 Found 79 hits of Enzyme Inhibition Constant Data
Found 79 hits of Enzyme Inhibition Constant Data Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50161477
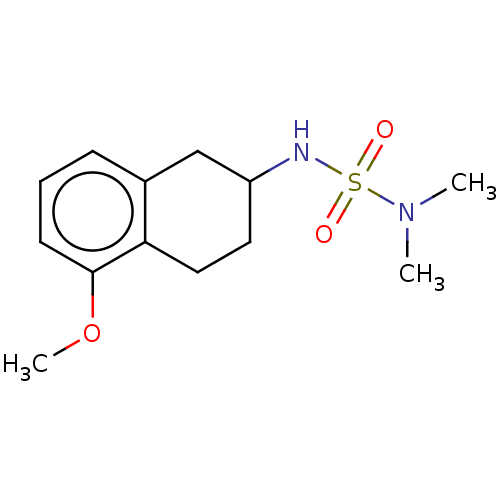
(CHEMBL3785269)Show InChI InChI=1S/C18H20N2O3S/c1-19(2)13-14-23-18-10-6-9-17-16(18)11-12-20(17)24(21,22)15-7-4-3-5-8-15/h3-12H,13-14H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Erzurum Technical University
Curated by ChEMBL
| Assay Description
Inhibition of AChE (unknown origin) using acetylcholine iodate as substrate preincubated for 10 mins followed by substrate addition by Lineweaver-Bur... |
Bioorg Med Chem 24: 2318-29 (2016)
Article DOI: 10.1016/j.bmc.2016.04.002
BindingDB Entry DOI: 10.7270/Q2930W3J |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50161487
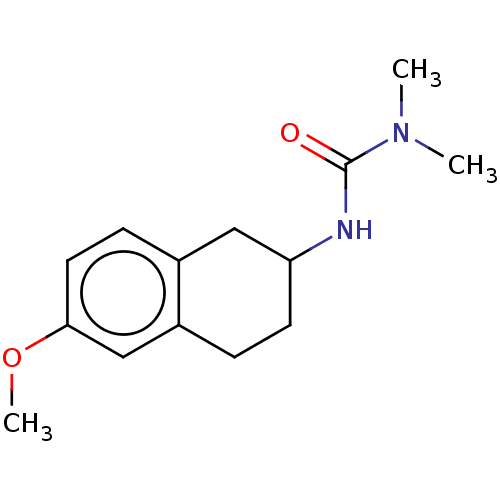
(CHEMBL3786516)Show InChI InChI=1S/C16H16N2O3S/c17-9-11-21-14-7-6-13-8-10-18(16(13)12-14)22(19,20)15-4-2-1-3-5-15/h1-8,10,12H,9,11,17H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.670 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Erzurum Technical University
Curated by ChEMBL
| Assay Description
Inhibition of AChE (unknown origin) using acetylcholine iodate as substrate preincubated for 10 mins followed by substrate addition by Lineweaver-Bur... |
Bioorg Med Chem 24: 2318-29 (2016)
Article DOI: 10.1016/j.bmc.2016.04.002
BindingDB Entry DOI: 10.7270/Q2930W3J |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50161485
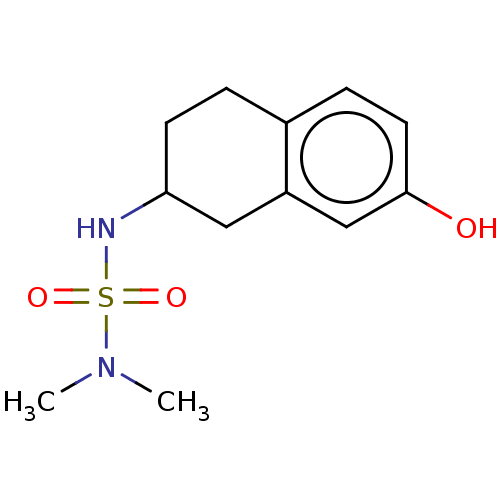
(CHEMBL3786719)Show InChI InChI=1S/C21H24N2O4S/c24-28(25,18-5-2-1-3-6-18)23-13-9-19-20(23)7-4-8-21(19)27-16-12-22-17-10-14-26-15-11-17/h1-9,13,17,22H,10-12,14-16H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.720 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Erzurum Technical University
Curated by ChEMBL
| Assay Description
Inhibition of AChE (unknown origin) using acetylcholine iodate as substrate preincubated for 10 mins followed by substrate addition by Lineweaver-Bur... |
Bioorg Med Chem 24: 2318-29 (2016)
Article DOI: 10.1016/j.bmc.2016.04.002
BindingDB Entry DOI: 10.7270/Q2930W3J |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50161479
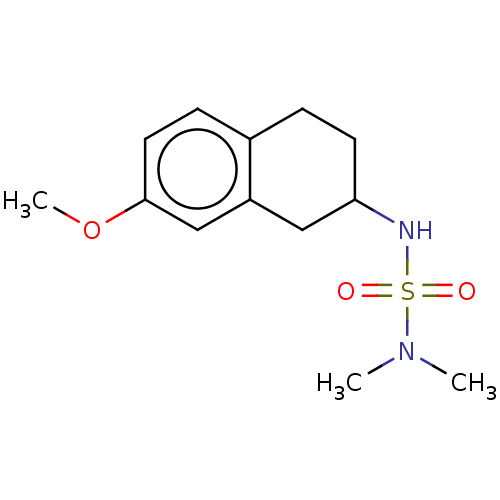
(CHEMBL3786448)Show InChI InChI=1S/C23H22N2O3S/c26-29(27,20-10-5-2-6-11-20)25-16-14-21-22(25)12-7-13-23(21)28-17-15-24-18-19-8-3-1-4-9-19/h1-14,16,24H,15,17-18H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.830 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Erzurum Technical University
Curated by ChEMBL
| Assay Description
Inhibition of AChE (unknown origin) using acetylcholine iodate as substrate preincubated for 10 mins followed by substrate addition by Lineweaver-Bur... |
Bioorg Med Chem 24: 2318-29 (2016)
Article DOI: 10.1016/j.bmc.2016.04.002
BindingDB Entry DOI: 10.7270/Q2930W3J |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50161491
(CHEMBL3786651)Show InChI InChI=1S/C14H20N2O2/c1-16(2)14(17)15-11-7-8-12-10(9-11)5-4-6-13(12)18-3/h4-6,11H,7-9H2,1-3H3,(H,15,17)/t11-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.980 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Erzurum Technical University
Curated by ChEMBL
| Assay Description
Inhibition of AChE (unknown origin) using acetylcholine iodate as substrate preincubated for 10 mins followed by substrate addition by Lineweaver-Bur... |
Bioorg Med Chem 24: 2318-29 (2016)
Article DOI: 10.1016/j.bmc.2016.04.002
BindingDB Entry DOI: 10.7270/Q2930W3J |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50161486

