

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50063581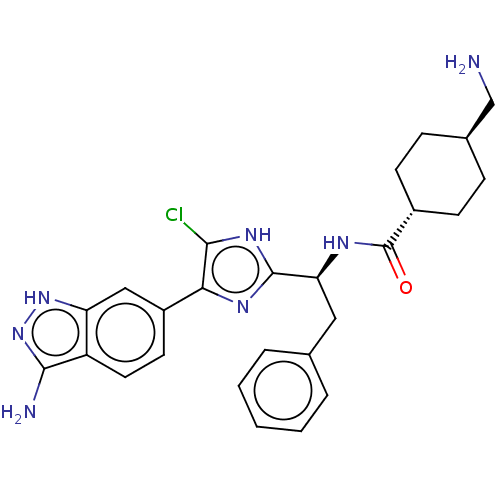 (CHEMBL3398612) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Competitive inhibition of human coagulation factor 11a assessed as reduction in release of p-nitroaniline after 10 to 120 mins by Michaelis-Menten eq... | Bioorg Med Chem 24: 2257-72 (2016) Article DOI: 10.1016/j.bmc.2016.03.062 BindingDB Entry DOI: 10.7270/Q2DN46X0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50153005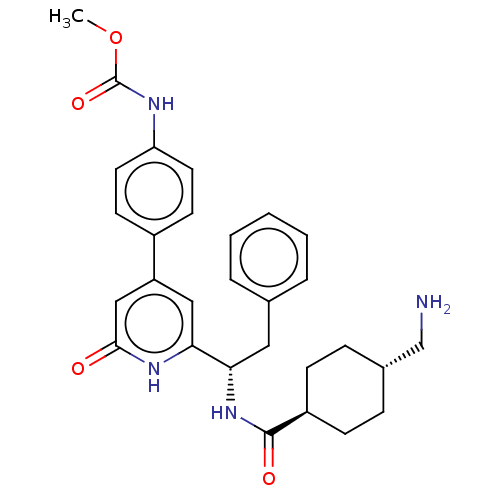 (CHEMBL3780342) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Competitive inhibition of human coagulation factor 11a assessed as reduction in release of p-nitroaniline after 10 to 120 mins by Michaelis-Menten eq... | Bioorg Med Chem 24: 2257-72 (2016) Article DOI: 10.1016/j.bmc.2016.03.062 BindingDB Entry DOI: 10.7270/Q2DN46X0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50153001 (CHEMBL3781742) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Competitive inhibition of human coagulation factor 11a assessed as reduction in release of p-nitroaniline after 10 to 120 mins by Michaelis-Menten eq... | Bioorg Med Chem 24: 2257-72 (2016) Article DOI: 10.1016/j.bmc.2016.03.062 BindingDB Entry DOI: 10.7270/Q2DN46X0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50153067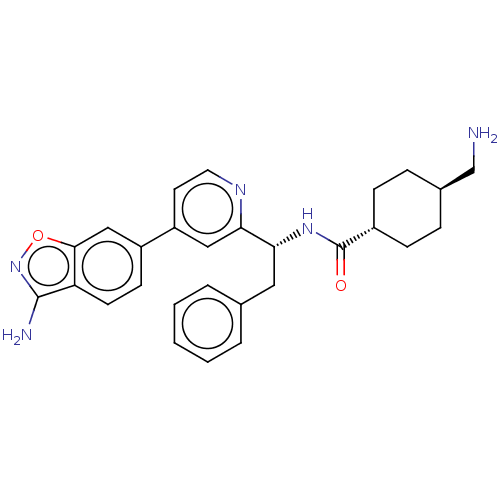 (CHEMBL3781202) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Competitive inhibition of human coagulation factor 11a assessed as reduction in release of p-nitroaniline after 10 to 120 mins by Michaelis-Menten eq... | Bioorg Med Chem 24: 2257-72 (2016) Article DOI: 10.1016/j.bmc.2016.03.062 BindingDB Entry DOI: 10.7270/Q2DN46X0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50153057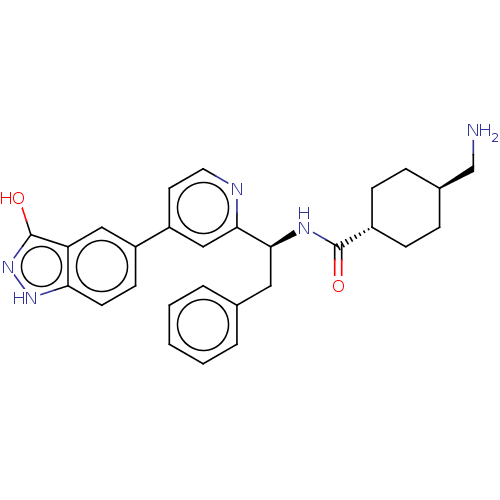 (CHEMBL3780324) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Competitive inhibition of human coagulation factor 11a assessed as reduction in release of p-nitroaniline after 10 to 120 mins by Michaelis-Menten eq... | Bioorg Med Chem 24: 2257-72 (2016) Article DOI: 10.1016/j.bmc.2016.03.062 BindingDB Entry DOI: 10.7270/Q2DN46X0 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50153063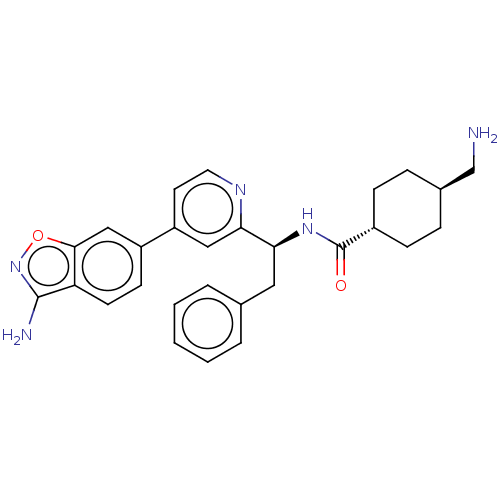 (CHEMBL3780922) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Competitive inhibition of human coagulation factor 11a assessed as reduction in release of p-nitroaniline after 10 to 120 mins by Michaelis-Menten eq... | Bioorg Med Chem 24: 2257-72 (2016) Article DOI: 10.1016/j.bmc.2016.03.062 BindingDB Entry DOI: 10.7270/Q2DN46X0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50153054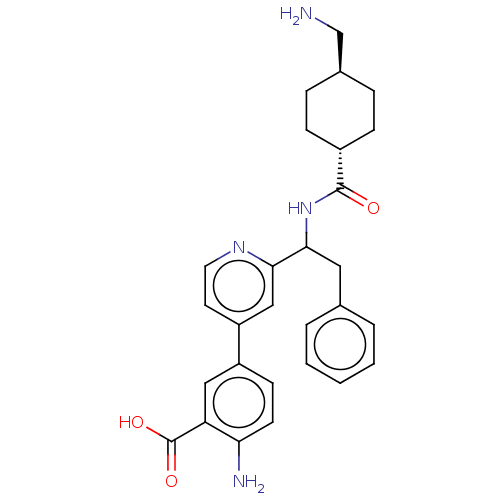 (CHEMBL3781553) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Competitive inhibition of human coagulation factor 11a assessed as reduction in release of p-nitroaniline after 10 to 120 mins by Michaelis-Menten eq... | Bioorg Med Chem 24: 2257-72 (2016) Article DOI: 10.1016/j.bmc.2016.03.062 BindingDB Entry DOI: 10.7270/Q2DN46X0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50153049 (CHEMBL3781788) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Competitive inhibition of human coagulation factor 11a assessed as reduction in release of p-nitroaniline after 10 to 120 mins by Michaelis-Menten eq... | Bioorg Med Chem 24: 2257-72 (2016) Article DOI: 10.1016/j.bmc.2016.03.062 BindingDB Entry DOI: 10.7270/Q2DN46X0 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50063582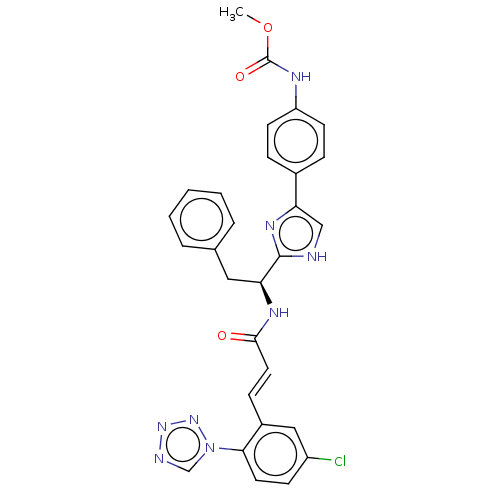 (CHEMBL3398642) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Competitive inhibition of human coagulation factor 11a assessed as reduction in release of p-nitroaniline after 10 to 120 mins by Michaelis-Menten eq... | Bioorg Med Chem 24: 2257-72 (2016) Article DOI: 10.1016/j.bmc.2016.03.062 BindingDB Entry DOI: 10.7270/Q2DN46X0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50153002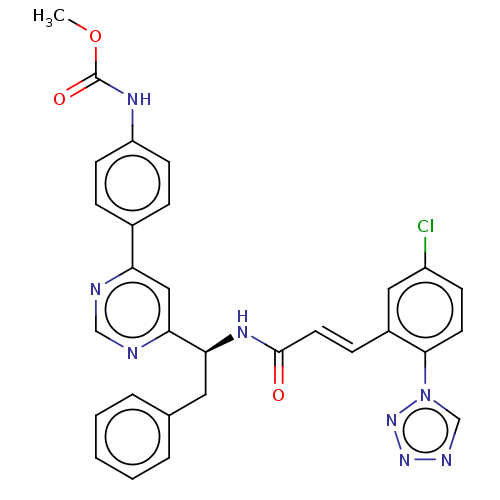 (CHEMBL3781363) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Competitive inhibition