 Found 121 hits of Enzyme Inhibition Constant Data
Found 121 hits of Enzyme Inhibition Constant Data Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50184464

(CHEMBL3822644)Show SMILES C[C@@H](N)C(=O)N1CCC(CC1)n1nc(C)c(Nc2ncc(Cl)c(n2)-c2cnn3ccccc23)c1C |r| Show InChI InChI=1S/C24H28ClN9O/c1-14(26)23(35)32-10-7-17(8-11-32)34-16(3)21(15(2)31-34)29-24-27-13-19(25)22(30-24)18-12-28-33-9-5-4-6-20(18)33/h4-6,9,12-14,17H,7-8,10-11,26H2,1-3H3,(H,27,29,30)/t14-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human IGF-1R using fluorescent labeled FL-KKSRGDYMTMQIG-CONH2 as substrate after 1 hr 50 mins |
J Med Chem 59: 4859-66 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00203
BindingDB Entry DOI: 10.7270/Q2KS6TG9 |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50184462

(CHEMBL3823154)Show SMILES Cc1nn(C2CCN(CC2)C(=O)CN)c(C)c1Nc1ncc(Cl)c(n1)-c1cnn2ccccc12 Show InChI InChI=1S/C23H26ClN9O/c1-14-21(15(2)33(30-14)16-6-9-31(10-7-16)20(34)11-25)28-23-26-13-18(24)22(29-23)17-12-27-32-8-4-3-5-19(17)32/h3-5,8,12-13,16H,6-7,9-11,25H2,1-2H3,(H,26,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human IGF-1R using fluorescent labeled FL-KKSRGDYMTMQIG-CONH2 as substrate after 1 hr 50 mins |
J Med Chem 59: 4859-66 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00203
BindingDB Entry DOI: 10.7270/Q2KS6TG9 |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50184475
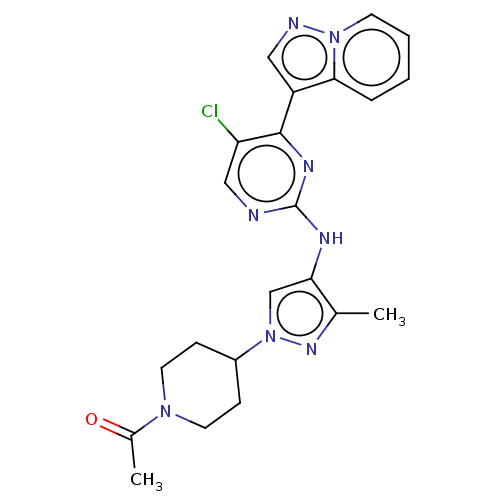
(CHEMBL3822989)Show SMILES CC(=O)N1CCC(CC1)n1cc(Nc2ncc(Cl)c(n2)-c2cnn3ccccc23)c(C)n1 Show InChI InChI=1S/C22H23ClN8O/c1-14-19(13-31(28-14)16-6-9-29(10-7-16)15(2)32)26-22-24-12-18(23)21(27-22)17-11-25-30-8-4-3-5-20(17)30/h3-5,8,11-13,16H,6-7,9-10H2,1-2H3,(H,24,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human IGF-1R using fluorescent labeled FL-KKSRGDYMTMQIG-CONH2 as substrate after 1 hr 50 mins |
J Med Chem 59: 4859-66 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00203
BindingDB Entry DOI: 10.7270/Q2KS6TG9 |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50184465

(CHEMBL3822976)Show SMILES CNCC(=O)N1CCC(CC1)n1nc(C)c(Nc2ncc(Cl)c(n2)-c2cnn3ccccc23)c1C Show InChI InChI=1S/C24H28ClN9O/c1-15-22(16(2)34(31-15)17-7-10-32(11-8-17)21(35)14-26-3)29-24-27-13-19(25)23(30-24)18-12-28-33-9-5-4-6-20(18)33/h4-6,9,12-13,17,26H,7-8,10-11,14H2,1-3H3,(H,27,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human IGF-1R using fluorescent labeled FL-KKSRGDYMTMQIG-CONH2 as substrate after 1 hr 50 mins |
J Med Chem 59: 4859-66 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00203
BindingDB Entry DOI: 10.7270/Q2KS6TG9 |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50184476

(CHEMBL3823301)Show SMILES CC(=O)N1CCC(CC1)n1ncc(Nc2ncc(Cl)c(n2)-c2cnn3ccccc23)c1C Show InChI InChI=1S/C22H23ClN8O/c1-14-19(13-26-31(14)16-6-9-29(10-7-16)15(2)32)27-22-24-12-18(23)21(28-22)17-11-25-30-8-4-3-5-20(17)30/h3-5,8,11-13,16H,6-7,9-10H2,1-2H3,(H,24,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human IGF-1R using fluorescent labeled FL-KKSRGDYMTMQIG-CONH2 as substrate after 1 hr 50 mins |
J Med Chem 59: 4859-66 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00203
BindingDB Entry DOI: 10.7270/Q2KS6TG9 |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50184463
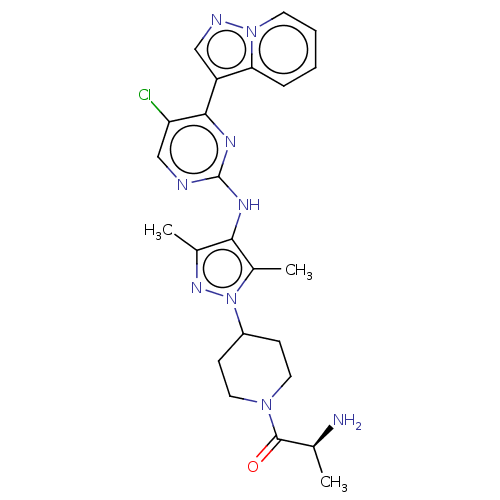
(CHEMBL3824103)Show SMILES C[C@H](N)C(=O)N1CCC(CC1)n1nc(C)c(Nc2ncc(Cl)c(n2)-c2cnn3ccccc23)c1C |r| Show InChI InChI=1S/C24H28ClN9O/c1-14(26)23(35)32-10-7-17(8-11-32)34-16(3)21(15(2)31-34)29-24-27-13-19(25)22(30-24)18-12-28-33-9-5-4-6-20(18)33/h4-6,9,12-14,17H,7-8,10-11,26H2,1-3H3,(H,27,29,30)/t14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human IGF-1R using fluorescent labeled FL-KKSRGDYMTMQIG-CONH2 as substrate after 1 hr 50 mins |
J Med Chem 59: 4859-66 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00203
BindingDB Entry DOI: 10.7270/Q2KS6TG9 |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50184455
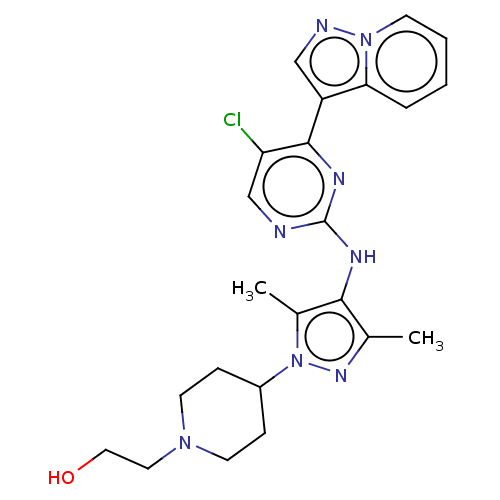
(CHEMBL3824323)Show SMILES Cc1nn(C2CCN(CCO)CC2)c(C)c1Nc1ncc(Cl)c(n1)-c1cnn2ccccc12 Show InChI InChI=1S/C23H27ClN8O/c1-15-21(16(2)32(29-15)17-6-9-30(10-7-17)11-12-33)27-23-25-14-19(24)22(28-23)18-13-26-31-8-4-3-5-20(18)31/h3-5,8,13-14,17,33H,6-7,9-12H2,1-2H3,(H,25,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human IGF-1R using fluorescent labeled FL-KKSRGDYMTMQIG-CONH2 as substrate after 1 hr 50 mins |
J Med Chem 59: 4859-66 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00203
BindingDB Entry DOI: 10.7270/Q2KS6TG9 |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50184452
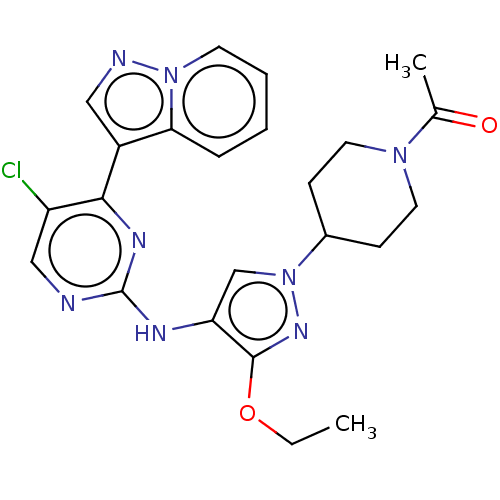
(CHEMBL3822943)Show SMILES CCOc1nn(cc1Nc1ncc(Cl)c(n1)-c1cnn2ccccc12)C1CCN(CC1)C(C)=O Show InChI InChI=1S/C23H25ClN8O2/c1-3-34-22-19(14-32(29-22)16-7-10-30(11-8-16)15(2)33)27-23-25-13-18(24)21(28-23)17-12-26-31-9-5-4-6-20(17)31/h4-6,9,12-14,16H,3,7-8,10-11H2,1-2H3,(H,25,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of IGF1-induced human IGF1R autophosphorylation expressed in IGF-1R knock-out mouse fibroblasts |
J Med Chem 59: 4859-66 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00203
BindingDB Entry DOI: 10.7270/Q2KS6TG9 |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50184457
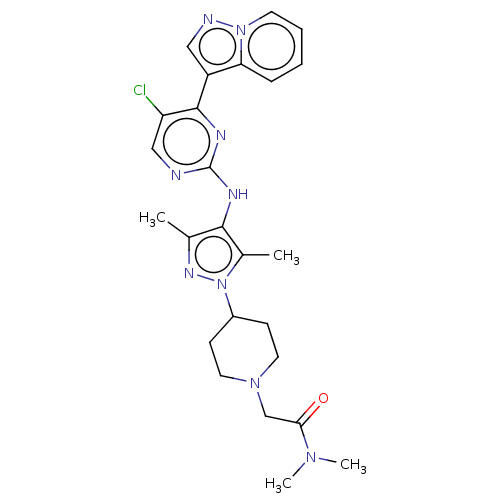
(CHEMBL3823095)Show SMILES CN(C)C(=O)CN1CCC(CC1)n1nc(C)c(Nc2ncc(Cl)c(n2)-c2cnn3ccccc23)c1C Show InChI InChI=1S/C25H30ClN9O/c1-16-23(17(2)35(31-16)18-8-11-33(12-9-18)15-22(36)32(3)4)29-25-27-14-20(26)24(30-25)19-13-28-34-10-6-5-7-21(19)34/h5-7,10,13-14,18H,8-9,11-12,15H2,1-4H3,(H,27,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human IGF-1R using fluorescent labeled FL-KKSRGDYMTMQIG-CONH2 as substrate after 1 hr 50 mins |
J Med Chem 59: 4859-66 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00203
BindingDB Entry DOI: 10.7270/Q2KS6TG9 |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50184467
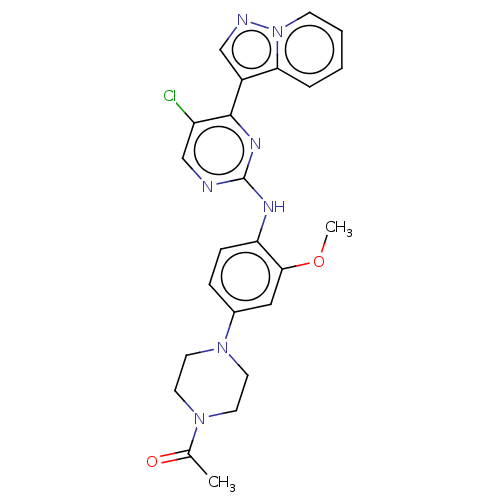
(CHEMBL3823297)Show SMILES COc1cc(ccc1Nc1ncc(Cl)c(n1)-c1cnn2ccccc12)N1CCN(CC1)C(C)=O Show InChI InChI=1S/C24H24ClN7O2/c1-16(33)30-9-11-31(12-10-30)17-6-7-20(22(13-17)34-2)28-24-26-15-19(25)23(29-24)18-14-27-32-8-4-3-5-21(18)32/h3-8,13-15H,9-12H2,1-2H3,(H,26,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human IGF-1R using fluorescent labeled FL-KKSRGDYMTMQIG-CONH2 as substrate after 1 hr 50 mins |
J Med Chem 59: 4859-66 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00203
BindingDB Entry DOI: 10.7270/Q2KS6TG9 |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50184460

