 Found 66 hits of Enzyme Inhibition Constant Data
Found 66 hits of Enzyme Inhibition Constant Data Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50205264
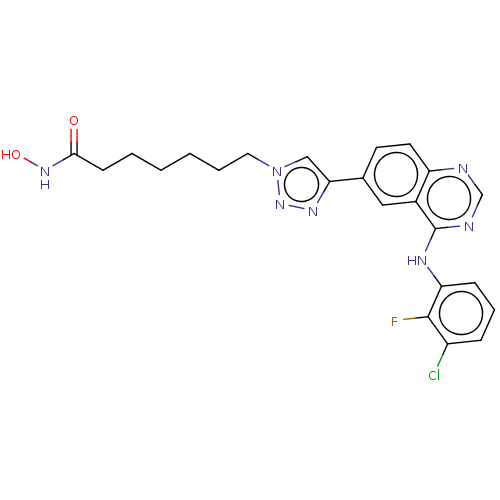
(CHEMBL3930620)Show SMILES ONC(=O)CCCCCCn1cc(nn1)-c1ccc2ncnc(Nc3cccc(Cl)c3F)c2c1 Show InChI InChI=1S/C23H23ClFN7O2/c24-17-6-5-7-19(22(17)25)28-23-16-12-15(9-10-18(16)26-14-27-23)20-13-32(31-29-20)11-4-2-1-3-8-21(33)30-34/h5-7,9-10,12-14,34H,1-4,8,11H2,(H,30,33)(H,26,27,28) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsinghua University
Curated by ChEMBL
| Assay Description
Inhibition of wild-type EGFR (unknown origin) assessed as remaining ATP level measured after 15 mins by luminescence analysis |
Bioorg Med Chem 25: 27-37 (2017)
Article DOI: 10.1016/j.bmc.2016.10.006
BindingDB Entry DOI: 10.7270/Q22N548X |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50205262
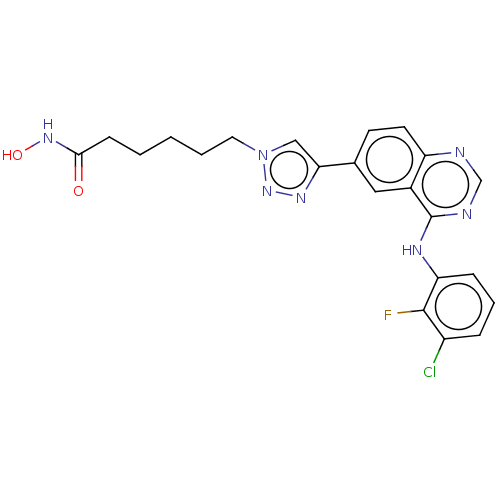
(CHEMBL3929484)Show SMILES ONC(=O)CCCCCn1cc(nn1)-c1ccc2ncnc(Nc3cccc(Cl)c3F)c2c1 Show InChI InChI=1S/C22H21ClFN7O2/c23-16-5-4-6-18(21(16)24)27-22-15-11-14(8-9-17(15)25-13-26-22)19-12-31(30-28-19)10-3-1-2-7-20(32)29-33/h4-6,8-9,11-13,33H,1-3,7,10H2,(H,29,32)(H,25,26,27) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsinghua University
Curated by ChEMBL
| Assay Description
Inhibition of wild-type EGFR (unknown origin) assessed as remaining ATP level measured after 15 mins by luminescence analysis |
Bioorg Med Chem 25: 27-37 (2017)
Article DOI: 10.1016/j.bmc.2016.10.006
BindingDB Entry DOI: 10.7270/Q22N548X |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50205266