(CHEMBL3787502)Show InChI InChI=1S/C19H22N2O3S/c1-15(2)20-12-14-24-19-10-6-9-18-17(19)11-13-21(18)25(22,23)16-7-4-3-5-8-16/h3-11,13,15,20H,12,14H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Erzurum Technical University
Curated by ChEMBL
| Assay Description
Inhibition of AChE (unknown origin) using acetylcholine iodate as substrate preincubated for 10 mins followed by substrate addition by Lineweaver-Bur... |
Bioorg Med Chem 24: 2318-29 (2016)
Article DOI: 10.1016/j.bmc.2016.04.002
BindingDB Entry DOI: 10.7270/Q2930W3J |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50161478
(CHEMBL3787223)Show InChI InChI=1S/C22H26N2O3S/c25-28(26,19-9-4-3-5-10-19)24-16-13-20-21(24)11-8-12-22(20)27-18-17-23-14-6-1-2-7-15-23/h3-5,8-13,16H,1-2,6-7,14-15,17-18H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Erzurum Technical University
Curated by ChEMBL
| Assay Description
Inhibition of AChE (unknown origin) using acetylcholine iodate as substrate preincubated for 10 mins followed by substrate addition by Lineweaver-Bur... |
Bioorg Med Chem 24: 2318-29 (2016)
Article DOI: 10.1016/j.bmc.2016.04.002
BindingDB Entry DOI: 10.7270/Q2930W3J |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM26190

(1,4-Dihydroxybenzene, XIII | 1,4-dihydroxybenzene ...)Show InChI InChI=1S/C6H6O2/c7-5-1-2-6(8)4-3-5/h1-4,7-8H | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Erzurum Technical University
Curated by ChEMBL
| Assay Description
Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition by Lineweaver-Bur... |
Bioorg Med Chem 24: 2318-29 (2016)
Article DOI: 10.1016/j.bmc.2016.04.002
BindingDB Entry DOI: 10.7270/Q2930W3J |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50161484
(CHEMBL3786442)Show InChI InChI=1S/C12H18N2O3S/c1-14(2)18(16,17)13-11-5-3-10-8-12(15)6-4-9(10)7-11/h4,6,8,11,13,15H,3,5,7H2,1-2H3/t11-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Erzurum Technical University
Curated by ChEMBL
| Assay Description
Inhibition of AChE (unknown origin) using acetylcholine iodate as substrate preincubated for 10 mins followed by substrate addition by Lineweaver-Bur... |
Bioorg Med Chem 24: 2318-29 (2016)
Article DOI: 10.1016/j.bmc.2016.04.002
BindingDB Entry DOI: 10.7270/Q2930W3J |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50161481
(CHEMBL3785391)Show InChI InChI=1S/C21H24N2O3S/c24-27(25,18-8-3-1-4-9-18)23-15-12-19-20(23)10-7-11-21(19)26-17-16-22-13-5-2-6-14-22/h1,3-4,7-12,15H,2,5-6,13-14,16-17H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Erzurum Technical University
Curated by ChEMBL
| Assay Description
Inhibition of AChE (unknown origin) using acetylcholine iodate as substrate preincubated for 10 mins followed by substrate addition by Lineweaver-Bur... |
Bioorg Med Chem 24: 2318-29 (2016)
Article DOI: 10.1016/j.bmc.2016.04.002
BindingDB Entry DOI: 10.7270/Q2930W3J |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50161483
(CHEMBL3786873)Show InChI InChI=1S/C20H22N2O3S/c23-26(24,17-7-2-1-3-8-17)22-14-11-18-19(22)9-6-10-20(18)25-16-15-21-12-4-5-13-21/h1-3,6-11,14H,4-5,12-13,15-16H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Erzurum Technical University
Curated by ChEMBL
| Assay Description
Inhibition of AChE (unknown origin) using acetylcholine iodate as substrate preincubated for 10 mins followed by substrate addition by Lineweaver-Bur... |
Bioorg Med Chem 24: 2318-29 (2016)
Article DOI: 10.1016/j.bmc.2016.04.002
BindingDB Entry DOI: 10.7270/Q2930W3J |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50161477

(CHEMBL3785269)Show InChI InChI=1S/C18H20N2O3S/c1-19(2)13-14-23-18-10-6-9-17-16(18)11-12-20(17)24(21,22)15-7-4-3-5-8-15/h3-12H,13-14H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Erzurum Technical University
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 2 using 4-nitrophenylacetate as substrate by Lineweaver-Burk plot analysis |
Bioorg Med Chem 24: 2318-29 (2016)
Article DOI: 10.1016/j.bmc.2016.04.002
BindingDB Entry DOI: 10.7270/Q2930W3J |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50161480
(CHEMBL3786937)Show InChI InChI=1S/C24H24N2O4S/c1-29-21-7-5-6-19(16-21)18-25-13-15-30-22-11-10-20-12-14-26(24(20)17-22)31(27,28)23-8-3-2-4-9-23/h2-12,14,16-17,25H,13,15,18H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Erzurum Technical University
Curated by ChEMBL
| Assay Description
Inhibition of AChE (unknown origin) using acetylcholine iodate as substrate preincubated for 10 mins followed by substrate addition by Lineweaver-Bur... |
Bioorg Med Chem 24: 2318-29 (2016)
Article DOI: 10.1016/j.bmc.2016.04.002
BindingDB Entry DOI: 10.7270/Q2930W3J |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50161482
(CHEMBL3785207)Show InChI InChI=1S/C18H20N2O3S/c1-2-19-12-14-23-18-10-6-9-17-16(18)11-13-20(17)24(21,22)15-7-4-3-5-8-15/h3-11,13,19H,2,12,14H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Erzurum Technical University
Curated by ChEMBL
| Assay Description
Inhibition of AChE (unknown origin) using acetylcholine iodate as substrate preincubated for 10 mins followed by substrate addition by Lineweaver-Bur... |
Bioorg Med Chem 24: 2318-29 (2016)
Article DOI: 10.1016/j.bmc.2016.04.002
BindingDB Entry DOI: 10.7270/Q2930W3J |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50161485

(CHEMBL3786719)Show InChI InChI=1S/C21H24N2O4S/c24-28(25,18-5-2-1-3-6-18)23-13-9-19-20(23)7-4-8-21(19)27-16-12-22-17-10-14-26-15-11-17/h1-9,13,17,22H,10-12,14-16H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Erzurum Technical University
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 2 using 4-nitrophenylacetate as substrate by Lineweaver-Burk plot analysis |
Bioorg Med Chem 24: 2318-29 (2016)
Article DOI: 10.1016/j.bmc.2016.04.002
BindingDB Entry DOI: 10.7270/Q2930W3J |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50161479