of human coagulation factor 11a assessed as reduction in release of p-nitroaniline after 10 to 120 mins by Michaelis-Menten eq... | Bioorg Med Chem 24: 2257-72 (2016) Article DOI: 10.1016/j.bmc.2016.03.062 BindingDB Entry DOI: 10.7270/Q2DN46X0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50103200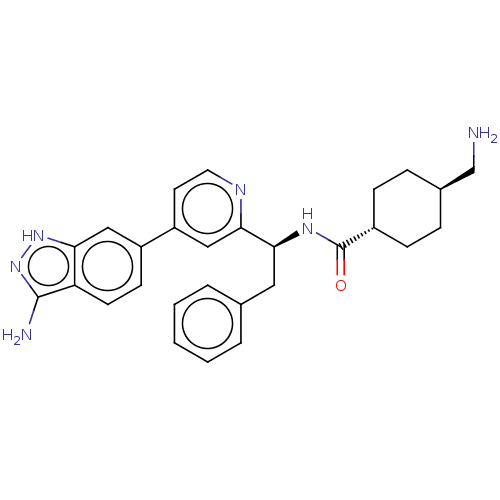 (CHEMBL3393386) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Competitive inhibition of human coagulation factor 11a assessed as reduction in release of p-nitroaniline after 10 to 120 mins by Michaelis-Menten eq... | Bioorg Med Chem 24: 2257-72 (2016) Article DOI: 10.1016/j.bmc.2016.03.062 BindingDB Entry DOI: 10.7270/Q2DN46X0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50153058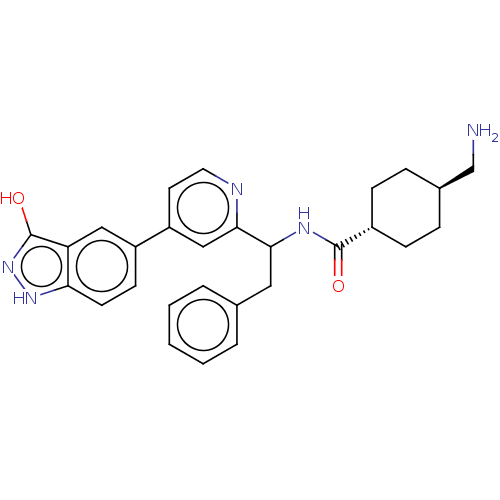 (CHEMBL3780189) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Competitive inhibition of human coagulation factor 11a assessed as reduction in release of p-nitroaniline after 10 to 120 mins by Michaelis-Menten eq... | Bioorg Med Chem 24: 2257-72 (2016) Article DOI: 10.1016/j.bmc.2016.03.062 BindingDB Entry DOI: 10.7270/Q2DN46X0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50153046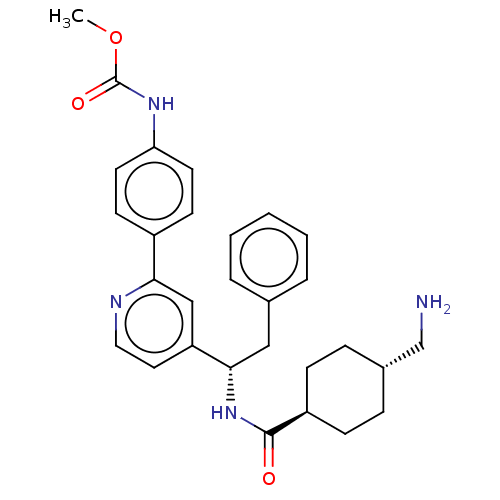 (CHEMBL3780777) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Competitive inhibition of human coagulation factor 11a assessed as reduction in release of p-nitroaniline after 10 to 120 mins by Michaelis-Menten eq... | Bioorg Med Chem 24: 2257-72 (2016) Article DOI: 10.1016/j.bmc.2016.03.062 BindingDB Entry DOI: 10.7270/Q2DN46X0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50153047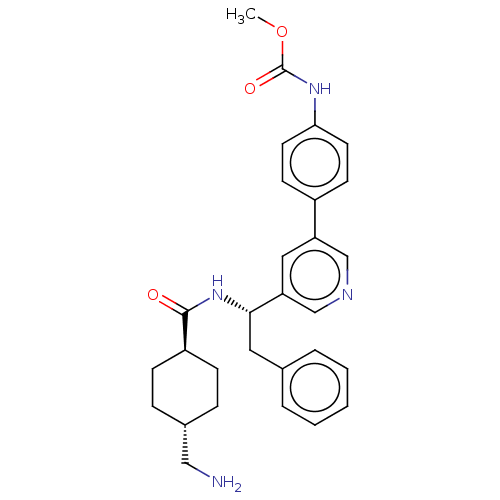 (CHEMBL3780030) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 8.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Competitive inhibition of human coagulation factor 11a assessed as reduction in release of p-nitroaniline after 10 to 120 mins by Michaelis-Menten eq... | Bioorg Med Chem 24: 2257-72 (2016) Article DOI: 10.1016/j.bmc.2016.03.062 BindingDB Entry DOI: 10.7270/Q2DN46X0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50153012 (CHEMBL3781550) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 9.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Competitive inhibition of human coagulation factor 11a assessed as reduction in release of p-nitroaniline after 10 to 120 mins by Michaelis-Menten eq... | Bioorg Med Chem 24: 2257-72 (2016) Article DOI: 10.1016/j.bmc.2016.03.062 BindingDB Entry DOI: 10.7270/Q2DN46X0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50153064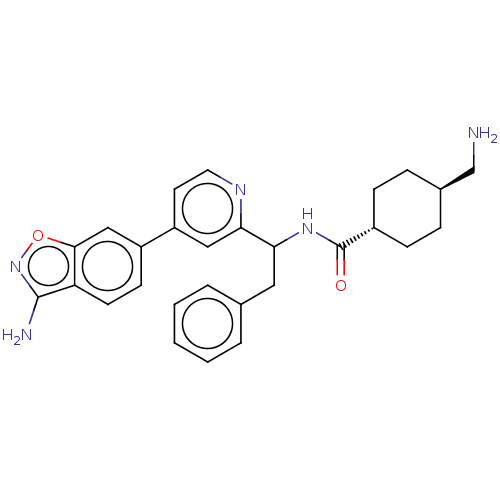 (CHEMBL3780285) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 9.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Competitive inhibition of human coagulation factor 11a assessed as reduction in release of p-nitroaniline after 10 to 120 mins by Michaelis-Menten eq... | Bioorg Med Chem 24: 2257-72 (2016) Article DOI: 10.1016/j.bmc.2016.03.062 BindingDB Entry DOI: 10.7270/Q2DN46X0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50153062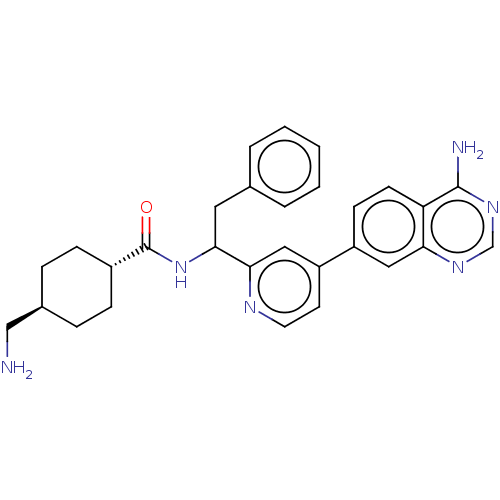 (CHEMBL3781505) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Competitive inhibition of human coagulation factor 11a assessed as reduction in release of p-nitroaniline after 10 to 120 mins by Michaelis-Menten eq... | Bioorg Med Chem 24: 2257-72 (2016) Article DOI: 10.1016/j.bmc.2016.03.062 BindingDB Entry DOI: 10.7270/Q2DN46X0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50153003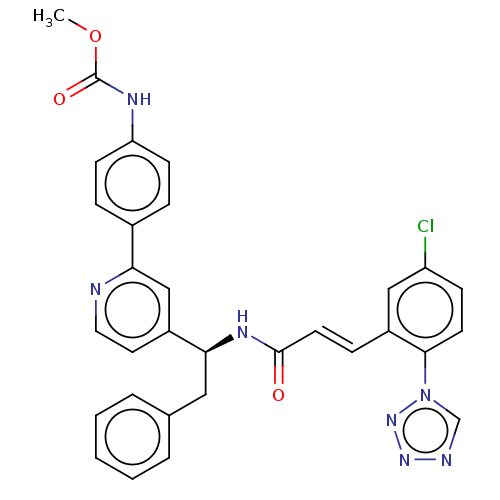 (CHEMBL3780001) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Competitive inhibition of human coagulation factor 11a assessed as reduction in release of p-nitroaniline after 10 to 120 mins by Michaelis-Menten eq... | Bioorg Med Chem 24: 2257-72 (2016) Article DOI: 10.1016/j.bmc.2016.03.062 BindingDB Entry DOI: 10.7270/Q2DN46X0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50153050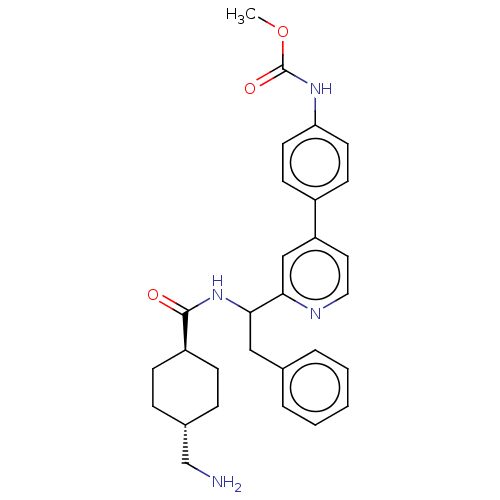 (CHEMBL3781580) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Competitive