

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50152733 (CHEMBL3781564) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Waterloo Curated by ChEMBL | Assay Description Inhibition of human AChE assessed as hydrolytic activity using acetylthiocholine iodide as substrate measured for 15 mins by Ellmans assay | Eur J Med Chem 113: 258-72 (2016) Article DOI: 10.1016/j.ejmech.2016.02.049 BindingDB Entry DOI: 10.7270/Q29W0HCH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50152733 (CHEMBL3781564) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Waterloo Curated by ChEMBL | Assay Description Inhibition of human BuChE assessed as hydrolytic activity using butyrylthiocholine iodide as substrate measured for 15 mins by Ellmans assay | Eur J Med Chem 113: 258-72 (2016) Article DOI: 10.1016/j.ejmech.2016.02.049 BindingDB Entry DOI: 10.7270/Q29W0HCH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amyloid-beta precursor protein (Homo sapiens (Human)) | BDBM50152738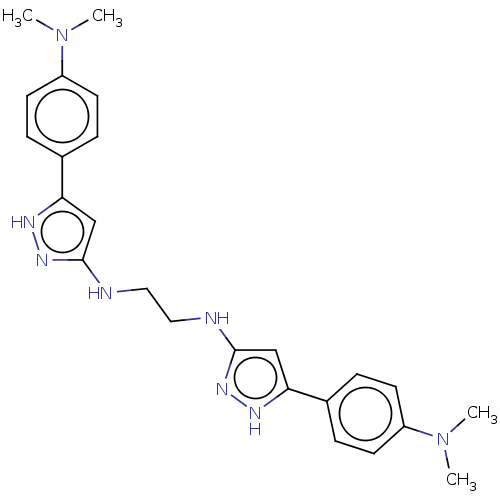 (CHEMBL3781022) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Waterloo Curated by ChEMBL | Assay Description Inhibition of amyloid beta (1 to 42) (unknown origin) aggregation measured after 24 hrs by Thioflavin T-based fluorometric assay | Eur J Med Chem 113: 258-72 (2016) Article DOI: 10.1016/j.ejmech.2016.02.049 BindingDB Entry DOI: 10.7270/Q29W0HCH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amyloid-beta precursor protein (Homo sapiens (Human)) | BDBM50152737 (CHEMBL3781419) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Waterloo Curated by ChEMBL | Assay Description Inhibition of amyloid beta (1 to 42) (unknown origin) aggregation measured after 24 hrs by Thioflavin T-based fluorometric assay | Eur J Med Chem 113: 258-72 (2016) Article DOI: 10.1016/j.ejmech.2016.02.049 BindingDB Entry DOI: 10.7270/Q29W0HCH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amyloid-beta precursor protein (Homo sapiens (Human)) | BDBM50152739 (CHEMBL3781026) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.05E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Waterloo Curated by ChEMBL | Assay Description Inhibition of amyloid beta (1 to 42) (unknown origin) aggregation measured after 24 hrs by Thioflavin T-based fluorometric assay | Eur J Med Chem 113: 258-72 (2016) Article DOI: 10.1016/j.ejmech.2016.02.049 BindingDB Entry DOI: 10.7270/Q29W0HCH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50152735 (CHEMBL3780448) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.08E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Waterloo Curated by ChEMBL | Assay Description Inhibition of human AChE assessed as hydrolytic activity using acetylthiocholine iodide as substrate measured for 15 mins by Ellmans assay | Eur J Med Chem 113: 258-72 (2016) Article DOI: 10.1016/j.ejmech.2016.02.049 BindingDB Entry DOI: 10.7270/Q29W0HCH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50152736 (CHEMBL3780131) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.11E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Waterloo Curated by ChEMBL | Assay Description Inhibition of human AChE assessed as hydrolytic activity using acetylthiocholine iodide as substrate measured for 15 mins by Ellmans assay | Eur J Med Chem 113: 258-72 (2016) Article DOI: 10.1016/j.ejmech.2016.02.049 BindingDB Entry DOI: 10.7270/Q29W0HCH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50152735 (CHEMBL3780448) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.64E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Waterloo Curated by ChEMBL | Assay Description Inhibition of human BuChE assessed as hydrolytic activity using butyrylthiocholine iodide as substrate measured for 15 mins by Ellmans assay | Eur J Med Chem 113: 258-72 (2016) Article DOI: 10.1016/j.ejmech.2016.02.049 BindingDB Entry DOI: 10.7270/Q29W0HCH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50152736 (CHEMBL3780131) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Waterloo Curated by ChEMBL | Assay Description Inhibition of human BuChE assessed as hydrolytic activity using butyrylthiocholine iodide as substrate measured for 15 mins by Ellmans assay | Eur J Med Chem 113: 258-72 (2016) Article DOI: 10.1016/j.ejmech.2016.02.049 BindingDB Entry DOI: 10.7270/Q29W0HCH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||