(CHEMBL3920583)Show SMILES ONC(=O)CCCCCn1cc(nn1)-c1ccc2ncnc(Nc3ccc(F)c(Cl)c3)c2c1 Show InChI InChI=1S/C22H21ClFN7O2/c23-17-11-15(6-7-18(17)24)27-22-16-10-14(5-8-19(16)25-13-26-22)20-12-31(30-28-20)9-3-1-2-4-21(32)29-33/h5-8,10-13,33H,1-4,9H2,(H,29,32)(H,25,26,27) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.650 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsinghua University
Curated by ChEMBL
| Assay Description
Inhibition of wild-type EGFR (unknown origin) assessed as remaining ATP level measured after 15 mins by luminescence analysis |
Bioorg Med Chem 25: 27-37 (2017)
Article DOI: 10.1016/j.bmc.2016.10.006
BindingDB Entry DOI: 10.7270/Q22N548X |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50205265
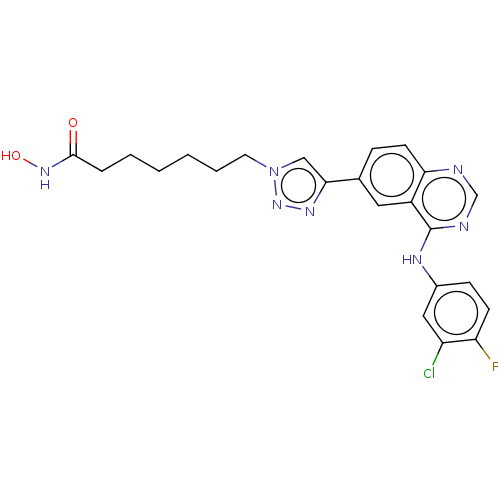
(CHEMBL3957055)Show SMILES ONC(=O)CCCCCCn1cc(nn1)-c1ccc2ncnc(Nc3ccc(F)c(Cl)c3)c2c1 Show InChI InChI=1S/C23H23ClFN7O2/c24-18-12-16(7-8-19(18)25)28-23-17-11-15(6-9-20(17)26-14-27-23)21-13-32(31-29-21)10-4-2-1-3-5-22(33)30-34/h6-9,11-14,34H,1-5,10H2,(H,30,33)(H,26,27,28) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.690 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsinghua University
Curated by ChEMBL
| Assay Description
Inhibition of wild-type EGFR (unknown origin) assessed as remaining ATP level measured after 15 mins by luminescence analysis |
Bioorg Med Chem 25: 27-37 (2017)
Article DOI: 10.1016/j.bmc.2016.10.006
BindingDB Entry DOI: 10.7270/Q22N548X |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50205264

(CHEMBL3930620)Show SMILES ONC(=O)CCCCCCn1cc(nn1)-c1ccc2ncnc(Nc3cccc(Cl)c3F)c2c1 Show InChI InChI=1S/C23H23ClFN7O2/c24-17-6-5-7-19(22(17)25)28-23-16-12-15(9-10-18(16)26-14-27-23)20-13-32(31-29-20)11-4-2-1-3-8-21(33)30-34/h5-7,9-10,12-14,34H,1-4,8,11H2,(H,30,33)(H,26,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.720 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsinghua University
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 (unknown origin) using acetylated peptide substrate preincubated for 15 mins followed by substrate addition measured after 60 min... |
Bioorg Med Chem 25: 27-37 (2017)
Article DOI: 10.1016/j.bmc.2016.10.006
BindingDB Entry DOI: 10.7270/Q22N548X |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50205262

(CHEMBL3929484)Show SMILES ONC(=O)CCCCCn1cc(nn1)-c1ccc2ncnc(Nc3cccc(Cl)c3F)c2c1 Show InChI InChI=1S/C22H21ClFN7O2/c23-16-5-4-6-18(21(16)24)27-22-15-11-14(8-9-17(15)25-13-26-22)19-12-31(30-28-19)10-3-1-2-7-20(32)29-33/h4-6,8-9,11-13,33H,1-3,7,10H2,(H,29,32)(H,25,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsinghua University
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 (unknown origin) using acetylated peptide substrate preincubated for 15 mins followed by substrate addition measured after 60 min... |
Bioorg Med Chem 25: 27-37 (2017)
Article DOI: 10.1016/j.bmc.2016.10.006
BindingDB Entry DOI: 10.7270/Q22N548X |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50205263

