

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50149957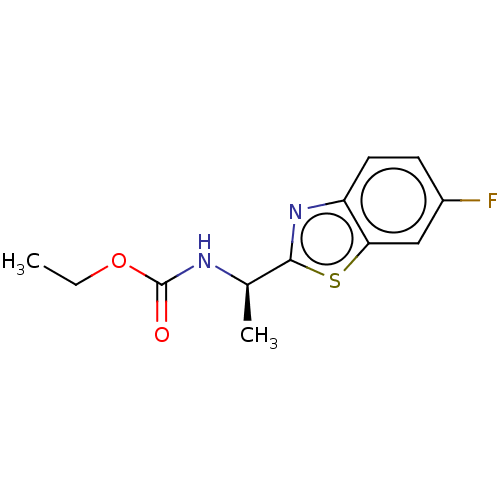 (CHEMBL3769730) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.06E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pardubice Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by spectrophotometric-based Ellman's method | Bioorg Med Chem 24: 1560-72 (2016) Article DOI: 10.1016/j.bmc.2016.02.033 BindingDB Entry DOI: 10.7270/Q2XW4MPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50149953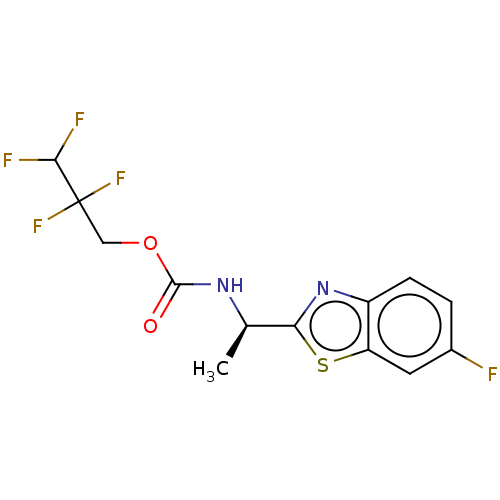 (CHEMBL3771027) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.75E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pardubice Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by spectrophotometric-based Ellman's method | Bioorg Med Chem 24: 1560-72 (2016) Article DOI: 10.1016/j.bmc.2016.02.033 BindingDB Entry DOI: 10.7270/Q2XW4MPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50149951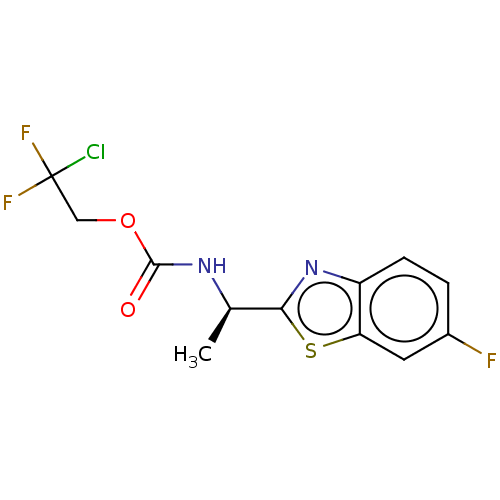 (CHEMBL3771191) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.14E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pardubice Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by spectrophotometric-based Ellman's method | Bioorg Med Chem 24: 1560-72 (2016) Article DOI: 10.1016/j.bmc.2016.02.033 BindingDB Entry DOI: 10.7270/Q2XW4MPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50149950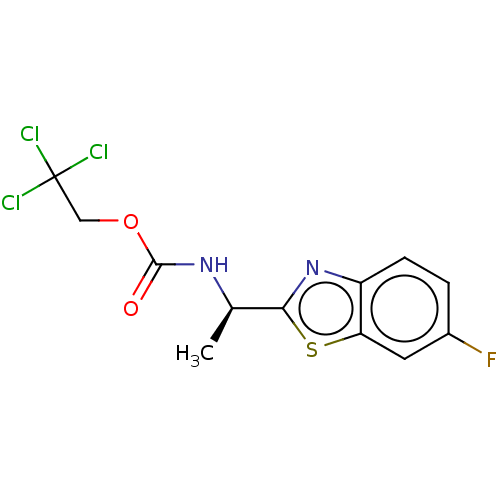 (CHEMBL3770572) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.31E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pardubice Curated by ChEMBL | Assay Description Inhibition of equine serum BChE by spectrophotometric-based Ellman's method | Bioorg Med Chem 24: 1560-72 (2016) Article DOI: 10.1016/j.bmc.2016.02.033 BindingDB Entry DOI: 10.7270/Q2XW4MPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50149959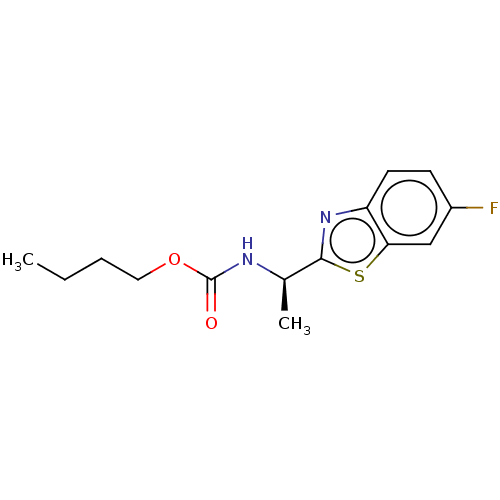 (CHEMBL3769959) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.26E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pardubice Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by spectrophotometric-based Ellman's method | Bioorg Med Chem 24: 1560-72 (2016) Article DOI: 10.1016/j.bmc.2016.02.033 BindingDB Entry DOI: 10.7270/Q2XW4MPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50149962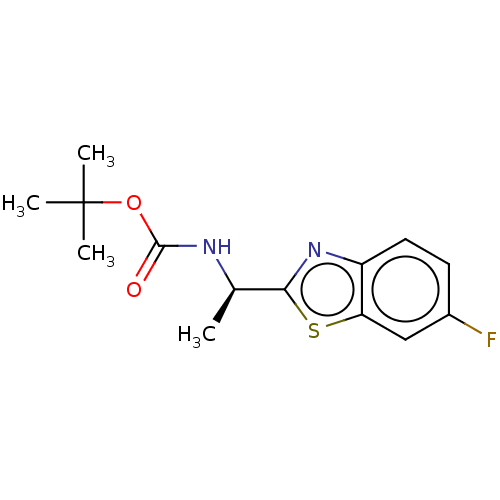 (CHEMBL3770856) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.05E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pardubice Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by spectrophotometric-based