 Found 38 hits of Enzyme Inhibition Constant Data
Found 38 hits of Enzyme Inhibition Constant Data Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University
Curated by ChEMBL
| Assay Description
Inhibition of JAK2 (unknown origin) by mobility shift assay |
Eur J Med Chem 117: 47-58 (2016)
Article DOI: 10.1016/j.ejmech.2016.03.074
BindingDB Entry DOI: 10.7270/Q2QJ7K5K |
More data for this
Ligand-Target Pair | |
Testis-specific serine/threonine-protein kinase 1
(Mus musculus) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University
Curated by ChEMBL
| Assay Description
Inhibition of mouse TSSK1 by luminescent ADP-Glo assay kit |
Eur J Med Chem 117: 47-58 (2016)
Article DOI: 10.1016/j.ejmech.2016.03.074
BindingDB Entry DOI: 10.7270/Q2QJ7K5K |
More data for this
Ligand-Target Pair | |
Rho-associated protein kinase 1
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.680 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University
Curated by ChEMBL
| Assay Description
Inhibition of ROCK1 (unknown origin) by mobility shift assay |
Eur J Med Chem 117: 47-58 (2016)
Article DOI: 10.1016/j.ejmech.2016.03.074
BindingDB Entry DOI: 10.7270/Q2QJ7K5K |
More data for this
Ligand-Target Pair | |
Ribosomal protein S6 kinase beta-1
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University
Curated by ChEMBL
| Assay Description
Inhibition of P70S6K (unknown origin) by mobility shift assay |
Eur J Med Chem 117: 47-58 (2016)
Article DOI: 10.1016/j.ejmech.2016.03.074
BindingDB Entry DOI: 10.7270/Q2QJ7K5K |
More data for this
Ligand-Target Pair | |
Ribosomal protein S6 kinase alpha-1
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University
Curated by ChEMBL
| Assay Description
Inhibition of RSK1 (unknown origin) by Z-LYTE kinase assay |
Eur J Med Chem 117: 47-58 (2016)
Article DOI: 10.1016/j.ejmech.2016.03.074
BindingDB Entry DOI: 10.7270/Q2QJ7K5K |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase Chk1
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal GST tagged recombinant full-length human CHK1 expressed in baculovirus Sf9 insect cells after 10 mins by luminescent ADP-Glo... |
Eur J Med Chem 117: 47-58 (2016)
Article DOI: 10.1016/j.ejmech.2016.03.074
BindingDB Entry DOI: 10.7270/Q2QJ7K5K |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Ribosomal protein S6 kinase alpha-5
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University
Curated by ChEMBL
| Assay Description
Inhibition of MSK1 (unknown origin) by mobility shift assay |
Eur J Med Chem 117: 47-58 (2016)
Article DOI: 10.1016/j.ejmech.2016.03.074