(CHEMBL3822947)Show SMILES C[C@H](O)C(=O)N1CCC(CC1)n1nc(C)c(Nc2ncc(Cl)c(n2)-c2cnn3ccccc23)c1C |r| Show InChI InChI=1S/C24H27ClN8O2/c1-14-21(15(2)33(30-14)17-7-10-31(11-8-17)23(35)16(3)34)28-24-26-13-19(25)22(29-24)18-12-27-32-9-5-4-6-20(18)32/h4-6,9,12-13,16-17,34H,7-8,10-11H2,1-3H3,(H,26,28,29)/t16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human IGF-1R using fluorescent labeled FL-KKSRGDYMTMQIG-CONH2 as substrate after 1 hr 50 mins |
J Med Chem 59: 4859-66 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00203
BindingDB Entry DOI: 10.7270/Q2KS6TG9 |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50184458

(CHEMBL3822587)Show SMILES Cc1nn(C2CCN(CC2)C(=O)CO)c(C)c1Nc1ncc(Cl)c(n1)-c1cnn2ccccc12 Show InChI InChI=1S/C23H25ClN8O2/c1-14-21(15(2)32(29-14)16-6-9-30(10-7-16)20(34)13-33)27-23-25-12-18(24)22(28-23)17-11-26-31-8-4-3-5-19(17)31/h3-5,8,11-12,16,33H,6-7,9-10,13H2,1-2H3,(H,25,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human IGF-1R using fluorescent labeled FL-KKSRGDYMTMQIG-CONH2 as substrate after 1 hr 50 mins |
J Med Chem 59: 4859-66 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00203
BindingDB Entry DOI: 10.7270/Q2KS6TG9 |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50184459

(CHEMBL3822651)Show SMILES COCC(=O)N1CCC(CC1)n1nc(C)c(Nc2ncc(Cl)c(n2)-c2cnn3ccccc23)c1C Show InChI InChI=1S/C24H27ClN8O2/c1-15-22(16(2)33(30-15)17-7-10-31(11-8-17)21(34)14-35-3)28-24-26-13-19(25)23(29-24)18-12-27-32-9-5-4-6-20(18)32/h4-6,9,12-13,17H,7-8,10-11,14H2,1-3H3,(H,26,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human IGF-1R using fluorescent labeled FL-KKSRGDYMTMQIG-CONH2 as substrate after 1 hr 50 mins |
J Med Chem 59: 4859-66 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00203
BindingDB Entry DOI: 10.7270/Q2KS6TG9 |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50184456
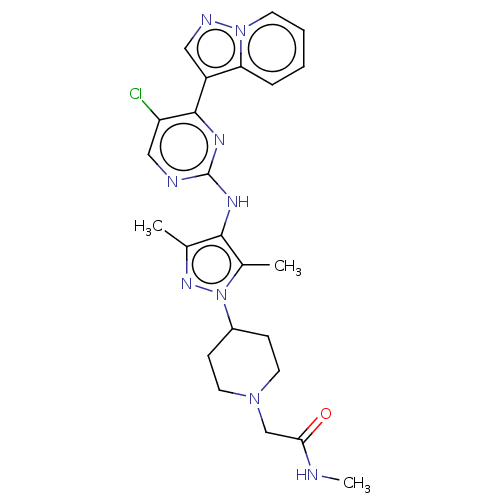
(CHEMBL3823962)Show SMILES CNC(=O)CN1CCC(CC1)n1nc(C)c(Nc2ncc(Cl)c(n2)-c2cnn3ccccc23)c1C Show InChI InChI=1S/C24H28ClN9O/c1-15-22(16(2)34(31-15)17-7-10-32(11-8-17)14-21(35)26-3)29-24-27-13-19(25)23(30-24)18-12-28-33-9-5-4-6-20(18)33/h4-6,9,12-13,17H,7-8,10-11,14H2,1-3H3,(H,26,35)(H,27,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human IGF-1R using fluorescent labeled FL-KKSRGDYMTMQIG-CONH2 as substrate after 1 hr 50 mins |
J Med Chem 59: 4859-66 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00203
BindingDB Entry DOI: 10.7270/Q2KS6TG9 |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50184472
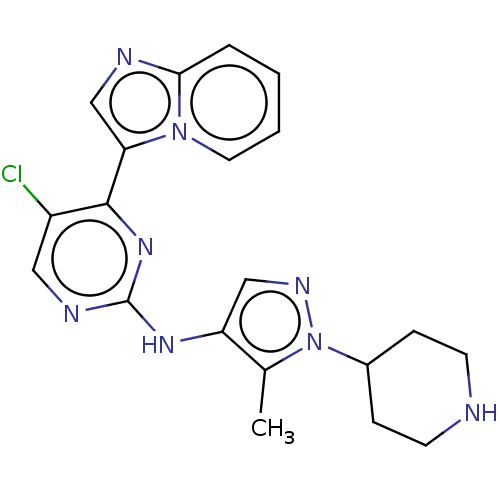
(CHEMBL3822543)Show SMILES Cc1c(Nc2ncc(Cl)c(n2)-c2cnc3ccccn23)cnn1C1CCNCC1 Show InChI InChI=1S/C20H21ClN8/c1-13-16(11-25-29(13)14-5-7-22-8-6-14)26-20-24-10-15(21)19(27-20)17-12-23-18-4-2-3-9-28(17)18/h2-4,9-12,14,22H,5-8H2,1H3,(H,24,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human IGF-1R using fluorescent labeled FL-KKSRGDYMTMQIG-CONH2 as substrate after 1 hr 50 mins |
J Med Chem 59: 4859-66 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00203
BindingDB Entry DOI: 10.7270/Q2KS6TG9 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50184450

(CHEMBL3824296)Show SMILES Cc1nn(C2CCNCC2)c(C)c1Nc1ncc(Cl)c(n1)-c1cnn2ccccc12 Show InChI InChI=1S/C21H23ClN8/c1-13-19(14(2)30(28-13)15-6-8-23-9-7-15)26-21-24-12-17(22)20(27-21)16-11-25-29-10-4-3-5-18(16)29/h3-5,10-12,15,23H,6-9H2,1-2H3,(H,24,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human IGF-1R using fluorescent labeled FL-KKSRGDYMTMQIG-CONH2 as substrate after 1 hr 50 mins |
J Med Chem 59: 4859-66 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00203
BindingDB Entry DOI: 10.7270/Q2KS6TG9 |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50184461

(CHEMBL3823030)Show SMILES C[C@@H](O)C(=O)N1CCC(CC1)n1nc(C)c(Nc2ncc(Cl)c(n2)-c2cnn3ccccc23)c1C |r| Show InChI InChI=1S/C24H27ClN8O2/c1-14-21(15(2)33(30-14)17-7-10-31(11-8-17)23(35)16(3)34)28-24-26-13-19(25)22(29-24)18-12-27-32-9-5-4-6-20(18)32/h4-6,9,12-13,16-17,34H,7-8,10-11H2,1-3H3,(H,26,28,29)/t16-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human IGF-1R using fluorescent labeled FL-KKSRGDYMTMQIG-CONH2 as substrate after 1 hr 50 mins |
J Med Chem 59: 4859-66 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00203
BindingDB Entry DOI: 10.7270/Q2KS6TG9 |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50184452

(CHEMBL3822943)Show SMILES CCOc1nn(cc1Nc1ncc(Cl)c(n1)-c1cnn2ccccc12)C1CCN(CC1)C(C)=O Show InChI InChI=1S/C23H25ClN8O2/c1-3-34-22-19(14-32(29-22)16-7-10-30(11-8-16)15(2)33)27-23-25-13-18(24)21(28-23)17-12-26-31-9-5-4-6-20(17)31/h4-6,9,12-14,16H,3,7-8,10-11H2,1-2H3,(H,25,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human IGF-1R using fluorescent labeled FL-KKSRGDYMTMQIG-CONH2 as substrate after 1 hr 50 mins |
J Med Chem 59: 4859-66 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00203
BindingDB Entry DOI: 10.7270/Q2KS6TG9 |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50184455

(CHEMBL3824323)Show SMILES Cc1nn(C2CCN(CCO)CC2)c(C)c1Nc1ncc(Cl)c(n1)-c1cnn2ccccc12 Show InChI InChI=1S/C23H27ClN8O/c1-15-21(16(2)32(29-15)17-6-9-30(10-7-17)11-12-33)27-23-25-14-19(24)22(28-23)18-13-26-31-8-4-3-5-20(18)31/h3-5,8,13-14,17,33H,6-7,9-12H2,1-2H3,(H,25,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of IGF1-induced human IGF1R autophosphorylation expressed in IGF-1R knock-out mouse fibroblasts |
J Med Chem 59: 4859-66 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00203
BindingDB Entry DOI: 10.7270/Q2KS6TG9 |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50184463

(CHEMBL3824103)Show SMILES C[C@H](N)C(=O)N1CCC(CC1)n1nc(C)c(Nc2ncc(Cl)c(n2)-c2cnn3ccccc23)c1C |r| Show InChI InChI=1S/C24H28ClN9O/c1-14(26)23(35)32-10-7-17(8-11-32)34-16(3)21(15(2)31-34)29-24-27-13-19(25)22(30-24)18-12-28-33-9-5-4-6-20(18)33/h4-6,9,12-14,17H,7-8,10-11,26H2,1-3H3,(H,27,29,30)/t14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of IGF1-induced human IGF1R autophosphorylation expressed in IGF-1R knock-out mouse fibroblasts |
J Med Chem 59: 4859-66 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00203
BindingDB Entry DOI: 10.7270/Q2KS6TG9 |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50184465

(CHEMBL3822976)Show SMILES CNCC(=O)N1CCC(CC1)n1nc(C)c(Nc2ncc(Cl)c(n2)-c2cnn3ccccc23)c1C Show InChI InChI=1S/C24H28ClN9O/c1-15-22(16(2)34(31-15)17-7-10-32(11-8-17)21(35)14-26-3)29-24-27-13-19(25)23(30-24)18-12-28-33-9-5-4-6-20(18)33/h4-6,9,12-13,17,26H,7-8,10-11,14H2,1-3H3,(H,27,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of IGF1-induced human IGF1R autophosphorylation expressed in IGF-1R knock-out mouse fibroblasts |
J Med Chem 59: 4859-66 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00203
BindingDB Entry DOI: 10.7270/Q2KS6TG9 |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50184450

(CHEMBL3824296)Show SMILES Cc1nn(C2CCNCC2)c(C)c1Nc1ncc(Cl)c(n1)-c1cnn2ccccc12 Show InChI InChI=1S/C21H23ClN8/c1-13-19(14(2)30(28-13)15-6-8-23-9-7-15)26-21-24-12-17(22)20(27-21)16-11-25-29-10-4-3-5-18(16)29/h3-5,10-12,15,23H,6-9H2,1-2H3,(H,24,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of IGF1-induced human IGF1R autophosphorylation expressed in IGF-1R knock-out mouse fibroblasts |
J Med Chem 59: 4859-66 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00203
BindingDB Entry DOI: 10.7270/Q2KS6TG9 |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50184467