(CHEMBL3786448)Show InChI InChI=1S/C23H22N2O3S/c26-29(27,20-10-5-2-6-11-20)25-16-14-21-22(25)12-7-13-23(21)28-17-15-24-18-19-8-3-1-4-9-19/h1-14,16,24H,15,17-18H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Erzurum Technical University
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 2 using 4-nitrophenylacetate as substrate by Lineweaver-Burk plot analysis |
Bioorg Med Chem 24: 2318-29 (2016)
Article DOI: 10.1016/j.bmc.2016.04.002
BindingDB Entry DOI: 10.7270/Q2930W3J |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50161486

(CHEMBL3787502)Show InChI InChI=1S/C19H22N2O3S/c1-15(2)20-12-14-24-19-10-6-9-18-17(19)11-13-21(18)25(22,23)16-7-4-3-5-8-16/h3-11,13,15,20H,12,14H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Erzurum Technical University
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 2 using 4-nitrophenylacetate as substrate by Lineweaver-Burk plot analysis |
Bioorg Med Chem 24: 2318-29 (2016)
Article DOI: 10.1016/j.bmc.2016.04.002
BindingDB Entry DOI: 10.7270/Q2930W3J |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50161478

(CHEMBL3787223)Show InChI InChI=1S/C22H26N2O3S/c25-28(26,19-9-4-3-5-10-19)24-16-13-20-21(24)11-8-12-22(20)27-18-17-23-14-6-1-2-7-15-23/h3-5,8-13,16H,1-2,6-7,14-15,17-18H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Erzurum Technical University
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 2 using 4-nitrophenylacetate as substrate by Lineweaver-Burk plot analysis |
Bioorg Med Chem 24: 2318-29 (2016)
Article DOI: 10.1016/j.bmc.2016.04.002
BindingDB Entry DOI: 10.7270/Q2930W3J |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50161487

(CHEMBL3786516)Show InChI InChI=1S/C16H16N2O3S/c17-9-11-21-14-7-6-13-8-10-18(16(13)12-14)22(19,20)15-4-2-1-3-5-15/h1-8,10,12H,9,11,17H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Erzurum Technical University
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 2 using 4-nitrophenylacetate as substrate by Lineweaver-Burk plot analysis |
Bioorg Med Chem 24: 2318-29 (2016)
Article DOI: 10.1016/j.bmc.2016.04.002
BindingDB Entry DOI: 10.7270/Q2930W3J |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50161484

(CHEMBL3786442)Show InChI InChI=1S/C12H18N2O3S/c1-14(2)18(16,17)13-11-5-3-10-8-12(15)6-4-9(10)7-11/h4,6,8,11,13,15H,3,5,7H2,1-2H3/t11-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Erzurum Technical University
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 2 using 4-nitrophenylacetate as substrate by Lineweaver-Burk plot analysis |
Bioorg Med Chem 24: 2318-29 (2016)
Article DOI: 10.1016/j.bmc.2016.04.002
BindingDB Entry DOI: 10.7270/Q2930W3J |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50161483

(CHEMBL3786873)Show InChI InChI=1S/C20H22N2O3S/c23-26(24,17-7-2-1-3-8-17)22-14-11-18-19(22)9-6-10-20(18)25-16-15-21-12-4-5-13-21/h1-3,6-11,14H,4-5,12-13,15-16H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Erzurum Technical University
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 2 using 4-nitrophenylacetate as substrate by Lineweaver-Burk plot analysis |
Bioorg Med Chem 24: 2318-29 (2016)
Article DOI: 10.1016/j.bmc.2016.04.002
BindingDB Entry DOI: 10.7270/Q2930W3J |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50161483

(CHEMBL3786873)Show InChI InChI=1S/C20H22N2O3S/c23-26(24,17-7-2-1-3-8-17)22-14-11-18-19(22)9-6-10-20(18)25-16-15-21-12-4-5-13-21/h1-3,6-11,14H,4-5,12-13,15-16H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Erzurum Technical University
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 1 using 4-nitrophenylacetate as substrate by Lineweaver-Burk plot analysis |
Bioorg Med Chem 24: 2318-29 (2016)
Article DOI: 10.1016/j.bmc.2016.04.002
BindingDB Entry DOI: 10.7270/Q2930W3J |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50161491

(CHEMBL3786651)Show InChI InChI=1S/C14H20N2O2/c1-16(2)14(17)15-11-7-8-12-10(9-11)5-4-6-13(12)18-3/h4-6,11H,7-9H2,1-3H3,(H,15,17)/t11-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Erzurum Technical University
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 2 using 4-nitrophenylacetate as substrate by Lineweaver-Burk plot analysis |
Bioorg Med Chem 24: 2318-29 (2016)
Article DOI: 10.1016/j.bmc.2016.04.002
BindingDB Entry DOI: 10.7270/Q2930W3J |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50161481

(CHEMBL3785391)Show InChI InChI=1S/C21H24N2O3S/c24-27(25,18-8-3-1-4-9-18)23-15-12-19-20(23)10-7-11-21(19)26-17-16-22-13-5-2-6-14-22/h1,3-4,7-12,15H,2,5-6,13-14,16-17H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Erzurum Technical University
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 2 using 4-nitrophenylacetate as substrate by Lineweaver-Burk plot analysis |
Bioorg Med Chem 24: 2318-29 (2016)
Article DOI: 10.1016/j.bmc.2016.04.002
BindingDB Entry DOI: 10.7270/Q2930W3J |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50161480

(CHEMBL3786937)Show InChI InChI=1S/C24H24N2O4S/c1-29-21-7-5-6-19(16-21)18-25-13-15-30-22-11-10-20-12-14-26(24(20)17-22)31(27,28)23-8-3-2-4-9-23/h2-12,14,16-17,25H,13,15,18H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Erzurum Technical University
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 2 using 4-nitrophenylacetate as substrate by Lineweaver-Burk plot analysis |
Bioorg Med Chem 24: 2318-29 (2016)
Article DOI: 10.1016/j.bmc.2016.04.002
BindingDB Entry DOI: 10.7270/Q2930W3J |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50161477

(CHEMBL3785269)Show InChI InChI=1S/C18H20N2O3S/c1-19(2)13-14-23-18-10-6-9-17-16(18)11-12-20(17)24(21,22)15-7-4-3-5-8-15/h3-12H,13-14H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Erzurum Technical University
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 1 using 4-nitrophenylacetate as substrate by Lineweaver-Burk plot analysis |
Bioorg Med Chem 24: 2318-29 (2016)
Article DOI: 10.1016/j.bmc.2016.04.002
BindingDB Entry DOI: 10.7270/Q2930W3J |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50161478