BindingDB Entry DOI: 10.7270/Q2QJ7K5K |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University
Curated by ChEMBL
| Assay Description
Inhibition of BRAF (unknown origin) after 1 hr by HTRF assay |
Eur J Med Chem 117: 47-58 (2016)
Article DOI: 10.1016/j.ejmech.2016.03.074
BindingDB Entry DOI: 10.7270/Q2QJ7K5K |
More data for this
Ligand-Target Pair | |
cGMP-dependent protein kinase 1
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University
Curated by ChEMBL
| Assay Description
Inhibition of PRKG1 (unknown origin) by mobility shift assay |
Eur J Med Chem 117: 47-58 (2016)
Article DOI: 10.1016/j.ejmech.2016.03.074
BindingDB Entry DOI: 10.7270/Q2QJ7K5K |
More data for this
Ligand-Target Pair | |
3-phosphoinositide-dependent protein kinase 1
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University
Curated by ChEMBL
| Assay Description
Inhibition of PDK1 (unknown origin) by mobility shift assay |
Eur J Med Chem 117: 47-58 (2016)
Article DOI: 10.1016/j.ejmech.2016.03.074
BindingDB Entry DOI: 10.7270/Q2QJ7K5K |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cyclin-A2/Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University
Curated by ChEMBL
| Assay Description
Inhibition of CDK2/cyclin A (unknown origin) after 60 mins by Z-LYTE kinase assay |
Eur J Med Chem 117: 47-58 (2016)
Article DOI: 10.1016/j.ejmech.2016.03.074
BindingDB Entry DOI: 10.7270/Q2QJ7K5K |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
ALK tyrosine kinase receptor
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University
Curated by ChEMBL
| Assay Description
Inhibition of ALK (unknown origin) by mobility shift assay |
Eur J Med Chem 117: 47-58 (2016)
Article DOI: 10.1016/j.ejmech.2016.03.074
BindingDB Entry DOI: 10.7270/Q2QJ7K5K |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University
Curated by ChEMBL
| Assay Description
Inhibition of Aurora A (unknown origin) after 1 hr by HTRF assay |
Eur J Med Chem 117: 47-58 (2016)
Article DOI: 10.1016/j.ejmech.2016.03.074
BindingDB Entry DOI: 10.7270/Q2QJ7K5K |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM25045

(3-(4-morpholin-4-ylpyrido[2,3]furo[2,4-b]pyrimidin...)Show InChI InChI=1S/C19H16N4O3/c24-13-4-1-3-12(11-13)17-21-15-14-5-2-6-20-19(14)26-16(15)18(22-17)23-7-9-25-10-8-23/h1-6,11,24H,7-10H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 8.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) after 10 mins by Kinase-Glo Luminescent Assay |
Eur J Med Chem 117: 47-58 (2016)
Article DOI: 10.1016/j.ejmech.2016.03.074
BindingDB Entry DOI: 10.7270/Q2QJ7K5K |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM25045

