

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM8960 ((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | 8.0 | n/a |
University of Karachi | Assay Description Inhibitory activities of AChE and BChE were evaluated by using the Ellman's method [34]. Herein compounds 2(a-h) and 5(a-h) were evaluated as inh... | Bioorg Chem 65: 38-47 (2016) Article DOI: 10.1016/j.bioorg.2016.01.004 BindingDB Entry DOI: 10.7270/Q27D2SX9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM175418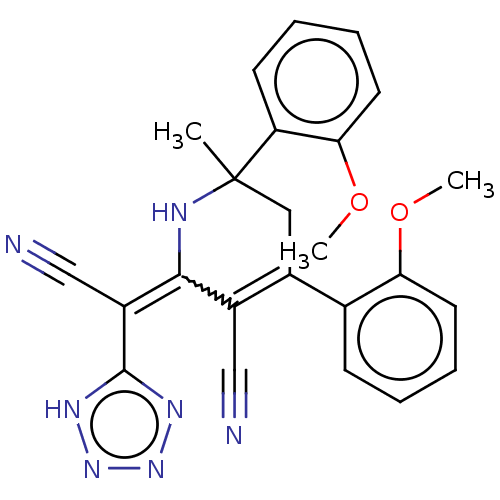 (2-(Cyano(1H-tetrazol-5-yl)methylene)-6-methyl-4,6-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 290 | n/a | n/a | n/a | n/a | 8.0 | n/a |
University of Karachi | Assay Description Inhibitory activities of AChE and BChE were evaluated by using the Ellman's method [34]. Herein compounds 2(a-h) and 5(a-h) were evaluated as inh... | Bioorg Chem 65: 38-47 (2016) Article DOI: 10.1016/j.bioorg.2016.01.004 BindingDB Entry DOI: 10.7270/Q27D2SX9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM175410 (2-[3-cyano-4,6-bis(3-methoxyphenyl)-6-methyl-1,2,5...) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.56E+3 | n/a | n/a | n/a | n/a | 8.0 | n/a |
University of Karachi | Assay Description Inhibitory activities of AChE and BChE were evaluated by using the Ellman's method [34]. Herein compounds 2(a-h) and 5(a-h) were evaluated as inh... | Bioorg Chem 65: 38-47 (2016) Article DOI: 10.1016/j.bioorg.2016.01.004 BindingDB Entry DOI: 10.7270/Q27D2SX9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM175415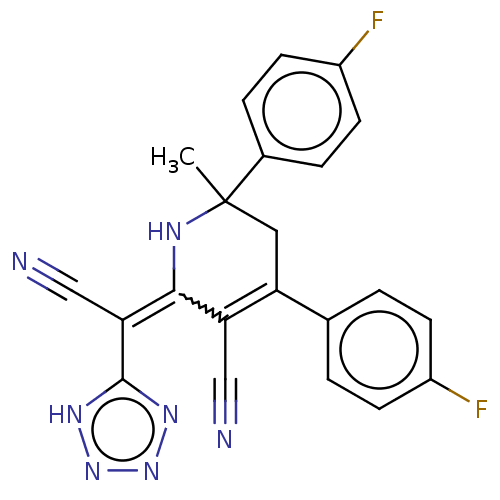 (2-(Cyano(1H-tetrazol-5-yl)methylene)-6-methyl-4,6-...) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.01E+3 | n/a | n/a | n/a | n/a | 8.0 | n/a |
University of Karachi | Assay Description Inhibitory activities of AChE and BChE were evaluated by using the Ellman's method [34]. Herein compounds 2(a-h) and 5(a-h) were evaluated as inh... | Bioorg Chem 65: 38-47 (2016) Article DOI: 10.1016/j.bioorg.2016.01.004 BindingDB Entry DOI: 10.7270/Q27D2SX9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM175416 (2-(Cyano(1H-tetrazol-5-yl)methylene)-6-methyl-4,6-...) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.32E+3 | n/a | n/a | n/a | n/a | 8.0 | n/a |
University of Karachi | Assay Description Inhibitory activities of AChE and BChE were evaluated by using the Ellman's method [34]. Herein compounds 2(a-h) and 5(a-h) were evaluated as inh... | Bioorg Chem 65: 38-47 (2016) Article DOI: 10.1016/j.bioorg.2016.01.004 BindingDB Entry DOI: 10.7270/Q27D2SX9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM175419 (2-(Cyano(1H-tetrazol-5-yl)methylene)-6-methyl-4,6-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.45E+3 | n/a | n/a | n/a | n/a | 8.0 | n/a |
University of Karachi | Assay Description Inhibitory activities of AChE and BChE were evaluated by using the Ellman's method [34]. Herein compounds 2(a-h) and 5(a-h) were evaluated as inh... | Bioorg Chem 65: 38-47 (2016) Article DOI: 10.1016/j.bioorg.2016.01.004 BindingDB Entry DOI: 10.7270/Q27D2SX9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM175416 (2-(Cyano(1H-tetrazol-5-yl)methylene)-6-methyl-4,6-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.78E+3 | n/a | n/a | n/a | n/a | 8.0 | n/a |