Ellman's method | Bioorg Med Chem 24: 1560-72 (2016) Article DOI: 10.1016/j.bmc.2016.02.033 BindingDB Entry DOI: 10.7270/Q2XW4MPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50149948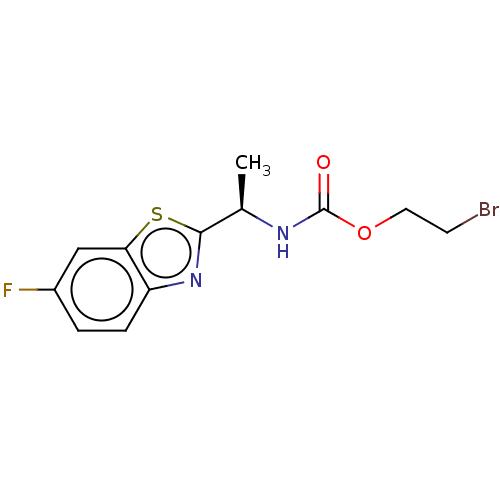 (CHEMBL3770816) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.14E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pardubice Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by spectrophotometric-based Ellman's method | Bioorg Med Chem 24: 1560-72 (2016) Article DOI: 10.1016/j.bmc.2016.02.033 BindingDB Entry DOI: 10.7270/Q2XW4MPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50149939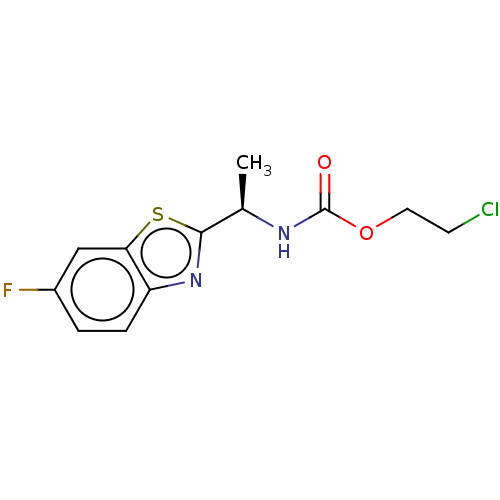 (CHEMBL3769807) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.27E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pardubice Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by spectrophotometric-based Ellman's method | Bioorg Med Chem 24: 1560-72 (2016) Article DOI: 10.1016/j.bmc.2016.02.033 BindingDB Entry DOI: 10.7270/Q2XW4MPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50149961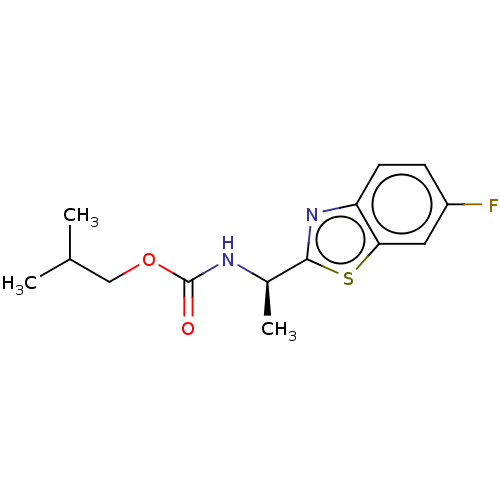 (CHEMBL3770054) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.29E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pardubice Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by spectrophotometric-based Ellman's method | Bioorg Med Chem 24: 1560-72 (2016) Article DOI: 10.1016/j.bmc.2016.02.033 BindingDB Entry DOI: 10.7270/Q2XW4MPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50149958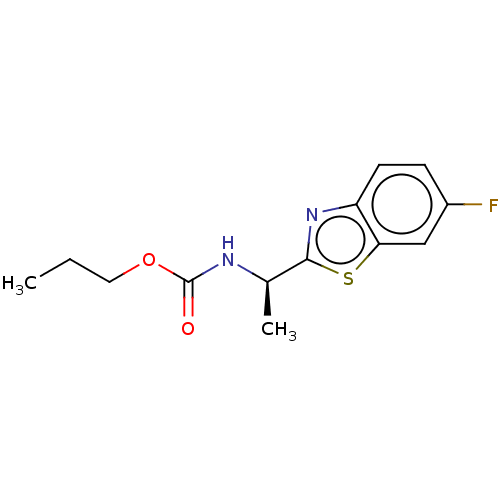 (CHEMBL3770152) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.31E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pardubice Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by spectrophotometric-based Ellman's method | Bioorg Med Chem 24: 1560-72 (2016) Article DOI: 10.1016/j.bmc.2016.02.033 BindingDB Entry DOI: 10.7270/Q2XW4MPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50149956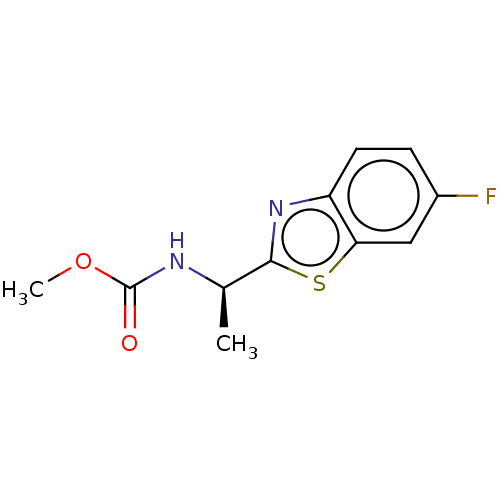 (CHEMBL3770401) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.36E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pardubice Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by spectrophotometric-based Ellman's method | Bioorg Med Chem 24: 1560-72 (2016) Article DOI: 10.1016/j.bmc.2016.02.033 BindingDB Entry DOI: 10.7270/Q2XW4MPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50149960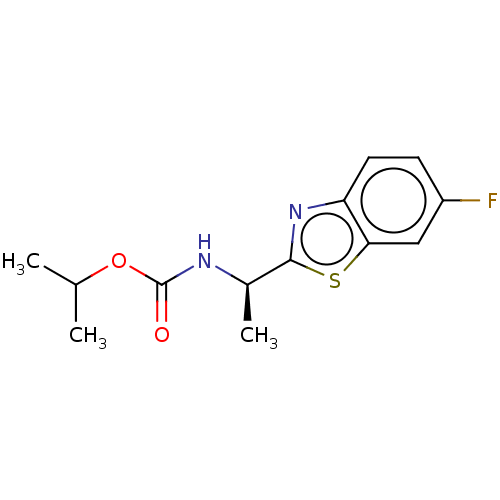 (CHEMBL3771281) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.65E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pardubice Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by