(CHEMBL3787223)Show InChI InChI=1S/C22H26N2O3S/c25-28(26,19-9-4-3-5-10-19)24-16-13-20-21(24)11-8-12-22(20)27-18-17-23-14-6-1-2-7-15-23/h3-5,8-13,16H,1-2,6-7,14-15,17-18H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Erzurum Technical University
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 1 using 4-nitrophenylacetate as substrate by Lineweaver-Burk plot analysis |
Bioorg Med Chem 24: 2318-29 (2016)
Article DOI: 10.1016/j.bmc.2016.04.002
BindingDB Entry DOI: 10.7270/Q2930W3J |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50161482

(CHEMBL3785207)Show InChI InChI=1S/C18H20N2O3S/c1-2-19-12-14-23-18-10-6-9-17-16(18)11-13-20(17)24(21,22)15-7-4-3-5-8-15/h3-11,13,19H,2,12,14H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Erzurum Technical University
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 2 using 4-nitrophenylacetate as substrate by Lineweaver-Burk plot analysis |
Bioorg Med Chem 24: 2318-29 (2016)
Article DOI: 10.1016/j.bmc.2016.04.002
BindingDB Entry DOI: 10.7270/Q2930W3J |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50161479

(CHEMBL3786448)Show InChI InChI=1S/C23H22N2O3S/c26-29(27,20-10-5-2-6-11-20)25-16-14-21-22(25)12-7-13-23(21)28-17-15-24-18-19-8-3-1-4-9-19/h1-14,16,24H,15,17-18H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Erzurum Technical University
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 1 using 4-nitrophenylacetate as substrate by Lineweaver-Burk plot analysis |
Bioorg Med Chem 24: 2318-29 (2016)
Article DOI: 10.1016/j.bmc.2016.04.002
BindingDB Entry DOI: 10.7270/Q2930W3J |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50161485

(CHEMBL3786719)Show InChI InChI=1S/C21H24N2O4S/c24-28(25,18-5-2-1-3-6-18)23-13-9-19-20(23)7-4-8-21(19)27-16-12-22-17-10-14-26-15-11-17/h1-9,13,17,22H,10-12,14-16H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Erzurum Technical University
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 1 using 4-nitrophenylacetate as substrate by Lineweaver-Burk plot analysis |
Bioorg Med Chem 24: 2318-29 (2016)
Article DOI: 10.1016/j.bmc.2016.04.002
BindingDB Entry DOI: 10.7270/Q2930W3J |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50161487

(CHEMBL3786516)Show InChI InChI=1S/C16H16N2O3S/c17-9-11-21-14-7-6-13-8-10-18(16(13)12-14)22(19,20)15-4-2-1-3-5-15/h1-8,10,12H,9,11,17H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Erzurum Technical University
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 1 using 4-nitrophenylacetate as substrate by Lineweaver-Burk plot analysis |
Bioorg Med Chem 24: 2318-29 (2016)
Article DOI: 10.1016/j.bmc.2016.04.002
BindingDB Entry DOI: 10.7270/Q2930W3J |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50161484

(CHEMBL3786442)Show InChI InChI=1S/C12H18N2O3S/c1-14(2)18(16,17)13-11-5-3-10-8-12(15)6-4-9(10)7-11/h4,6,8,11,13,15H,3,5,7H2,1-2H3/t11-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Erzurum Technical University
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 1 using 4-nitrophenylacetate as substrate by Lineweaver-Burk plot analysis |
Bioorg Med Chem 24: 2318-29 (2016)
Article DOI: 10.1016/j.bmc.2016.04.002
BindingDB Entry DOI: 10.7270/Q2930W3J |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50161486

(CHEMBL3787502)Show InChI InChI=1S/C19H22N2O3S/c1-15(2)20-12-14-24-19-10-6-9-18-17(19)11-13-21(18)25(22,23)16-7-4-3-5-8-16/h3-11,13,15,20H,12,14H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Erzurum Technical University
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 1 using 4-nitrophenylacetate as substrate by Lineweaver-Burk plot analysis |
Bioorg Med Chem 24: 2318-29 (2016)
Article DOI: 10.1016/j.bmc.2016.04.002
BindingDB Entry DOI: 10.7270/Q2930W3J |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50161482

(CHEMBL3785207)Show InChI InChI=1S/C18H20N2O3S/c1-2-19-12-14-23-18-10-6-9-17-16(18)11-13-20(17)24(21,22)15-7-4-3-5-8-15/h3-11,13,19H,2,12,14H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Erzurum Technical University
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 1 using 4-nitrophenylacetate as substrate by Lineweaver-Burk plot analysis |
Bioorg Med Chem 24: 2318-29 (2016)
Article DOI: 10.1016/j.bmc.2016.04.002
BindingDB Entry DOI: 10.7270/Q2930W3J |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50161481

(CHEMBL3785391)Show InChI InChI=1S/C21H24N2O3S/c24-27(25,18-8-3-1-4-9-18)23-15-12-19-20(23)10-7-11-21(19)26-17-16-22-13-5-2-6-14-22/h1,3-4,7-12,15H,2,5-6,13-14,16-17H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Erzurum Technical University
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 1 using 4-nitrophenylacetate as substrate by Lineweaver-Burk plot analysis |
Bioorg Med Chem 24: 2318-29 (2016)
Article DOI: 10.1016/j.bmc.2016.04.002
BindingDB Entry DOI: 10.7270/Q2930W3J |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50161480

(CHEMBL3786937)Show InChI InChI=1S/C24H24N2O4S/c1-29-21-7-5-6-19(16-21)18-25-13-15-30-22-11-10-20-12-14-26(24(20)17-22)31(27,28)23-8-3-2-4-9-23/h2-12,14,16-17,25H,13,15,18H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Erzurum Technical University
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 1 using 4-nitrophenylacetate as substrate by Lineweaver-Burk plot analysis |
Bioorg Med Chem 24: 2318-29 (2016)
Article DOI: 10.1016/j.bmc.2016.04.002
BindingDB Entry DOI: 10.7270/Q2930W3J |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50161491

(CHEMBL3786651)Show InChI InChI=1S/C14H20N2O2/c1-16(2)14(17)15-11-7-8-12-10(9-11)5-4-6-13(12)18-3/h4-6,11H,7-9H2,1-3H3,(H,15,17)/t11-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Erzurum Technical University
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 1 using 4-nitrophenylacetate as substrate by Lineweaver-Burk plot analysis |
Bioorg Med Chem 24: 2318-29 (2016)
Article DOI: 10.1016/j.bmc.2016.04.002
BindingDB Entry DOI: 10.7270/Q2930W3J |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM8961