University of Karachi | Assay Description Inhibitory activities of AChE and BChE were evaluated by using the Ellman's method [34]. Herein compounds 2(a-h) and 5(a-h) were evaluated as inh... | Bioorg Chem 65: 38-47 (2016) Article DOI: 10.1016/j.bioorg.2016.01.004 BindingDB Entry DOI: 10.7270/Q27D2SX9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM175413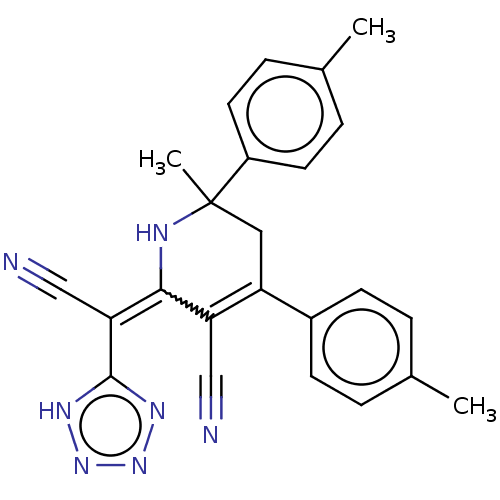 (2-(Cyano(1H-tetrazol-5-yl)methylene)-6-methyl-4,6-...) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.45E+3 | n/a | n/a | n/a | n/a | 8.0 | n/a |
University of Karachi | Assay Description Inhibitory activities of AChE and BChE were evaluated by using the Ellman's method [34]. Herein compounds 2(a-h) and 5(a-h) were evaluated as inh... | Bioorg Chem 65: 38-47 (2016) Article DOI: 10.1016/j.bioorg.2016.01.004 BindingDB Entry DOI: 10.7270/Q27D2SX9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM175417 (2-(Cyano(1H-tetrazol-5-yl)methylene)-6-methyl-4,6-...) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.11E+3 | n/a | n/a | n/a | n/a | 8.0 | n/a |
University of Karachi | Assay Description Inhibitory activities of AChE and BChE were evaluated by using the Ellman's method [34]. Herein compounds 2(a-h) and 5(a-h) were evaluated as inh... | Bioorg Chem 65: 38-47 (2016) Article DOI: 10.1016/j.bioorg.2016.01.004 BindingDB Entry DOI: 10.7270/Q27D2SX9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM175406 (2-[3-cyano-6-methyl-4,6-bis(3-methylphenyl)-1,2,5,...) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.21E+3 | n/a | n/a | n/a | n/a | 8.0 | n/a |
University of Karachi | Assay Description Inhibitory activities of AChE and BChE were evaluated by using the Ellman's method [34]. Herein compounds 2(a-h) and 5(a-h) were evaluated as inh... | Bioorg Chem 65: 38-47 (2016) Article DOI: 10.1016/j.bioorg.2016.01.004 BindingDB Entry DOI: 10.7270/Q27D2SX9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM175411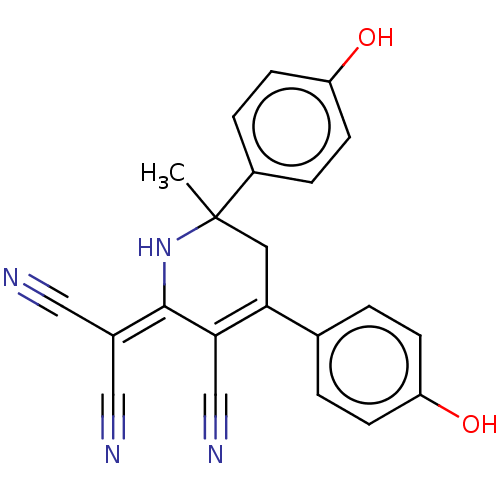 (2-[3-cyano-4,6-bis(4-hydroxyphenyl)-6-methyl-1,2,5...) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.38E+3 | n/a | n/a | n/a | n/a | 8.0 | n/a |
University of Karachi | Assay Description Inhibitory activities of AChE and BChE were evaluated by using the Ellman's method [34]. Herein compounds 2(a-h) and 5(a-h) were evaluated as inh... | Bioorg Chem 65: 38-47 (2016) Article DOI: 10.1016/j.bioorg.2016.01.004 BindingDB Entry DOI: 10.7270/Q27D2SX9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM175413 (2-(Cyano(1H-tetrazol-5-yl)methylene)-6-methyl-4,6-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.87E+3 | n/a | n/a | n/a | n/a | 8.0 | n/a |
University of Karachi | Assay Description Inhibitory activities of AChE and BChE were evaluated by using the Ellman's method [34]. Herein compounds 2(a-h) and 5(a-h) were evaluated as inh... | Bioorg Chem 65: 38-47 (2016) Article DOI: 10.1016/j.bioorg.2016.01.004 BindingDB Entry DOI: 10.7270/Q27D2SX9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM175412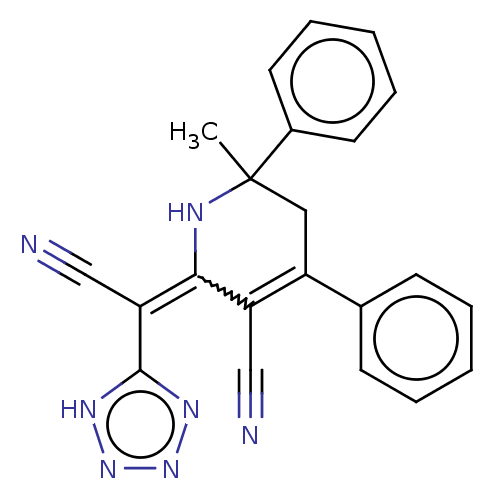 (2-(Cyano(1H-tetrazol-5-yl)methylene)-6-methyl-4,6-...) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.02E+3 | n/a | n/a | n/a | n/a | 8.0 | n/a |
