

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50164512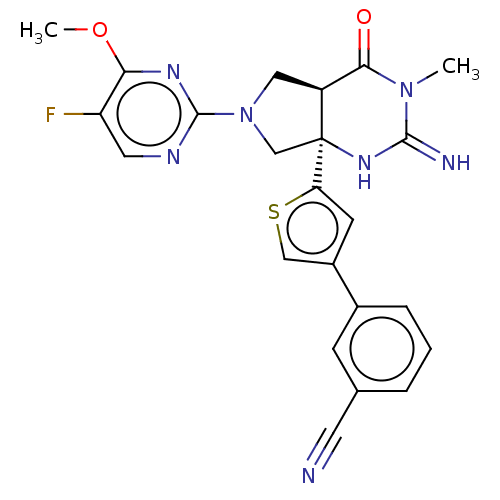 (CHEMBL3800286) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of recombinant human BACE1 preincubated for 30 mins followed QSY7EISEVNLDAEFC-Eu-amide substrate addition measured after 90 mins by FRET a... | J Med Chem 59: 3231-48 (2016) Article DOI: 10.1021/acs.jmedchem.5b01995 BindingDB Entry DOI: 10.7270/Q2CR5W8V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50398693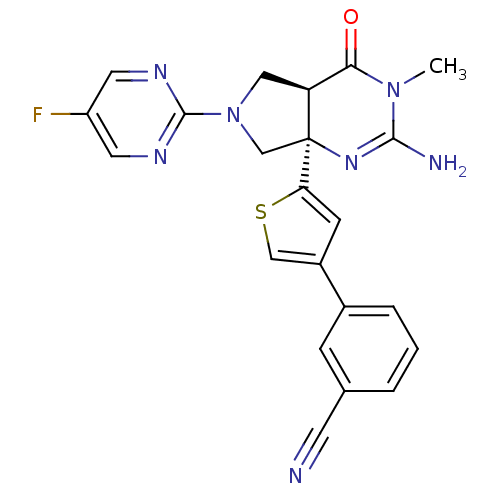 (CHEMBL2178718) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of recombinant human BACE1 preincubated for 30 mins followed QSY7EISEVNLDAEFC-Eu-amide substrate addition measured after 90 mins by FRET a... | J Med Chem 59: 3231-48 (2016) Article DOI: 10.1021/acs.jmedchem.5b01995 BindingDB Entry DOI: 10.7270/Q2CR5W8V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM9488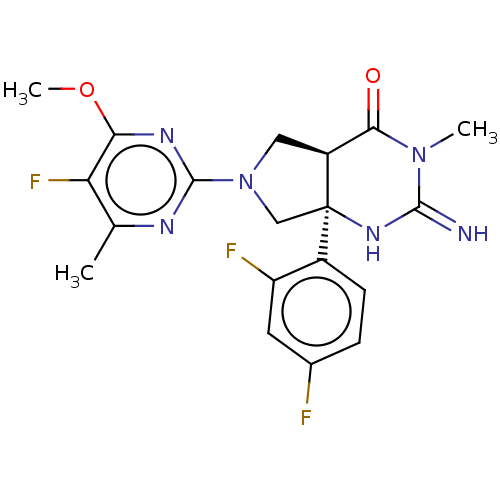 (US8541427, 1) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | Article PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of recombinant human BACE1 preincubated for 30 mins followed QSY7EISEVNLDAEFC-Eu-amide substrate addition measured after 90 mins by FRET a... | J Med Chem 59: 3231-48 (2016) Article DOI: 10.1021/acs.jmedchem.5b01995 BindingDB Entry DOI: 10.7270/Q2CR5W8V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM102945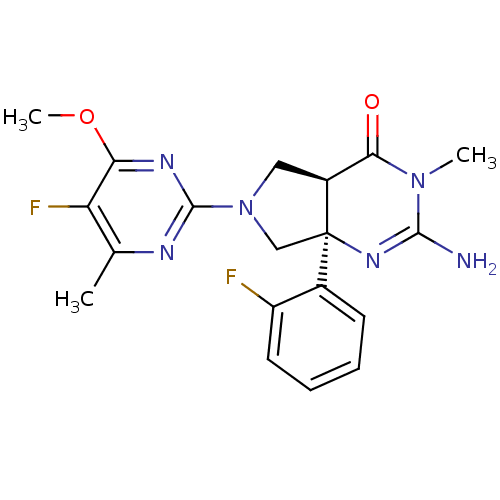 (US8541427, 16) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of recombinant human BACE1 preincubated for 30 mins followed QSY7EISEVNLDAEFC-Eu-amide substrate addition measured after 90 mins by FRET a... | J Med Chem 59: 3231-48 (2016) Article DOI: 10.1021/acs.jmedchem.5b01995 BindingDB Entry DOI: 10.7270/Q2CR5W8V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50398689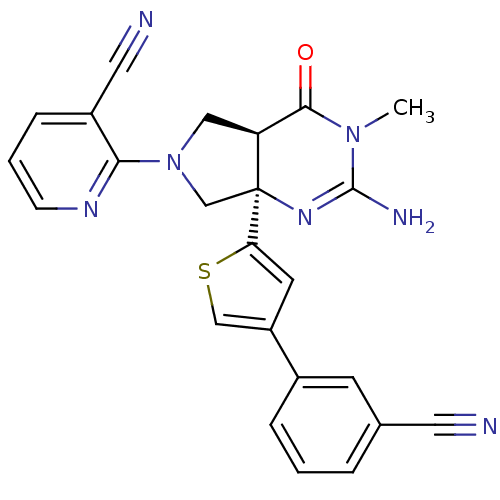 (CHEMBL2178713) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem Similars | Article PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of recombinant human BACE1 preincubated for 30 mins followed QSY7EISEVNLDAEFC-Eu-amide substrate addition measured after 90 mins by FRET a... | J Med Chem 59: 3231-48 (2016) Article DOI: 10.1021/acs.jmedchem.5b01995 BindingDB Entry DOI: 10.7270/Q2CR5W8V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM102946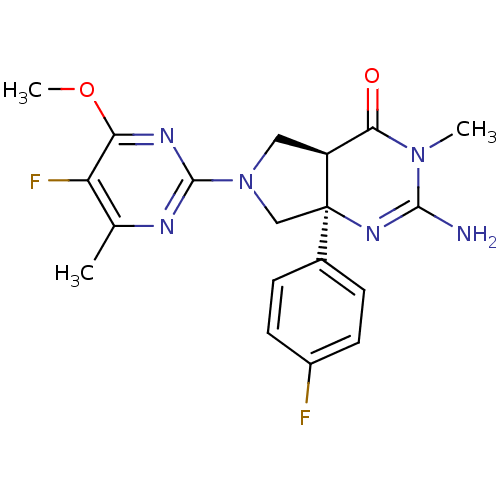 (US8541427, 17) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of recombinant human BACE1 preincubated for 30 mins followed QSY7EISEVNLDAEFC-Eu-amide substrate addition measured after 90 mins by FRET a... | J Med Chem 59: 3231-48 (2016) Article DOI: 10.1021/acs.jmedchem.5b01995 BindingDB Entry DOI: 10.7270/Q2CR5W8V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM102937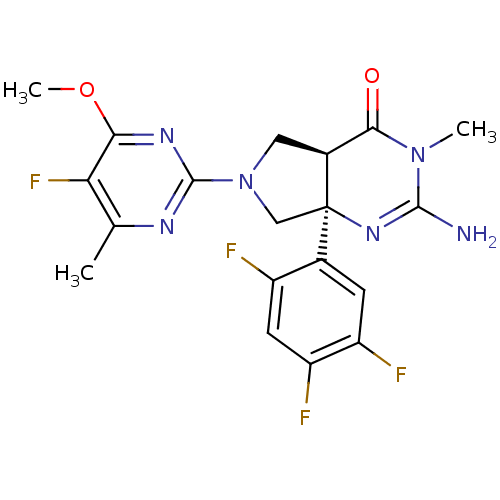 (US8541427, 8) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of recombinant human BACE1 preincubated for 30 mins followed QSY7EISEVNLDAEFC-Eu-amide substrate addition measured after 90 mins by FRET a... | J Med Chem 59: 3231-48 (2016) Article DOI: 10.1021/acs.jmedchem.5b01995 BindingDB Entry DOI: 10.7270/Q2CR5W8V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM102944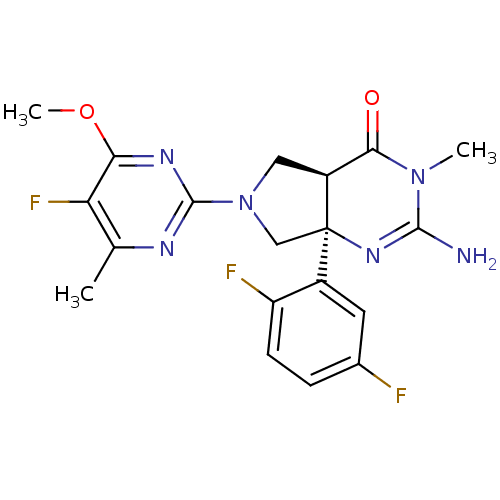 (US8541427, 15 | US8541427, 206 | US8541427, 44 | U...