(1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...)Show InChI InChI=1S/C13H14N2/c14-13-9-5-1-3-7-11(9)15-12-8-4-2-6-10(12)13/h1,3,5,7H,2,4,6,8H2,(H2,14,15) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 7.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Erzurum Technical University
Curated by ChEMBL
| Assay Description
Inhibition of AChE (unknown origin) using acetylcholine iodate as substrate preincubated for 10 mins followed by substrate addition by Lineweaver-Bur... |
Bioorg Med Chem 24: 2318-29 (2016)
Article DOI: 10.1016/j.bmc.2016.04.002
BindingDB Entry DOI: 10.7270/Q2930W3J |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM10880

(AZA | AZA2 | AZM acetazolamide | Acerazolamide, AA...)Show InChI InChI=1S/C4H6N4O3S2/c1-2(9)6-3-7-8-4(12-3)13(5,10)11/h1H3,(H2,5,10,11)(H,6,7,9) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| 33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Erzurum Technical University
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 2 using 4-nitrophenylacetate as substrate by Lineweaver-Burk plot analysis |
Bioorg Med Chem 24: 2318-29 (2016)
Article DOI: 10.1016/j.bmc.2016.04.002
BindingDB Entry DOI: 10.7270/Q2930W3J |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM10880

(AZA | AZA2 | AZM acetazolamide | Acerazolamide, AA...)Show InChI InChI=1S/C4H6N4O3S2/c1-2(9)6-3-7-8-4(12-3)13(5,10)11/h1H3,(H2,5,10,11)(H,6,7,9) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 204 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Erzurum Technical University
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 1 using 4-nitrophenylacetate as substrate by Lineweaver-Burk plot analysis |
Bioorg Med Chem 24: 2318-29 (2016)
Article DOI: 10.1016/j.bmc.2016.04.002
BindingDB Entry DOI: 10.7270/Q2930W3J |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50161481

(CHEMBL3785391)Show InChI InChI=1S/C21H24N2O3S/c24-27(25,18-8-3-1-4-9-18)23-15-12-19-20(23)10-7-11-21(19)26-17-16-22-13-5-2-6-14-22/h1,3-4,7-12,15H,2,5-6,13-14,16-17H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Erzurum Technical University
Curated by ChEMBL
| Assay Description
Inhibition of AChE (unknown origin) using acetylcholine iodate as substrate preincubated for 10 mins followed by substrate addition by Ellman's metho... |
Bioorg Med Chem 24: 2318-29 (2016)
Article DOI: 10.1016/j.bmc.2016.04.002
BindingDB Entry DOI: 10.7270/Q2930W3J |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM8961

(1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...)Show InChI InChI=1S/C13H14N2/c14-13-9-5-1-3-7-11(9)15-12-8-4-2-6-10(12)13/h1,3,5,7H,2,4,6,8H2,(H2,14,15) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 7.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Erzurum Technical University
Curated by ChEMBL
| Assay Description
Inhibition of AChE (unknown origin) using acetylcholine iodate as substrate preincubated for 10 mins followed by substrate addition by Ellman's metho... |
Bioorg Med Chem 24: 2318-29 (2016)
Article DOI: 10.1016/j.bmc.2016.04.002
BindingDB Entry DOI: 10.7270/Q2930W3J |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50161486

(CHEMBL3787502)Show InChI InChI=1S/C19H22N2O3S/c1-15(2)20-12-14-24-19-10-6-9-18-17(19)11-13-21(18)25(22,23)16-7-4-3-5-8-16/h3-11,13,15,20H,12,14H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Erzurum Technical University
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 1 using 4-nitrophenylacetate as substrate by spectrophotometric analysis |
Bioorg Med Chem 24: 2318-29 (2016)
Article DOI: 10.1016/j.bmc.2016.04.002
BindingDB Entry DOI: 10.7270/Q2930W3J |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50161487

(CHEMBL3786516)Show InChI InChI=1S/C16H16N2O3S/c17-9-11-21-14-7-6-13-8-10-18(16(13)12-14)22(19,20)15-4-2-1-3-5-15/h1-8,10,12H,9,11,17H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Erzurum Technical University
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 1 using 4-nitrophenylacetate as substrate by spectrophotometric analysis |
Bioorg Med Chem 24: 2318-29 (2016)
Article DOI: 10.1016/j.bmc.2016.04.002
BindingDB Entry DOI: 10.7270/Q2930W3J |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50161491

(CHEMBL3786651)Show InChI InChI=1S/C14H20N2O2/c1-16(2)14(17)15-11-7-8-12-10(9-11)5-4-6-13(12)18-3/h4-6,11H,7-9H2,1-3H3,(H,15,17)/t11-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Erzurum Technical University
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 1 using 4-nitrophenylacetate as substrate by spectrophotometric analysis |
Bioorg Med Chem 24: 2318-29 (2016)
Article DOI: 10.1016/j.bmc.2016.04.002
BindingDB Entry DOI: 10.7270/Q2930W3J |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50161478

(CHEMBL3787223)Show InChI InChI=1S/C22H26N2O3S/c25-28(26,19-9-4-3-5-10-19)24-16-13-20-21(24)11-8-12-22(20)27-18-17-23-14-6-1-2-7-15-23/h3-5,8-13,16H,1-2,6-7,14-15,17-18H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Erzurum Technical University
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 2 using 4-nitrophenylacetate as substrate by spectrophotometric analysis |
Bioorg Med Chem 24: 2318-29 (2016)
Article DOI: 10.1016/j.bmc.2016.04.002
BindingDB Entry DOI: 10.7270/Q2930W3J |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50161479

(CHEMBL3786448)Show InChI InChI=1S/C23H22N2O3S/c26-29(27,20-10-5-2-6-11-20)25-16-14-21-22(25)12-7-13-23(21)28-17-15-24-18-19-8-3-1-4-9-19/h1-14,16,24H,15,17-18H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Erzurum Technical University
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 2 using 4-nitrophenylacetate as substrate by spectrophotometric analysis |
Bioorg Med Chem 24: 2318-29 (2016)
Article DOI: 10.1016/j.bmc.2016.04.002
BindingDB Entry DOI: 10.7270/Q2930W3J |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50161486

(CHEMBL3787502)Show InChI InChI=1S/C19H22N2O3S/c1-15(2)20-12-14-24-19-10-6-9-18-17(19)11-13-21(18)25(22,23)16-7-4-3-5-8-16/h3-11,13,15,20H,12,14H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Erzurum Technical University
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 2 using 4-nitrophenylacetate as substrate by spectrophotometric analysis |
Bioorg Med Chem 24: 2318-29 (2016)
Article DOI: 10.1016/j.bmc.2016.04.002
BindingDB Entry DOI: 10.7270/Q2930W3J |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50161481