(3-(4-morpholin-4-ylpyrido[2,3]furo[2,4-b]pyrimidin...)Show InChI InChI=1S/C19H16N4O3/c24-13-4-1-3-12(11-13)17-21-15-14-5-2-6-20-19(14)26-16(15)18(22-17)23-7-9-25-10-8-23/h1-6,11,24H,7-10H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 8.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University
Curated by ChEMBL
| Assay Description
Inhibition of mTOR (unknown origin) by lance ultra assay |
Eur J Med Chem 117: 47-58 (2016)
Article DOI: 10.1016/j.ejmech.2016.03.074
BindingDB Entry DOI: 10.7270/Q2QJ7K5K |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
RAC-gamma serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University
Curated by ChEMBL
| Assay Description
Inhibition of Akt3 (unknown origin) by mobility shift assay |
Eur J Med Chem 117: 47-58 (2016)
Article DOI: 10.1016/j.ejmech.2016.03.074
BindingDB Entry DOI: 10.7270/Q2QJ7K5K |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase Sgk1
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University
Curated by ChEMBL
| Assay Description
Inhibition of SGK (unknown origin) by mobility shift assay |
Eur J Med Chem 117: 47-58 (2016)
Article DOI: 10.1016/j.ejmech.2016.03.074
BindingDB Entry DOI: 10.7270/Q2QJ7K5K |
More data for this
Ligand-Target Pair | |
RAC-beta serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University
Curated by ChEMBL
| Assay Description
Inhibition of Akt2 (unknown origin) by mobility shift assay |
Eur J Med Chem 117: 47-58 (2016)
Article DOI: 10.1016/j.ejmech.2016.03.074
BindingDB Entry DOI: 10.7270/Q2QJ7K5K |
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University
Curated by ChEMBL
| Assay Description
Inhibition of Akt1 (unknown origin) after 1 hr by HTRF assay |
Eur J Med Chem 117: 47-58 (2016)
Article DOI: 10.1016/j.ejmech.2016.03.074
BindingDB Entry DOI: 10.7270/Q2QJ7K5K |
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50170284
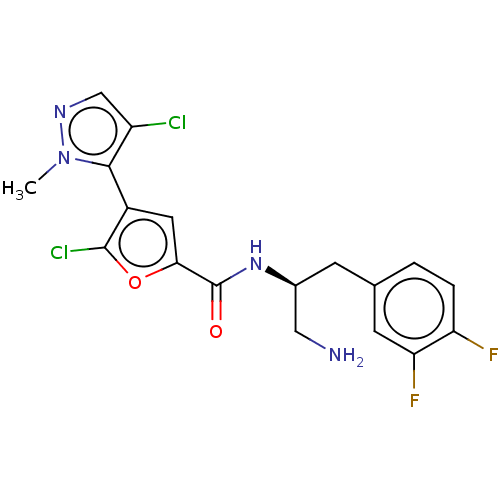
(GSK2141795 | GSK2141795C | Uprosertib)Show SMILES Cn1ncc(Cl)c1-c1cc(oc1Cl)C(=O)N[C@H](CN)Cc1ccc(F)c(F)c1 |r,wD:16.18,(2.37,-.54,;3.52,.49,;3.36,2.02,;4.77,2.65,;5.8,1.51,;7.33,1.67,;5.03,.17,;5.65,-1.23,;7.16,-1.55,;7.32,-3.09,;5.91,-3.71,;4.88,-2.57,;3.35,-2.73,;8.65,-3.86,;9.99,-3.09,;8.65,-5.4,;9.99,-6.17,;11.32,-5.4,;12.65,-6.17,;9.99,-7.71,;11.32,-8.48,;12.65,-7.71,;13.99,-8.48,;13.99,-10.02,;15.32,-10.79,;12.65,-10.79,;12.65,-12.33,;11.32,-10.02,)| Show InChI InChI=1S/C18H16Cl2F2N4O2/c1-26-16(12(19)8-24-26)11-6-15(28-17(11)20)18(27)25-10(7-23)4-9-2-3-13(21)14(22)5-9/h2-3,5-6,8,10H,4,7,23H2,1H3,(H,25,27)/t10-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University
Curated by ChEMBL
| Assay Description