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of recombinant human BACE1 preincubated for 30 mins followed QSY7EISEVNLDAEFC-Eu-amide substrate addition measured after 90 mins by FRET a... | J Med Chem 59: 3231-48 (2016) Article DOI: 10.1021/acs.jmedchem.5b01995 BindingDB Entry DOI: 10.7270/Q2CR5W8V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50164509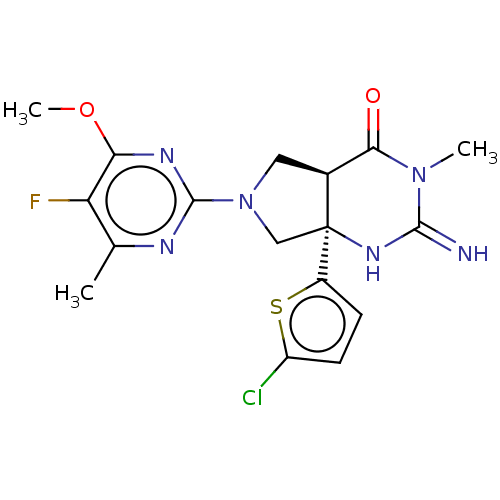 (CHEMBL3798199) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of recombinant human BACE1 preincubated for 30 mins followed QSY7EISEVNLDAEFC-Eu-amide substrate addition measured after 90 mins by FRET a... | J Med Chem 59: 3231-48 (2016) Article DOI: 10.1021/acs.jmedchem.5b01995 BindingDB Entry DOI: 10.7270/Q2CR5W8V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50164508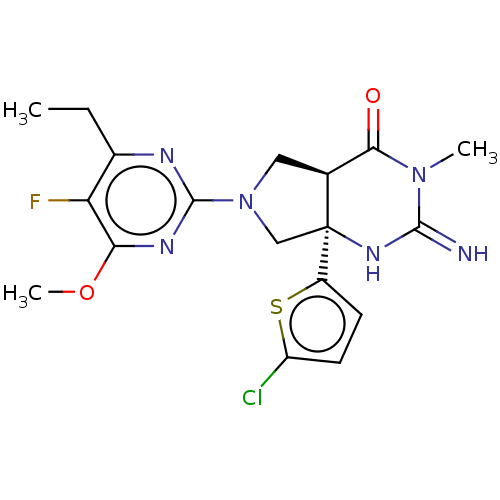 (CHEMBL3797321) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of recombinant human BACE1 preincubated for 30 mins followed QSY7EISEVNLDAEFC-Eu-amide substrate addition measured after 90 mins by FRET a... | J Med Chem 59: 3231-48 (2016) Article DOI: 10.1021/acs.jmedchem.5b01995 BindingDB Entry DOI: 10.7270/Q2CR5W8V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50164507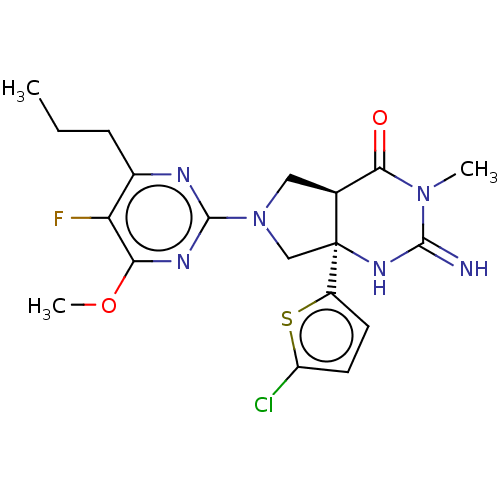 (CHEMBL3798760) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of recombinant human BACE1 preincubated for 30 mins followed QSY7EISEVNLDAEFC-Eu-amide substrate addition measured after 90 mins by FRET a... | J Med Chem 59: 3231-48 (2016) Article DOI: 10.1021/acs.jmedchem.5b01995 BindingDB Entry DOI: 10.7270/Q2CR5W8V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50164510 (CHEMBL3798544) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of recombinant human BACE1 preincubated for 30 mins followed QSY7EISEVNLDAEFC-Eu-amide substrate addition measured after 90 mins by FRET a... | J Med Chem 59: 3231-48 (2016) Article DOI: 10.1021/acs.jmedchem.5b01995 BindingDB Entry DOI: 10.7270/Q2CR5W8V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM102940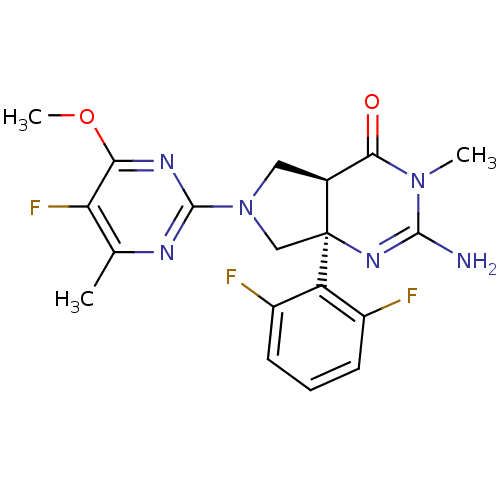 (US8541427, 11) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of recombinant human BACE1 preincubated for 30 mins followed QSY7EISEVNLDAEFC-Eu-amide substrate addition measured after 90 mins by FRET a... | J Med Chem 59: 3231-48 (2016) Article DOI: 10.1021/acs.jmedchem.5b01995 BindingDB Entry DOI: 10.7270/Q2CR5W8V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM102958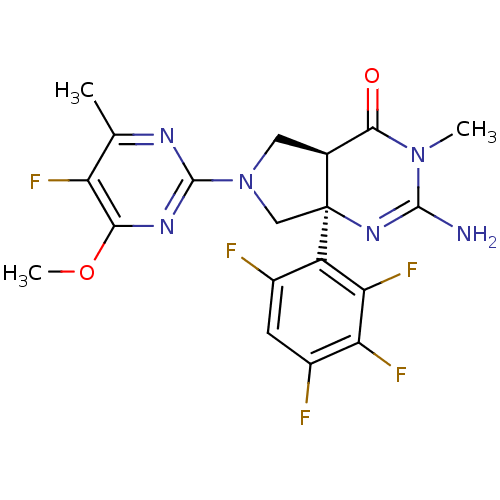 (US8541427, 29) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of recombinant human BACE1 preincubated for 30 mins followed QSY7EISEVNLDAEFC-Eu-amide substrate addition measured after 90 mins by FRET a... | J Med Chem 59: 3231-48 (2016) Article DOI: 10.1021/acs.jmedchem.5b01995 BindingDB Entry DOI: 10.7270/Q2CR5W8V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM102941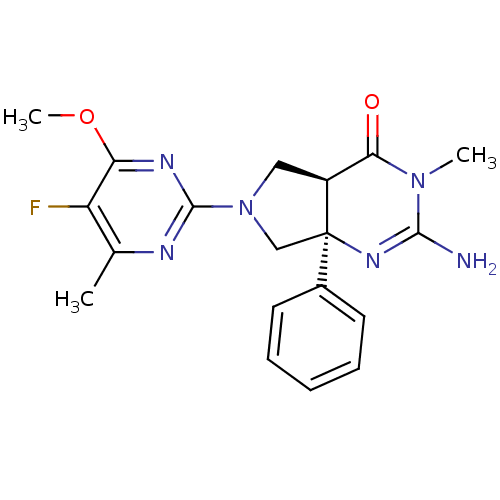 (US8541427, 12) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 36 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of recombinant human BACE1 preincubated for 30 mins followed QSY7EISEVNLDAEFC-Eu-amide substrate addition measured after 90 mins by FRET a... | J Med Chem 59: 3231-48 (2016) Article DOI: 10.1021/acs.jmedchem.5b01995 BindingDB Entry DOI: 10.7270/Q2CR5W8V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50164505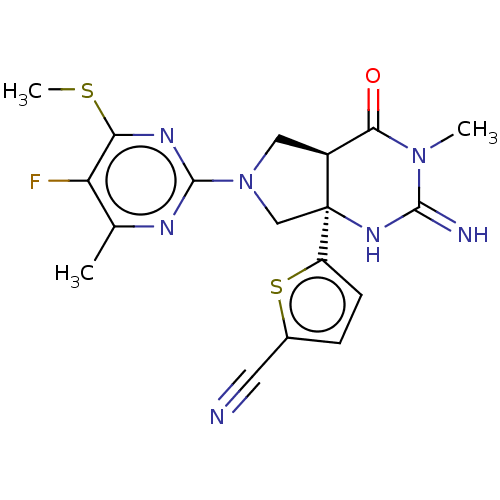 (CHEMBL3797680) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | 45 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of recombinant human BACE1 preincubated for 30 mins followed QSY7EISEVNLDAEFC-Eu-amide substrate addition measured after 90 mins by FRET a... | J Med Chem 59: 3231-48 (2016) Article DOI: 10.1021/acs.jmedchem.5b01995 BindingDB Entry DOI: 10.7270/Q2CR5W8V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50164506 (CHEMBL3799339) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | 48 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of recombinant human BACE1 preincubated for 30 mins followed QSY7EISEVNLDAEFC-Eu-amide substrate addition measured after 90 mins by FRET a... | J Med Chem 59: 3231-48 (2016) Article DOI: 10.1021/acs.jmedchem.5b01995 BindingDB Entry DOI: 10.7270/Q2CR5W8V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM102969 (US8541427, 40) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 53 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of recombinant human BACE1 preincubated for 30 mins followed QSY7EISEVNLDAEFC-Eu-amide substrate addition measured after 90 mins by FRET a... | J Med Chem 59: 3231-48 (2016) Article DOI: 10.1021/acs.jmedchem.5b01995 BindingDB Entry DOI: 10.7270/Q2CR5W8V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50164511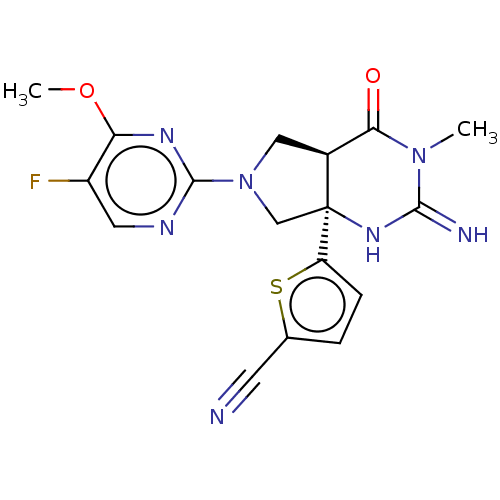 (CHEMBL3800447) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 57 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of recombinant human BACE1 preincubated for 30 mins followed QSY7EISEVNLDAEFC-Eu-amide substrate addition measured after 90 mins by FRET a... | J Med Chem 59: 3231-48 (2016) Article DOI: 10.1021/acs.jmedchem.5b01995 BindingDB Entry DOI: 10.7270/Q2CR5W8V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50164504 (CHEMBL3799879) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | 336 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of