(CHEMBL3785391)Show InChI InChI=1S/C21H24N2O3S/c24-27(25,18-8-3-1-4-9-18)23-15-12-19-20(23)10-7-11-21(19)26-17-16-22-13-5-2-6-14-22/h1,3-4,7-12,15H,2,5-6,13-14,16-17H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Erzurum Technical University
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 1 using 4-nitrophenylacetate as substrate by spectrophotometric analysis |
Bioorg Med Chem 24: 2318-29 (2016)
Article DOI: 10.1016/j.bmc.2016.04.002
BindingDB Entry DOI: 10.7270/Q2930W3J |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50161480

(CHEMBL3786937)Show InChI InChI=1S/C24H24N2O4S/c1-29-21-7-5-6-19(16-21)18-25-13-15-30-22-11-10-20-12-14-26(24(20)17-22)31(27,28)23-8-3-2-4-9-23/h2-12,14,16-17,25H,13,15,18H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Erzurum Technical University
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 1 using 4-nitrophenylacetate as substrate by spectrophotometric analysis |
Bioorg Med Chem 24: 2318-29 (2016)
Article DOI: 10.1016/j.bmc.2016.04.002
BindingDB Entry DOI: 10.7270/Q2930W3J |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50161482

(CHEMBL3785207)Show InChI InChI=1S/C18H20N2O3S/c1-2-19-12-14-23-18-10-6-9-17-16(18)11-13-20(17)24(21,22)15-7-4-3-5-8-15/h3-11,13,19H,2,12,14H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Erzurum Technical University
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 1 using 4-nitrophenylacetate as substrate by spectrophotometric analysis |
Bioorg Med Chem 24: 2318-29 (2016)
Article DOI: 10.1016/j.bmc.2016.04.002
BindingDB Entry DOI: 10.7270/Q2930W3J |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50161485

(CHEMBL3786719)Show InChI InChI=1S/C21H24N2O4S/c24-28(25,18-5-2-1-3-6-18)23-13-9-19-20(23)7-4-8-21(19)27-16-12-22-17-10-14-26-15-11-17/h1-9,13,17,22H,10-12,14-16H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Erzurum Technical University
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 2 using 4-nitrophenylacetate as substrate by spectrophotometric analysis |
Bioorg Med Chem 24: 2318-29 (2016)
Article DOI: 10.1016/j.bmc.2016.04.002
BindingDB Entry DOI: 10.7270/Q2930W3J |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50161487

(CHEMBL3786516)Show InChI InChI=1S/C16H16N2O3S/c17-9-11-21-14-7-6-13-8-10-18(16(13)12-14)22(19,20)15-4-2-1-3-5-15/h1-8,10,12H,9,11,17H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Erzurum Technical University
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 2 using 4-nitrophenylacetate as substrate by spectrophotometric analysis |
Bioorg Med Chem 24: 2318-29 (2016)
Article DOI: 10.1016/j.bmc.2016.04.002
BindingDB Entry DOI: 10.7270/Q2930W3J |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50161477

(CHEMBL3785269)Show InChI InChI=1S/C18H20N2O3S/c1-19(2)13-14-23-18-10-6-9-17-16(18)11-12-20(17)24(21,22)15-7-4-3-5-8-15/h3-12H,13-14H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Erzurum Technical University
Curated by ChEMBL
| Assay Description
Inhibition of AChE (unknown origin) using acetylcholine iodate as substrate preincubated for 10 mins followed by substrate addition by Ellman's metho... |
Bioorg Med Chem 24: 2318-29 (2016)
Article DOI: 10.1016/j.bmc.2016.04.002
BindingDB Entry DOI: 10.7270/Q2930W3J |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50161484

(CHEMBL3786442)Show InChI InChI=1S/C12H18N2O3S/c1-14(2)18(16,17)13-11-5-3-10-8-12(15)6-4-9(10)7-11/h4,6,8,11,13,15H,3,5,7H2,1-2H3/t11-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Erzurum Technical University
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 2 using 4-nitrophenylacetate as substrate by spectrophotometric analysis |
Bioorg Med Chem 24: 2318-29 (2016)
Article DOI: 10.1016/j.bmc.2016.04.002
BindingDB Entry DOI: 10.7270/Q2930W3J |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50161483

(CHEMBL3786873)Show InChI InChI=1S/C20H22N2O3S/c23-26(24,17-7-2-1-3-8-17)22-14-11-18-19(22)9-6-10-20(18)25-16-15-21-12-4-5-13-21/h1-3,6-11,14H,4-5,12-13,15-16H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Erzurum Technical University
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 2 using 4-nitrophenylacetate as substrate by spectrophotometric analysis |
Bioorg Med Chem 24: 2318-29 (2016)
Article DOI: 10.1016/j.bmc.2016.04.002
BindingDB Entry DOI: 10.7270/Q2930W3J |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50161479

(CHEMBL3786448)Show InChI InChI=1S/C23H22N2O3S/c26-29(27,20-10-5-2-6-11-20)25-16-14-21-22(25)12-7-13-23(21)28-17-15-24-18-19-8-3-1-4-9-19/h1-14,16,24H,15,17-18H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Erzurum Technical University
Curated by ChEMBL
| Assay Description
Inhibition of AChE (unknown origin) using acetylcholine iodate as substrate preincubated for 10 mins followed by substrate addition by Ellman's metho... |
Bioorg Med Chem 24: 2318-29 (2016)
Article DOI: 10.1016/j.bmc.2016.04.002
BindingDB Entry DOI: 10.7270/Q2930W3J |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50161487

(CHEMBL3786516)Show InChI InChI=1S/C16H16N2O3S/c17-9-11-21-14-7-6-13-8-10-18(16(13)12-14)22(19,20)15-4-2-1-3-5-15/h1-8,10,12H,9,11,17H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Erzurum Technical University
Curated by ChEMBL
| Assay Description
Inhibition of AChE (unknown origin) using acetylcholine iodate as substrate preincubated for 10 mins followed by substrate addition by Ellman's metho... |
Bioorg Med Chem 24: 2318-29 (2016)
Article DOI: 10.1016/j.bmc.2016.04.002
BindingDB Entry DOI: 10.7270/Q2930W3J |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50161478

(CHEMBL3787223)Show InChI InChI=1S/C22H26N2O3S/c25-28(26,19-9-4-3-5-10-19)24-16-13-20-21(24)11-8-12-22(20)27-18-17-23-14-6-1-2-7-15-23/h3-5,8-13,16H,1-2,6-7,14-15,17-18H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Erzurum Technical University
Curated by ChEMBL
| Assay Description
Inhibition of AChE (unknown origin) using acetylcholine iodate as substrate preincubated for 10 mins followed by substrate addition by Ellman's metho... |
Bioorg Med Chem 24: 2318-29 (2016)
Article DOI: 10.1016/j.bmc.2016.04.002
BindingDB Entry DOI: 10.7270/Q2930W3J |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50161491