inhibition of human coagulation factor 11a assessed as reduction in release of p-nitroaniline after 10 to 120 mins by Michaelis-Menten eq... | Bioorg Med Chem 24: 2257-72 (2016) Article DOI: 10.1016/j.bmc.2016.03.062 BindingDB Entry DOI: 10.7270/Q2DN46X0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50103204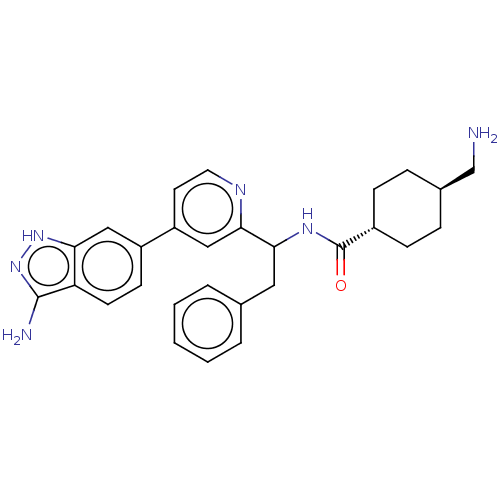 (CHEMBL3393385) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Competitive inhibition of human coagulation factor 11a assessed as reduction in release of p-nitroaniline after 10 to 120 mins by Michaelis-Menten eq... | Bioorg Med Chem 24: 2257-72 (2016) Article DOI: 10.1016/j.bmc.2016.03.062 BindingDB Entry DOI: 10.7270/Q2DN46X0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50153048 (CHEMBL3780127) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Competitive inhibition of human coagulation factor 11a assessed as reduction in release of p-nitroaniline after 10 to 120 mins by Michaelis-Menten eq... | Bioorg Med Chem 24: 2257-72 (2016) Article DOI: 10.1016/j.bmc.2016.03.062 BindingDB Entry DOI: 10.7270/Q2DN46X0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50153004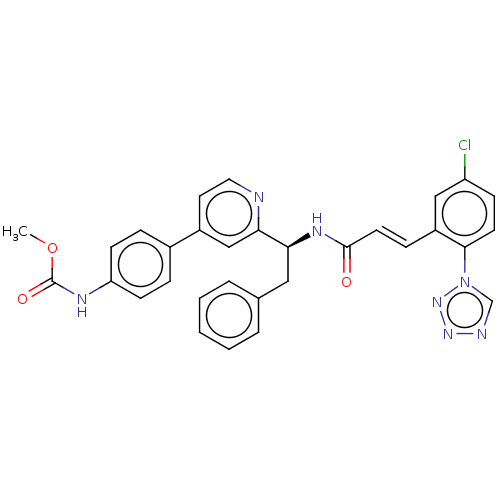 (CHEMBL3781319) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Competitive inhibition of human coagulation factor 11a assessed as reduction in release of p-nitroaniline after 10 to 120 mins by Michaelis-Menten eq... | Bioorg Med Chem 24: 2257-72 (2016) Article DOI: 10.1016/j.bmc.2016.03.062 BindingDB Entry DOI: 10.7270/Q2DN46X0 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50153051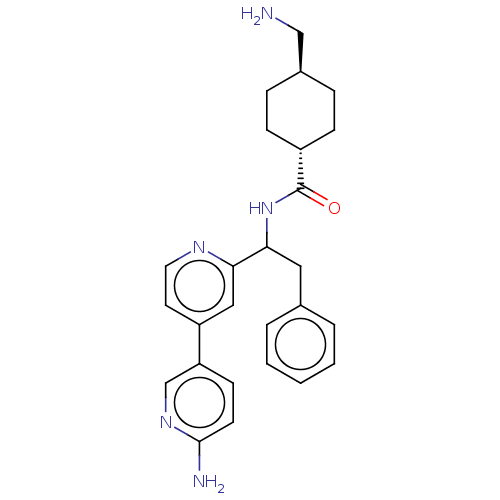 (CHEMBL3781277) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Competitive inhibition of human coagulation factor 11a assessed as reduction in release of p-nitroaniline after 10 to 120 mins by Michaelis-Menten eq... | Bioorg Med Chem 24: 2257-72 (2016) Article DOI: 10.1016/j.bmc.2016.03.062 BindingDB Entry DOI: 10.7270/Q2DN46X0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM50103200 (CHEMBL3393386) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human plasma kallikrein assessed as reduction in release of p-nitroaniline after 10 to 120 mins by Michaelis-Menten equation analysis | Bioorg Med Chem 24: 2257-72 (2016) Article DOI: 10.1016/j.bmc.2016.03.062 BindingDB Entry DOI: 10.7270/Q2DN46X0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM50153049 (CHEMBL3781788) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 42 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human plasma kallikrein assessed as reduction in release of p-nitroaniline after 10 to 120 mins by Michaelis-Menten equation analysis | Bioorg Med Chem 24: 2257-72 (2016) Article DOI: 10.1016/j.bmc.2016.03.062 BindingDB Entry DOI: 10.7270/Q2DN46X0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50153060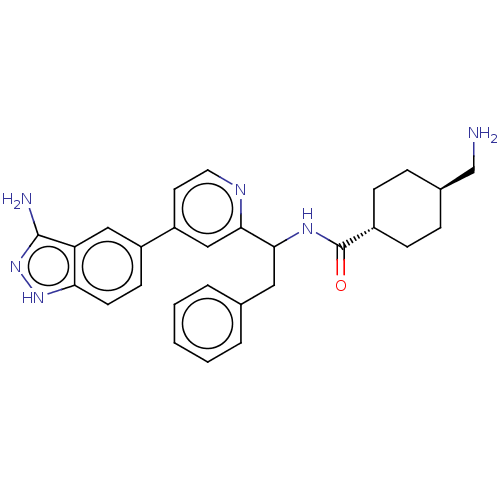 (CHEMBL3780751) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 53 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Competitive inhibition of human coagulation factor 11a assessed as reduction in release of p-nitroaniline after 10 to 120 mins by Michaelis-Menten eq... | Bioorg Med Chem 24: 2257-72 (2016) Article DOI: 10.1016/j.bmc.2016.03.062 BindingDB Entry DOI: 10.7270/Q2DN46X0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50153052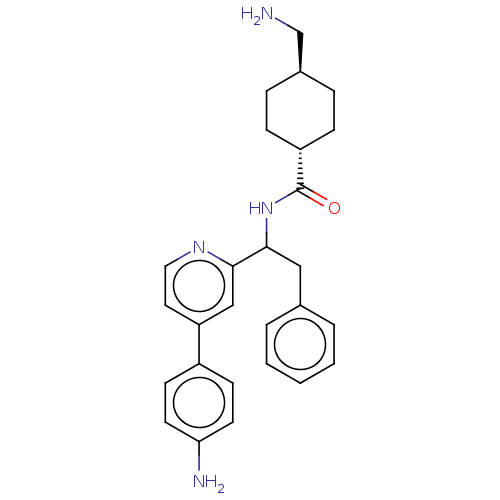 (CHEMBL3781981) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 56 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Competitive inhibition of human coagulation factor 11a assessed as reduction in release of p-nitroaniline after 10 to 120 mins by Michaelis-Menten eq... | Bioorg Med Chem 24: 2257-72 (2016) Article DOI: 10.1016/j.bmc.2016.03.062 BindingDB Entry DOI: 10.7270/Q2DN46X0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50153059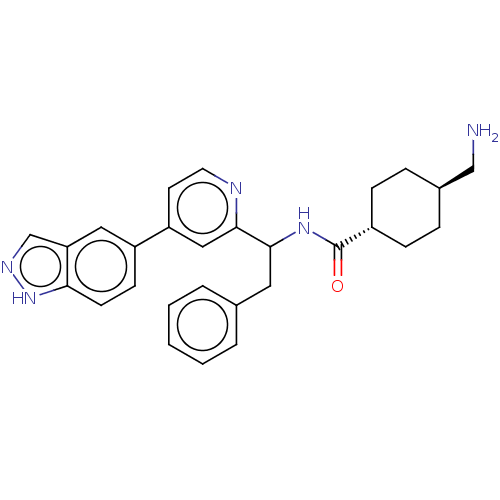 (CHEMBL3780930) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 61 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Competitive inhibition of human coagulation