University of Karachi | Assay Description Inhibitory activities of AChE and BChE were evaluated by using the Ellman's method [34]. Herein compounds 2(a-h) and 5(a-h) were evaluated as inh... | Bioorg Chem 65: 38-47 (2016) Article DOI: 10.1016/j.bioorg.2016.01.004 BindingDB Entry DOI: 10.7270/Q27D2SX9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM175419 (2-(Cyano(1H-tetrazol-5-yl)methylene)-6-methyl-4,6-...) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.42E+3 | n/a | n/a | n/a | n/a | 8.0 | n/a |
University of Karachi | Assay Description Inhibitory activities of AChE and BChE were evaluated by using the Ellman's method [34]. Herein compounds 2(a-h) and 5(a-h) were evaluated as inh... | Bioorg Chem 65: 38-47 (2016) Article DOI: 10.1016/j.bioorg.2016.01.004 BindingDB Entry DOI: 10.7270/Q27D2SX9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM175409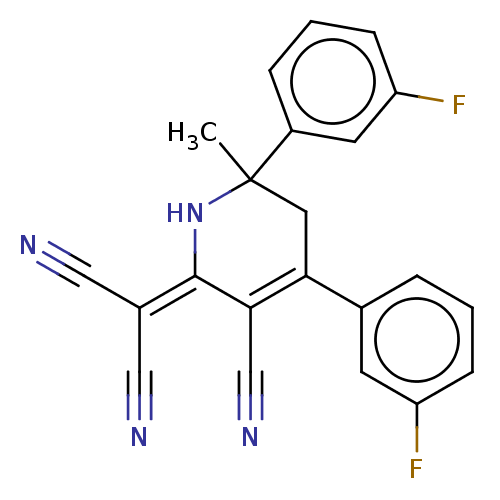 (2-[3-cyano-4,6-bis(3-fluorophenyl)-6-methyl-1,2,5,...) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.01E+3 | n/a | n/a | n/a | n/a | 8.0 | n/a |
University of Karachi | Assay Description Inhibitory activities of AChE and BChE were evaluated by using the Ellman's method [34]. Herein compounds 2(a-h) and 5(a-h) were evaluated as inh... | Bioorg Chem 65: 38-47 (2016) Article DOI: 10.1016/j.bioorg.2016.01.004 BindingDB Entry DOI: 10.7270/Q27D2SX9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM8960 ((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 8.41E+3 | n/a | n/a | n/a | n/a | 8.0 | n/a |
University of Karachi | Assay Description Inhibitory activities of AChE and BChE were evaluated by using the Ellman's method [34]. Herein compounds 2(a-h) and 5(a-h) were evaluated as inh... | Bioorg Chem 65: 38-47 (2016) Article DOI: 10.1016/j.bioorg.2016.01.004 BindingDB Entry DOI: 10.7270/Q27D2SX9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM175408 (2-[3-cyano-4,6-bis(4-iodophenyl)-6-methyl-1,2,5,6-...) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.12E+4 | n/a | n/a | n/a | n/a | 8.0 | n/a |
University of Karachi | Assay Description Inhibitory activities of AChE and BChE were evaluated by using the Ellman's method [34]. Herein compounds 2(a-h) and 5(a-h) were evaluated as inh... | Bioorg Chem 65: 38-47 (2016) Article DOI: 10.1016/j.bioorg.2016.01.004 BindingDB Entry DOI: 10.7270/Q27D2SX9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM175407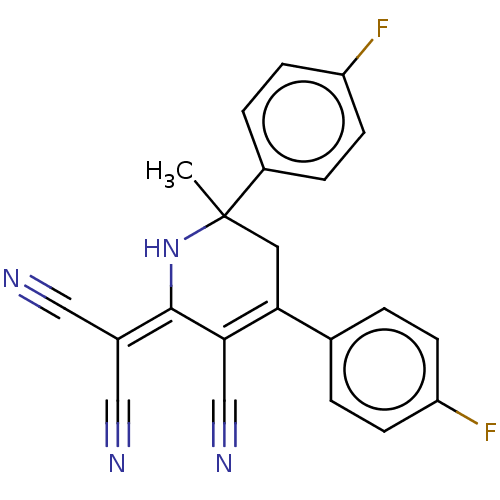 (2-[3-cyano-4,6-bis(4-fluorophenyl)-6-methyl-1,2,5,...) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.19E+4 | n/a | n/a | n/a | n/a | 8.0 | n/a |