Inhibition of Akt1 (unknown origin) expressed in Escherichia coli using peptide substrate after 1 hr by HTRF assay |
Eur J Med Chem 117: 47-58 (2016)
Article DOI: 10.1016/j.ejmech.2016.03.074
BindingDB Entry DOI: 10.7270/Q2QJ7K5K |
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM256836
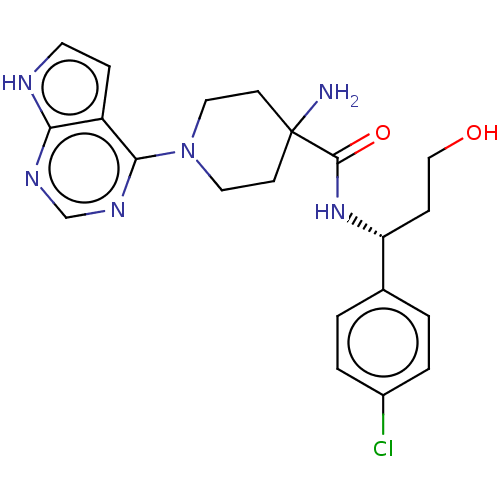
(BDBM50427349 | US10654855, Example 15 | US11236095...)Show SMILES NC1(CCN(CC1)c1ncnc2[nH]ccc12)C(=O)N[C@H](CCO)c1ccc(Cl)cc1 |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University
Curated by ChEMBL
| Assay Description
Inhibition of Akt1 (unknown origin) expressed in Escherichia coli using peptide substrate after 1 hr by HTRF assay |
Eur J Med Chem 117: 47-58 (2016)
Article DOI: 10.1016/j.ejmech.2016.03.074
BindingDB Entry DOI: 10.7270/Q2QJ7K5K |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 52 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University
Curated by ChEMBL
| Assay Description
Inhibition of GSK3beta (unknown origin) after 60 mins by Z-LYTE kinase assay |
Eur J Med Chem 117: 47-58 (2016)
Article DOI: 10.1016/j.ejmech.2016.03.074
BindingDB Entry DOI: 10.7270/Q2QJ7K5K |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50170430

(CHEMBL3805228)Show SMILES Cn1ncc(Cl)c1-c1cc(oc1Cl)C(=O)NC1(CCNCC1)c1ccc(Cl)c(Cl)c1 |(.16,9.88,;-1.04,9.6,;-2.21,10.59,;-3.53,9.79,;-3.16,8.29,;-3.96,7.35,;-1.64,8.19,;-.74,6.95,;-1.21,5.48,;.04,4.6,;1.28,5.49,;.8,6.95,;1.53,7.95,;.04,3.06,;1.1,2.44,;-1.3,2.29,;-1.33,.77,;,1.54,;1.33,.77,;1.33,-.77,;,-1.54,;-1.33,-.77,;-2.63,-.02,;-2.59,-1.56,;-3.91,-2.36,;-5.26,-1.63,;-6.31,-2.27,;-5.3,-.09,;-6.38,.5,;-3.98,.71,)| Show InChI InChI=1S/C20H18Cl4N4O2/c1-28-17(15(23)10-26-28)12-9-16(30-18(12)24)19(29)27-20(4-6-25-7-5-20)11-2-3-13(21)14(22)8-11/h2-3,8-10,25H,4-7H2,1H3,(H,27,29) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 61 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University
Curated by ChEMBL
| Assay Description
Inhibition of Akt1 (unknown origin) expressed in Escherichia coli using peptide substrate after 1 hr by HTRF assay |
Eur J Med Chem 117: 47-58 (2016)
Article DOI: 10.1016/j.ejmech.2016.03.074
BindingDB Entry DOI: 10.7270/Q2QJ7K5K |
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50170444

(CHEMBL3805163)Show SMILES Cn1nccc1-c1csc(c1)C(=O)NC1(CCNCC1)c1ccc(Cl)c(Cl)c1 Show InChI InChI=1S/C20H20Cl2N4OS/c1-26-17(4-7-24-26)13-10-18(28-12-13)19(27)25-20(5-8-23-9-6-20)14-2-3-15(21)16(22)11-14/h2-4,7,10-12,23H,5-6,8-9H2,1H3,(H,25,27) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 71 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University
Curated by ChEMBL
| Assay Description