factor 11a assessed as reduction in release of p-nitroaniline after 10 to 120 mins by Michaelis-Menten eq... | Bioorg Med Chem 24: 2257-72 (2016) Article DOI: 10.1016/j.bmc.2016.03.062 BindingDB Entry DOI: 10.7270/Q2DN46X0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypsin (Homo sapiens (Human)) | BDBM50103200 (CHEMBL3393386) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 64 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human coagulation trypsin assessed as reduction in release of p-nitroaniline after 10 to 120 mins by Michaelis-Menten equation analysis | Bioorg Med Chem 24: 2257-72 (2016) Article DOI: 10.1016/j.bmc.2016.03.062 BindingDB Entry DOI: 10.7270/Q2DN46X0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50153056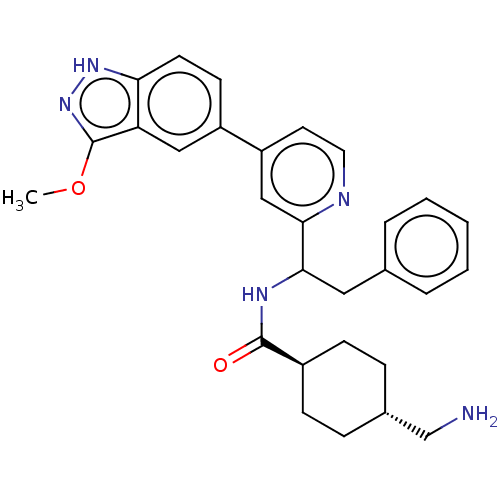 (CHEMBL3780501) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 87 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Competitive inhibition of human coagulation factor 11a assessed as reduction in release of p-nitroaniline after 10 to 120 mins by Michaelis-Menten eq... | Bioorg Med Chem 24: 2257-72 (2016) Article DOI: 10.1016/j.bmc.2016.03.062 BindingDB Entry DOI: 10.7270/Q2DN46X0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50153066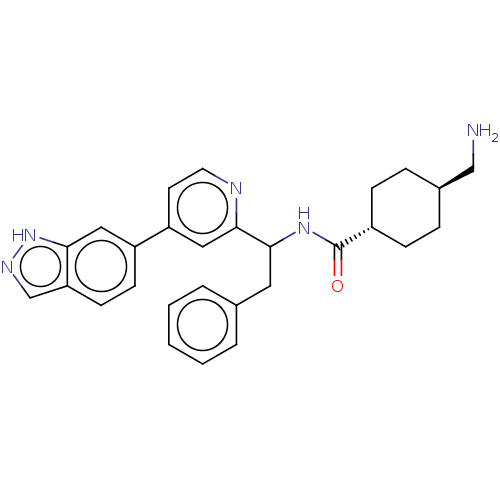 (CHEMBL3781679) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 87 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Competitive inhibition of human coagulation factor 11a assessed as reduction in release of p-nitroaniline after 10 to 120 mins by Michaelis-Menten eq... | Bioorg Med Chem 24: 2257-72 (2016) Article DOI: 10.1016/j.bmc.2016.03.062 BindingDB Entry DOI: 10.7270/Q2DN46X0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50153065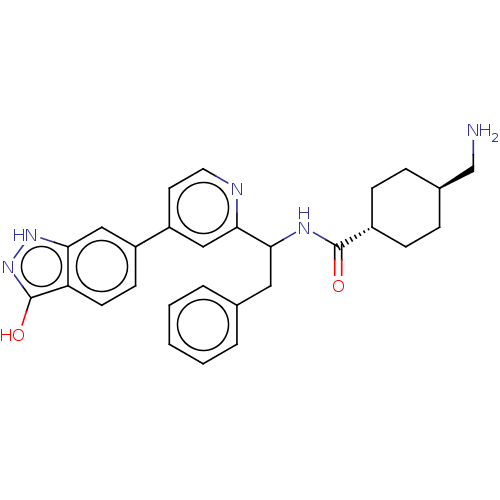 (CHEMBL3781581) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 97 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Competitive inhibition of human coagulation factor 11a assessed as reduction in release of p-nitroaniline after 10 to 120 mins by Michaelis-Menten eq... | Bioorg Med Chem 24: 2257-72 (2016) Article DOI: 10.1016/j.bmc.2016.03.062 BindingDB Entry DOI: 10.7270/Q2DN46X0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypsin (Homo sapiens (Human)) | BDBM50153049 (CHEMBL3781788) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 101 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human coagulation trypsin assessed as reduction in release of p-nitroaniline after 10 to 120 mins by Michaelis-Menten equation analysis | Bioorg Med Chem 24: 2257-72 (2016) Article DOI: 10.1016/j.bmc.2016.03.062 BindingDB Entry DOI: 10.7270/Q2DN46X0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50153055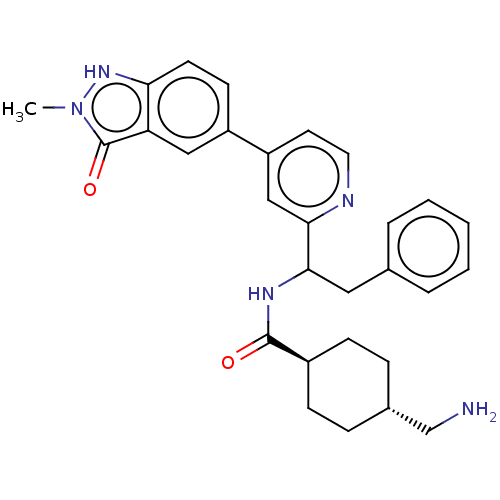 (CHEMBL3781691) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Competitive inhibition of human coagulation factor 11a assessed as reduction in release of p-nitroaniline after 10 to 120 mins by Michaelis-Menten eq... | Bioorg Med Chem 24: 2257-72 (2016) Article DOI: 10.1016/j.bmc.2016.03.062 BindingDB Entry DOI: 10.7270/Q2DN46X0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50153061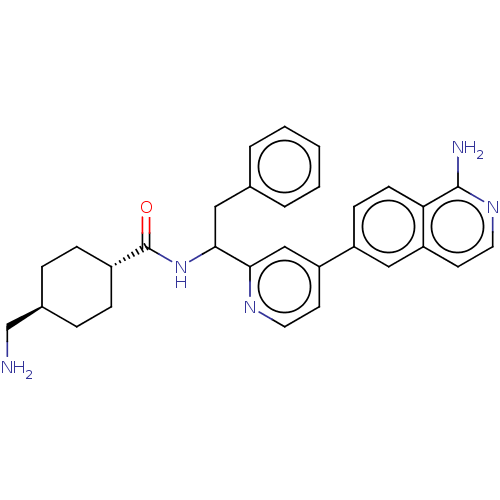 (CHEMBL3781468) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 144 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Competitive inhibition of human coagulation factor 11a assessed as reduction in release of p-nitroaniline after 10 to 120 mins by Michaelis-Menten eq... | Bioorg Med Chem 24: 2257-72 (2016) Article DOI: 10.1016/j.bmc.2016.03.062 BindingDB Entry DOI: 10.7270/Q2DN46X0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50153053 (CHEMBL3780389) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 205 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Competitive inhibition of human coagulation factor 11a assessed as reduction in release of p-nitroaniline after 10 to 120 mins by Michaelis-Menten eq... | Bioorg Med Chem 24: 2257-72 (2016) Article DOI: 10.1016/j.bmc.2016.03.062 BindingDB Entry DOI: 