recombinant human BACE1 preincubated for 30 mins followed QSY7EISEVNLDAEFC-Eu-amide substrate addition measured after 90 mins by FRET a... | J Med Chem 59: 3231-48 (2016) Article DOI: 10.1021/acs.jmedchem.5b01995 BindingDB Entry DOI: 10.7270/Q2CR5W8V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H2 receptor (Homo sapiens (Human)) | BDBM9488 (US8541427, 1) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | Article PubMed | 4.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human histamine H2 receptor | J Med Chem 59: 3231-48 (2016) Article DOI: 10.1021/acs.jmedchem.5b01995 BindingDB Entry DOI: 10.7270/Q2CR5W8V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Motilin receptor (Homo sapiens (Human)) | BDBM9488 (US8541427, 1) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | Article PubMed | 1.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human motilin receptor | J Med Chem 59: 3231-48 (2016) Article DOI: 10.1021/acs.jmedchem.5b01995 BindingDB Entry DOI: 10.7270/Q2CR5W8V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM9488 (US8541427, 1) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of BACE1 in human HEK293 cells transfected with human APP Swe/Lon mutations assessed as amyloid beta 40 level after 4 hrs by electrochemil... | J Med Chem 59: 3231-48 (2016) Article DOI: 10.1021/acs.jmedchem.5b01995 BindingDB Entry DOI: 10.7270/Q2CR5W8V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM102946 (US8541427, 17) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of BACE1 in human HEK293 cells transfected with human APP Swe/Lon mutations assessed as amyloid beta 40 level after 4 hrs by electrochemil... | J Med Chem 59: 3231-48 (2016) Article DOI: 10.1021/acs.jmedchem.5b01995 BindingDB Entry DOI: 10.7270/Q2CR5W8V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM102945 (US8541427, 16) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of BACE1 in human HEK293 cells transfected with human APP Swe/Lon mutations assessed as amyloid beta 40 level after 4 hrs by electrochemil... | J Med Chem 59: 3231-48 (2016) Article DOI: 10.1021/acs.jmedchem.5b01995 BindingDB Entry DOI: 10.7270/Q2CR5W8V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50164512 (CHEMBL3800286) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of BACE1 in human HEK293 cells transfected with human APP Swe/Lon mutations assessed as amyloid beta 40 level after 4 hrs by electrochemil... | J Med Chem 59: 3231-48 (2016) Article DOI: 10.1021/acs.jmedchem.5b01995 BindingDB Entry DOI: 10.7270/Q2CR5W8V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM102944 (US8541427, 15 | US8541427, 206 | US8541427, 44 | U...