Inhibition of Akt1 (unknown origin) expressed in Escherichia coli using peptide substrate after 1 hr by HTRF assay |
Eur J Med Chem 117: 47-58 (2016)
Article DOI: 10.1016/j.ejmech.2016.03.074
BindingDB Entry DOI: 10.7270/Q2QJ7K5K |
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50170282

(CHEMBL3806131)Show SMILES Cn1ncc(Cl)c1-c1csc(c1)C(=O)NC1(CCNCC1)c1ccc(F)cc1 Show InChI InChI=1S/C20H20ClFN4OS/c1-26-18(16(21)11-24-26)13-10-17(28-12-13)19(27)25-20(6-8-23-9-7-20)14-2-4-15(22)5-3-14/h2-5,10-12,23H,6-9H2,1H3,(H,25,27) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 320 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University
Curated by ChEMBL
| Assay Description
Inhibition of Akt1 (unknown origin) expressed in Escherichia coli using peptide substrate after 1 hr by HTRF assay |
Eur J Med Chem 117: 47-58 (2016)
Article DOI: 10.1016/j.ejmech.2016.03.074
BindingDB Entry DOI: 10.7270/Q2QJ7K5K |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 339 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University
Curated by ChEMBL
| Assay Description
Inhibition of ABL (unknown origin) by mobility shift assay |
Eur J Med Chem 117: 47-58 (2016)
Article DOI: 10.1016/j.ejmech.2016.03.074
BindingDB Entry DOI: 10.7270/Q2QJ7K5K |
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50170281

(CHEMBL3805232)Show SMILES Cn1ncc(Br)c1-c1csc(c1)C(=O)NC1(CCNCC1)c1ccc(F)cc1 Show InChI InChI=1S/C20H20BrFN4OS/c1-26-18(16(21)11-24-26)13-10-17(28-12-13)19(27)25-20(6-8-23-9-7-20)14-2-4-15(22)5-3-14/h2-5,10-12,23H,6-9H2,1H3,(H,25,27) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 360 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University
Curated by ChEMBL
| Assay Description
Inhibition of Akt1 (unknown origin) expressed in Escherichia coli using peptide substrate after 1 hr by HTRF assay |
Eur J Med Chem 117: 47-58 (2016)
Article DOI: 10.1016/j.ejmech.2016.03.074
BindingDB Entry DOI: 10.7270/Q2QJ7K5K |
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50170432

(CHEMBL3805691)Show SMILES Cn1ncc(Cl)c1-c1cc(oc1Cl)C(=O)NC1(CCNCC1)c1ccc(F)cc1 |(.16,9.88,;-1.04,9.6,;-2.21,10.59,;-3.53,9.79,;-3.16,8.29,;-3.96,7.35,;-1.64,8.19,;-.74,6.95,;-1.21,5.48,;.04,4.6,;1.28,5.49,;.8,6.95,;1.53,7.95,;.04,3.06,;1.1,2.44,;-1.3,2.29,;-1.33,.77,;,1.54,;1.33,.77,;1.33,-.77,;,-1.54,;-1.33,-.77,;-2.63,-.02,;-2.59,-1.56,;-3.91,-2.36,;-5.26,-1.63,;-6.31,-2.27,;-5.3,-.09,;-3.98,.71,)| Show InChI InChI=1S/C20H19Cl2FN4O2/c1-27-17(15(21)11-25-27)14-10-16(29-18(14)22)19(28)26-20(6-8-24-9-7-20)12-2-4-13(23)5-3-12/h2-5,10-11,24H,6-9H2,1H3,(H,26,28) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 390 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University