(CHEMBL3964033)Show SMILES ONC(=O)CCCCCn1cc(nn1)-c1ccc2ncnc(Nc3ccc(OCc4cccc(F)c4)c(Cl)c3)c2c1 Show InChI InChI=1S/C29H27ClFN7O3/c30-24-15-22(9-11-27(24)41-17-19-5-4-6-21(31)13-19)34-29-23-14-20(8-10-25(23)32-18-33-29)26-16-38(37-35-26)12-3-1-2-7-28(39)36-40/h4-6,8-11,13-16,18,40H,1-3,7,12,17H2,(H,36,39)(H,32,33,34) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsinghua University
Curated by ChEMBL
| Assay Description
Inhibition of wild-type EGFR (unknown origin) assessed as remaining ATP level measured after 15 mins by luminescence analysis |
Bioorg Med Chem 25: 27-37 (2017)
Article DOI: 10.1016/j.bmc.2016.10.006
BindingDB Entry DOI: 10.7270/Q22N548X |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM50205268
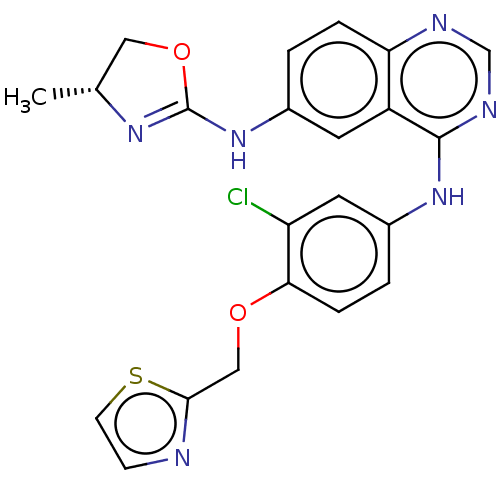
(ARRY-334543 | ARRY-543 | ASLAN-001 | Varlitinib)Show SMILES C[C@@H]1COC(Nc2ccc3ncnc(Nc4ccc(OCc5nccs5)c(Cl)c4)c3c2)=N1 |r,c:34| Show InChI InChI=1S/C22H19ClN6O2S/c1-13-10-31-22(27-13)29-14-2-4-18-16(8-14)21(26-12-25-18)28-15-3-5-19(17(23)9-15)30-11-20-24-6-7-32-20/h2-9,12-13H,10-11H2,1H3,(H,27,29)(H,25,26,28)/t13-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsinghua University
Curated by ChEMBL
| Assay Description
Inhibition of HER2 (unknown origin) |
Bioorg Med Chem 25: 27-37 (2017)
Article DOI: 10.1016/j.bmc.2016.10.006
BindingDB Entry DOI: 10.7270/Q22N548X |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50307768

(7-(4-(3-Ethynylphenylamino)-7-methoxyquinazolin-6-...)Show SMILES COc1cc2ncnc(Nc3cccc(c3)C#C)c2cc1OCCCCCCC(=O)NO Show InChI InChI=1S/C24H26N4O4/c1-3-17-9-8-10-18(13-17)27-24-19-14-22(21(31-2)15-20(19)25-16-26-24)32-12-7-5-4-6-11-23(29)28-30/h1,8-10,13-16,30H,4-7,11-12H2,2H3,(H,28,29)(H,25,26,27) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsinghua University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human amino-terminal GST-His6-fused EGFR expressed in baculovirus expression system assessed as phosphorylation using Bioti... |
Bioorg Med Chem 25: 27-37 (2017)
Article DOI: 10.1016/j.bmc.2016.10.006
BindingDB Entry DOI: 10.7270/Q22N548X |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50205265

(CHEMBL3957055)Show SMILES ONC(=O)CCCCCCn1cc(nn1)-c1ccc2ncnc(Nc3ccc(F)c(Cl)c3)c2c1 Show InChI InChI=1S/C23H23ClFN7O2/c24-18-12-16(7-8-19(18)25)28-23-17-11-15(6-9-20(17)26-14-27-23)21-13-32(31-29-21)10-4-2-1-3-5-22(33)30-34/h6-9,11-14,34H,1-5,10H2,(H,30,33)(H,26,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsinghua University
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 (unknown origin) using acetylated peptide substrate preincubated for 15 mins followed by substrate addition measured after 60 min... |
Bioorg Med Chem 25: 27-37 (2017)
Article DOI: 10.1016/j.bmc.2016.10.006
BindingDB Entry DOI: 10.7270/Q22N548X |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50205264