(CHEMBL3786651)Show InChI InChI=1S/C14H20N2O2/c1-16(2)14(17)15-11-7-8-12-10(9-11)5-4-6-13(12)18-3/h4-6,11H,7-9H2,1-3H3,(H,15,17)/t11-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Erzurum Technical University
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 2 using 4-nitrophenylacetate as substrate by spectrophotometric analysis |
Bioorg Med Chem 24: 2318-29 (2016)
Article DOI: 10.1016/j.bmc.2016.04.002
BindingDB Entry DOI: 10.7270/Q2930W3J |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50161478

(CHEMBL3787223)Show InChI InChI=1S/C22H26N2O3S/c25-28(26,19-9-4-3-5-10-19)24-16-13-20-21(24)11-8-12-22(20)27-18-17-23-14-6-1-2-7-15-23/h3-5,8-13,16H,1-2,6-7,14-15,17-18H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Erzurum Technical University
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 1 using 4-nitrophenylacetate as substrate by spectrophotometric analysis |
Bioorg Med Chem 24: 2318-29 (2016)
Article DOI: 10.1016/j.bmc.2016.04.002
BindingDB Entry DOI: 10.7270/Q2930W3J |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50161491

(CHEMBL3786651)Show InChI InChI=1S/C14H20N2O2/c1-16(2)14(17)15-11-7-8-12-10(9-11)5-4-6-13(12)18-3/h4-6,11H,7-9H2,1-3H3,(H,15,17)/t11-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Erzurum Technical University
Curated by ChEMBL
| Assay Description
Inhibition of AChE (unknown origin) using acetylcholine iodate as substrate preincubated for 10 mins followed by substrate addition by Ellman's metho... |
Bioorg Med Chem 24: 2318-29 (2016)
Article DOI: 10.1016/j.bmc.2016.04.002
BindingDB Entry DOI: 10.7270/Q2930W3J |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50161486

(CHEMBL3787502)Show InChI InChI=1S/C19H22N2O3S/c1-15(2)20-12-14-24-19-10-6-9-18-17(19)11-13-21(18)25(22,23)16-7-4-3-5-8-16/h3-11,13,15,20H,12,14H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Erzurum Technical University
Curated by ChEMBL
| Assay Description
Inhibition of AChE (unknown origin) using acetylcholine iodate as substrate preincubated for 10 mins followed by substrate addition by Ellman's metho... |
Bioorg Med Chem 24: 2318-29 (2016)
Article DOI: 10.1016/j.bmc.2016.04.002
BindingDB Entry DOI: 10.7270/Q2930W3J |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50161484

(CHEMBL3786442)Show InChI InChI=1S/C12H18N2O3S/c1-14(2)18(16,17)13-11-5-3-10-8-12(15)6-4-9(10)7-11/h4,6,8,11,13,15H,3,5,7H2,1-2H3/t11-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Erzurum Technical University
Curated by ChEMBL
| Assay Description
Inhibition of AChE (unknown origin) using acetylcholine iodate as substrate preincubated for 10 mins followed by substrate addition by Ellman's metho... |
Bioorg Med Chem 24: 2318-29 (2016)
Article DOI: 10.1016/j.bmc.2016.04.002
BindingDB Entry DOI: 10.7270/Q2930W3J |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50161485

(CHEMBL3786719)Show InChI InChI=1S/C21H24N2O4S/c24-28(25,18-5-2-1-3-6-18)23-13-9-19-20(23)7-4-8-21(19)27-16-12-22-17-10-14-26-15-11-17/h1-9,13,17,22H,10-12,14-16H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Erzurum Technical University
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 1 using 4-nitrophenylacetate as substrate by spectrophotometric analysis |
Bioorg Med Chem 24: 2318-29 (2016)
Article DOI: 10.1016/j.bmc.2016.04.002
BindingDB Entry DOI: 10.7270/Q2930W3J |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50161484

(CHEMBL3786442)Show InChI InChI=1S/C12H18N2O3S/c1-14(2)18(16,17)13-11-5-3-10-8-12(15)6-4-9(10)7-11/h4,6,8,11,13,15H,3,5,7H2,1-2H3/t11-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Erzurum Technical University
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 1 using 4-nitrophenylacetate as substrate by spectrophotometric analysis |
Bioorg Med Chem 24: 2318-29 (2016)
Article DOI: 10.1016/j.bmc.2016.04.002
BindingDB Entry DOI: 10.7270/Q2930W3J |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50161483

(CHEMBL3786873)Show InChI InChI=1S/C20H22N2O3S/c23-26(24,17-7-2-1-3-8-17)22-14-11-18-19(22)9-6-10-20(18)25-16-15-21-12-4-5-13-21/h1-3,6-11,14H,4-5,12-13,15-16H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Erzurum Technical University
Curated by ChEMBL
| Assay Description
Inhibition of AChE (unknown origin) using acetylcholine iodate as substrate preincubated for 10 mins followed by substrate addition by Ellman's metho... |
Bioorg Med Chem 24: 2318-29 (2016)
Article DOI: 10.1016/j.bmc.2016.04.002
BindingDB Entry DOI: 10.7270/Q2930W3J |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50161485

(CHEMBL3786719)Show InChI InChI=1S/C21H24N2O4S/c24-28(25,18-5-2-1-3-6-18)23-13-9-19-20(23)7-4-8-21(19)27-16-12-22-17-10-14-26-15-11-17/h1-9,13,17,22H,10-12,14-16H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
Erzurum Technical University
Curated by ChEMBL
| Assay Description
Inhibition of AChE (unknown origin) using acetylcholine iodate as substrate preincubated for 10 mins followed by substrate addition by Ellman's metho... |
Bioorg Med Chem 24: 2318-29 (2016)
Article DOI: 10.1016/j.bmc.2016.04.002
BindingDB Entry DOI: 10.7270/Q2930W3J |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM10880

(AZA | AZA2 | AZM acetazolamide | Acerazolamide, AA...)Show InChI InChI=1S/C4H6N4O3S2/c1-2(9)6-3-7-8-4(12-3)13(5,10)11/h1H3,(H2,5,10,11)(H,6,7,9) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
Erzurum Technical University
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 2 using 4-nitrophenylacetate as substrate by spectrophotometric analysis |
Bioorg Med Chem 24: 2318-29 (2016)
Article DOI: 10.1016/j.bmc.2016.04.002
BindingDB Entry DOI: 10.7270/Q2930W3J |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50161481