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of BACE1 in human HEK293 cells transfected with human APP Swe/Lon mutations assessed as amyloid beta 40 level after 4 hrs by electrochemil... | J Med Chem 59: 3231-48 (2016) Article DOI: 10.1021/acs.jmedchem.5b01995 BindingDB Entry DOI: 10.7270/Q2CR5W8V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM102937 (US8541427, 8) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of BACE1 in human HEK293 cells transfected with human APP Swe/Lon mutations assessed as amyloid beta 40 level after 4 hrs by electrochemil... | J Med Chem 59: 3231-48 (2016) Article DOI: 10.1021/acs.jmedchem.5b01995 BindingDB Entry DOI: 10.7270/Q2CR5W8V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50164505 (CHEMBL3797680) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of BACE1 in human HEK293 cells transfected with human APP Swe/Lon mutations assessed as amyloid beta 40 level after 4 hrs by electrochemil... | J Med Chem 59: 3231-48 (2016) Article DOI: 10.1021/acs.jmedchem.5b01995 BindingDB Entry DOI: 10.7270/Q2CR5W8V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50164510 (CHEMBL3798544) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of BACE1 in human HEK293 cells transfected with human APP Swe/Lon mutations assessed as amyloid beta 40 level after 4 hrs by electrochemil... | J Med Chem 59: 3231-48 (2016) Article DOI: 10.1021/acs.jmedchem.5b01995 BindingDB Entry DOI: 10.7270/Q2CR5W8V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM102940 (US8541427, 11) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 49 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of BACE1 in human HEK293 cells transfected with human APP Swe/Lon mutations assessed as amyloid beta 40 level after 4 hrs by electrochemil... | J Med Chem 59: 3231-48 (2016) Article DOI: 10.1021/acs.jmedchem.5b01995 BindingDB Entry DOI: 10.7270/Q2CR5W8V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50164509 (CHEMBL3798199) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 51 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of BACE1 in human HEK293 cells transfected with human APP Swe/Lon mutations assessed as amyloid beta 40 level after 4 hrs by electrochemil... | J Med Chem 59: 3231-48 (2016) Article DOI: 10.1021/acs.jmedchem.5b01995 BindingDB Entry DOI: 10.7270/Q2CR5W8V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM102941 (US8541427, 12) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 58 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of BACE1 in human HEK293 cells transfected with human APP Swe/Lon mutations assessed as amyloid beta 40 level after 4 hrs by electrochemil... | J Med Chem 59: 3231-48 (2016) Article DOI: 10.1021/acs.jmedchem.5b01995 BindingDB Entry DOI: 10.7270/Q2CR5W8V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM102958 (US8541427, 29) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 93 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of BACE1 in human HEK293 cells transfected with human APP Swe/Lon mutations assessed as