Curated by ChEMBL
| Assay Description
Inhibition of Akt1 (unknown origin) expressed in Escherichia coli using peptide substrate after 1 hr by HTRF assay |
Eur J Med Chem 117: 47-58 (2016)
Article DOI: 10.1016/j.ejmech.2016.03.074
BindingDB Entry DOI: 10.7270/Q2QJ7K5K |
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50170436

(CHEMBL3805855)Show SMILES Cn1ncc(Br)c1-c1coc(c1)C(=O)NC1(CCNCC1)c1ccc(Cl)c(Cl)c1 Show InChI InChI=1S/C20H19BrCl2N4O2/c1-27-18(14(21)10-25-27)12-8-17(29-11-12)19(28)26-20(4-6-24-7-5-20)13-2-3-15(22)16(23)9-13/h2-3,8-11,24H,4-7H2,1H3,(H,26,28) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 440 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University
Curated by ChEMBL
| Assay Description
Inhibition of Akt1 (unknown origin) expressed in Escherichia coli using peptide substrate after 1 hr by HTRF assay |
Eur J Med Chem 117: 47-58 (2016)
Article DOI: 10.1016/j.ejmech.2016.03.074
BindingDB Entry DOI: 10.7270/Q2QJ7K5K |
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50170421

(CHEMBL3805319)Show SMILES Cn1ncc(Cl)c1-c1c(Cl)c(C(=O)NC2(CCNCC2)c2ccc(Cl)c(Cl)c2)n(C)c1Cl |(.16,9.88,;-1.04,9.6,;-2.21,10.59,;-3.53,9.79,;-3.16,8.29,;-3.96,7.35,;-1.64,8.19,;-.74,6.95,;-1.21,5.48,;-2.38,5.09,;.04,4.6,;.04,3.06,;1.1,2.44,;-1.3,2.29,;-1.33,.77,;,1.54,;1.33,.77,;1.33,-.77,;,-1.54,;-1.33,-.77,;-2.63,-.02,;-3.98,.71,;-5.3,-.09,;-5.26,-1.63,;-6.31,-2.27,;-3.91,-2.36,;-3.88,-3.6,;-2.59,-1.56,;1.28,5.49,;2.45,5.1,;.8,6.95,;1.53,7.95,)| Show InChI InChI=1S/C21H20Cl5N5O/c1-30-18(16(25)15(19(30)26)17-14(24)10-28-31(17)2)20(32)29-21(5-7-27-8-6-21)11-3-4-12(22)13(23)9-11/h3-4,9-10,27H,5-8H2,1-2H3,(H,29,32) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 850 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University
Curated by ChEMBL
| Assay Description
Inhibition of Akt1 (unknown origin) expressed in Escherichia coli using peptide substrate after 1 hr by HTRF assay |
Eur J Med Chem 117: 47-58 (2016)
Article DOI: 10.1016/j.ejmech.2016.03.074
BindingDB Entry DOI: 10.7270/Q2QJ7K5K |
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50170283

(CHEMBL3805292)Show SMILES Cn1nccc1-c1csc(c1)C(=O)NC1(CCNCC1)c1ccc(F)cc1 Show InChI InChI=1S/C20H21FN4OS/c1-25-17(6-9-23-25)14-12-18(27-13-14)19(26)24-20(7-10-22-11-8-20)15-2-4-16(21)5-3-15/h2-6,9,12-13,22H,7-8,10-11H2,1H3,(H,24,26) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 920 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University
Curated by ChEMBL
| Assay Description
Inhibition of Akt1 (unknown origin) expressed in Escherichia coli using peptide substrate after 1 hr by HTRF assay |
