

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50196073 (CHEMBL3919081) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku Medical and Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of recombinant human PTP1B (1 to 322 residues) expressed in Escherichia coli using pNPP as substrate preincubated for 10 mins followed by ... | J Nat Prod 79: 1842-7 (2016) Article DOI: 10.1021/acs.jnatprod.6b00367 BindingDB Entry DOI: 10.7270/Q20V8FRH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50196066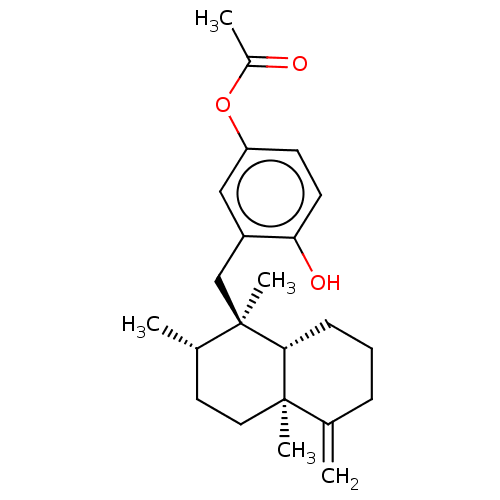 (20-O-Acetylneoavarol | CHEMBL516014) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku Medical and Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of recombinant human PTP1B (1 to 322 residues) expressed in Escherichia coli using pNPP as substrate preincubated for 10 mins followed by ... | J Nat Prod 79: 1842-7 (2016) Article DOI: 10.1021/acs.jnatprod.6b00367 BindingDB Entry DOI: 10.7270/Q20V8FRH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50196071 (CHEMBL3971802) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku Medical and Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of recombinant human PTP1B (1 to 322 residues) expressed in Escherichia coli using pNPP as substrate preincubated for 10 mins followed by ... | J Nat Prod 79: 1842-7 (2016) Article DOI: 10.1021/acs.jnatprod.6b00367 BindingDB Entry DOI: 10.7270/Q20V8FRH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50196070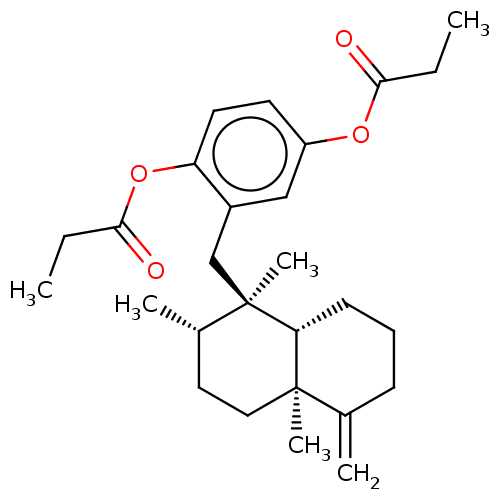 (CHEMBL3962935) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku Medical and Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of recombinant human PTP1B (1 to 322 residues) expressed in Escherichia coli using pNPP as substrate preincubated for 10 mins followed by ... | J Nat Prod 79: 1842-7 (2016) Article DOI: 10.1021/acs.jnatprod.6b00367 BindingDB Entry DOI: 10.7270/Q20V8FRH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50196064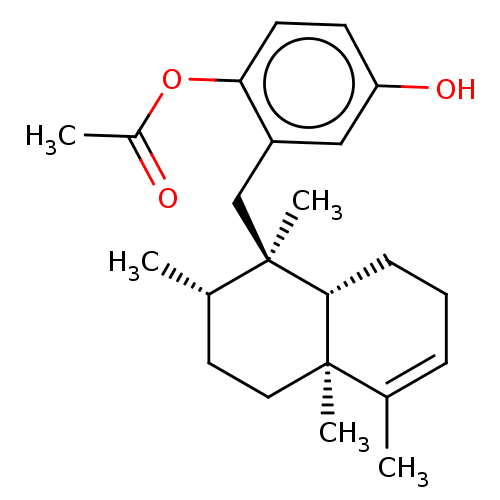 (CHEMBL521017) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku Medical and Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of recombinant human PTP1B (1 to 322 residues) expressed in Escherichia coli using pNPP as substrate preincubated for 10 mins followed by ... | J Nat Prod 79: 1842-7 (2016) Article DOI: 10.1021/acs.jnatprod.6b00367 BindingDB Entry DOI: 10.7270/Q20V8FRH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50196067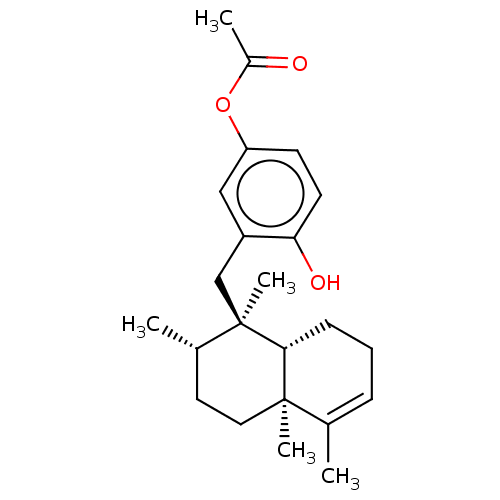 (CHEMBL3893783) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku Medical and Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of recombinant human PTP1B (1 to 322 residues) expressed