amyloid beta 40 level after 4 hrs by electrochemil... | J Med Chem 59: 3231-48 (2016) Article DOI: 10.1021/acs.jmedchem.5b01995 BindingDB Entry DOI: 10.7270/Q2CR5W8V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50164504 (CHEMBL3799879) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 157 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of BACE1 in human HEK293 cells transfected with human APP Swe/Lon mutations assessed as amyloid beta 40 level after 4 hrs by electrochemil... | J Med Chem 59: 3231-48 (2016) Article DOI: 10.1021/acs.jmedchem.5b01995 BindingDB Entry DOI: 10.7270/Q2CR5W8V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50164507 (CHEMBL3798760) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of BACE1 in human HEK293 cells transfected with human APP Swe/Lon mutations assessed as amyloid beta 40 level after 4 hrs by electrochemil... | J Med Chem 59: 3231-48 (2016) Article DOI: 10.1021/acs.jmedchem.5b01995 BindingDB Entry DOI: 10.7270/Q2CR5W8V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50164508 (CHEMBL3797321) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 203 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of BACE1 in human HEK293 cells transfected with human APP Swe/Lon mutations assessed as amyloid beta 40 level after 4 hrs by electrochemil... | J Med Chem 59: 3231-48 (2016) Article DOI: 10.1021/acs.jmedchem.5b01995 BindingDB Entry DOI: 10.7270/Q2CR5W8V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM102969 (US8541427, 40) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 234 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of BACE1 in human HEK293 cells transfected with human APP Swe/Lon mutations assessed as amyloid beta 40 level after 4 hrs by electrochemil... | J Med Chem 59: 3231-48 (2016) Article DOI: 10.1021/acs.jmedchem.5b01995 BindingDB Entry DOI: 10.7270/Q2CR5W8V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50164511 (CHEMBL3800447) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 238 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of BACE1 in human HEK293 cells transfected with human APP Swe/Lon mutations assessed as amyloid beta 40 level after 4 hrs by electrochemil... | J Med Chem 59: 3231-48 (2016) Article DOI: 10.1021/acs.jmedchem.5b01995 BindingDB Entry DOI: 10.7270/Q2CR5W8V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50164506 (CHEMBL3799339) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 361 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of BACE1 in human HEK293 cells transfected with human APP Swe/Lon mutations assessed as amyloid beta 40 level after 4 hrs by electrochemil... | J Med Chem 59: 3231-48 (2016) Article DOI: 10.1021/acs.jmedchem.5b01995 BindingDB Entry DOI: 10.7270/Q2CR5W8V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50398693 (CHEMBL2178718) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of