Eur J Med Chem 117: 47-58 (2016)
Article DOI: 10.1016/j.ejmech.2016.03.074
BindingDB Entry DOI: 10.7270/Q2QJ7K5K |
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50170442

(CHEMBL3806229)Show SMILES Cn1ncc(Cl)c1-c1coc(c1)C(=O)NC1(CCNCC1)c1ccc(F)cc1 Show InChI InChI=1S/C20H20ClFN4O2/c1-26-18(16(21)11-24-26)13-10-17(28-12-13)19(27)25-20(6-8-23-9-7-20)14-2-4-15(22)5-3-14/h2-5,10-12,23H,6-9H2,1H3,(H,25,27) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 920 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University
Curated by ChEMBL
| Assay Description
Inhibition of Akt1 (unknown origin) expressed in Escherichia coli using peptide substrate after 1 hr by HTRF assay |
Eur J Med Chem 117: 47-58 (2016)
Article DOI: 10.1016/j.ejmech.2016.03.074
BindingDB Entry DOI: 10.7270/Q2QJ7K5K |
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50170422
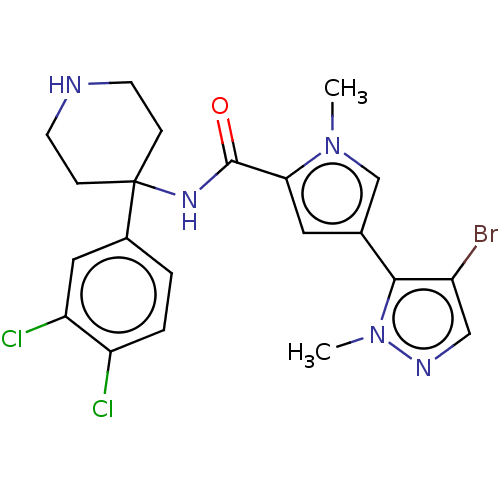
(CHEMBL3805443)Show SMILES Cn1cc(cc1C(=O)NC1(CCNCC1)c1ccc(Cl)c(Cl)c1)-c1c(Br)cnn1C Show InChI InChI=1S/C21H22BrCl2N5O/c1-28-12-13(19-15(22)11-26-29(19)2)9-18(28)20(30)27-21(5-7-25-8-6-21)14-3-4-16(23)17(24)10-14/h3-4,9-12,25H,5-8H2,1-2H3,(H,27,30) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.26E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University
Curated by ChEMBL
| Assay Description
Inhibition of Akt1 (unknown origin) expressed in Escherichia coli using peptide substrate after 1 hr by HTRF assay |
Eur J Med Chem 117: 47-58 (2016)
Article DOI: 10.1016/j.ejmech.2016.03.074
BindingDB Entry DOI: 10.7270/Q2QJ7K5K |
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50170285
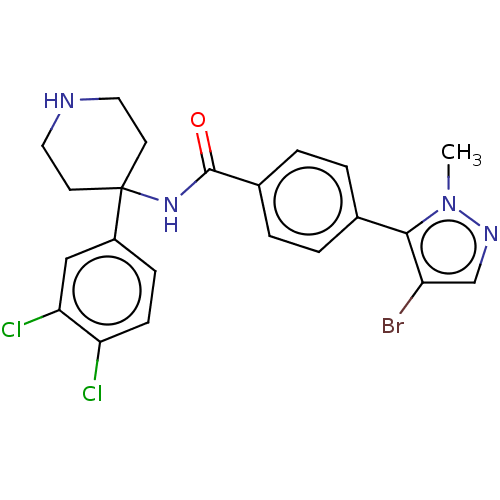
(CHEMBL3805037)Show SMILES Cn1ncc(Br)c1-c1ccc(cc1)C(=O)NC1(CCNCC1)c1ccc(Cl)c(Cl)c1 Show InChI InChI=1S/C22H21BrCl2N4O/c1-29-20(17(23)13-27-29)14-2-4-15(5-3-14)21(30)28-22(8-10-26-11-9-22)16-6-7-18(24)19(25)12-16/h2-7,12-13,26H,8-11H2,1H3,(H,28,30) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University
Curated by ChEMBL
| Assay Description
Inhibition of Akt1 (unknown origin) expressed in Escherichia coli using peptide substrate after 1 hr by HTRF assay |
Eur J Med Chem 117: 47-58 (2016)
Article DOI: 10.1016/j.ejmech.2016.03.074
BindingDB Entry DOI: 10.7270/Q2QJ7K5K |
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50170423
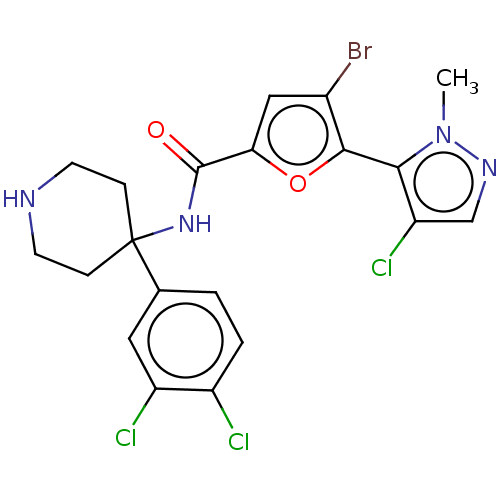
