

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Endothelin receptor type B (Homo sapiens (Human)) | BDBM50197953 (CHEMBL3980643) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Perugia Curated by ChEMBL | Assay Description Agonist activity at human recombinant ETB receptor expressed in CHOK1 cells assessed as induction of Ca2+ mobilization by fluorimetric method | Eur J Med Chem 121: 658-670 (2016) Article DOI: 10.1016/j.ejmech.2016.06.006 BindingDB Entry DOI: 10.7270/Q2T155MF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin receptor type B (Homo sapiens (Human)) | BDBM50197955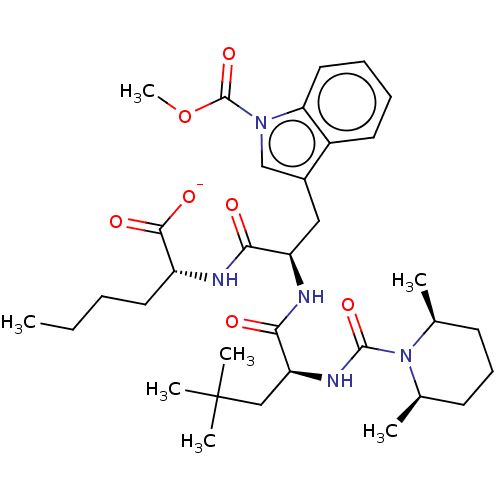 (CHEMBL1515091) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.410 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Perugia Curated by ChEMBL | Assay Description Antagonist activity at ETB receptor in human BSMC assessed as inhibition of endothelin-1 or 3-mediated Ca2+ mobilization preincubated for 5 mins foll... | Eur J Med Chem 121: 658-670 (2016) Article DOI: 10.1016/j.ejmech.2016.06.006 BindingDB Entry DOI: 10.7270/Q2T155MF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50197954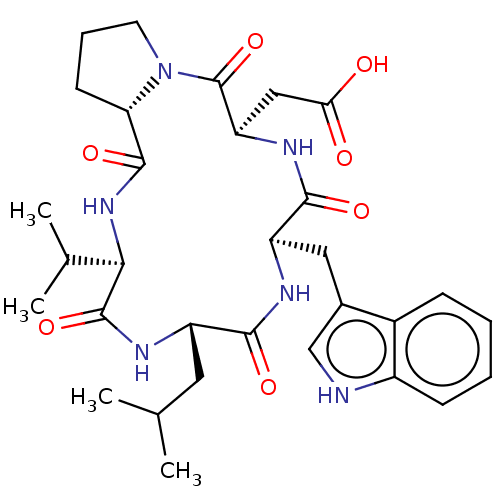 (CHEBI:2965 | CHEMBL314691) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.620 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Perugia Curated by ChEMBL | Assay Description Antagonist activity at ETA receptor in human SKNMC cells assessed as inhibition of endothelin-1-mediated Ca2+ mobilization by fura-2/AM dye-based spe... | Eur J Med Chem 121: 658-670 (2016) Article DOI: 10.1016/j.ejmech.2016.06.006 BindingDB Entry DOI: 10.7270/Q2T155MF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50197953 (CHEMBL3980643) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.670 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Perugia Curated by ChEMBL | Assay Description Agonist activity at ETA receptor in human SKNMC cells assessed as induction of Ca2+ mobilization by fluorimetric method | Eur J Med Chem 121: 658-670 (2016) Article DOI: 10.1016/j.ejmech.2016.06.006 BindingDB Entry DOI: 10.7270/Q2T155MF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50061101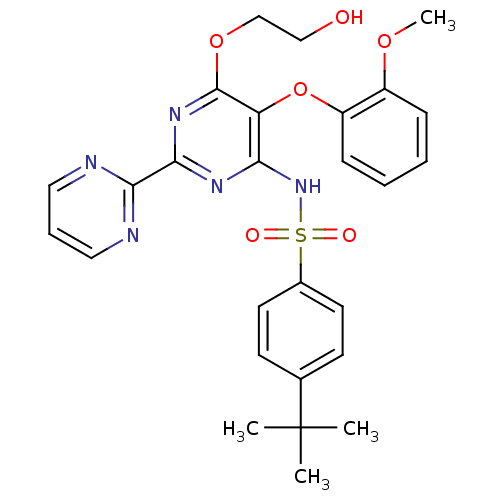 (4-(1,1-Dimethylethyl)-N-(6-(2-hydroxyethoxy)-5-(2-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Perugia Curated by ChEMBL | Assay Description Binding affinity to human ETA receptor | Eur J Med Chem 121: 658-670 (2016) Article DOI: 10.1016/j.ejmech.2016.06.006 BindingDB Entry DOI: 10.7270/Q2T155MF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50197957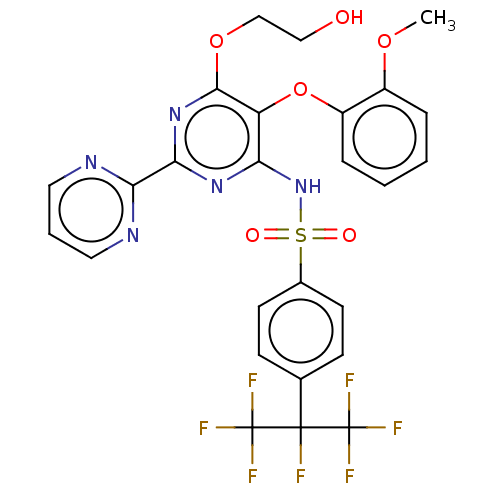 (CHEMBL3891334) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 75 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Perugia Curated by ChEMBL | Assay Description Agonist activity at ETA receptor in human SKNMC cells assessed as induction of Ca2+ mobilization by fluorimetric method | Eur J Med Chem 121: 658-670 (2016) Article DOI: 10.1016/j.ejmech.2016.06.006 BindingDB Entry DOI: 10.7270/Q2T155MF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin receptor type B (Homo sapiens (Human)) | BDBM50061101 (4-(1,1-Dimethylethyl)-N-(6-(2-hydroxyethoxy)-5-(2-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 95 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Perugia Curated by ChEMBL | Assay Description Binding affinity to human ETB receptor | Eur J Med Chem 121: 658-670 (2016) Article DOI: 10.1016/j.ejmech.2016.06.006 BindingDB Entry DOI: 10.7270/Q2T155MF | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50197956 (CHEMBL3918612) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.93E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Perugia Curated by ChEMBL | Assay Description Agonist activity at ETA receptor in human SKNMC cells assessed as induction of Ca2+ mobilization by fluorimetric method | Eur J Med Chem 121: 658-670 (2016) Article DOI: 10.1016/j.ejmech.2016.06.006 BindingDB Entry DOI: 10.7270/Q2T155MF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||