(CHEMBL3823297)Show SMILES COc1cc(ccc1Nc1ncc(Cl)c(n1)-c1cnn2ccccc12)N1CCN(CC1)C(C)=O Show InChI InChI=1S/C24H24ClN7O2/c1-16(33)30-9-11-31(12-10-30)17-6-7-20(22(13-17)34-2)28-24-26-15-19(25)23(29-24)18-14-27-32-8-4-3-5-21(18)32/h3-8,13-15H,9-12H2,1-2H3,(H,26,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of IGF1-induced human IGF1R autophosphorylation expressed in IGF-1R knock-out mouse fibroblasts |
J Med Chem 59: 4859-66 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00203
BindingDB Entry DOI: 10.7270/Q2KS6TG9 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 7
(Homo sapiens (Human)) | BDBM50184466
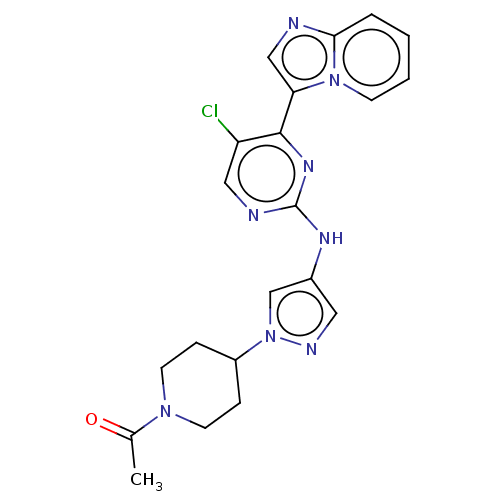
(CHEMBL3823659)Show SMILES CC(=O)N1CCC(CC1)n1cc(Nc2ncc(Cl)c(n2)-c2cnc3ccccn23)cn1 Show InChI InChI=1S/C21H21ClN8O/c1-14(31)28-8-5-16(6-9-28)30-13-15(10-25-30)26-21-24-11-17(22)20(27-21)18-12-23-19-4-2-3-7-29(18)19/h2-4,7,10-13,16H,5-6,8-9H2,1H3,(H,24,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of CDK7 (unknown origin) |
J Med Chem 59: 4859-66 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00203
BindingDB Entry DOI: 10.7270/Q2KS6TG9 |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50184475

(CHEMBL3822989)Show SMILES CC(=O)N1CCC(CC1)n1cc(Nc2ncc(Cl)c(n2)-c2cnn3ccccc23)c(C)n1 Show InChI InChI=1S/C22H23ClN8O/c1-14-19(13-31(28-14)16-6-9-29(10-7-16)15(2)32)26-22-24-12-18(23)21(27-22)17-11-25-30-8-4-3-5-20(17)30/h3-5,8,11-13,16H,6-7,9-10H2,1-2H3,(H,24,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of IGF1-induced human IGF1R autophosphorylation expressed in IGF-1R knock-out mouse fibroblasts |
J Med Chem 59: 4859-66 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00203
BindingDB Entry DOI: 10.7270/Q2KS6TG9 |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50184462

(CHEMBL3823154)Show SMILES Cc1nn(C2CCN(CC2)C(=O)CN)c(C)c1Nc1ncc(Cl)c(n1)-c1cnn2ccccc12 Show InChI InChI=1S/C23H26ClN9O/c1-14-21(15(2)33(30-14)16-6-9-31(10-7-16)20(34)11-25)28-23-26-13-18(24)22(29-23)17-12-27-32-8-4-3-5-19(17)32/h3-5,8,12-13,16H,6-7,9-11,25H2,1-2H3,(H,26,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of IGF1-induced human IGF1R autophosphorylation expressed in IGF-1R knock-out mouse fibroblasts |
J Med Chem 59: 4859-66 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00203
BindingDB Entry DOI: 10.7270/Q2KS6TG9 |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50184471

(CHEMBL3822583)Show SMILES CC(=O)N1CCC(CC1)n1ncc(Nc2ncc(Cl)c(n2)-c2cnc3ccccn23)c1C Show InChI InChI=1S/C22H23ClN8O/c1-14-18(12-26-31(14)16-6-9-29(10-7-16)15(2)32)27-22-25-11-17(23)21(28-22)19-13-24-20-5-3-4-8-30(19)20/h3-5,8,11-13,16H,6-7,9-10H2,1-2H3,(H,25,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human IGF-1R using fluorescent labeled FL-KKSRGDYMTMQIG-CONH2 as substrate after 1 hr 50 mins |
J Med Chem 59: 4859-66 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00203
BindingDB Entry DOI: 10.7270/Q2KS6TG9 |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50184457

(CHEMBL3823095)Show SMILES CN(C)C(=O)CN1CCC(CC1)n1nc(C)c(Nc2ncc(Cl)c(n2)-c2cnn3ccccc23)c1C Show InChI InChI=1S/C25H30ClN9O/c1-16-23(17(2)35(31-16)18-8-11-33(12-9-18)15-22(36)32(3)4)29-25-27-14-20(26)24(30-25)19-13-28-34-10-6-5-7-21(19)34/h5-7,10,13-14,18H,8-9,11-12,15H2,1-4H3,(H,27,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of IGF1-induced human IGF1R autophosphorylation expressed in IGF-1R knock-out mouse fibroblasts |
J Med Chem 59: 4859-66 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00203
BindingDB Entry DOI: 10.7270/Q2KS6TG9 |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50184464

(CHEMBL3822644)Show SMILES C[C@@H](N)C(=O)N1CCC(CC1)n1nc(C)c(Nc2ncc(Cl)c(n2)-c2cnn3ccccc23)c1C |r| Show InChI InChI=1S/C24H28ClN9O/c1-14(26)23(35)32-10-7-17(8-11-32)34-16(3)21(15(2)31-34)29-24-27-13-19(25)22(30-24)18-12-28-33-9-5-4-6-20(18)33/h4-6,9,12-14,17H,7-8,10-11,26H2,1-3H3,(H,27,29,30)/t14-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of IGF1-induced human IGF1R autophosphorylation expressed in IGF-1R knock-out mouse fibroblasts |
J Med Chem 59: 4859-66 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00203
BindingDB Entry DOI: 10.7270/Q2KS6TG9 |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50184456

(CHEMBL3823962)Show SMILES CNC(=O)CN1CCC(CC1)n1nc(C)c(Nc2ncc(Cl)c(n2)-c2cnn3ccccc23)c1C Show InChI InChI=1S/C24H28ClN9O/c1-15-22(16(2)34(31-15)17-7-10-32(11-8-17)14-21(35)26-3)29-24-27-13-19(25)23(30-24)18-12-28-33-9-5-4-6-20(18)33/h4-6,9,12-13,17H,7-8,10-11,14H2,1-3H3,(H,26,35)(H,27,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of IGF1-induced human IGF1R autophosphorylation expressed in IGF-1R knock-out mouse fibroblasts |
J Med Chem 59: 4859-66 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00203
BindingDB Entry DOI: 10.7270/Q2KS6TG9 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50184466

(CHEMBL3823659)Show SMILES CC(=O)N1CCC(CC1)n1cc(Nc2ncc(Cl)c(n2)-c2cnc3ccccn23)cn1 Show InChI InChI=1S/C21H21ClN8O/c1-14(31)28-8-5-16(6-9-28)30-13-15(10-25-30)26-21-24-11-17(22)20(27-21)18-12-23-19-4-2-3-7-29(18)19/h2-4,7,10-13,16H,5-6,8-9H2,1H3,(H,24,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of CDK2 (unknown origin) |
J Med Chem 59: 4859-66 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00203
BindingDB Entry DOI: 10.7270/Q2KS6TG9 |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50184451

(CHEMBL3823914)Show SMILES CC(=O)N1CCC(CC1)n1nc(C)c(Nc2ncc(Cl)c(n2)-c2cnn3ccccc23)c1C Show InChI InChI=1S/C23H25ClN8O/c1-14-21(15(2)32(29-14)17-7-10-30(11-8-17)16(3)33)27-23-25-13-19(24)22(28-23)18-12-26-31-9-5-4-6-20(18)31/h4-6,9,12-13,17H,7-8,10-11H2,1-3H3,(H,25,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of IGF1-induced human IGF1R autophosphorylation expressed in IGF-1R knock-out mouse fibroblasts |
J Med Chem 59: 4859-66 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00203
BindingDB Entry DOI: 10.7270/Q2KS6TG9 |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50184458

(CHEMBL3822587)Show SMILES Cc1nn(C2CCN(CC2)C(=O)CO)c(C)c1Nc1ncc(Cl)c(n1)-c1cnn2ccccc12 Show InChI InChI=1S/C23H25ClN8O2/c1-14-21(15(2)32(29-14)16-6-9-30(10-7-16)20(34)13-33)27-23-25-12-18(24)22(28-23)17-11-26-31-8-4-3-5-19(17)31/h3-5,8,11-12,16,33H,6-7,9-10,13H2,1-2H3,(H,25,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of IGF1-induced human IGF1R autophosphorylation expressed in IGF-1R knock-out mouse fibroblasts |
J Med Chem 59: 4859-66 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00203
BindingDB Entry DOI: 10.7270/Q2KS6TG9 |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50184451

(CHEMBL3823914)Show SMILES CC(=O)N1CCC(CC1)n1nc(C)c(Nc2ncc(Cl)c(n2)-c2cnn3ccccc23)c1C Show InChI InChI=1S/C23H25ClN8O/c1-14-21(15(2)32(29-14)17-7-10-30(11-8-17)16(3)33)27-23-25-13-19(24)22(28-23)18-12-26-31-9-5-4-6-20(18)31/h4-6,9,12-13,17H,7-8,10-11H2,1-3H3,(H,25,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human IGF-1R using fluorescent labeled FL-KKSRGDYMTMQIG-CONH2 as substrate after 1 hr 50 mins |
J Med Chem 59: 4859-66 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00203
BindingDB Entry DOI: 10.7270/Q2KS6TG9 |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50184453
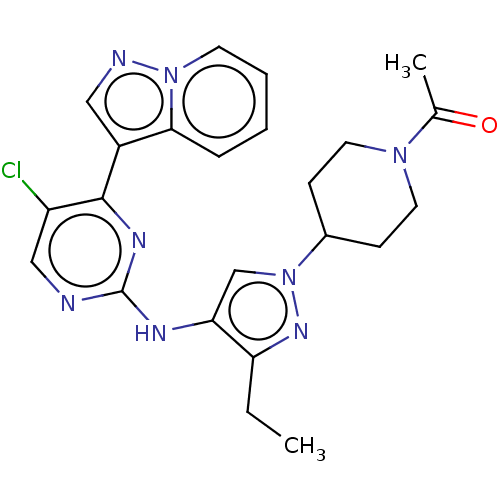
(CHEMBL3822619)Show SMILES CCc1nn(cc1Nc1ncc(Cl)c(n1)-c1cnn2ccccc12)C1CCN(CC1)C(C)=O Show InChI InChI=1S/C23H25ClN8O/c1-3-19-20(14-32(29-19)16-7-10-30(11-8-16)15(2)33)27-23-25-13-18(24)22(28-23)17-12-26-31-9-5-4-6-21(17)31/h4-6,9,12-14,16H,3,7-8,10-11H2,1-2H3,(H,25,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human IGF-1R using fluorescent labeled FL-KKSRGDYMTMQIG-CONH2 as substrate after 1 hr 50 mins |
J Med Chem 59: 4859-66 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00203
BindingDB Entry DOI: 10.7270/Q2KS6TG9 |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50184476

(CHEMBL3823301)Show SMILES CC(=O)N1CCC(CC1)n1ncc(Nc2ncc(Cl)c(n2)-c2cnn3ccccc23)c1C Show InChI InChI=1S/C22H23ClN8O/c1-14-19(13-26-31(14)16-6-9-29(10-7-16)15(2)32)27-22-24-12-18(23)21(28-22)17-11-25-30-8-4-3-5-20(17)30/h3-5,8,11-13,16H,6-7,9-10H2,1-2H3,(H,24,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of IGF1-induced human IGF1R autophosphorylation expressed in IGF-1R knock-out mouse fibroblasts |
J Med Chem 59: 4859-66 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00203
BindingDB Entry DOI: 10.7270/Q2KS6TG9 |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50184469
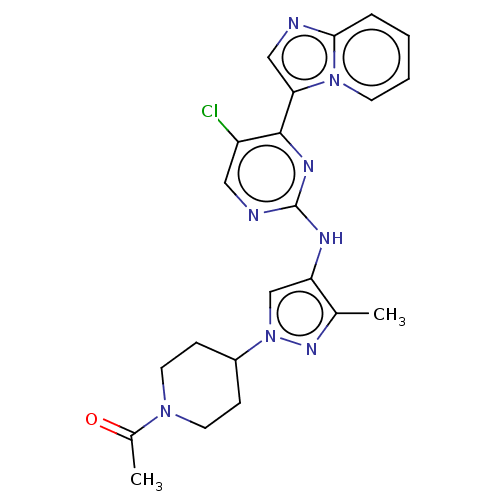
(CHEMBL3823278)Show SMILES CC(=O)N1CCC(CC1)n1cc(Nc2ncc(Cl)c(n2)-c2cnc3ccccn23)c(C)n1 Show InChI InChI=1S/C22H23ClN8O/c1-14-18(13-31(28-14)16-6-9-29(10-7-16)15(2)32)26-22-25-11-17(23)21(27-22)19-12-24-20-5-3-4-8-30(19)20/h3-5,8,11-13,16H,6-7,9-10H2,1-2H3,(H,25,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of IGF1-induced human IGF1R autophosphorylation expressed in IGF-1R knock-out mouse fibroblasts |
J Med Chem 59: 4859-66 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00203
BindingDB Entry DOI: 10.7270/Q2KS6TG9 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 9
(Homo sapiens (Human)) | BDBM50184466