in Escherichia coli using pNPP as substrate preincubated for 10 mins followed by ... | J Nat Prod 79: 1842-7 (2016) Article DOI: 10.1021/acs.jnatprod.6b00367 BindingDB Entry DOI: 10.7270/Q20V8FRH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50196068 (CHEMBL3892140) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku Medical and Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of recombinant human PTP1B (1 to 322 residues) expressed in Escherichia coli using pNPP as substrate preincubated for 10 mins followed by ... | J Nat Prod 79: 1842-7 (2016) Article DOI: 10.1021/acs.jnatprod.6b00367 BindingDB Entry DOI: 10.7270/Q20V8FRH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50242257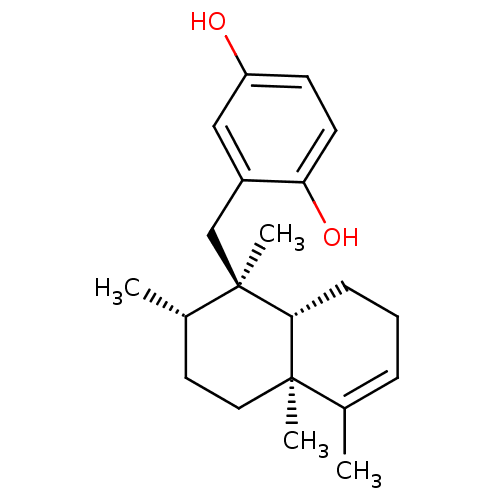 (2-((1R,2S,4aS)-1,2,4a,5-Tetramethyl-1,2,3,4,4a,7,8...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku Medical and Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of recombinant human PTP1B (1 to 322 residues) expressed in Escherichia coli using pNPP as substrate preincubated for 10 mins followed by ... | J Nat Prod 79: 1842-7 (2016) Article DOI: 10.1021/acs.jnatprod.6b00367 BindingDB Entry DOI: 10.7270/Q20V8FRH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50196065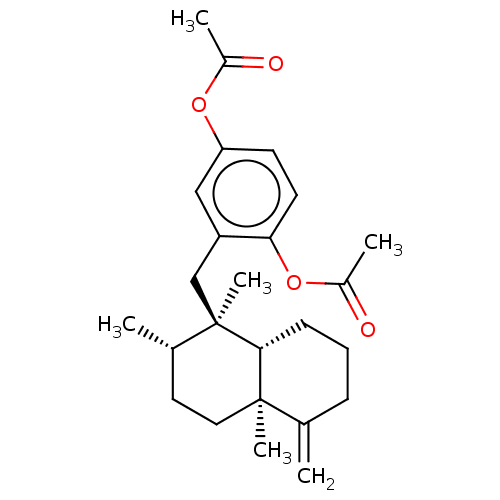 (CHEMBL3973897) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku Medical and Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of recombinant human PTP1B (1 to 322 residues) expressed in Escherichia coli using pNPP as substrate preincubated for 10 mins followed by ... | J Nat Prod 79: 1842-7 (2016) Article DOI: 10.1021/acs.jnatprod.6b00367 BindingDB Entry DOI: 10.7270/Q20V8FRH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50196072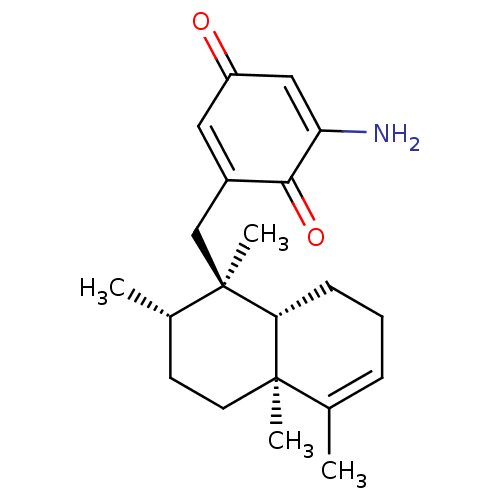 (3''-Amino-Avarone | CHEMBL464646) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku Medical and Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of recombinant human PTP1B (1 to 322 residues) expressed in Escherichia coli using pNPP as substrate preincubated for 10 mins followed by ... | J Nat Prod 79: 1842-7 (2016) Article DOI: 10.1021/acs.jnatprod.6b00367 BindingDB Entry DOI: 10.7270/Q20V8FRH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50196069 (CHEMBL3965313) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku Medical and Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of recombinant human PTP1B (1 to 322 residues) expressed in Escherichia coli using pNPP as substrate preincubated for 10 mins followed by ... | J Nat Prod 79: 1842-7 (2016) Article DOI: 10.1021/acs.jnatprod.6b00367 BindingDB Entry DOI: 10.7270/Q20V8FRH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||