 (CHEMBL3765708) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceuticals International Co. 40 Landsdowne Street, Cambridge, MA 02139, United States. Curated by ChEMBL | Assay Description Inhibition of CDK2/Cyclin E (unknown origin) expressed in baculoviral infected insect Sf9 cells using histone H1 as substrate in presence of [gamma-3... | Bioorg Med Chem Lett 26: 1156-60 (2016) Article DOI: 10.1016/j.bmcl.2016.01.049 BindingDB Entry DOI: 10.7270/Q29P33HD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase B-raf (Homo sapiens (Human)) | BDBM50139148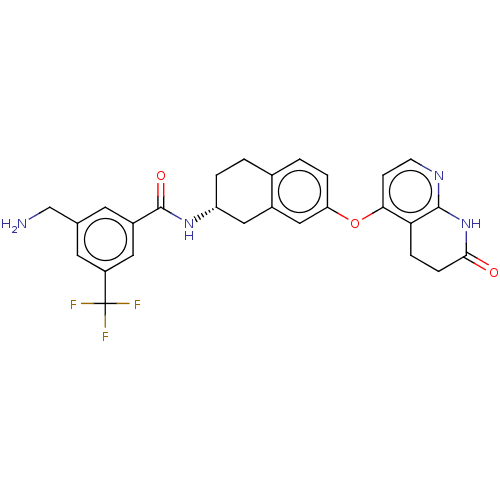 (CHEMBL3764101) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceuticals International Co. 40 Landsdowne Street, Cambridge, MA 02139, United States. Curated by ChEMBL | Assay Description Inhibition of CDK2/Cyclin E (unknown origin) expressed in baculoviral infected insect Sf9 cells using histone H1 as substrate in presence of [gamma-3... | Bioorg Med Chem Lett 26: 1156-60 (2016) Article DOI: 10.1016/j.bmcl.2016.01.049 BindingDB Entry DOI: 10.7270/Q29P33HD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase B-raf (Homo sapiens (Human)) | BDBM50139159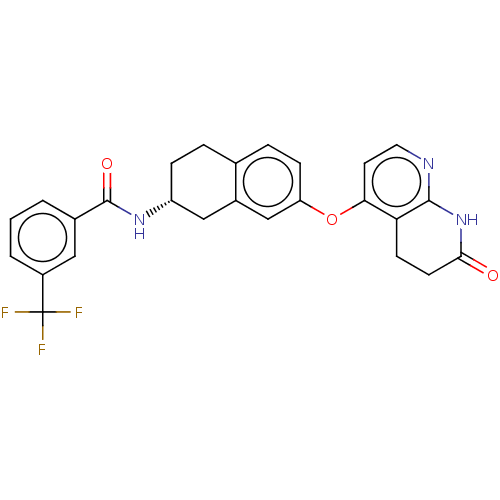 (CHEMBL3765596) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceuticals International Co. 40 Landsdowne Street, Cambridge, MA 02139, United States. Curated by ChEMBL | Assay Description Inhibition of CDK2/Cyclin E (unknown origin) | Bioorg Med Chem Lett 26: 1156-60 (2016) Article DOI: 10.1016/j.bmcl.2016.01.049 BindingDB Entry DOI: 10.7270/Q29P33HD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase B-raf (Homo sapiens (Human)) | BDBM50139152 (CHEMBL3764275) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceuticals International Co. 40 Landsdowne Street, Cambridge, MA 02139, United States. Curated by ChEMBL | Assay Description Inhibition of CDK2/Cyclin E (unknown origin) expressed in baculoviral infected insect Sf9 cells using histone H1 as substrate in presence of [gamma-3... | Bioorg Med Chem Lett 26: 1156-60 (2016) Article DOI: 10.1016/j.bmcl.2016.01.049 BindingDB Entry DOI: 10.7270/Q29P33HD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase B-raf (Homo sapiens (Human)) | BDBM50139154 (CHEMBL3765074) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceuticals International Co. 40 Landsdowne Street, Cambridge, MA 02139, United States. Curated by ChEMBL | Assay Description Inhibition of CDK2/Cyclin E (unknown origin) expressed in baculoviral infected insect Sf9 cells using histone H1 as substrate in presence of [gamma-3... | Bioorg Med Chem Lett 26: 1156-60 (2016) Article DOI: 10.1016/j.bmcl.2016.01.049 BindingDB Entry DOI: 10.7270/Q29P33HD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase B-raf (Homo sapiens (Human)) | BDBM50139158 (CHEMBL3763646) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceuticals International Co. 40 Landsdowne Street, Cambridge, MA 02139, United States. Curated by ChEMBL | Assay Description Inhibition of CDK5/p35NCK (unknown origin) using histone H1 as substrate in presence of [gamma-33P]ATP | Bioorg Med Chem Lett 26: 1156-60 (2016) Article DOI: 10.1016/j.bmcl.2016.01.049 BindingDB Entry DOI: 10.7270/Q29P33HD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase B-raf (Homo sapiens (Human)) | BDBM50139153 (CHEMBL3765355) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceuticals International Co. 40 Landsdowne Street, Cambridge, MA 02139, United States. Curated by ChEMBL | Assay Description Inhibition of CDK4/Cyclin D1 (unknown origin) using RPPTLSPIPHIPR peptide as substrate in presence of [gamma-33P]ATP | Bioorg Med Chem Lett 26: 1156-60 (2016) Article DOI: 10.1016/j.bmcl.2016.01.049 BindingDB Entry DOI: 10.7270/Q29P33HD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase B-raf (Homo sapiens (Human)) | BDBM50339621 (3-[(methylamino)methyl]-N-{(2R)-7-[(7-oxo-5,6,7,8-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceuticals International Co. 40 Landsdowne Street, Cambridge, MA 02139, United States. Curated by ChEMBL | Assay Description Inhibition of CDK2/Cyclin E (unknown origin) expressed in baculoviral infected insect Sf9 cells using histone H1 as substrate in presence of [gamma-3... | Bioorg Med Chem Lett 26: 1156-60 (2016) Article DOI: 10.1016/j.bmcl.2016.01.049 BindingDB Entry DOI: 10.7270/Q29P33HD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase B-raf (Homo sapiens (Human)) | BDBM50339609 (3-[(Dimethylamino)methyl]-N-{(2R)-7-[(7-oxo-5,6,7,...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceuticals International Co. 40 Landsdowne Street, Cambridge, MA 02139, United States. Curated by ChEMBL | Assay Description Inhibition of CDK2/Cyclin E (unknown origin) expressed in baculoviral infected insect Sf9 cells using histone H1 as substrate in presence of [gamma-3... | Bioorg Med Chem Lett 26: 1156-60 (2016) Article DOI: 10.1016/j.bmcl.2016.01.049 BindingDB Entry DOI: 10.7270/Q29P33HD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase B-raf (Homo sapiens (Human)) | BDBM50139155 (CHEMBL3765708) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceuticals International Co. 40 Landsdowne Street, Cambridge, MA 02139, United States. Curated by ChEMBL | Assay Description Inhibition of CDK5/p35NCK (unknown origin) using histone H1 as substrate in presence of [gamma-33P]ATP | Bioorg Med Chem Lett 26: 1156-60 (2016) Article DOI: 10.1016/j.bmcl.2016.01.049 BindingDB Entry DOI: 10.7270/Q29P33HD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase B-raf (Homo sapiens (Human)) | BDBM50139147 (CHEMBL3765509) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 56 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceuticals International Co. 40 Landsdowne Street, Cambridge, MA 02139, United States. Curated by ChEMBL | Assay Description Inhibition of CDK2/Cyclin E (unknown origin) expressed in baculoviral infected insect Sf9 cells using histone H1 as substrate in presence of [gamma-3... | Bioorg Med Chem Lett 26: 1156-60 (2016) Article DOI: 10.1016/j.bmcl.2016.01.049 BindingDB Entry DOI: 10.7270/Q29P33HD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase B-raf (Homo sapiens (Human)) | BDBM50339608 (3-(1-Amino-1-methylethyl)-N-{(2R)-7-[(7-oxo-5,6,7,...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 56 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceuticals International Co. 40 Landsdowne Street, Cambridge, MA 02139, United States. Curated by ChEMBL | Assay Description Inhibition of CDK1/Cyclin B (unknown origin) expressed in baculoviral infected insect Sf9 cells using histone H1 as substrate in presence of [gamma-3... | Bioorg Med Chem Lett 26: 1156-60 (2016) Article DOI: 10.1016/j.bmcl.2016.01.049 BindingDB Entry DOI: 10.7270/Q29P33HD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase B-raf (Homo sapiens (Human)) | BDBM50139149 (CHEMBL3765012) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 67 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceuticals International Co. 40 Landsdowne Street, Cambridge, MA 02139, United States. Curated by ChEMBL | Assay Description Inhibition of CDK1/Cyclin B (unknown origin) expressed in baculoviral infected insect Sf9 cells using histone H1 as substrate in presence of [gamma-3... | Bioorg Med Chem Lett 26: 1156-60 (2016) Article DOI: 10.1016/j.bmcl.2016.01.049 BindingDB Entry DOI: 10.7270/Q29P33HD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase B-raf (Homo sapiens (Human)) | BDBM50139157 (CHEMBL3764588) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 74 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceuticals International Co. 40 Landsdowne Street, Cambridge, MA 02139, United States. Curated by ChEMBL | Assay Description Inhibition of CDK5/p35NCK (unknown origin) using histone H1 as substrate in presence of [gamma-33P]ATP | Bioorg Med Chem Lett 26: 1156-60 (2016) Article DOI: 10.1016/j.bmcl.2016.01.049 BindingDB Entry DOI: 10.7270/Q29P33HD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase B-raf (Homo sapiens (Human)) | BDBM50139154 (CHEMBL3765074) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 121 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceuticals International Co. 40 Landsdowne Street, Cambridge, MA 02139, United States. Curated by ChEMBL | Assay Description Inhibition of CDK4/Cyclin D1 (unknown origin) using RPPTLSPIPHIPR peptide as substrate in presence of [gamma-33P]ATP | Bioorg Med Chem Lett 26: 1156-60 (2016) Article DOI: 10.1016/j.bmcl.2016.01.049 BindingDB Entry DOI: 10.7270/Q29P33HD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase B-raf (Homo sapiens (Human)) | BDBM50139156 (CHEMBL3763694) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 123 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceuticals International Co. 40 Landsdowne Street, Cambridge, MA 02139, United States. Curated by ChEMBL | Assay Description Inhibition of CDK5/p35NCK (unknown origin) using histone H1 as substrate in presence of [gamma-33P]ATP | Bioorg Med Chem Lett 26: 1156-60 (2016) Article DOI: 10.1016/j.bmcl.2016.01.049 BindingDB Entry DOI: 10.7270/Q29P33HD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase B-raf (Homo sapiens (Human)) | BDBM50139150 (CHEMBL3763999) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 207 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceuticals International Co. 40 Landsdowne Street, Cambridge, MA 02139, United States. Curated by ChEMBL | Assay Description Inhibition of CDK4/Cyclin D1 (unknown origin) using RPPTLSPIPHIPR peptide as substrate in presence of [gamma-33P]ATP | Bioorg Med Chem Lett 26: 1156-60 (2016) Article DOI: 10.1016/j.bmcl.2016.01.049 BindingDB Entry DOI: 10.7270/Q29P33HD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase B-raf (Homo sapiens (Human)) | BDBM50139152 (CHEMBL3764275) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 254 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceuticals International Co. 40 Landsdowne Street, Cambridge, MA 02139, United States. Curated by ChEMBL | Assay Description Inhibition of CDK4/Cyclin D1 (unknown origin) using RPPTLSPIPHIPR peptide as substrate in presence of [gamma-33P]ATP | Bioorg Med Chem Lett 26: 1156-60 (2016) Article DOI: 10.1016/j.bmcl.2016.01.049 BindingDB Entry DOI: 10.7270/Q29P33HD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase B-raf (Homo sapiens (Human)) | BDBM50139148 (CHEMBL3764101) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 274 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceuticals International Co. 40 Landsdowne Street, Cambridge, MA 02139, United States. Curated by ChEMBL | Assay Description Inhibition of CDK2/Cyclin E (unknown origin) expressed in baculoviral infected insect Sf9 cells using histone H1 as substrate in presence of [gamma-3... | Bioorg Med Chem Lett 26: 1156-60 (2016) Article DOI: 10.1016/j.bmcl.2016.01.049 BindingDB Entry DOI: 10.7270/Q29P33HD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase B-raf (Homo sapiens (Human)) | BDBM50139159 (CHEMBL3765596) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 487 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceuticals International Co. 40 Landsdowne Street, Cambridge, MA 02139, United States. Curated by ChEMBL | Assay Description Inhibition of CDK2/Cyclin E (unknown origin) expressed in baculoviral infected insect Sf9 cells using histone H1 as substrate in presence of [gamma-3... | Bioorg Med Chem Lett 26: 1156-60 (2016) Article DOI: 10.1016/j.bmcl.2016.01.049 BindingDB Entry DOI: 10.7270/Q29P33HD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase B-raf (Homo sapiens (Human)) | BDBM50139151 (CHEMBL3764870) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 854 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceuticals International Co. 40 Landsdowne Street, Cambridge, MA 02139, United States. Curated by ChEMBL | Assay Description Inhibition of CDK4/Cyclin D1 (unknown origin) using RPPTLSPIPHIPR peptide as substrate in presence of [gamma-33P]ATP | Bioorg Med Chem Lett 26: 1156-60 (2016) Article DOI: 10.1016/j.bmcl.2016.01.049 BindingDB Entry DOI: 10.7270/Q29P33HD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50139149 (CHEMBL3765012) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceuticals International Co. 40 Landsdowne Street, Cambridge, MA 02139, United States. Curated by ChEMBL | Assay Description Inhibition of CDK2/Cyclin E (unknown origin) | Bioorg Med Chem Lett 26: 1156-60 (2016) Article DOI: 10.1016/j.bmcl.2016.01.049 BindingDB Entry DOI: 10.7270/Q29P33HD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50139151 (CHEMBL3764870) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceuticals International Co. 40 Landsdowne Street, Cambridge, MA 02139, United States. Curated by ChEMBL | Assay Description Inhibition of CDK2/Cyclin E (unknown origin) | Bioorg Med Chem Lett 26: 1156-60 (2016) Article DOI: 10.1016/j.bmcl.2016.01.049 BindingDB Entry DOI: 10.7270/Q29P33HD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50139147 (CHEMBL3765509) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceuticals International Co. 40 Landsdowne Street, Cambridge, MA 02139, United States. Curated by ChEMBL | Assay Description Inhibition of CDK2/Cyclin E (unknown origin) | Bioorg Med Chem Lett 26: 1156-60 (2016) Article DOI: 10.1016/j.bmcl.2016.01.049 BindingDB Entry DOI: 10.7270/Q29P33HD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50339609 (3-[(Dimethylamino)methyl]-N-{(2R)-7-[(7-oxo-5,6,7,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceuticals International Co. 40 Landsdowne Street, Cambridge, MA 02139, United States. Curated by ChEMBL | Assay Description Inhibition of CDK5/p35NCK (unknown origin) using histone H1 as substrate in presence of [gamma-33P]ATP | Bioorg Med Chem Lett 26: 1156-60 (2016) Article DOI: 10.1016/j.bmcl.2016.01.049 BindingDB Entry DOI: 10.7270/Q29P33HD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50139148 (CHEMBL3764101) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceuticals International Co. 40 Landsdowne Street, Cambridge, MA 02139, United States. Curated by ChEMBL | Assay Description Inhibition of CDK2/Cyclin E (unknown origin) | Bioorg Med Chem Lett 26: 1156-60 (2016) Article DOI: 10.1016/j.bmcl.2016.01.049 BindingDB Entry DOI: 10.7270/Q29P33HD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50339608 (3-(1-Amino-1-methylethyl)-N-{(2R)-7-[(7-oxo-5,6,7,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceuticals International Co. 40 Landsdowne Street, Cambridge, MA 02139, United States. Curated by ChEMBL | Assay Description Inhibition of CDK2/Cyclin E (unknown origin) | Bioorg Med Chem Lett 26: 1156-60 (2016) Article DOI: 10.1016/j.bmcl.2016.01.049 BindingDB Entry DOI: 10.7270/Q29P33HD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50339621 (3-[(methylamino)methyl]-N-{(2R)-7-[(7-oxo-5,6,7,8-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceuticals International Co. 40 Landsdowne Street, Cambridge, MA 02139, United States. Curated by ChEMBL | Assay Description Inhibition of CDK2/Cyclin E (unknown origin) | Bioorg Med Chem Lett 26: 1156-60 (2016) Article DOI: 10.1016/j.bmcl.2016.01.049 BindingDB Entry DOI: 10.7270/Q29P33HD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50139159 (CHEMBL3765596) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.11E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceuticals International Co. 40 Landsdowne Street, Cambridge, MA 02139, United States. Curated by ChEMBL | Assay Description Inhibition of CDK2/Cyclin E (unknown origin) | Bioorg Med Chem Lett 26: 1156-60 (2016) Article DOI: 10.1016/j.bmcl.2016.01.049 BindingDB Entry DOI: 10.7270/Q29P33HD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50139157 (CHEMBL3764588) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.11E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceuticals International Co. 40 Landsdowne Street, Cambridge, MA 02139, United States. Curated by ChEMBL | Assay Description Inhibition of CDK2/Cyclin E (unknown origin) | Bioorg Med Chem Lett 26: 1156-60 (2016) Article DOI: 10.1016/j.bmcl.2016.01.049 BindingDB Entry DOI: 10.7270/Q29P33HD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50139155 (CHEMBL3765708) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.11E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceuticals International Co. 40 Landsdowne Street, Cambridge, MA 02139, United States. Curated by ChEMBL | Assay Description Inhibition of CDK2/Cyclin E (unknown origin) | Bioorg Med Chem Lett 26: 1156-60 (2016) Article DOI: 10.1016/j.bmcl.2016.01.049 BindingDB Entry DOI: 10.7270/Q29P33HD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50139150 (CHEMBL3763999) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.38E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceuticals International Co. 40 Landsdowne Street, Cambridge, MA 02139, United States. Curated by ChEMBL | Assay Description Inhibition of CDK2/Cyclin E (unknown origin) | Bioorg Med Chem Lett 26: 1156-60 (2016) Article DOI: 10.1016/j.bmcl.2016.01.049 BindingDB Entry DOI: 10.7270/Q29P33HD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50139153 (CHEMBL3765355) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceuticals International Co. 40 Landsdowne Street, Cambridge, MA 02139, United States. Curated by ChEMBL | Assay Description Inhibition of CDK2/Cyclin E (unknown origin) | Bioorg Med Chem Lett 26: 1156-60 (2016) Article DOI: 10.1016/j.bmcl.2016.01.049 BindingDB Entry DOI: 10.7270/Q29P33HD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50139154 (CHEMBL3765074) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceuticals International Co. 40 Landsdowne Street, Cambridge, MA 02139, United States. Curated by ChEMBL | Assay Description Inhibition of CDK2/Cyclin E (unknown origin) | Bioorg Med Chem Lett 26: 1156-60 (2016) Article DOI: 10.1016/j.bmcl.2016.01.049 BindingDB Entry DOI: 10.7270/Q29P33HD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50139158 (CHEMBL3763646) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceuticals International Co. 40 Landsdowne Street, Cambridge, MA 02139, United States. Curated by ChEMBL | Assay Description Inhibition of CDK2/Cyclin E (unknown origin) | Bioorg Med Chem Lett 26: 1156-60 (2016) Article DOI: 10.1016/j.bmcl.2016.01.049 BindingDB Entry DOI: 10.7270/Q29P33HD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50139152 (CHEMBL3764275) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceuticals International Co. 40 Landsdowne Street, Cambridge, MA 02139, United States. Curated by ChEMBL | Assay Description Inhibition of CDK2/Cyclin E (unknown origin) | Bioorg Med Chem Lett 26: 1156-60 (2016) Article DOI: 10.1016/j.bmcl.2016.01.049 BindingDB Entry DOI: 10.7270/Q29P33HD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50139156 (CHEMBL3763694) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceuticals International Co. 40 Landsdowne Street, Cambridge, MA 02139, United States. Curated by ChEMBL | Assay Description Inhibition of CDK2/Cyclin E (unknown origin) | Bioorg Med Chem Lett 26: 1156-60 (2016) Article DOI: 10.1016/j.bmcl.2016.01.049 BindingDB Entry DOI: 10.7270/Q29P33HD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||