(CHEMBL3823659)Show SMILES CC(=O)N1CCC(CC1)n1cc(Nc2ncc(Cl)c(n2)-c2cnc3ccccn23)cn1 Show InChI InChI=1S/C21H21ClN8O/c1-14(31)28-8-5-16(6-9-28)30-13-15(10-25-30)26-21-24-11-17(22)20(27-21)18-12-23-19-4-2-3-7-29(18)19/h2-4,7,10-13,16H,5-6,8-9H2,1H3,(H,24,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of CDK9 (unknown origin) |
J Med Chem 59: 4859-66 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00203
BindingDB Entry DOI: 10.7270/Q2KS6TG9 |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50184470
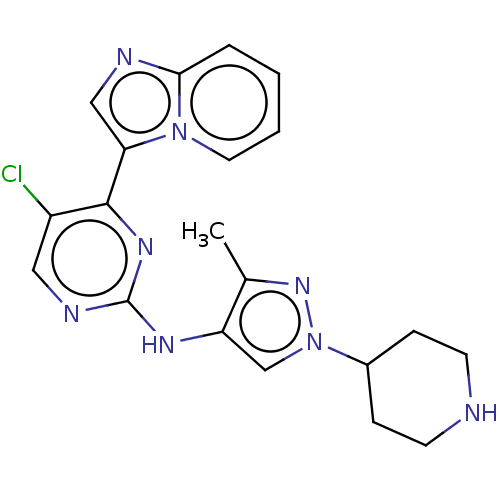
(CHEMBL3823007)Show SMILES Cc1nn(cc1Nc1ncc(Cl)c(n1)-c1cnc2ccccn12)C1CCNCC1 Show InChI InChI=1S/C20H21ClN8/c1-13-16(12-29(27-13)14-5-7-22-8-6-14)25-20-24-10-15(21)19(26-20)17-11-23-18-4-2-3-9-28(17)18/h2-4,9-12,14,22H,5-8H2,1H3,(H,24,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human IGF-1R using fluorescent labeled FL-KKSRGDYMTMQIG-CONH2 as substrate after 1 hr 50 mins |
J Med Chem 59: 4859-66 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00203
BindingDB Entry DOI: 10.7270/Q2KS6TG9 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50184454

(CHEMBL3822962)Show SMILES CCc1c(Nc2ncc(Cl)c(n2)-c2cnn3ccccc23)cnn1C1CCN(CC1)C(C)=O Show InChI InChI=1S/C23H25ClN8O/c1-3-20-19(14-27-32(20)16-7-10-30(11-8-16)15(2)33)28-23-25-13-18(24)22(29-23)17-12-26-31-9-5-4-6-21(17)31/h4-6,9,12-14,16H,3,7-8,10-11H2,1-2H3,(H,25,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human IGF-1R using fluorescent labeled FL-KKSRGDYMTMQIG-CONH2 as substrate after 1 hr 50 mins |
J Med Chem 59: 4859-66 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00203
BindingDB Entry DOI: 10.7270/Q2KS6TG9 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 8
(Homo sapiens (Human)) | BDBM50184466

(CHEMBL3823659)Show SMILES CC(=O)N1CCC(CC1)n1cc(Nc2ncc(Cl)c(n2)-c2cnc3ccccn23)cn1 Show InChI InChI=1S/C21H21ClN8O/c1-14(31)28-8-5-16(6-9-28)30-13-15(10-25-30)26-21-24-11-17(22)20(27-21)18-12-23-19-4-2-3-7-29(18)19/h2-4,7,10-13,16H,5-6,8-9H2,1H3,(H,24,26,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of CDK8 (unknown origin) |
J Med Chem 59: 4859-66 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00203
BindingDB Entry DOI: 10.7270/Q2KS6TG9 |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50184466

(CHEMBL3823659)Show SMILES CC(=O)N1CCC(CC1)n1cc(Nc2ncc(Cl)c(n2)-c2cnc3ccccn23)cn1 Show InChI InChI=1S/C21H21ClN8O/c1-14(31)28-8-5-16(6-9-28)30-13-15(10-25-30)26-21-24-11-17(22)20(27-21)18-12-23-19-4-2-3-7-29(18)19/h2-4,7,10-13,16H,5-6,8-9H2,1H3,(H,24,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human IGF-1R using fluorescent labeled FL-KKSRGDYMTMQIG-CONH2 as substrate after 1 hr 50 mins |
J Med Chem 59: 4859-66 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00203
BindingDB Entry DOI: 10.7270/Q2KS6TG9 |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50184460

(CHEMBL3822947)Show SMILES C[C@H](O)C(=O)N1CCC(CC1)n1nc(C)c(Nc2ncc(Cl)c(n2)-c2cnn3ccccc23)c1C |r| Show InChI InChI=1S/C24H27ClN8O2/c1-14-21(15(2)33(30-14)17-7-10-31(11-8-17)23(35)16(3)34)28-24-26-13-19(25)22(29-24)18-12-27-32-9-5-4-6-20(18)32/h4-6,9,12-13,16-17,34H,7-8,10-11H2,1-3H3,(H,26,28,29)/t16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of IGF1-induced human IGF1R autophosphorylation expressed in IGF-1R knock-out mouse fibroblasts |
J Med Chem 59: 4859-66 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00203
BindingDB Entry DOI: 10.7270/Q2KS6TG9 |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50184474

(CHEMBL3823522)Show SMILES COc1nn(cc1Nc1ncc(Cl)c(n1)-c1cnn2ccccc12)C1CCN(CC1)C(C)=O Show InChI InChI=1S/C22H23ClN8O2/c1-14(32)29-9-6-15(7-10-29)31-13-18(21(28-31)33-2)26-22-24-12-17(23)20(27-22)16-11-25-30-8-4-3-5-19(16)30/h3-5,8,11-13,15H,6-7,9-10H2,1-2H3,(H,24,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 46 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of IGF1-induced human IGF1R autophosphorylation expressed in IGF-1R knock-out mouse fibroblasts |
J Med Chem 59: 4859-66 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00203
BindingDB Entry DOI: 10.7270/Q2KS6TG9 |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50184461

(CHEMBL3823030)Show SMILES C[C@@H](O)C(=O)N1CCC(CC1)n1nc(C)c(Nc2ncc(Cl)c(n2)-c2cnn3ccccc23)c1C |r| Show InChI InChI=1S/C24H27ClN8O2/c1-14-21(15(2)33(30-14)17-7-10-31(11-8-17)23(35)16(3)34)28-24-26-13-19(25)22(29-24)18-12-27-32-9-5-4-6-20(18)32/h4-6,9,12-13,16-17,34H,7-8,10-11H2,1-3H3,(H,26,28,29)/t16-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of IGF1-induced human IGF1R autophosphorylation expressed in IGF-1R knock-out mouse fibroblasts |
J Med Chem 59: 4859-66 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00203
BindingDB Entry DOI: 10.7270/Q2KS6TG9 |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50184453

(CHEMBL3822619)Show SMILES CCc1nn(cc1Nc1ncc(Cl)c(n1)-c1cnn2ccccc12)C1CCN(CC1)C(C)=O Show InChI InChI=1S/C23H25ClN8O/c1-3-19-20(14-32(29-19)16-7-10-30(11-8-16)15(2)33)27-23-25-13-18(24)22(28-23)17-12-26-31-9-5-4-6-21(17)31/h4-6,9,12-14,16H,3,7-8,10-11H2,1-2H3,(H,25,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 49 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of IGF1-induced human IGF1R autophosphorylation expressed in IGF-1R knock-out mouse fibroblasts |
J Med Chem 59: 4859-66 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00203
BindingDB Entry DOI: 10.7270/Q2KS6TG9 |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50184474

(CHEMBL3823522)Show SMILES COc1nn(cc1Nc1ncc(Cl)c(n1)-c1cnn2ccccc12)C1CCN(CC1)C(C)=O Show InChI InChI=1S/C22H23ClN8O2/c1-14(32)29-9-6-15(7-10-29)31-13-18(21(28-31)33-2)26-22-24-12-17(23)20(27-22)16-11-25-30-8-4-3-5-19(16)30/h3-5,8,11-13,15H,6-7,9-10H2,1-2H3,(H,24,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 49 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human IGF-1R using fluorescent labeled FL-KKSRGDYMTMQIG-CONH2 as substrate after 1 hr 50 mins |
J Med Chem 59: 4859-66 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00203
BindingDB Entry DOI: 10.7270/Q2KS6TG9 |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50184459

(CHEMBL3822651)Show SMILES COCC(=O)N1CCC(CC1)n1nc(C)c(Nc2ncc(Cl)c(n2)-c2cnn3ccccc23)c1C Show InChI InChI=1S/C24H27ClN8O2/c1-15-22(16(2)33(30-15)17-7-10-31(11-8-17)21(34)14-35-3)28-24-26-13-19(25)23(29-24)18-12-27-32-9-5-4-6-20(18)32/h4-6,9,12-13,17H,7-8,10-11,14H2,1-3H3,(H,26,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 49 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of IGF1-induced human IGF1R autophosphorylation expressed in IGF-1R knock-out mouse fibroblasts |
J Med Chem 59: 4859-66 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00203
BindingDB Entry DOI: 10.7270/Q2KS6TG9 |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50184473

(CHEMBL3823929)Show SMILES CC(=O)N1CCC(CC1)n1nc(C)c(Nc2ncc(Cl)c(n2)-c2cnc3ccccn23)c1C Show InChI InChI=1S/C23H25ClN8O/c1-14-21(15(2)32(29-14)17-7-10-30(11-8-17)16(3)33)27-23-26-12-18(24)22(28-23)19-13-25-20-6-4-5-9-31(19)20/h4-6,9,12-13,17H,7-8,10-11H2,1-3H3,(H,26,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 49 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human IGF-1R using fluorescent labeled FL-KKSRGDYMTMQIG-CONH2 as substrate after 1 hr 50 mins |
J Med Chem 59: 4859-66 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00203
BindingDB Entry DOI: 10.7270/Q2KS6TG9 |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50184469

(CHEMBL3823278)Show SMILES CC(=O)N1CCC(CC1)n1cc(Nc2ncc(Cl)c(n2)-c2cnc3ccccn23)c(C)n1 Show InChI InChI=1S/C22H23ClN8O/c1-14-18(13-31(28-14)16-6-9-29(10-7-16)15(2)32)26-22-25-11-17(23)21(27-22)19-12-24-20-5-3-4-8-30(19)20/h3-5,8,11-13,16H,6-7,9-10H2,1-2H3,(H,25,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human IGF-1R using fluorescent labeled FL-KKSRGDYMTMQIG-CONH2 as substrate after 1 hr 50 mins |
J Med Chem 59: 4859-66 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00203
BindingDB Entry DOI: 10.7270/Q2KS6TG9 |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50184470

(CHEMBL3823007)Show SMILES Cc1nn(cc1Nc1ncc(Cl)c(n1)-c1cnc2ccccn12)C1CCNCC1 Show InChI InChI=1S/C20H21ClN8/c1-13-16(12-29(27-13)14-5-7-22-8-6-14)25-20-24-10-15(21)19(26-20)17-11-23-18-4-2-3-9-28(17)18/h2-4,9-12,14,22H,5-8H2,1H3,(H,24,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 54 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of IGF1-induced human IGF1R autophosphorylation expressed in IGF-1R knock-out mouse fibroblasts |
J Med Chem 59: 4859-66 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00203
BindingDB Entry DOI: 10.7270/Q2KS6TG9 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50184472