(CHEMBL3785391)Show InChI InChI=1S/C21H24N2O3S/c24-27(25,18-8-3-1-4-9-18)23-15-12-19-20(23)10-7-11-21(19)26-17-16-22-13-5-2-6-14-22/h1,3-4,7-12,15H,2,5-6,13-14,16-17H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 52 | n/a | n/a | n/a | n/a | n/a | n/a |
Erzurum Technical University
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 2 using 4-nitrophenylacetate as substrate by spectrophotometric analysis |
Bioorg Med Chem 24: 2318-29 (2016)
Article DOI: 10.1016/j.bmc.2016.04.002
BindingDB Entry DOI: 10.7270/Q2930W3J |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50161482

(CHEMBL3785207)Show InChI InChI=1S/C18H20N2O3S/c1-2-19-12-14-23-18-10-6-9-17-16(18)11-13-20(17)24(21,22)15-7-4-3-5-8-15/h3-11,13,19H,2,12,14H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Erzurum Technical University
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 2 using 4-nitrophenylacetate as substrate by spectrophotometric analysis |
Bioorg Med Chem 24: 2318-29 (2016)
Article DOI: 10.1016/j.bmc.2016.04.002
BindingDB Entry DOI: 10.7270/Q2930W3J |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50161477

(CHEMBL3785269)Show InChI InChI=1S/C18H20N2O3S/c1-19(2)13-14-23-18-10-6-9-17-16(18)11-12-20(17)24(21,22)15-7-4-3-5-8-15/h3-12H,13-14H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Erzurum Technical University
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 2 using 4-nitrophenylacetate as substrate by spectrophotometric analysis |
Bioorg Med Chem 24: 2318-29 (2016)
Article DOI: 10.1016/j.bmc.2016.04.002
BindingDB Entry DOI: 10.7270/Q2930W3J |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50161480

(CHEMBL3786937)Show InChI InChI=1S/C24H24N2O4S/c1-29-21-7-5-6-19(16-21)18-25-13-15-30-22-11-10-20-12-14-26(24(20)17-22)31(27,28)23-8-3-2-4-9-23/h2-12,14,16-17,25H,13,15,18H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 75 | n/a | n/a | n/a | n/a | n/a | n/a |
Erzurum Technical University
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 2 using 4-nitrophenylacetate as substrate by spectrophotometric analysis |
Bioorg Med Chem 24: 2318-29 (2016)
Article DOI: 10.1016/j.bmc.2016.04.002
BindingDB Entry DOI: 10.7270/Q2930W3J |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50161479

(CHEMBL3786448)Show InChI InChI=1S/C23H22N2O3S/c26-29(27,20-10-5-2-6-11-20)25-16-14-21-22(25)12-7-13-23(21)28-17-15-24-18-19-8-3-1-4-9-19/h1-14,16,24H,15,17-18H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 79 | n/a | n/a | n/a | n/a | n/a | n/a |
Erzurum Technical University
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 1 using 4-nitrophenylacetate as substrate by spectrophotometric analysis |
Bioorg Med Chem 24: 2318-29 (2016)
Article DOI: 10.1016/j.bmc.2016.04.002
BindingDB Entry DOI: 10.7270/Q2930W3J |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50161482

(CHEMBL3785207)Show InChI InChI=1S/C18H20N2O3S/c1-2-19-12-14-23-18-10-6-9-17-16(18)11-13-20(17)24(21,22)15-7-4-3-5-8-15/h3-11,13,19H,2,12,14H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 103 | n/a | n/a | n/a | n/a | n/a | n/a |
Erzurum Technical University
Curated by ChEMBL
| Assay Description
Inhibition of AChE (unknown origin) using acetylcholine iodate as substrate preincubated for 10 mins followed by substrate addition by Ellman's metho... |
Bioorg Med Chem 24: 2318-29 (2016)
Article DOI: 10.1016/j.bmc.2016.04.002
BindingDB Entry DOI: 10.7270/Q2930W3J |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50161477

(CHEMBL3785269)Show InChI InChI=1S/C18H20N2O3S/c1-19(2)13-14-23-18-10-6-9-17-16(18)11-12-20(17)24(21,22)15-7-4-3-5-8-15/h3-12H,13-14H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 114 | n/a | n/a | n/a | n/a | n/a | n/a |
Erzurum Technical University
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 1 using 4-nitrophenylacetate as substrate by spectrophotometric analysis |
Bioorg Med Chem 24: 2318-29 (2016)
Article DOI: 10.1016/j.bmc.2016.04.002
BindingDB Entry DOI: 10.7270/Q2930W3J |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50161480

(CHEMBL3786937)Show InChI InChI=1S/C24H24N2O4S/c1-29-21-7-5-6-19(16-21)18-25-13-15-30-22-11-10-20-12-14-26(24(20)17-22)31(27,28)23-8-3-2-4-9-23/h2-12,14,16-17,25H,13,15,18H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 132 | n/a | n/a | n/a | n/a | n/a | n/a |
Erzurum Technical University
Curated by ChEMBL
| Assay Description
Inhibition of AChE (unknown origin) using acetylcholine iodate as substrate preincubated for 10 mins followed by substrate addition by Ellman's metho... |
Bioorg Med Chem 24: 2318-29 (2016)
Article DOI: 10.1016/j.bmc.2016.04.002
BindingDB Entry DOI: 10.7270/Q2930W3J |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50161483

(CHEMBL3786873)Show InChI InChI=1S/C20H22N2O3S/c23-26(24,17-7-2-1-3-8-17)22-14-11-18-19(22)9-6-10-20(18)25-16-15-21-12-4-5-13-21/h1-3,6-11,14H,4-5,12-13,15-16H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 158 | n/a | n/a | n/a | n/a | n/a | n/a |
Erzurum Technical University
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 1 using 4-nitrophenylacetate as substrate by spectrophotometric analysis |
Bioorg Med Chem 24: 2318-29 (2016)
Article DOI: 10.1016/j.bmc.2016.04.002
BindingDB Entry DOI: 10.7270/Q2930W3J |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM10880

(AZA | AZA2 | AZM acetazolamide | Acerazolamide, AA...)Show InChI InChI=1S/C4H6N4O3S2/c1-2(9)6-3-7-8-4(12-3)13(5,10)11/h1H3,(H2,5,10,11)(H,6,7,9) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
Erzurum Technical University
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 1 using 4-nitrophenylacetate as substrate by spectrophotometric analysis |
Bioorg Med Chem 24: 2318-29 (2016)
Article DOI: 10.1016/j.bmc.2016.04.002
BindingDB Entry DOI: 10.7270/Q2930W3J |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data