10.7270/Q2DN46X0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM50153057 (CHEMBL3780324) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human plasma kallikrein assessed as reduction in release of p-nitroaniline after 10 to 120 mins by Michaelis-Menten equation analysis | Bioorg Med Chem 24: 2257-72 (2016) Article DOI: 10.1016/j.bmc.2016.03.062 BindingDB Entry DOI: 10.7270/Q2DN46X0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypsin (Homo sapiens (Human)) | BDBM50153057 (CHEMBL3780324) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 999 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human coagulation trypsin assessed as reduction in release of p-nitroaniline after 10 to 120 mins by Michaelis-Menten equation analysis | Bioorg Med Chem 24: 2257-72 (2016) Article DOI: 10.1016/j.bmc.2016.03.062 BindingDB Entry DOI: 10.7270/Q2DN46X0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50153004 (CHEMBL3781319) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | 1.13E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human coagulation factor 10a assessed as reduction in release of p-nitroaniline after 10 to 120 mins by Michaelis-Menten equation analy... | Bioorg Med Chem 24: 2257-72 (2016) Article DOI: 10.1016/j.bmc.2016.03.062 BindingDB Entry DOI: 10.7270/Q2DN46X0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM50153004 (CHEMBL3781319) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | 1.85E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human plasma kallikrein assessed as reduction in release of p-nitroaniline after 10 to 120 mins by Michaelis-Menten equation analysis | Bioorg Med Chem 24: 2257-72 (2016) Article DOI: 10.1016/j.bmc.2016.03.062 BindingDB Entry DOI: 10.7270/Q2DN46X0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypsin (Homo sapiens (Human)) | BDBM50153004 (CHEMBL3781319) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | >6.22E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human coagulation trypsin assessed as reduction in release of p-nitroaniline after 10 to 120 mins by Michaelis-Menten equation analysis | Bioorg Med Chem 24: 2257-72 (2016) Article DOI: 10.1016/j.bmc.2016.03.062 BindingDB Entry DOI: 10.7270/Q2DN46X0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50153057 (CHEMBL3780324) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | >9.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human coagulation factor 10a assessed as reduction in release of p-nitroaniline after 10 to 120 mins by Michaelis-Menten equation analy... | Bioorg Med Chem 24: 2257-72 (2016) Article DOI: 10.1016/j.bmc.2016.03.062 BindingDB Entry DOI: 10.7270/Q2DN46X0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50153049 (CHEMBL3781788) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | >9.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human coagulation factor 10a assessed as reduction in release of p-nitroaniline after 10 to 120 mins by Michaelis-Menten equation analy... | Bioorg Med Chem 24: 2257-72 (2016) Article DOI: 10.1016/j.bmc.2016.03.062 BindingDB Entry DOI: 10.7270/Q2DN46X0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50103200 (CHEMBL3393386) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >9.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human coagulation factor 10a assessed as reduction in release of p-nitroaniline after 10 to 120 mins by Michaelis-Menten equation analy... | Bioorg Med Chem 24: 2257-72 (2016) Article DOI: 10.1016/j.bmc.2016.03.062 BindingDB Entry DOI: 10.7270/Q2DN46X0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tissue-type plasminogen activator (Homo sapiens (Human)) | BDBM50153004 (CHEMBL3781319) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | 1.05E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human TPA assessed as reduction in release of p-nitroaniline after 10 to 120 mins by Michaelis-Menten equation analysis | Bioorg Med Chem 24: 2257-72 (2016) Article DOI: 10.1016/j.bmc.2016.03.062 BindingDB Entry DOI: 10.7270/Q2DN46X0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor VII (Homo sapiens (Human)) | BDBM50103200 (CHEMBL3393386) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.09E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human coagulation factor 7a assessed as reduction in release of p-nitroaniline after 10 to 120 mins by Michaelis-Menten equation analys... | Bioorg Med Chem 24: 2257-72 (2016) Article DOI: 10.1016/j.bmc.2016.03.062 BindingDB Entry DOI: 10.7270/Q2DN46X0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor VII (Homo sapiens (Human)) | BDBM50153049 (CHEMBL3781788) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | >1.22E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human coagulation factor 7a assessed as reduction in release of p-nitroaniline after 10 to 120 mins by Michaelis-Menten equation analys... | Bioorg Med Chem 24: 2257-72 (2016) Article DOI: 10.1016/j.bmc.2016.03.062 BindingDB Entry DOI: 10.7270/Q2DN46X0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Homo sapiens (Human)) | BDBM50153004 (CHEMBL3781319) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | 1.25E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human plasmin assessed as reduction in release of p-nitroaniline after 10 to 120 mins by Michaelis-Menten equation analysis | Bioorg Med Chem 24: 2257-72 (2016) Article DOI: 10.1016/j.bmc.2016.03.062 BindingDB Entry DOI: 10.7270/Q2DN46X0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50153049 (CHEMBL3781788) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | >1.26E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human coagulation thrombin assessed as reduction in release of p-nitroaniline after 10 to 120 mins by Michaelis-Menten equation analysi... | Bioorg Med Chem 24: 2257-72 (2016) Article DOI: 10.1016/j.bmc.2016.03.062 BindingDB Entry DOI: 10.7270/Q2DN46X0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50103200 (CHEMBL3393386) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.26E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human coagulation thrombin assessed as reduction in release of p-nitroaniline after 10 to 120 mins by Michaelis-Menten equation analysi... | Bioorg Med Chem 24: 2257-72 (2016) Article DOI: 10.1016/j.bmc.2016.03.062 BindingDB Entry DOI: 10.7270/Q2DN46X0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50153057 (CHEMBL3780324) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | >1.26E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human coagulation thrombin assessed as reduction in release of p-nitroaniline after 10 to 120 mins by Michaelis-Menten equation analysi... | Bioorg Med Chem 24: 2257-72 (2016) Article DOI: 10.1016/j.bmc.2016.03.062 BindingDB Entry DOI: 10.7270/Q2DN46X0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50153004 (CHEMBL3781319) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | >1.33E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human coagulation thrombin assessed as reduction in release of p-nitroaniline after 10 to 120 mins by Michaelis-Menten equation analysi... | Bioorg Med Chem 24: 2257-72 (2016) Article DOI: 10.1016/j.bmc.2016.03.062 BindingDB Entry DOI: 10.7270/Q2DN46X0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor VII (Homo sapiens (Human)) | BDBM50153004 (CHEMBL3781319) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | >1.34E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human coagulation factor 7a assessed as reduction in release of p-nitroaniline after 10 to 120 mins by Michaelis-Menten equation analys... | Bioorg Med Chem 24: 2257-72 (2016) Article DOI: 10.1016/j.bmc.2016.03.062 BindingDB Entry DOI: 10.7270/Q2DN46X0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM50103200 (CHEMBL3393386) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.41E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human urokinase assessed as reduction in release of p-nitroaniline after 10 to 120 mins by Michaelis-Menten equation analysis | Bioorg Med Chem 24: 2257-72 (2016) Article DOI: 10.1016/j.bmc.2016.03.062 BindingDB Entry DOI: 