(CHEMBL3822543)Show SMILES Cc1c(Nc2ncc(Cl)c(n2)-c2cnc3ccccn23)cnn1C1CCNCC1 Show InChI InChI=1S/C20H21ClN8/c1-13-16(11-25-29(13)14-5-7-22-8-6-14)26-20-24-10-15(21)19(27-20)17-12-23-18-4-2-3-9-28(17)18/h2-4,9-12,14,22H,5-8H2,1H3,(H,24,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of IGF1-induced human IGF1R autophosphorylation expressed in IGF-1R knock-out mouse fibroblasts |
J Med Chem 59: 4859-66 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00203
BindingDB Entry DOI: 10.7270/Q2KS6TG9 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50184475

(CHEMBL3822989)Show SMILES CC(=O)N1CCC(CC1)n1cc(Nc2ncc(Cl)c(n2)-c2cnn3ccccc23)c(C)n1 Show InChI InChI=1S/C22H23ClN8O/c1-14-19(13-31(28-14)16-6-9-29(10-7-16)15(2)32)26-22-24-12-18(23)21(27-22)17-11-25-30-8-4-3-5-20(17)30/h3-5,8,11-13,16H,6-7,9-10H2,1-2H3,(H,24,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 63 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of CDK2 (unknown origin) |
J Med Chem 59: 4859-66 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00203
BindingDB Entry DOI: 10.7270/Q2KS6TG9 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 1
(Homo sapiens (Human)) | BDBM50184466

(CHEMBL3823659)Show SMILES CC(=O)N1CCC(CC1)n1cc(Nc2ncc(Cl)c(n2)-c2cnc3ccccn23)cn1 Show InChI InChI=1S/C21H21ClN8O/c1-14(31)28-8-5-16(6-9-28)30-13-15(10-25-30)26-21-24-11-17(22)20(27-21)18-12-23-19-4-2-3-7-29(18)19/h2-4,7,10-13,16H,5-6,8-9H2,1H3,(H,24,26,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 66 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of CDK1 (unknown origin) |
J Med Chem 59: 4859-66 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00203
BindingDB Entry DOI: 10.7270/Q2KS6TG9 |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50184468
(CHEMBL3822858)Show SMILES COc1nn(cc1Nc1ncc(Cl)c(n1)-c1cnc2ccccn12)C1CCN(CC1)C(C)=O Show InChI InChI=1S/C22H23ClN8O2/c1-14(32)29-9-6-15(7-10-29)31-13-17(21(28-31)33-2)26-22-25-11-16(23)20(27-22)18-12-24-19-5-3-4-8-30(18)19/h3-5,8,11-13,15H,6-7,9-10H2,1-2H3,(H,25,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 76 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human IGF-1R using fluorescent labeled FL-KKSRGDYMTMQIG-CONH2 as substrate after 1 hr 50 mins |
J Med Chem 59: 4859-66 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00203
BindingDB Entry DOI: 10.7270/Q2KS6TG9 |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50184454

(CHEMBL3822962)Show SMILES CCc1c(Nc2ncc(Cl)c(n2)-c2cnn3ccccc23)cnn1C1CCN(CC1)C(C)=O Show InChI InChI=1S/C23H25ClN8O/c1-3-20-19(14-27-32(20)16-7-10-30(11-8-16)15(2)33)28-23-25-13-18(24)22(29-23)17-12-26-31-9-5-4-6-21(17)31/h4-6,9,12-14,16H,3,7-8,10-11H2,1-2H3,(H,25,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 85 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of IGF1-induced human IGF1R autophosphorylation expressed in IGF-1R knock-out mouse fibroblasts |
J Med Chem 59: 4859-66 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00203
BindingDB Entry DOI: 10.7270/Q2KS6TG9 |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50184473

(CHEMBL3823929)Show SMILES CC(=O)N1CCC(CC1)n1nc(C)c(Nc2ncc(Cl)c(n2)-c2cnc3ccccn23)c1C Show InChI InChI=1S/C23H25ClN8O/c1-14-21(15(2)32(29-14)17-7-10-30(11-8-17)16(3)33)27-23-26-12-18(24)22(28-23)19-13-25-20-6-4-5-9-31(19)20/h4-6,9,12-13,17H,7-8,10-11H2,1-3H3,(H,26,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of IGF1-induced human IGF1R autophosphorylation expressed in IGF-1R knock-out mouse fibroblasts |
J Med Chem 59: 4859-66 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00203
BindingDB Entry DOI: 10.7270/Q2KS6TG9 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50184453

(CHEMBL3822619)Show SMILES CCc1nn(cc1Nc1ncc(Cl)c(n1)-c1cnn2ccccc12)C1CCN(CC1)C(C)=O Show InChI InChI=1S/C23H25ClN8O/c1-3-19-20(14-32(29-19)16-7-10-30(11-8-16)15(2)33)27-23-25-13-18(24)22(28-23)17-12-26-31-9-5-4-6-21(17)31/h4-6,9,12-14,16H,3,7-8,10-11H2,1-2H3,(H,25,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 113 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of CDK2 (unknown origin) |
J Med Chem 59: 4859-66 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00203
BindingDB Entry DOI: 10.7270/Q2KS6TG9 |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50184471

(CHEMBL3822583)Show SMILES CC(=O)N1CCC(CC1)n1ncc(Nc2ncc(Cl)c(n2)-c2cnc3ccccn23)c1C Show InChI InChI=1S/C22H23ClN8O/c1-14-18(12-26-31(14)16-6-9-29(10-7-16)15(2)32)27-22-25-11-17(23)21(28-22)19-13-24-20-5-3-4-8-30(19)20/h3-5,8,11-13,16H,6-7,9-10H2,1-2H3,(H,25,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 126 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of IGF1-induced human IGF1R autophosphorylation expressed in IGF-1R knock-out mouse fibroblasts |
J Med Chem 59: 4859-66 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00203
BindingDB Entry DOI: 10.7270/Q2KS6TG9 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50184469

(CHEMBL3823278)Show SMILES CC(=O)N1CCC(CC1)n1cc(Nc2ncc(Cl)c(n2)-c2cnc3ccccn23)c(C)n1 Show InChI InChI=1S/C22H23ClN8O/c1-14-18(13-31(28-14)16-6-9-29(10-7-16)15(2)32)26-22-25-11-17(23)21(27-22)19-12-24-20-5-3-4-8-30(19)20/h3-5,8,11-13,16H,6-7,9-10H2,1-2H3,(H,25,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 135 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of CDK2 (unknown origin) |
J Med Chem 59: 4859-66 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00203
BindingDB Entry DOI: 10.7270/Q2KS6TG9 |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50184466

(CHEMBL3823659)Show SMILES CC(=O)N1CCC(CC1)n1cc(Nc2ncc(Cl)c(n2)-c2cnc3ccccn23)cn1 Show InChI InChI=1S/C21H21ClN8O/c1-14(31)28-8-5-16(6-9-28)30-13-15(10-25-30)26-21-24-11-17(22)20(27-21)18-12-23-19-4-2-3-7-29(18)19/h2-4,7,10-13,16H,5-6,8-9H2,1H3,(H,24,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 181 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of IGF1-induced human IGF1R autophosphorylation expressed in IGF-1R knock-out mouse fibroblasts |
J Med Chem 59: 4859-66 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00203
BindingDB Entry DOI: 10.7270/Q2KS6TG9 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50184471

(CHEMBL3822583)Show SMILES CC(=O)N1CCC(CC1)n1ncc(Nc2ncc(Cl)c(n2)-c2cnc3ccccn23)c1C Show InChI InChI=1S/C22H23ClN8O/c1-14-18(12-26-31(14)16-6-9-29(10-7-16)15(2)32)27-22-25-11-17(23)21(28-22)19-13-24-20-5-3-4-8-30(19)20/h3-5,8,11-13,16H,6-7,9-10H2,1-2H3,(H,25,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 232 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of CDK2 (unknown origin) |
J Med Chem 59: 4859-66 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00203
BindingDB Entry DOI: 10.7270/Q2KS6TG9 |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50184468

(CHEMBL3822858)Show SMILES COc1nn(cc1Nc1ncc(Cl)c(n1)-c1cnc2ccccn12)C1CCN(CC1)C(C)=O Show InChI InChI=1S/C22H23ClN8O2/c1-14(32)29-9-6-15(7-10-29)31-13-17(21(28-31)33-2)26-22-25-11-16(23)20(27-22)18-12-24-19-5-3-4-8-30(18)19/h3-5,8,11-13,15H,6-7,9-10H2,1-2H3,(H,25,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 246 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of IGF1-induced human IGF1R autophosphorylation expressed in IGF-1R knock-out mouse fibroblasts |
J Med Chem 59: 4859-66 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00203
BindingDB Entry DOI: 10.7270/Q2KS6TG9 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50184476

(CHEMBL3823301)Show SMILES CC(=O)N1CCC(CC1)n1ncc(Nc2ncc(Cl)c(n2)-c2cnn3ccccc23)c1C Show InChI InChI=1S/C22H23ClN8O/c1-14-19(13-26-31(14)16-6-9-29(10-7-16)15(2)32)27-22-24-12-18(23)21(28-22)17-11-25-30-8-4-3-5-20(17)30/h3-5,8,11-13,16H,6-7,9-10H2,1-2H3,(H,24,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 254 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of CDK2 (unknown origin) |
J Med Chem 59: 4859-66 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00203
BindingDB Entry DOI: 10.7270/Q2KS6TG9 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 7
(Homo sapiens (Human)) | BDBM50184461

(CHEMBL3823030)Show SMILES C[C@@H](O)C(=O)N1CCC(CC1)n1nc(C)c(Nc2ncc(Cl)c(n2)-c2cnn3ccccc23)c1C |r| Show InChI InChI=1S/C24H27ClN8O2/c1-14-21(15(2)33(30-14)17-7-10-31(11-8-17)23(35)16(3)34)28-24-26-13-19(25)22(29-24)18-12-27-32-9-5-4-6-20(18)32/h4-6,9,12-13,16-17,34H,7-8,10-11H2,1-3H3,(H,26,28,29)/t16-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of CDK7 (unknown origin) |
J Med Chem 59: 4859-66 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00203
BindingDB Entry DOI: 10.7270/Q2KS6TG9 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50184463

(CHEMBL3824103)Show SMILES C[C@H](N)C(=O)N1CCC(CC1)n1nc(C)c(Nc2ncc(Cl)c(n2)-c2cnn3ccccc23)c1C |r| Show InChI InChI=1S/C24H28ClN9O/c1-14(26)23(35)32-10-7-17(8-11-32)34-16(3)21(15(2)31-34)29-24-27-13-19(25)22(30-24)18-12-28-33-9-5-4-6-20(18)33/h4-6,9,12-14,17H,7-8,10-11,26H2,1-3H3,(H,27,29,30)/t14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
J Med Chem 59: 4859-66 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00203
BindingDB Entry DOI: 10.7270/Q2KS6TG9 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50184474

(CHEMBL3823522)Show SMILES COc1nn(cc1Nc1ncc(Cl)c(n1)-c1cnn2ccccc12)C1CCN(CC1)C(C)=O Show InChI InChI=1S/C22H23ClN8O2/c1-14(32)29-9-6-15(7-10-29)31-13-18(21(28-31)33-2)26-22-24-12-17(23)20(27-22)16-11-25-30-8-4-3-5-19(16)30/h3-5,8,11-13,15H,6-7,9-10H2,1-2H3,(H,24,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 361 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of CDK2 (unknown origin) |
J Med Chem 59: 4859-66 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00203
BindingDB Entry DOI: 10.7270/Q2KS6TG9 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50184470

(CHEMBL3823007)Show SMILES Cc1nn(cc1Nc1ncc(Cl)c(n1)-c1cnc2ccccn12)C1CCNCC1 Show InChI InChI=1S/C20H21ClN8/c1-13-16(12-29(27-13)14-5-7-22-8-6-14)25-20-24-10-15(21)19(26-20)17-11-23-18-4-2-3-9-28(17)18/h2-4,9-12,14,22H,5-8H2,1H3,(H,24,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 392 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of CDK2 (unknown origin) |
J Med Chem 59: 4859-66 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00203
BindingDB Entry DOI: 10.7270/Q2KS6TG9 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50184472

(CHEMBL3822543)Show SMILES Cc1c(Nc2ncc(Cl)c(n2)-c2cnc3ccccn23)cnn1C1CCNCC1 Show InChI InChI=1S/C20H21ClN8/c1-13-16(11-25-29(13)14-5-7-22-8-6-14)26-20-24-10-15(21)19(27-20)17-12-23-18-4-2-3-9-28(17)18/h2-4,9-12,14,22H,5-8H2,1H3,(H,24,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 392 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of CDK2 (unknown origin) |
J Med Chem 59: 4859-66 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00203
BindingDB Entry DOI: 10.7270/Q2KS6TG9 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 1
(Homo sapiens (Human)) | BDBM50184461