(CHEMBL3930620)Show SMILES ONC(=O)CCCCCCn1cc(nn1)-c1ccc2ncnc(Nc3cccc(Cl)c3F)c2c1 Show InChI InChI=1S/C23H23ClFN7O2/c24-17-6-5-7-19(22(17)25)28-23-16-12-15(9-10-18(16)26-14-27-23)20-13-32(31-29-20)11-4-2-1-3-8-21(33)30-34/h5-7,9-10,12-14,34H,1-4,8,11H2,(H,30,33)(H,26,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsinghua University
Curated by ChEMBL
| Assay Description
Inhibition of HDAC6 (unknown origin) using acetylated peptide substrate preincubated for 15 mins followed by substrate addition measured after 60 min... |
Bioorg Med Chem 25: 27-37 (2017)
Article DOI: 10.1016/j.bmc.2016.10.006
BindingDB Entry DOI: 10.7270/Q22N548X |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50205260
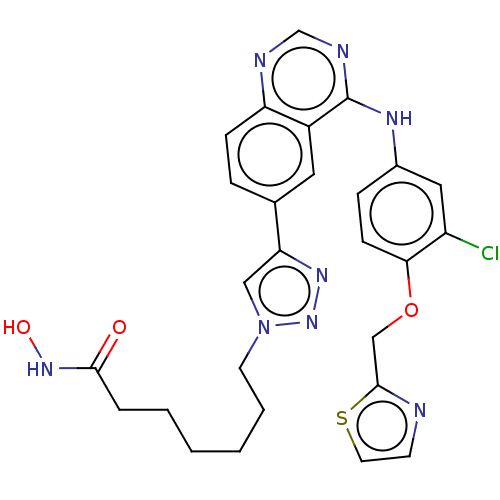
(CHEMBL3902778)Show SMILES ONC(=O)CCCCCCn1cc(nn1)-c1ccc2ncnc(Nc3ccc(OCc4nccs4)c(Cl)c3)c2c1 Show InChI InChI=1S/C27H27ClN8O3S/c28-21-14-19(7-9-24(21)39-16-26-29-10-12-40-26)32-27-20-13-18(6-8-22(20)30-17-31-27)23-15-36(35-33-23)11-4-2-1-3-5-25(37)34-38/h6-10,12-15,17,38H,1-5,11,16H2,(H,34,37)(H,30,31,32) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsinghua University
Curated by ChEMBL
| Assay Description
Inhibition of wild-type EGFR (unknown origin) assessed as remaining ATP level measured after 15 mins by luminescence analysis |
Bioorg Med Chem 25: 27-37 (2017)
Article DOI: 10.1016/j.bmc.2016.10.006
BindingDB Entry DOI: 10.7270/Q22N548X |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50205262

(CHEMBL3929484)Show SMILES ONC(=O)CCCCCn1cc(nn1)-c1ccc2ncnc(Nc3cccc(Cl)c3F)c2c1 Show InChI InChI=1S/C22H21ClFN7O2/c23-16-5-4-6-18(21(16)24)27-22-15-11-14(8-9-17(15)25-13-26-22)19-12-31(30-28-19)10-3-1-2-7-20(32)29-33/h4-6,8-9,11-13,33H,1-3,7,10H2,(H,29,32)(H,25,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsinghua University
Curated by ChEMBL
| Assay Description
Inhibition of HDAC6 (unknown origin) using acetylated peptide substrate preincubated for 15 mins followed by substrate addition measured after 60 min... |
Bioorg Med Chem 25: 27-37 (2017)
Article DOI: 10.1016/j.bmc.2016.10.006
BindingDB Entry DOI: 10.7270/Q22N548X |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50205256
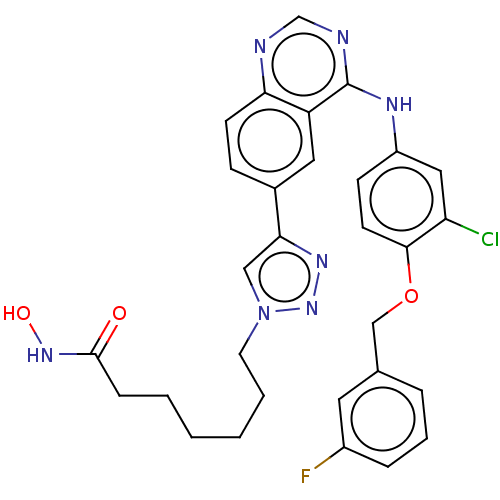
(CHEMBL3911796)Show SMILES ONC(=O)CCCCCCn1cc(nn1)-c1ccc2ncnc(Nc3ccc(OCc4cccc(F)c4)c(Cl)c3)c2c1 Show InChI InChI=1S/C30H29ClFN7O3/c31-25-16-23(10-12-28(25)42-18-20-6-5-7-22(32)14-20)35-30-24-15-21(9-11-26(24)33-19-34-30)27-17-39(38-36-27)13-4-2-1-3-8-29(40)37-41/h5-7,9-12,14-17,19,41H,1-4,8,13,18H2,(H,37,40)(H,33,34,35) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsinghua University
Curated by ChEMBL
| Assay Description
Inhibition of wild-type EGFR (unknown origin) assessed as remaining ATP level measured after 15 mins by luminescence analysis |
Bioorg Med Chem 25: 27-37 (2017)
Article DOI: 10.1016/j.bmc.2016.10.006
BindingDB Entry DOI: 10.7270/Q22N548X |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50205258
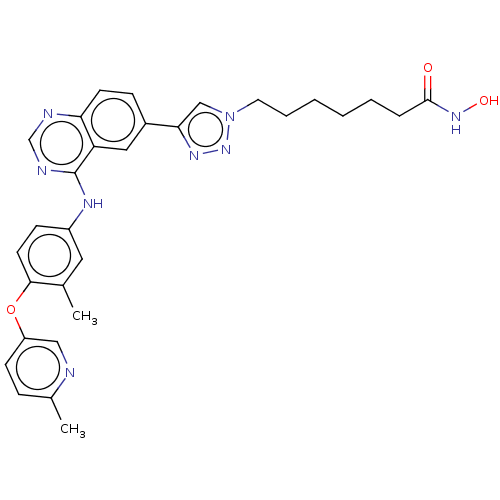
(CHEMBL3893822)Show SMILES Cc1ccc(Oc2ccc(Nc3ncnc4ccc(cc34)-c3cn(CCCCCCC(=O)NO)nn3)cc2C)cn1 Show InChI InChI=1S/C30H32N8O3/c1-20-15-23(10-13-28(20)41-24-11-8-21(2)31-17-24)34-30-25-16-22(9-12-26(25)32-19-33-30)27-18-38(37-35-27)14-6-4-3-5-7-29(39)36-40/h8-13,15-19,40H,3-7,14H2,1-2H3,(H,36,39)(H,32,33,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsinghua University
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 (unknown origin) using acetylated peptide substrate preincubated for 15 mins followed by substrate addition measured after 60 min... |
Bioorg Med Chem 25: 27-37 (2017)
Article DOI: 10.1016/j.bmc.2016.10.006
BindingDB Entry DOI: 10.7270/Q22N548X |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50205260