spectrophotometric-based Ellman's method | Bioorg Med Chem 24: 1560-72 (2016) Article DOI: 10.1016/j.bmc.2016.02.033 BindingDB Entry DOI: 10.7270/Q2XW4MPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50149957 (CHEMBL3769730) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.01E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pardubice Curated by ChEMBL | Assay Description Inhibition of equine serum BChE by spectrophotometric-based Ellman's method | Bioorg Med Chem 24: 1560-72 (2016) Article DOI: 10.1016/j.bmc.2016.02.033 BindingDB Entry DOI: 10.7270/Q2XW4MPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50149962 (CHEMBL3770856) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.31E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pardubice Curated by ChEMBL | Assay Description Inhibition of equine serum BChE by spectrophotometric-based Ellman's method | Bioorg Med Chem 24: 1560-72 (2016) Article DOI: 10.1016/j.bmc.2016.02.033 BindingDB Entry DOI: 10.7270/Q2XW4MPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50149953 (CHEMBL3771027) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pardubice Curated by ChEMBL | Assay Description Inhibition of equine serum BChE by spectrophotometric-based Ellman's method | Bioorg Med Chem 24: 1560-72 (2016) Article DOI: 10.1016/j.bmc.2016.02.033 BindingDB Entry DOI: 10.7270/Q2XW4MPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50149951 (CHEMBL3771191) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.56E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pardubice Curated by ChEMBL | Assay Description Inhibition of equine serum BChE by spectrophotometric-based Ellman's method | Bioorg Med Chem 24: 1560-72 (2016) Article DOI: 10.1016/j.bmc.2016.02.033 BindingDB Entry DOI: 10.7270/Q2XW4MPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50149960 (CHEMBL3771281) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.27E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pardubice Curated by ChEMBL | Assay Description Inhibition of equine serum BChE by spectrophotometric-based Ellman's method | Bioorg Med Chem 24: 1560-72 (2016) Article DOI: 10.1016/j.bmc.2016.02.033 BindingDB Entry DOI: 10.7270/Q2XW4MPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50149958 (CHEMBL3770152) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.46E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pardubice Curated by ChEMBL | Assay Description Inhibition of equine serum