(CHEMBL3823030)Show SMILES C[C@@H](O)C(=O)N1CCC(CC1)n1nc(C)c(Nc2ncc(Cl)c(n2)-c2cnn3ccccc23)c1C |r| Show InChI InChI=1S/C24H27ClN8O2/c1-14-21(15(2)33(30-14)17-7-10-31(11-8-17)23(35)16(3)34)28-24-26-13-19(25)22(29-24)18-12-27-32-9-5-4-6-20(18)32/h4-6,9,12-13,16-17,34H,7-8,10-11H2,1-3H3,(H,26,28,29)/t16-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 430 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of CDK1 (unknown origin) |
J Med Chem 59: 4859-66 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00203
BindingDB Entry DOI: 10.7270/Q2KS6TG9 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 9
(Homo sapiens (Human)) | BDBM50184461

(CHEMBL3823030)Show SMILES C[C@@H](O)C(=O)N1CCC(CC1)n1nc(C)c(Nc2ncc(Cl)c(n2)-c2cnn3ccccc23)c1C |r| Show InChI InChI=1S/C24H27ClN8O2/c1-14-21(15(2)33(30-14)17-7-10-31(11-8-17)23(35)16(3)34)28-24-26-13-19(25)22(29-24)18-12-27-32-9-5-4-6-20(18)32/h4-6,9,12-13,16-17,34H,7-8,10-11H2,1-3H3,(H,26,28,29)/t16-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 450 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of CDK9 (unknown origin) |
J Med Chem 59: 4859-66 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00203
BindingDB Entry DOI: 10.7270/Q2KS6TG9 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50184468

(CHEMBL3822858)Show SMILES COc1nn(cc1Nc1ncc(Cl)c(n1)-c1cnc2ccccn12)C1CCN(CC1)C(C)=O Show InChI InChI=1S/C22H23ClN8O2/c1-14(32)29-9-6-15(7-10-29)31-13-17(21(28-31)33-2)26-22-25-11-16(23)20(27-22)18-12-24-19-5-3-4-8-30(18)19/h3-5,8,11-13,15H,6-7,9-10H2,1-2H3,(H,25,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 494 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of CDK2 (unknown origin) |
J Med Chem 59: 4859-66 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00203
BindingDB Entry DOI: 10.7270/Q2KS6TG9 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50184462

(CHEMBL3823154)Show SMILES Cc1nn(C2CCN(CC2)C(=O)CN)c(C)c1Nc1ncc(Cl)c(n1)-c1cnn2ccccc12 Show InChI InChI=1S/C23H26ClN9O/c1-14-21(15(2)33(30-14)16-6-9-31(10-7-16)20(34)11-25)28-23-26-13-18(24)22(29-23)17-12-27-32-8-4-3-5-19(17)32/h3-5,8,12-13,16H,6-7,9-11,25H2,1-2H3,(H,26,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
J Med Chem 59: 4859-66 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00203
BindingDB Entry DOI: 10.7270/Q2KS6TG9 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50184452

(CHEMBL3822943)Show SMILES CCOc1nn(cc1Nc1ncc(Cl)c(n1)-c1cnn2ccccc12)C1CCN(CC1)C(C)=O Show InChI InChI=1S/C23H25ClN8O2/c1-3-34-22-19(14-32(29-22)16-7-10-30(11-8-16)15(2)33)27-23-25-13-18(24)21(28-23)17-12-26-31-9-5-4-6-20(17)31/h4-6,9,12-14,16H,3,7-8,10-11H2,1-2H3,(H,25,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 545 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of CDK2 (unknown origin) |
J Med Chem 59: 4859-66 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00203
BindingDB Entry DOI: 10.7270/Q2KS6TG9 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50184461

(CHEMBL3823030)Show SMILES C[C@@H](O)C(=O)N1CCC(CC1)n1nc(C)c(Nc2ncc(Cl)c(n2)-c2cnn3ccccc23)c1C |r| Show InChI InChI=1S/C24H27ClN8O2/c1-14-21(15(2)33(30-14)17-7-10-31(11-8-17)23(35)16(3)34)28-24-26-13-19(25)22(29-24)18-12-27-32-9-5-4-6-20(18)32/h4-6,9,12-13,16-17,34H,7-8,10-11H2,1-3H3,(H,26,28,29)/t16-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERK2 (2 to 360 residues) |
J Med Chem 59: 4859-66 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00203
BindingDB Entry DOI: 10.7270/Q2KS6TG9 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50184461

(CHEMBL3823030)Show SMILES C[C@@H](O)C(=O)N1CCC(CC1)n1nc(C)c(Nc2ncc(Cl)c(n2)-c2cnn3ccccc23)c1C |r| Show InChI InChI=1S/C24H27ClN8O2/c1-14-21(15(2)33(30-14)17-7-10-31(11-8-17)23(35)16(3)34)28-24-26-13-19(25)22(29-24)18-12-27-32-9-5-4-6-20(18)32/h4-6,9,12-13,16-17,34H,7-8,10-11H2,1-3H3,(H,26,28,29)/t16-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of CDK2 (unknown origin) |
J Med Chem 59: 4859-66 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00203
BindingDB Entry DOI: 10.7270/Q2KS6TG9 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 8
(Homo sapiens (Human)) | BDBM50184461

(CHEMBL3823030)Show SMILES C[C@@H](O)C(=O)N1CCC(CC1)n1nc(C)c(Nc2ncc(Cl)c(n2)-c2cnn3ccccc23)c1C |r| Show InChI InChI=1S/C24H27ClN8O2/c1-14-21(15(2)33(30-14)17-7-10-31(11-8-17)23(35)16(3)34)28-24-26-13-19(25)22(29-24)18-12-27-32-9-5-4-6-20(18)32/h4-6,9,12-13,16-17,34H,7-8,10-11H2,1-3H3,(H,26,28,29)/t16-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 810 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of CDK8 (unknown origin) |
J Med Chem 59: 4859-66 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00203
BindingDB Entry DOI: 10.7270/Q2KS6TG9 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50184454

(CHEMBL3822962)Show SMILES CCc1c(Nc2ncc(Cl)c(n2)-c2cnn3ccccc23)cnn1C1CCN(CC1)C(C)=O Show InChI InChI=1S/C23H25ClN8O/c1-3-20-19(14-27-32(20)16-7-10-30(11-8-16)15(2)33)28-23-25-13-18(24)22(29-23)17-12-26-31-9-5-4-6-21(17)31/h4-6,9,12-14,16H,3,7-8,10-11H2,1-2H3,(H,25,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 832 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of CDK2 (unknown origin) |
J Med Chem 59: 4859-66 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00203
BindingDB Entry DOI: 10.7270/Q2KS6TG9 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50184467

(CHEMBL3823297)Show SMILES COc1cc(ccc1Nc1ncc(Cl)c(n1)-c1cnn2ccccc12)N1CCN(CC1)C(C)=O Show InChI InChI=1S/C24H24ClN7O2/c1-16(33)30-9-11-31(12-10-30)17-6-7-20(22(13-17)34-2)28-24-26-15-19(25)23(29-24)18-14-27-32-8-4-3-5-21(18)32/h3-8,13-15H,9-12H2,1-2H3,(H,26,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
J Med Chem 59: 4859-66 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00203
BindingDB Entry DOI: 10.7270/Q2KS6TG9 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50184464

(CHEMBL3822644)Show SMILES C[C@@H](N)C(=O)N1CCC(CC1)n1nc(C)c(Nc2ncc(Cl)c(n2)-c2cnn3ccccc23)c1C |r| Show InChI InChI=1S/C24H28ClN9O/c1-14(26)23(35)32-10-7-17(8-11-32)34-16(3)21(15(2)31-34)29-24-27-13-19(25)22(30-24)18-12-28-33-9-5-4-6-20(18)33/h4-6,9,12-14,17H,7-8,10-11,26H2,1-3H3,(H,27,29,30)/t14-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
J Med Chem 59: 4859-66 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00203
BindingDB Entry DOI: 10.7270/Q2KS6TG9 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50184467

(CHEMBL3823297)Show SMILES COc1cc(ccc1Nc1ncc(Cl)c(n1)-c1cnn2ccccc12)N1CCN(CC1)C(C)=O Show InChI InChI=1S/C24H24ClN7O2/c1-16(33)30-9-11-31(12-10-30)17-6-7-20(22(13-17)34-2)28-24-26-15-19(25)23(29-24)18-14-27-32-8-4-3-5-21(18)32/h3-8,13-15H,9-12H2,1-2H3,(H,26,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.03E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of CDK2 (unknown origin) |
J Med Chem 59: 4859-66 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00203
BindingDB Entry DOI: 10.7270/Q2KS6TG9 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50184451

(CHEMBL3823914)Show SMILES CC(=O)N1CCC(CC1)n1nc(C)c(Nc2ncc(Cl)c(n2)-c2cnn3ccccc23)c1C Show InChI InChI=1S/C23H25ClN8O/c1-14-21(15(2)32(29-14)17-7-10-30(11-8-17)16(3)33)27-23-25-13-19(24)22(28-23)18-12-26-31-9-5-4-6-20(18)31/h4-6,9,12-13,17H,7-8,10-11H2,1-3H3,(H,25,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.38E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of CDK2 (unknown origin) |
J Med Chem 59: 4859-66 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00203
BindingDB Entry DOI: 10.7270/Q2KS6TG9 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50184450

(CHEMBL3824296)Show SMILES Cc1nn(C2CCNCC2)c(C)c1Nc1ncc(Cl)c(n1)-c1cnn2ccccc12 Show InChI InChI=1S/C21H23ClN8/c1-13-19(14(2)30(28-13)15-6-8-23-9-7-15)26-21-24-12-17(22)20(27-21)16-11-25-29-10-4-3-5-18(16)29/h3-5,10-12,15,23H,6-9H2,1-2H3,(H,24,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.52E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of CDK2 (unknown origin) |
J Med Chem 59: 4859-66 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00203
BindingDB Entry DOI: 10.7270/Q2KS6TG9 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50184473

(CHEMBL3823929)Show SMILES CC(=O)N1CCC(CC1)n1nc(C)c(Nc2ncc(Cl)c(n2)-c2cnc3ccccn23)c1C Show InChI InChI=1S/C23H25ClN8O/c1-14-21(15(2)32(29-14)17-7-10-30(11-8-17)16(3)33)27-23-26-12-18(24)22(28-23)19-13-25-20-6-4-5-9-31(19)20/h4-6,9,12-13,17H,7-8,10-11H2,1-3H3,(H,26,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.68E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of CDK2 (unknown origin) |
J Med Chem 59: 4859-66 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00203
BindingDB Entry DOI: 10.7270/Q2KS6TG9 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50184465

(CHEMBL3822976)Show SMILES CNCC(=O)N1CCC(CC1)n1nc(C)c(Nc2ncc(Cl)c(n2)-c2cnn3ccccc23)c1C Show InChI InChI=1S/C24H28ClN9O/c1-15-22(16(2)34(31-15)17-7-10-32(11-8-17)21(35)14-26-3)29-24-27-13-19(25)23(30-24)18-12-28-33-9-5-4-6-20(18)33/h4-6,9,12-13,17,26H,7-8,10-11,14H2,1-3H3,(H,27,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
J Med Chem 59: 4859-66 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00203
BindingDB Entry DOI: 10.7270/Q2KS6TG9 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50184450

(CHEMBL3824296)Show SMILES Cc1nn(C2CCNCC2)c(C)c1Nc1ncc(Cl)c(n1)-c1cnn2ccccc12 Show InChI InChI=1S/C21H23ClN8/c1-13-19(14(2)30(28-13)15-6-8-23-9-7-15)26-21-24-12-17(22)20(27-21)16-11-25-29-10-4-3-5-18(16)29/h3-5,10-12,15,23H,6-9H2,1-2H3,(H,24,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
J Med Chem 59: 4859-66 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00203
BindingDB Entry DOI: 10.7270/Q2KS6TG9 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50184453