University of Karachi | Assay Description Inhibitory activities of AChE and BChE were evaluated by using the Ellman's method [34]. Herein compounds 2(a-h) and 5(a-h) were evaluated as inh... | Bioorg Chem 65: 38-47 (2016) Article DOI: 10.1016/j.bioorg.2016.01.004 BindingDB Entry DOI: 10.7270/Q27D2SX9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM175405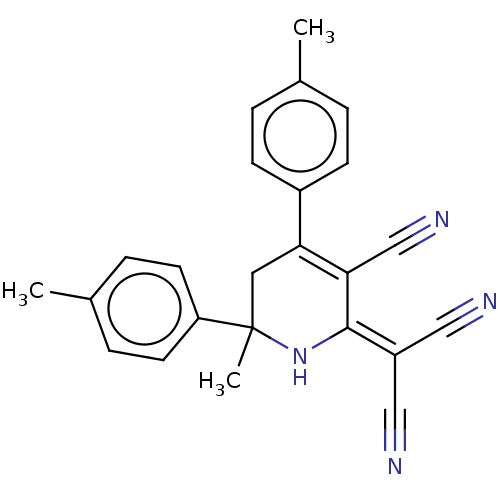 (2-[3-cyano-6-methyl-4,6-bis(4-methylphenyl)-1,2,5,...) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.69E+4 | n/a | n/a | n/a | n/a | 8.0 | n/a |
University of Karachi | Assay Description Inhibitory activities of AChE and BChE were evaluated by using the Ellman's method [34]. Herein compounds 2(a-h) and 5(a-h) were evaluated as inh... | Bioorg Chem 65: 38-47 (2016) Article DOI: 10.1016/j.bioorg.2016.01.004 BindingDB Entry DOI: 10.7270/Q27D2SX9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50022775 ((m-Hydroxyphenyl)trimethylammonium dimethylcarbama...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.93E+4 | n/a | n/a | n/a | n/a | 8.0 | n/a |
University of Karachi | Assay Description Inhibitory activities of AChE and BChE were evaluated by using the Ellman's method [34]. Herein compounds 2(a-h) and 5(a-h) were evaluated as inh... | Bioorg Chem 65: 38-47 (2016) Article DOI: 10.1016/j.bioorg.2016.01.004 BindingDB Entry DOI: 10.7270/Q27D2SX9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM175418 (2-(Cyano(1H-tetrazol-5-yl)methylene)-6-methyl-4,6-...) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.03E+4 | n/a | n/a | n/a | n/a | 8.0 | n/a |
University of Karachi | Assay Description Inhibitory activities of AChE and BChE were evaluated by using the Ellman's method [34]. Herein compounds 2(a-h) and 5(a-h) were evaluated as inh... | Bioorg Chem 65: 38-47 (2016) Article DOI: 10.1016/j.bioorg.2016.01.004 BindingDB Entry DOI: 10.7270/Q27D2SX9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM175404 (2-Dicyanomethylene-6-methyl-4,6-bis(phenyl)-1,2,5,...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.03E+4 | n/a | n/a | n/a | n/a | 8.0 | n/a |
University of Karachi | Assay Description Inhibitory activities of AChE and BChE were evaluated by using the Ellman's method [34]. Herein compounds 2(a-h) and 5(a-h) were evaluated as inh... | Bioorg Chem 65: 38-47 (2016) Article DOI: 10.1016/j.bioorg.2016.01.004 BindingDB Entry DOI: 10.7270/Q27D2SX9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM175414 (2-(Cyano(1H-tetrazol-5-yl)methylene)-6-methyl-4,6-...) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.08E+4 | n/a | n/a | n/a | n/a | 8.0 | n/a |
University of Karachi | Assay Description Inhibitory activities of AChE and BChE were evaluated by using the Ellman's method [34]. Herein compounds 2(a-h) and 5(a-h) were evaluated as inh... | Bioorg Chem 65: 38-47 (2016) Article DOI: 10.1016/j.bioorg.2016.01.004 BindingDB Entry DOI: 10.7270/Q27D2SX9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM175412 (2-(Cyano(1H-tetrazol-5-yl)methylene)-6-methyl-4,6-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.21E+4 | n/a | n/a | n/a | n/a | 8.0 | n/a |
University of Karachi | Assay Description Inhibitory activities of AChE and BChE were evaluated by using the Ellman's method [34]. Herein compounds 2(a-h) and 5(a-h) were evaluated as inh... | Bioorg Chem 65: 38-47 (2016) Article DOI: 10.1016/j.bioorg.2016.01.004 BindingDB Entry DOI: 10.7270/Q27D2SX9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM175406 (2-[3-cyano-6-methyl-4,6-bis(3-methylphenyl)-1,2,5,...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.59E+4 | n/a | n/a | n/a | n/a | 8.0 | n/a |