10.7270/Q2DN46X0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM50153057 (CHEMBL3780324) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | >1.41E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human urokinase assessed as reduction in release of p-nitroaniline after 10 to 120 mins by Michaelis-Menten equation analysis | Bioorg Med Chem 24: 2257-72 (2016) Article DOI: 10.1016/j.bmc.2016.03.062 BindingDB Entry DOI: 10.7270/Q2DN46X0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM50153049 (CHEMBL3781788) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | >1.41E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human urokinase assessed as reduction in release of p-nitroaniline after 10 to 120 mins by Michaelis-Menten equation analysis | Bioorg Med Chem 24: 2257-72 (2016) Article DOI: 10.1016/j.bmc.2016.03.062 BindingDB Entry DOI: 10.7270/Q2DN46X0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor VII (Homo sapiens (Human)) | BDBM50153057 (CHEMBL3780324) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | >1.52E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human coagulation factor 7a assessed as reduction in release of p-nitroaniline after 10 to 120 mins by Michaelis-Menten equation analys... | Bioorg Med Chem 24: 2257-72 (2016) Article DOI: 10.1016/j.bmc.2016.03.062 BindingDB Entry DOI: 10.7270/Q2DN46X0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin K-dependent protein C (Homo sapiens (Human)) | BDBM50103200 (CHEMBL3393386) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >2.14E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human activated protein C assessed as reduction in release of p-nitroaniline after 10 to 120 mins by Michaelis-Menten equation analysis | Bioorg Med Chem 24: 2257-72 (2016) Article DOI: 10.1016/j.bmc.2016.03.062 BindingDB Entry DOI: 10.7270/Q2DN46X0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tissue-type plasminogen activator (Homo sapiens (Human)) | BDBM50153057 (CHEMBL3780324) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | >2.19E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human TPA assessed as reduction in release of p-nitroaniline after 10 to 120 mins by Michaelis-Menten equation analysis | Bioorg Med Chem 24: 2257-72 (2016) Article DOI: 10.1016/j.bmc.2016.03.062 BindingDB Entry DOI: 10.7270/Q2DN46X0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tissue-type plasminogen activator (Homo sapiens (Human)) | BDBM50153049 (CHEMBL3781788) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | >2.19E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human TPA assessed as reduction in release of p-nitroaniline after 10 to 120 mins by Michaelis-Menten equation analysis | Bioorg Med Chem 24: 2257-72 (2016) Article DOI: 10.1016/j.bmc.2016.03.062 BindingDB Entry DOI: 10.7270/Q2DN46X0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tissue-type plasminogen activator (Homo sapiens (Human)) | BDBM50103200 (CHEMBL3393386) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >2.19E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human TPA assessed as reduction in release of p-nitroaniline after 10 to 120 mins by Michaelis-Menten equation analysis | Bioorg Med Chem 24: 2257-72 (2016) Article DOI: 10.1016/j.bmc.2016.03.062 BindingDB Entry DOI: 10.7270/Q2DN46X0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Homo sapiens (Human)) | BDBM50153049 (CHEMBL3781788) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | >2.21E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human plasmin assessed as reduction in release of p-nitroaniline after 10 to 120 mins by Michaelis-Menten equation analysis | Bioorg Med Chem 24: 2257-72 (2016) Article DOI: 10.1016/j.bmc.2016.03.062 BindingDB Entry DOI: 10.7270/Q2DN46X0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Homo sapiens (Human)) | BDBM50153057 (CHEMBL3780324) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | >2.21E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human plasmin assessed as reduction in release of