(CHEMBL3822619)Show SMILES CCc1nn(cc1Nc1ncc(Cl)c(n1)-c1cnn2ccccc12)C1CCN(CC1)C(C)=O Show InChI InChI=1S/C23H25ClN8O/c1-3-19-20(14-32(29-19)16-7-10-30(11-8-16)15(2)33)27-23-25-13-18(24)22(28-23)17-12-26-31-9-5-4-6-21(17)31/h4-6,9,12-14,16H,3,7-8,10-11H2,1-2H3,(H,25,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG expressed in CHOK1 cells after 6 mins by electrophysiology assay |
J Med Chem 59: 4859-66 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00203
BindingDB Entry DOI: 10.7270/Q2KS6TG9 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50184469

(CHEMBL3823278)Show SMILES CC(=O)N1CCC(CC1)n1cc(Nc2ncc(Cl)c(n2)-c2cnc3ccccn23)c(C)n1 Show InChI InChI=1S/C22H23ClN8O/c1-14-18(13-31(28-14)16-6-9-29(10-7-16)15(2)32)26-22-25-11-17(23)21(27-22)19-12-24-20-5-3-4-8-30(19)20/h3-5,8,11-13,16H,6-7,9-10H2,1-2H3,(H,25,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG expressed in CHOK1 cells after 6 mins by electrophysiology assay |
J Med Chem 59: 4859-66 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00203
BindingDB Entry DOI: 10.7270/Q2KS6TG9 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50184466

(CHEMBL3823659)Show SMILES CC(=O)N1CCC(CC1)n1cc(Nc2ncc(Cl)c(n2)-c2cnc3ccccn23)cn1 Show InChI InChI=1S/C21H21ClN8O/c1-14(31)28-8-5-16(6-9-28)30-13-15(10-25-30)26-21-24-11-17(22)20(27-21)18-12-23-19-4-2-3-7-29(18)19/h2-4,7,10-13,16H,5-6,8-9H2,1H3,(H,24,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG expressed in CHOK1 cells after 6 mins by electrophysiology assay |
J Med Chem 59: 4859-66 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00203
BindingDB Entry DOI: 10.7270/Q2KS6TG9 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50184452

(CHEMBL3822943)Show SMILES CCOc1nn(cc1Nc1ncc(Cl)c(n1)-c1cnn2ccccc12)C1CCN(CC1)C(C)=O Show InChI InChI=1S/C23H25ClN8O2/c1-3-34-22-19(14-32(29-22)16-7-10-30(11-8-16)15(2)33)27-23-25-13-18(24)21(28-23)17-12-26-31-9-5-4-6-20(17)31/h4-6,9,12-14,16H,3,7-8,10-11H2,1-2H3,(H,25,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG expressed in CHOK1 cells after 6 mins by electrophysiology assay |
J Med Chem 59: 4859-66 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00203
BindingDB Entry DOI: 10.7270/Q2KS6TG9 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50184468

(CHEMBL3822858)Show SMILES COc1nn(cc1Nc1ncc(Cl)c(n1)-c1cnc2ccccn12)C1CCN(CC1)C(C)=O Show InChI InChI=1S/C22H23ClN8O2/c1-14(32)29-9-6-15(7-10-29)31-13-17(21(28-31)33-2)26-22-25-11-16(23)20(27-22)18-12-24-19-5-3-4-8-30(18)19/h3-5,8,11-13,15H,6-7,9-10H2,1-2H3,(H,25,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG expressed in CHOK1 cells after 6 mins by electrophysiology assay |
J Med Chem 59: 4859-66 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00203
BindingDB Entry DOI: 10.7270/Q2KS6TG9 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50184475

(CHEMBL3822989)Show SMILES CC(=O)N1CCC(CC1)n1cc(Nc2ncc(Cl)c(n2)-c2cnn3ccccc23)c(C)n1 Show InChI InChI=1S/C22H23ClN8O/c1-14-19(13-31(28-14)16-6-9-29(10-7-16)15(2)32)26-22-24-12-18(23)21(27-22)17-11-25-30-8-4-3-5-20(17)30/h3-5,8,11-13,16H,6-7,9-10H2,1-2H3,(H,24,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG expressed in CHOK1 cells after 6 mins by electrophysiology assay |
J Med Chem 59: 4859-66 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00203
BindingDB Entry DOI: 10.7270/Q2KS6TG9 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50184457

(CHEMBL3823095)Show SMILES CN(C)C(=O)CN1CCC(CC1)n1nc(C)c(Nc2ncc(Cl)c(n2)-c2cnn3ccccc23)c1C Show InChI InChI=1S/C25H30ClN9O/c1-16-23(17(2)35(31-16)18-8-11-33(12-9-18)15-22(36)32(3)4)29-25-27-14-20(26)24(30-25)19-13-28-34-10-6-5-7-21(19)34/h5-7,10,13-14,18H,8-9,11-12,15H2,1-4H3,(H,27,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG expressed in CHOK1 cells after 6 mins by electrophysiology assay |
J Med Chem 59: 4859-66 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00203
BindingDB Entry DOI: 10.7270/Q2KS6TG9 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50184455

(CHEMBL3824323)Show SMILES Cc1nn(C2CCN(CCO)CC2)c(C)c1Nc1ncc(Cl)c(n1)-c1cnn2ccccc12 Show InChI InChI=1S/C23H27ClN8O/c1-15-21(16(2)32(29-15)17-6-9-30(10-7-17)11-12-33)27-23-25-14-19(24)22(28-23)18-13-26-31-8-4-3-5-20(18)31/h3-5,8,13-14,17,33H,6-7,9-12H2,1-2H3,(H,25,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG expressed in CHOK1 cells after 6 mins by electrophysiology assay |
J Med Chem 59: 4859-66 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00203
BindingDB Entry DOI: 10.7270/Q2KS6TG9 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50184457

(CHEMBL3823095)Show SMILES CN(C)C(=O)CN1CCC(CC1)n1nc(C)c(Nc2ncc(Cl)c(n2)-c2cnn3ccccc23)c1C Show InChI InChI=1S/C25H30ClN9O/c1-16-23(17(2)35(31-16)18-8-11-33(12-9-18)15-22(36)32(3)4)29-25-27-14-20(26)24(30-25)19-13-28-34-10-6-5-7-21(19)34/h5-7,10,13-14,18H,8-9,11-12,15H2,1-4H3,(H,27,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
J Med Chem 59: 4859-66 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00203
BindingDB Entry DOI: 10.7270/Q2KS6TG9 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50184456

(CHEMBL3823962)Show SMILES CNC(=O)CN1CCC(CC1)n1nc(C)c(Nc2ncc(Cl)c(n2)-c2cnn3ccccc23)c1C Show InChI InChI=1S/C24H28ClN9O/c1-15-22(16(2)34(31-15)17-7-10-32(11-8-17)14-21(35)26-3)29-24-27-13-19(25)23(30-24)18-12-28-33-9-5-4-6-20(18)33/h4-6,9,12-13,17H,7-8,10-11,14H2,1-3H3,(H,26,35)(H,27,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
J Med Chem 59: 4859-66 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00203
BindingDB Entry DOI: 10.7270/Q2KS6TG9 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50184455

(CHEMBL3824323)Show SMILES Cc1nn(C2CCN(CCO)CC2)c(C)c1Nc1ncc(Cl)c(n1)-c1cnn2ccccc12 Show InChI InChI=1S/C23H27ClN8O/c1-15-21(16(2)32(29-15)17-6-9-30(10-7-17)11-12-33)27-23-25-14-19(24)22(28-23)18-13-26-31-8-4-3-5-20(18)31/h3-5,8,13-14,17,33H,6-7,9-12H2,1-2H3,(H,25,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
J Med Chem 59: 4859-66 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00203
BindingDB Entry DOI: 10.7270/Q2KS6TG9 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50184458

(CHEMBL3822587)Show SMILES Cc1nn(C2CCN(CC2)C(=O)CO)c(C)c1Nc1ncc(Cl)c(n1)-c1cnn2ccccc12 Show InChI InChI=1S/C23H25ClN8O2/c1-14-21(15(2)32(29-14)16-6-9-30(10-7-16)20(34)13-33)27-23-25-12-18(24)22(28-23)17-11-26-31-8-4-3-5-19(17)31/h3-5,8,11-12,16,33H,6-7,9-10,13H2,1-2H3,(H,25,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
J Med Chem 59: 4859-66 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00203
BindingDB Entry DOI: 10.7270/Q2KS6TG9 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50184451

(CHEMBL3823914)Show SMILES CC(=O)N1CCC(CC1)n1nc(C)c(Nc2ncc(Cl)c(n2)-c2cnn3ccccc23)c1C Show InChI InChI=1S/C23H25ClN8O/c1-14-21(15(2)32(29-14)17-7-10-30(11-8-17)16(3)33)27-23-25-13-19(24)22(28-23)18-12-26-31-9-5-4-6-20(18)31/h4-6,9,12-13,17H,7-8,10-11H2,1-3H3,(H,25,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
J Med Chem 59: 4859-66 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00203
BindingDB Entry DOI: 10.7270/Q2KS6TG9 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50184474

(CHEMBL3823522)Show SMILES COc1nn(cc1Nc1ncc(Cl)c(n1)-c1cnn2ccccc12)C1CCN(CC1)C(C)=O Show InChI InChI=1S/C22H23ClN8O2/c1-14(32)29-9-6-15(7-10-29)31-13-18(21(28-31)33-2)26-22-24-12-17(23)20(27-22)16-11-25-30-8-4-3-5-19(16)30/h3-5,8,11-13,15H,6-7,9-10H2,1-2H3,(H,24,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG expressed in CHOK1 cells after 6 mins by electrophysiology assay |
J Med Chem 59: 4859-66 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00203
BindingDB Entry DOI: 10.7270/Q2KS6TG9 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50184460

(CHEMBL3822947)Show SMILES C[C@H](O)C(=O)N1CCC(CC1)n1nc(C)c(Nc2ncc(Cl)c(n2)-c2cnn3ccccc23)c1C |r| Show InChI InChI=1S/C24H27ClN8O2/c1-14-21(15(2)33(30-14)17-7-10-31(11-8-17)23(35)16(3)34)28-24-26-13-19(25)22(29-24)18-12-27-32-9-5-4-6-20(18)32/h4-6,9,12-13,16-17,34H,7-8,10-11H2,1-3H3,(H,26,28,29)/t16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
J Med Chem 59: 4859-66 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00203
BindingDB Entry DOI: 10.7270/Q2KS6TG9 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50184459

(CHEMBL3822651)Show SMILES COCC(=O)N1CCC(CC1)n1nc(C)c(Nc2ncc(Cl)c(n2)-c2cnn3ccccc23)c1C Show InChI InChI=1S/C24H27ClN8O2/c1-15-22(16(2)33(30-15)17-7-10-31(11-8-17)21(34)14-35-3)28-24-26-13-19(25)23(29-24)18-12-27-32-9-5-4-6-20(18)32/h4-6,9,12-13,17H,7-8,10-11,14H2,1-3H3,(H,26,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
J Med Chem 59: 4859-66 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00203
BindingDB Entry DOI: 10.7270/Q2KS6TG9 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50184456

(CHEMBL3823962)Show SMILES CNC(=O)CN1CCC(CC1)n1nc(C)c(Nc2ncc(Cl)c(n2)-c2cnn3ccccc23)c1C Show InChI InChI=1S/C24H28ClN9O/c1-15-22(16(2)34(31-15)17-7-10-32(11-8-17)14-21(35)26-3)29-24-27-13-19(25)23(30-24)18-12-28-33-9-5-4-6-20(18)33/h4-6,9,12-13,17H,7-8,10-11,14H2,1-3H3,(H,26,35)(H,27,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG expressed in CHOK1 cells after 6 mins by electrophysiology assay |
J Med Chem 59: 4859-66 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00203
BindingDB Entry DOI: 10.7270/Q2KS6TG9 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50184454

(CHEMBL3822962)Show SMILES CCc1c(Nc2ncc(Cl)c(n2)-c2cnn3ccccc23)cnn1C1CCN(CC1)C(C)=O Show InChI InChI=1S/C23H25ClN8O/c1-3-20-19(14-27-32(20)16-7-10-30(11-8-16)15(2)33)28-23-25-13-18(24)22(29-23)17-12-26-31-9-5-4-6-21(17)31/h4-6,9,12-14,16H,3,7-8,10-11H2,1-2H3,(H,25,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG expressed in CHOK1 cells after 6 mins by electrophysiology assay |
J Med Chem 59: 4859-66 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00203
BindingDB Entry DOI: 10.7270/Q2KS6TG9 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50184459