CYP3A4 in human liver microsomes using testosterone and midazolam as substrate preincubated for 30 mins followed substrate addition by ... | J Med Chem 59: 3231-48 (2016) Article DOI: 10.1021/acs.jmedchem.5b01995 BindingDB Entry DOI: 10.7270/Q2CR5W8V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM9488 (US8541427, 1) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human ERG | J Med Chem 59: 3231-48 (2016) Article DOI: 10.1021/acs.jmedchem.5b01995 BindingDB Entry DOI: 10.7270/Q2CR5W8V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50164512 (CHEMBL3800286) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of CYP3A4 in human liver microsomes using testosterone and midazolam as substrate preincubated for 30 mins followed substrate addition by ... | J Med Chem 59: 3231-48 (2016) Article DOI: 10.1021/acs.jmedchem.5b01995 BindingDB Entry DOI: 10.7270/Q2CR5W8V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50164509 (CHEMBL3798199) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of CYP3A4 in human liver microsomes using testosterone and midazolam as substrate preincubated for 30 mins followed substrate addition by ... | J Med Chem 59: 3231-48 (2016) Article DOI: 10.1021/acs.jmedchem.5b01995 BindingDB Entry DOI: 10.7270/Q2CR5W8V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM102940 (US8541427, 11) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of CYP3A4 in human liver microsomes using testosterone and midazolam as substrate preincubated for 30 mins followed substrate addition by ... | J Med Chem 59: 3231-48 (2016) Article DOI: 10.1021/acs.jmedchem.5b01995 BindingDB Entry DOI: 10.7270/Q2CR5W8V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM102937 (US8541427, 8) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of CYP3A4 in human liver microsomes using testosterone and midazolam as substrate preincubated for 30 mins followed substrate addition by ... | J Med Chem 59: 3231-48 (2016) Article DOI: 10.1021/acs.jmedchem.5b01995 BindingDB Entry DOI: 10.7270/Q2CR5W8V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM102969 (US8541427, 40) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of CYP3A4 in human liver microsomes using testosterone and midazolam as substrate preincubated for 30 mins followed substrate addition by ... | J Med Chem 59: 3231-48 (2016) Article DOI: 10.1021/acs.jmedchem.5b01995 BindingDB Entry DOI: 10.7270/Q2CR5W8V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50164510 (CHEMBL3798544) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 2.