p-nitroaniline after 10 to 120 mins by Michaelis-Menten equation analysis | Bioorg Med Chem 24: 2257-72 (2016) Article DOI: 10.1016/j.bmc.2016.03.062 BindingDB Entry DOI: 10.7270/Q2DN46X0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Homo sapiens (Human)) | BDBM50103200 (CHEMBL3393386) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >2.21E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human plasmin assessed as reduction in release of p-nitroaniline after 10 to 120 mins by Michaelis-Menten equation analysis | Bioorg Med Chem 24: 2257-72 (2016) Article DOI: 10.1016/j.bmc.2016.03.062 BindingDB Entry DOI: 10.7270/Q2DN46X0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM50153004 (CHEMBL3781319) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | >2.97E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human urokinase assessed as reduction in release of p-nitroaniline after 10 to 120 mins by Michaelis-Menten equation analysis | Bioorg Med Chem 24: 2257-72 (2016) Article DOI: 10.1016/j.bmc.2016.03.062 BindingDB Entry DOI: 10.7270/Q2DN46X0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin K-dependent protein C (Homo sapiens (Human)) | BDBM50153049 (CHEMBL3781788) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | >3.02E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human activated protein C assessed as reduction in release of p-nitroaniline after 10 to 120 mins by Michaelis-Menten equation analysis | Bioorg Med Chem 24: 2257-72 (2016) Article DOI: 10.1016/j.bmc.2016.03.062 BindingDB Entry DOI: 10.7270/Q2DN46X0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin K-dependent protein C (Homo sapiens (Human)) | BDBM50153004 (CHEMBL3781319) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | >3.02E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human activated protein C assessed as reduction in release of p-nitroaniline after 10 to 120 mins by Michaelis-Menten equation analysis | Bioorg Med Chem 24: 2257-72 (2016) Article DOI: 10.1016/j.bmc.2016.03.062 BindingDB Entry DOI: 10.7270/Q2DN46X0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin K-dependent protein C (Homo sapiens (Human)) | BDBM50153057 (CHEMBL3780324) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | >3.02E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human activated protein C assessed as reduction in release of p-nitroaniline after 10 to 120 mins by Michaelis-Menten equation analysis | Bioorg Med Chem 24: 2257-72 (2016) Article DOI: 10.1016/j.bmc.2016.03.062 BindingDB Entry DOI: 10.7270/Q2DN46X0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor IX (Homo sapiens (Human)) | BDBM50153057 (CHEMBL3780324) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | >3.49E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human coagulation factor 9a assessed as reduction in release of p-nitroaniline after 10 to 120 mins by Michaelis-Menten equation analys... | Bioorg Med Chem 24: 2257-72 (2016) Article DOI: 10.1016/j.bmc.2016.03.062 BindingDB Entry DOI: 10.7270/Q2DN46X0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor IX (Homo sapiens (Human)) | BDBM50103200 (CHEMBL3393386) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >3.49E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human coagulation factor 9a assessed as reduction in release of p-nitroaniline after 10 to 120 mins by Michaelis-Menten equation analys... | Bioorg Med Chem 24: 2257-72 (2016) Article DOI: 10.1016/j.bmc.2016.03.062 BindingDB Entry DOI: 10.7270/Q2DN46X0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor IX (Homo sapiens (Human)) | BDBM50153049 (CHEMBL3781788) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | >3.49E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human coagulation factor 9a assessed as reduction in release of p-nitroaniline after 10 to 120 mins by Michaelis-Menten equation analys... | Bioorg Med Chem 24: 2257-72 (2016) Article DOI: 10.1016/j.bmc.2016.03.062 BindingDB Entry DOI: 10.7270/Q2DN46X0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||