BChE by spectrophotometric-based Ellman's method | Bioorg Med Chem 24: 1560-72 (2016) Article DOI: 10.1016/j.bmc.2016.02.033 BindingDB Entry DOI: 10.7270/Q2XW4MPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50149948 (CHEMBL3770816) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.84E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pardubice Curated by ChEMBL | Assay Description Inhibition of equine serum BChE by spectrophotometric-based Ellman's method | Bioorg Med Chem 24: 1560-72 (2016) Article DOI: 10.1016/j.bmc.2016.02.033 BindingDB Entry DOI: 10.7270/Q2XW4MPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM10620 ((S)-3-(1-(dimethylamino)ethyl)phenyl ethyl(methyl)...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.84E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pardubice Curated by ChEMBL | Assay Description Inhibition of equine serum BChE by spectrophotometric-based Ellman's method | Bioorg Med Chem 24: 1560-72 (2016) Article DOI: 10.1016/j.bmc.2016.02.033 BindingDB Entry DOI: 10.7270/Q2XW4MPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50149949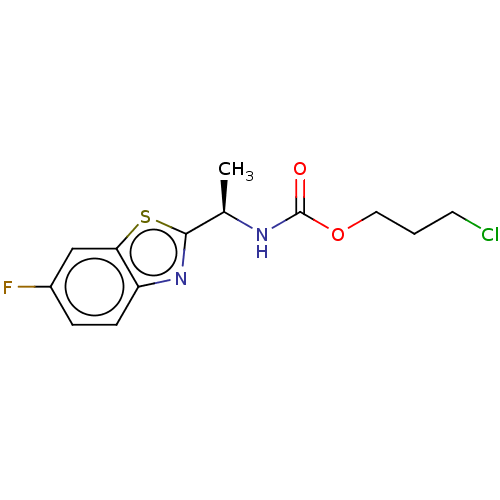 (CHEMBL3769638) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.88E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pardubice Curated by ChEMBL | Assay Description Inhibition of equine serum BChE by spectrophotometric-based Ellman's method | Bioorg Med Chem 24: 1560-72 (2016) Article DOI: 10.1016/j.bmc.2016.02.033 BindingDB Entry DOI: 10.7270/Q2XW4MPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50149961 (CHEMBL3770054) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.02E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pardubice Curated by ChEMBL | Assay Description Inhibition of equine serum BChE by spectrophotometric-based Ellman's method | Bioorg Med Chem 24: 1560-72 (2016) Article DOI: 10.1016/j.bmc.2016.02.033 BindingDB Entry DOI: 10.7270/Q2XW4MPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50149952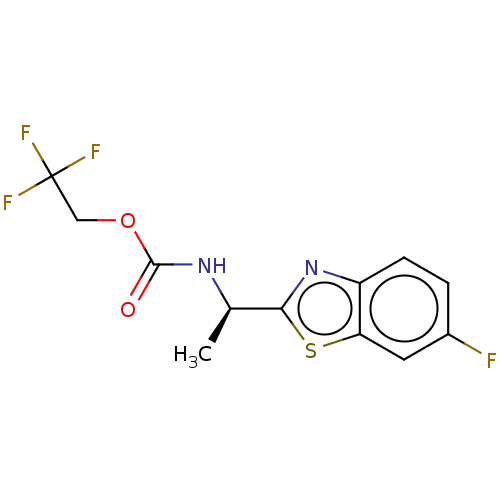 (CHEMBL3770851) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.05E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pardubice Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by spectrophotometric-based Ellman's method | Bioorg Med Chem 24: 1560-72 (2016) Article DOI: 10.1016/j.bmc.2016.02.033 BindingDB Entry DOI: 10.7270/Q2XW4MPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50149959 (CHEMBL3769959) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.09E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pardubice