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of CYP3A4 in human liver microsomes using testosterone and midazolam as substrate preincubated for 30 mins followed substrate addition by ... | J Med Chem 59: 3231-48 (2016) Article DOI: 10.1021/acs.jmedchem.5b01995 BindingDB Entry DOI: 10.7270/Q2CR5W8V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM9488 (US8541427, 1) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of CYP3A4 in human liver microsomes using testosterone and midazolam as substrate preincubated for 30 mins followed substrate addition by ... | J Med Chem 59: 3231-48 (2016) Article DOI: 10.1021/acs.jmedchem.5b01995 BindingDB Entry DOI: 10.7270/Q2CR5W8V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM102941 (US8541427, 12) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of CYP3A4 in human liver microsomes using testosterone and midazolam as substrate preincubated for 30 mins followed substrate addition by ... | J Med Chem 59: 3231-48 (2016) Article DOI: 10.1021/acs.jmedchem.5b01995 BindingDB Entry DOI: 10.7270/Q2CR5W8V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50398689 (CHEMBL2178713) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of CYP3A4 in human liver microsomes using testosterone and midazolam as substrate preincubated for 30 mins followed substrate addition by ... | J Med Chem 59: 3231-48 (2016) Article DOI: 10.1021/acs.jmedchem.5b01995 BindingDB Entry DOI: 10.7270/Q2CR5W8V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM102945 (US8541427, 16) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of CYP3A4 in human liver microsomes using testosterone and midazolam as substrate preincubated for 30 mins followed substrate addition by ... | J Med Chem 59: 3231-48 (2016) Article DOI: 10.1021/acs.jmedchem.5b01995 BindingDB Entry DOI: 10.7270/Q2CR5W8V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM102958 (US8541427, 29) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of CYP3A4 in human liver microsomes using testosterone and midazolam as substrate preincubated for 30 mins followed substrate addition by ... | J Med Chem 59: 3231-48 (2016) Article DOI: 10.1021/acs.jmedchem.5b01995 BindingDB Entry DOI: 10.7270/Q2CR5W8V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM102944 (US8541427, 15 | US8541427, 206 | US8541427, 44 | U...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of CYP3A4 in human liver microsomes using testosterone and midazolam as substrate preincubated for 30 mins followed substrate addition by ... | J Med Chem 59: 3231-48 (2016) Article DOI: 10.1021/acs.jmedchem.5b01995 BindingDB Entry DOI: 10.7270/Q2CR5W8V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM102946 (US8541427, 17) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.41E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of CYP3A4 in human liver microsomes using testosterone and midazolam as substrate preincubated for 30 mins followed substrate addition by ... | J Med Chem 59: 3231-48 (2016) Article DOI: 10.1021/acs.jmedchem.5b01995 BindingDB Entry DOI: 10.7270/Q2CR5W8V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||