(CHEMBL3805457)Show SMILES Cn1ncc(Cl)c1-c1oc(cc1Br)C(=O)NC1(CCNCC1)c1ccc(Cl)c(Cl)c1 |(3.87,7,;3.23,8.06,;3.82,9.48,;2.64,10.48,;1.33,9.67,;.19,10.14,;1.71,8.2,;.8,6.95,;1.28,5.49,;.04,4.6,;-1.21,5.48,;-.74,6.95,;-1.46,7.94,;.04,3.06,;1.1,2.44,;-1.3,2.29,;-1.33,.77,;,1.54,;1.33,.77,;1.33,-.77,;,-1.54,;-1.33,-.77,;-2.63,-.02,;-3.98,.71,;-5.3,-.09,;-5.26,-1.63,;-6.31,-2.27,;-3.91,-2.36,;-3.88,-3.6,;-2.59,-1.56,)| Show InChI InChI=1S/C20H18BrCl3N4O2/c1-28-17(15(24)10-26-28)18-12(21)9-16(30-18)19(29)27-20(4-6-25-7-5-20)11-2-3-13(22)14(23)8-11/h2-3,8-10,25H,4-7H2,1H3,(H,27,29) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.48E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University
Curated by ChEMBL
| Assay Description
Inhibition of Akt1 (unknown origin) expressed in Escherichia coli using peptide substrate after 1 hr by HTRF assay |
Eur J Med Chem 117: 47-58 (2016)
Article DOI: 10.1016/j.ejmech.2016.03.074
BindingDB Entry DOI: 10.7270/Q2QJ7K5K |
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50170418
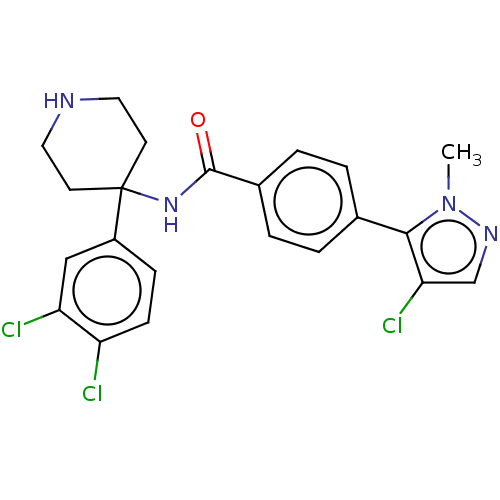
(CHEMBL3805006)Show SMILES Cn1ncc(Cl)c1-c1ccc(cc1)C(=O)NC1(CCNCC1)c1ccc(Cl)c(Cl)c1 Show InChI InChI=1S/C22H21Cl3N4O/c1-29-20(19(25)13-27-29)14-2-4-15(5-3-14)21(30)28-22(8-10-26-11-9-22)16-6-7-17(23)18(24)12-16/h2-7,12-13,26H,8-11H2,1H3,(H,28,30) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.91E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University
Curated by ChEMBL
| Assay Description
Inhibition of Akt1 (unknown origin) expressed in Escherichia coli using peptide substrate after 1 hr by HTRF assay |
Eur J Med Chem 117: 47-58 (2016)
Article DOI: 10.1016/j.ejmech.2016.03.074
BindingDB Entry DOI: 10.7270/Q2QJ7K5K |
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50170437
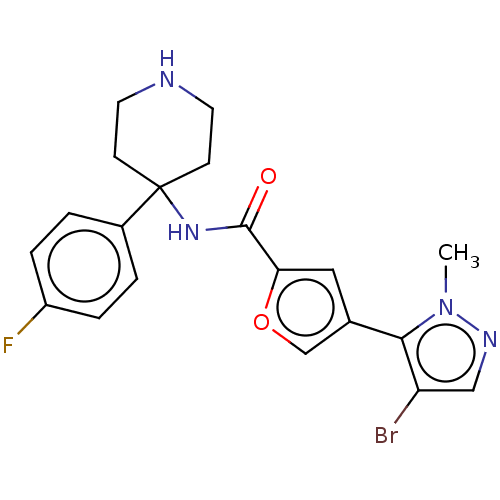
(CHEMBL3805617)Show SMILES Cn1ncc(Br)c1-c1coc(c1)C(=O)NC1(CCNCC1)c1ccc(F)cc1 Show InChI InChI=1S/C20H20BrFN4O2/c1-26-18(16(21)11-24-26)13-10-17(28-12-13)19(27)25-20(6-8-23-9-7-20)14-2-4-15(22)5-3-14/h2-5,10-12,23H,6-9H2,1H3,(H,25,27) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.02E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University
Curated by ChEMBL
| Assay Description
Inhibition of Akt1 (unknown origin) expressed in Escherichia coli using peptide substrate after 1 hr by HTRF assay |
Eur J Med Chem 117: 47-58 (2016)
Article DOI: 10.1016/j.ejmech.2016.03.074
BindingDB Entry DOI: 10.7270/Q2QJ7K5K |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 9
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.27E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University
Curated by ChEMBL
| Assay Description
Inhibition of JNK2 (unknown origin) by mobility shift assay |
Eur J Med Chem 117: 47-58 (2016)
Article DOI: 10.1016/j.ejmech.2016.03.074
BindingDB Entry DOI: 10.7270/Q2QJ7K5K |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data