University of Karachi | Assay Description Inhibitory activities of AChE and BChE were evaluated by using the Ellman's method [34]. Herein compounds 2(a-h) and 5(a-h) were evaluated as inh... | Bioorg Chem 65: 38-47 (2016) Article DOI: 10.1016/j.bioorg.2016.01.004 BindingDB Entry DOI: 10.7270/Q27D2SX9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM175405 (2-[3-cyano-6-methyl-4,6-bis(4-methylphenyl)-1,2,5,...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.65E+4 | n/a | n/a | n/a | n/a | 8.0 | n/a |
University of Karachi | Assay Description Inhibitory activities of AChE and BChE were evaluated by using the Ellman's method [34]. Herein compounds 2(a-h) and 5(a-h) were evaluated as inh... | Bioorg Chem 65: 38-47 (2016) Article DOI: 10.1016/j.bioorg.2016.01.004 BindingDB Entry DOI: 10.7270/Q27D2SX9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM175415 (2-(Cyano(1H-tetrazol-5-yl)methylene)-6-methyl-4,6-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.88E+4 | n/a | n/a | n/a | n/a | 8.0 | n/a |
University of Karachi | Assay Description Inhibitory activities of AChE and BChE were evaluated by using the Ellman's method [34]. Herein compounds 2(a-h) and 5(a-h) were evaluated as inh... | Bioorg Chem 65: 38-47 (2016) Article DOI: 10.1016/j.bioorg.2016.01.004 BindingDB Entry DOI: 10.7270/Q27D2SX9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM175417 (2-(Cyano(1H-tetrazol-5-yl)methylene)-6-methyl-4,6-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.92E+4 | n/a | n/a | n/a | n/a | 8.0 | n/a |
University of Karachi | Assay Description Inhibitory activities of AChE and BChE were evaluated by using the Ellman's method [34]. Herein compounds 2(a-h) and 5(a-h) were evaluated as inh... | Bioorg Chem 65: 38-47 (2016) Article DOI: 10.1016/j.bioorg.2016.01.004 BindingDB Entry DOI: 10.7270/Q27D2SX9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM175411 (2-[3-cyano-4,6-bis(4-hydroxyphenyl)-6-methyl-1,2,5...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.22E+4 | n/a | n/a | n/a | n/a | 8.0 | n/a |
University of Karachi | Assay Description Inhibitory activities of AChE and BChE were evaluated by using the Ellman's method [34]. Herein compounds 2(a-h) and 5(a-h) were evaluated as inh... | Bioorg Chem 65: 38-47 (2016) Article DOI: 10.1016/j.bioorg.2016.01.004 BindingDB Entry DOI: 10.7270/Q27D2SX9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM50022775 ((m-Hydroxyphenyl)trimethylammonium dimethylcarbama...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.32E+4 | n/a | n/a | n/a | n/a | 8.0 | n/a |
University of Karachi | Assay Description Inhibitory activities of AChE and BChE were evaluated by using the Ellman's method [34]. Herein compounds 2(a-h) and 5(a-h) were evaluated as inh... | Bioorg Chem 65: 38-47 (2016) Article DOI: 10.1016/j.bioorg.2016.01.004 BindingDB Entry DOI: 10.7270/Q27D2SX9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM175408 (2-[3-cyano-4,6-bis(4-iodophenyl)-6-methyl-1,2,5,6-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.53E+4 | n/a | n/a | n/a | n/a | 8.0 | n/a |