Curated by ChEMBL | Assay Description Inhibition of equine serum BChE by spectrophotometric-based Ellman's method | Bioorg Med Chem 24: 1560-72 (2016) Article DOI: 10.1016/j.bmc.2016.02.033 BindingDB Entry DOI: 10.7270/Q2XW4MPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50149939 (CHEMBL3769807) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.12E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pardubice Curated by ChEMBL | Assay Description Inhibition of equine serum BChE by spectrophotometric-based Ellman's method | Bioorg Med Chem 24: 1560-72 (2016) Article DOI: 10.1016/j.bmc.2016.02.033 BindingDB Entry DOI: 10.7270/Q2XW4MPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM10620 ((S)-3-(1-(dimethylamino)ethyl)phenyl ethyl(methyl)...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5.61E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pardubice Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by spectrophotometric-based Ellman's method | Bioorg Med Chem 24: 1560-72 (2016) Article DOI: 10.1016/j.bmc.2016.02.033 BindingDB Entry DOI: 10.7270/Q2XW4MPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50149955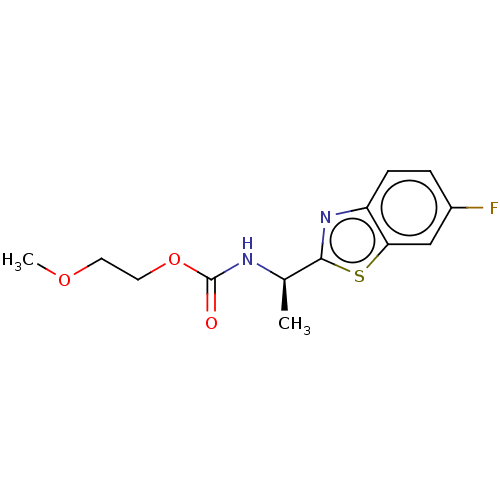 (CHEMBL3770297) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.62E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pardubice Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by spectrophotometric-based Ellman's method | Bioorg Med Chem 24: 1560-72 (2016) Article DOI: 10.1016/j.bmc.2016.02.033 BindingDB Entry DOI: 10.7270/Q2XW4MPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50149952 (CHEMBL3770851) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.34E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pardubice Curated by ChEMBL | Assay Description Inhibition of equine serum BChE by spectrophotometric-based Ellman's method | Bioorg Med Chem 24: 1560-72 (2016) Article DOI: 10.1016/j.bmc.2016.02.033 BindingDB Entry DOI: 10.7270/Q2XW4MPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50149955 (CHEMBL3770297) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.63E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pardubice Curated by ChEMBL | Assay Description Inhibition of equine serum BChE by spectrophotometric-based Ellman's method | Bioorg Med Chem 24: 1560-72 (2016) Article DOI: 10.1016/j.bmc.2016.02.033 BindingDB Entry DOI: 10.7270/Q2XW4MPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50149954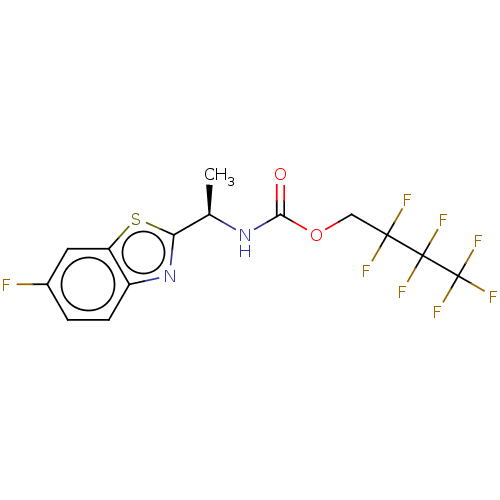 (CHEMBL3770408) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.45E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pardubice Curated by ChEMBL | Assay Description Inhibition of equine serum BChE by spectrophotometric-based Ellman's method | Bioorg Med Chem 24: 1560-72 (2016) Article DOI: 10.1016/j.bmc.2016.02.033 BindingDB Entry DOI: 10.7270/Q2XW4MPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50149949 (CHEMBL3769638) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.05E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pardubice Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by spectrophotometric-based Ellman's method | Bioorg Med Chem 24: 1560-72 (2016) Article DOI: 10.1016/j.bmc.2016.02.033 BindingDB Entry DOI: 10.7270/Q2XW4MPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50149954 (CHEMBL3770408) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.19E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pardubice Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by spectrophotometric-based Ellman's method | Bioorg Med Chem 24: 1560-72 (2016) Article DOI: 10.1016/j.bmc.2016.02.033 BindingDB Entry DOI: 10.7270/Q2XW4MPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50149956 (CHEMBL3770401) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.03E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pardubice Curated by ChEMBL | Assay Description Inhibition of equine serum BChE by spectrophotometric-based Ellman's method | Bioorg Med Chem 24: 1560-72 (2016) Article DOI: 10.1016/j.bmc.2016.02.033 BindingDB Entry DOI: 10.7270/Q2XW4MPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50149950 (CHEMBL3770572) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.22E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pardubice Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by spectrophotometric-based Ellman's method | Bioorg Med Chem 24: 1560-72 (2016) Article DOI: 10.1016/j.bmc.2016.02.033 BindingDB Entry DOI: 10.7270/Q2XW4MPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Tetronarce californica (Pacific electric ray) (Tor...) | BDBM10620 ((S)-3-(1-(dimethylamino)ethyl)phenyl ethyl(methyl)...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | n/a | n/a | 0.0333 | n/a | n/a |
University of Pardubice Curated by ChEMBL | Assay Description Pseudo-irreversible inhibition of Torpedo californica AChE using acetylthiocholine as substrate by cornish bowden plot analysis | Bioorg Med Chem 24: 1560-72 (2016) Article DOI: 10.1016/j.bmc.2016.02.033 BindingDB Entry DOI: 10.7270/Q2XW4MPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||