(CHEMBL3822651)Show SMILES COCC(=O)N1CCC(CC1)n1nc(C)c(Nc2ncc(Cl)c(n2)-c2cnn3ccccc23)c1C Show InChI InChI=1S/C24H27ClN8O2/c1-15-22(16(2)33(30-15)17-7-10-31(11-8-17)21(34)14-35-3)28-24-26-13-19(25)23(29-24)18-12-27-32-9-5-4-6-20(18)32/h4-6,9,12-13,17H,7-8,10-11,14H2,1-3H3,(H,26,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG expressed in CHOK1 cells after 6 mins by electrophysiology assay |
J Med Chem 59: 4859-66 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00203
BindingDB Entry DOI: 10.7270/Q2KS6TG9 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50184471

(CHEMBL3822583)Show SMILES CC(=O)N1CCC(CC1)n1ncc(Nc2ncc(Cl)c(n2)-c2cnc3ccccn23)c1C Show InChI InChI=1S/C22H23ClN8O/c1-14-18(12-26-31(14)16-6-9-29(10-7-16)15(2)32)27-22-25-11-17(23)21(28-22)19-13-24-20-5-3-4-8-30(19)20/h3-5,8,11-13,16H,6-7,9-10H2,1-2H3,(H,25,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG expressed in CHOK1 cells after 6 mins by electrophysiology assay |
J Med Chem 59: 4859-66 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00203
BindingDB Entry DOI: 10.7270/Q2KS6TG9 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50184461

(CHEMBL3823030)Show SMILES C[C@@H](O)C(=O)N1CCC(CC1)n1nc(C)c(Nc2ncc(Cl)c(n2)-c2cnn3ccccc23)c1C |r| Show InChI InChI=1S/C24H27ClN8O2/c1-14-21(15(2)33(30-14)17-7-10-31(11-8-17)23(35)16(3)34)28-24-26-13-19(25)22(29-24)18-12-27-32-9-5-4-6-20(18)32/h4-6,9,12-13,16-17,34H,7-8,10-11H2,1-3H3,(H,26,28,29)/t16-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
J Med Chem 59: 4859-66 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00203
BindingDB Entry DOI: 10.7270/Q2KS6TG9 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50184458

(CHEMBL3822587)Show SMILES Cc1nn(C2CCN(CC2)C(=O)CO)c(C)c1Nc1ncc(Cl)c(n1)-c1cnn2ccccc12 Show InChI InChI=1S/C23H25ClN8O2/c1-14-21(15(2)32(29-14)16-6-9-30(10-7-16)20(34)13-33)27-23-25-12-18(24)22(28-23)17-11-26-31-8-4-3-5-19(17)31/h3-5,8,11-12,16,33H,6-7,9-10,13H2,1-2H3,(H,25,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG expressed in CHOK1 cells after 6 mins by electrophysiology assay |
J Med Chem 59: 4859-66 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00203
BindingDB Entry DOI: 10.7270/Q2KS6TG9 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50184451

(CHEMBL3823914)Show SMILES CC(=O)N1CCC(CC1)n1nc(C)c(Nc2ncc(Cl)c(n2)-c2cnn3ccccc23)c1C Show InChI InChI=1S/C23H25ClN8O/c1-14-21(15(2)32(29-14)17-7-10-30(11-8-17)16(3)33)27-23-25-13-19(24)22(28-23)18-12-26-31-9-5-4-6-20(18)31/h4-6,9,12-13,17H,7-8,10-11H2,1-3H3,(H,25,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG expressed in CHOK1 cells after 6 mins by electrophysiology assay |
J Med Chem 59: 4859-66 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00203
BindingDB Entry DOI: 10.7270/Q2KS6TG9 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50184460

(CHEMBL3822947)Show SMILES C[C@H](O)C(=O)N1CCC(CC1)n1nc(C)c(Nc2ncc(Cl)c(n2)-c2cnn3ccccc23)c1C |r| Show InChI InChI=1S/C24H27ClN8O2/c1-14-21(15(2)33(30-14)17-7-10-31(11-8-17)23(35)16(3)34)28-24-26-13-19(25)22(29-24)18-12-27-32-9-5-4-6-20(18)32/h4-6,9,12-13,16-17,34H,7-8,10-11H2,1-3H3,(H,26,28,29)/t16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG expressed in CHOK1 cells after 6 mins by electrophysiology assay |
J Med Chem 59: 4859-66 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00203
BindingDB Entry DOI: 10.7270/Q2KS6TG9 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50184450

(CHEMBL3824296)Show SMILES Cc1nn(C2CCNCC2)c(C)c1Nc1ncc(Cl)c(n1)-c1cnn2ccccc12 Show InChI InChI=1S/C21H23ClN8/c1-13-19(14(2)30(28-13)15-6-8-23-9-7-15)26-21-24-12-17(22)20(27-21)16-11-25-29-10-4-3-5-18(16)29/h3-5,10-12,15,23H,6-9H2,1-2H3,(H,24,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG expressed in CHOK1 cells after 6 mins by electrophysiology assay |
J Med Chem 59: 4859-66 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00203
BindingDB Entry DOI: 10.7270/Q2KS6TG9 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50184473

(CHEMBL3823929)Show SMILES CC(=O)N1CCC(CC1)n1nc(C)c(Nc2ncc(Cl)c(n2)-c2cnc3ccccn23)c1C Show InChI InChI=1S/C23H25ClN8O/c1-14-21(15(2)32(29-14)17-7-10-30(11-8-17)16(3)33)27-23-26-12-18(24)22(28-23)19-13-25-20-6-4-5-9-31(19)20/h4-6,9,12-13,17H,7-8,10-11H2,1-3H3,(H,26,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG expressed in CHOK1 cells after 6 mins by electrophysiology assay |
J Med Chem 59: 4859-66 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00203
BindingDB Entry DOI: 10.7270/Q2KS6TG9 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50184461

(CHEMBL3823030)Show SMILES C[C@@H](O)C(=O)N1CCC(CC1)n1nc(C)c(Nc2ncc(Cl)c(n2)-c2cnn3ccccc23)c1C |r| Show InChI InChI=1S/C24H27ClN8O2/c1-14-21(15(2)33(30-14)17-7-10-31(11-8-17)23(35)16(3)34)28-24-26-13-19(25)22(29-24)18-12-27-32-9-5-4-6-20(18)32/h4-6,9,12-13,16-17,34H,7-8,10-11H2,1-3H3,(H,26,28,29)/t16-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG expressed in CHOK1 cells after 6 mins by electrophysiology assay |
J Med Chem 59: 4859-66 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00203
BindingDB Entry DOI: 10.7270/Q2KS6TG9 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50184470

(CHEMBL3823007)Show SMILES Cc1nn(cc1Nc1ncc(Cl)c(n1)-c1cnc2ccccn12)C1CCNCC1 Show InChI InChI=1S/C20H21ClN8/c1-13-16(12-29(27-13)14-5-7-22-8-6-14)25-20-24-10-15(21)19(26-20)17-11-23-18-4-2-3-9-28(17)18/h2-4,9-12,14,22H,5-8H2,1H3,(H,24,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG expressed in CHOK1 cells after 6 mins by electrophysiology assay |
J Med Chem 59: 4859-66 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00203
BindingDB Entry DOI: 10.7270/Q2KS6TG9 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50184472

(CHEMBL3822543)Show SMILES Cc1c(Nc2ncc(Cl)c(n2)-c2cnc3ccccn23)cnn1C1CCNCC1 Show InChI InChI=1S/C20H21ClN8/c1-13-16(11-25-29(13)14-5-7-22-8-6-14)26-20-24-10-15(21)19(27-20)17-12-23-18-4-2-3-9-28(17)18/h2-4,9-12,14,22H,5-8H2,1H3,(H,24,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG expressed in CHOK1 cells after 6 mins by electrophysiology assay |
J Med Chem 59: 4859-66 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00203
BindingDB Entry DOI: 10.7270/Q2KS6TG9 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50184465

(CHEMBL3822976)Show SMILES CNCC(=O)N1CCC(CC1)n1nc(C)c(Nc2ncc(Cl)c(n2)-c2cnn3ccccc23)c1C Show InChI InChI=1S/C24H28ClN9O/c1-15-22(16(2)34(31-15)17-7-10-32(11-8-17)21(35)14-26-3)29-24-27-13-19(25)23(30-24)18-12-28-33-9-5-4-6-20(18)33/h4-6,9,12-13,17,26H,7-8,10-11,14H2,1-3H3,(H,27,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG expressed in CHOK1 cells after 6 mins by electrophysiology assay |
J Med Chem 59: 4859-66 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00203
BindingDB Entry DOI: 10.7270/Q2KS6TG9 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50184463

(CHEMBL3824103)Show SMILES C[C@H](N)C(=O)N1CCC(CC1)n1nc(C)c(Nc2ncc(Cl)c(n2)-c2cnn3ccccc23)c1C |r| Show InChI InChI=1S/C24H28ClN9O/c1-14(26)23(35)32-10-7-17(8-11-32)34-16(3)21(15(2)31-34)29-24-27-13-19(25)22(30-24)18-12-28-33-9-5-4-6-20(18)33/h4-6,9,12-14,17H,7-8,10-11,26H2,1-3H3,(H,27,29,30)/t14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG expressed in CHOK1 cells after 6 mins by electrophysiology assay |
J Med Chem 59: 4859-66 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00203
BindingDB Entry DOI: 10.7270/Q2KS6TG9 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50184464

(CHEMBL3822644)Show SMILES C[C@@H](N)C(=O)N1CCC(CC1)n1nc(C)c(Nc2ncc(Cl)c(n2)-c2cnn3ccccc23)c1C |r| Show InChI InChI=1S/C24H28ClN9O/c1-14(26)23(35)32-10-7-17(8-11-32)34-16(3)21(15(2)31-34)29-24-27-13-19(25)22(30-24)18-12-28-33-9-5-4-6-20(18)33/h4-6,9,12-14,17H,7-8,10-11,26H2,1-3H3,(H,27,29,30)/t14-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG expressed in CHOK1 cells after 6 mins by electrophysiology assay |
J Med Chem 59: 4859-66 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00203
BindingDB Entry DOI: 10.7270/Q2KS6TG9 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50184476

(CHEMBL3823301)Show SMILES CC(=O)N1CCC(CC1)n1ncc(Nc2ncc(Cl)c(n2)-c2cnn3ccccc23)c1C Show InChI InChI=1S/C22H23ClN8O/c1-14-19(13-26-31(14)16-6-9-29(10-7-16)15(2)32)27-22-24-12-18(23)21(28-22)17-11-25-30-8-4-3-5-20(17)30/h3-5,8,11-13,16H,6-7,9-10H2,1-2H3,(H,24,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG expressed in CHOK1 cells after 6 mins by electrophysiology assay |
J Med Chem 59: 4859-66 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00203
BindingDB Entry DOI: 10.7270/Q2KS6TG9 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50184467

(CHEMBL3823297)Show SMILES COc1cc(ccc1Nc1ncc(Cl)c(n1)-c1cnn2ccccc12)N1CCN(CC1)C(C)=O Show InChI InChI=1S/C24H24ClN7O2/c1-16(33)30-9-11-31(12-10-30)17-6-7-20(22(13-17)34-2)28-24-26-15-19(25)23(29-24)18-14-27-32-8-4-3-5-21(18)32/h3-8,13-15H,9-12H2,1-2H3,(H,26,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG expressed in CHOK1 cells after 6 mins by electrophysiology assay |
J Med Chem 59: 4859-66 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00203
BindingDB Entry DOI: 10.7270/Q2KS6TG9 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50184462

(CHEMBL3823154)Show SMILES Cc1nn(C2CCN(CC2)C(=O)CN)c(C)c1Nc1ncc(Cl)c(n1)-c1cnn2ccccc12 Show InChI InChI=1S/C23H26ClN9O/c1-14-21(15(2)33(30-14)16-6-9-31(10-7-16)20(34)11-25)28-23-26-13-18(24)22(29-23)17-12-27-32-8-4-3-5-19(17)32/h3-5,8,12-13,16H,6-7,9-11,25H2,1-2H3,(H,26,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG expressed in CHOK1 cells after 6 mins by electrophysiology assay |
J Med Chem 59: 4859-66 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00203
BindingDB Entry DOI: 10.7270/Q2KS6TG9 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data