University of Karachi | Assay Description Inhibitory activities of AChE and BChE were evaluated by using the Ellman's method [34]. Herein compounds 2(a-h) and 5(a-h) were evaluated as inh... | Bioorg Chem 65: 38-47 (2016) Article DOI: 10.1016/j.bioorg.2016.01.004 BindingDB Entry DOI: 10.7270/Q27D2SX9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM175404 (2-Dicyanomethylene-6-methyl-4,6-bis(phenyl)-1,2,5,...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.81E+4 | n/a | n/a | n/a | n/a | 8.0 | n/a |
University of Karachi | Assay Description Inhibitory activities of AChE and BChE were evaluated by using the Ellman's method [34]. Herein compounds 2(a-h) and 5(a-h) were evaluated as inh... | Bioorg Chem 65: 38-47 (2016) Article DOI: 10.1016/j.bioorg.2016.01.004 BindingDB Entry DOI: 10.7270/Q27D2SX9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM175410 (2-[3-cyano-4,6-bis(3-methoxyphenyl)-6-methyl-1,2,5...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.82E+4 | n/a | n/a | n/a | n/a | 8.0 | n/a |
University of Karachi | Assay Description Inhibitory activities of AChE and BChE were evaluated by using the Ellman's method [34]. Herein compounds 2(a-h) and 5(a-h) were evaluated as inh... | Bioorg Chem 65: 38-47 (2016) Article DOI: 10.1016/j.bioorg.2016.01.004 BindingDB Entry DOI: 10.7270/Q27D2SX9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM175407 (2-[3-cyano-4,6-bis(4-fluorophenyl)-6-methyl-1,2,5,...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.52E+4 | n/a | n/a | n/a | n/a | 8.0 | n/a |
University of Karachi | Assay Description Inhibitory activities of AChE and BChE were evaluated by using the Ellman's method [34]. Herein compounds 2(a-h) and 5(a-h) were evaluated as inh... | Bioorg Chem 65: 38-47 (2016) Article DOI: 10.1016/j.bioorg.2016.01.004 BindingDB Entry DOI: 10.7270/Q27D2SX9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM175414 (2-(Cyano(1H-tetrazol-5-yl)methylene)-6-methyl-4,6-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.22E+4 | n/a | n/a | n/a | n/a | 8.0 | n/a |
University of Karachi | Assay Description Inhibitory activities of AChE and BChE were evaluated by using the Ellman's method [34]. Herein compounds 2(a-h) and 5(a-h) were evaluated as inh... | Bioorg Chem 65: 38-47 (2016) Article DOI: 10.1016/j.bioorg.2016.01.004 BindingDB Entry DOI: 10.7270/Q27D2SX9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM175409 (2-[3-cyano-4,6-bis(3-fluorophenyl)-6-methyl-1,2,5,...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.88E+4 | n/a | n/a | n/a | n/a | 8.0 | n/a |
University of Karachi | Assay Description Inhibitory activities of AChE and BChE were evaluated by using the Ellman's method [34]. Herein compounds 2(a-h) and 5(a-h) were evaluated as inh... | Bioorg Chem 65: 38-47 (2016) Article DOI: 10.1016/j.bioorg.2016.01.004 BindingDB Entry DOI: 10.7270/Q27D2SX9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||