(CHEMBL3902778)Show SMILES ONC(=O)CCCCCCn1cc(nn1)-c1ccc2ncnc(Nc3ccc(OCc4nccs4)c(Cl)c3)c2c1 Show InChI InChI=1S/C27H27ClN8O3S/c28-21-14-19(7-9-24(21)39-16-26-29-10-12-40-26)32-27-20-13-18(6-8-22(20)30-17-31-27)23-15-36(35-33-23)11-4-2-1-3-5-25(37)34-38/h6-10,12-15,17,38H,1-5,11,16H2,(H,34,37)(H,30,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsinghua University
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 (unknown origin) using acetylated peptide substrate preincubated for 15 mins followed by substrate addition measured after 60 min... |
Bioorg Med Chem 25: 27-37 (2017)
Article DOI: 10.1016/j.bmc.2016.10.006
BindingDB Entry DOI: 10.7270/Q22N548X |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50205257
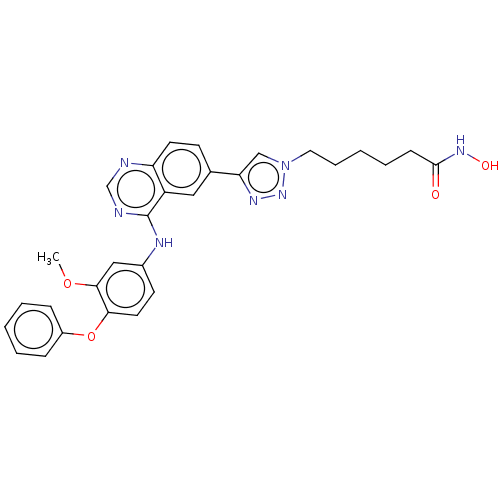
(CHEMBL3921816)Show SMILES COc1cc(Nc2ncnc3ccc(cc23)-c2cn(CCCCCC(=O)NO)nn2)ccc1Oc1ccccc1 Show InChI InChI=1S/C29H29N7O4/c1-39-27-17-21(12-14-26(27)40-22-8-4-2-5-9-22)32-29-23-16-20(11-13-24(23)30-19-31-29)25-18-36(35-33-25)15-7-3-6-10-28(37)34-38/h2,4-5,8-9,11-14,16-19,38H,3,6-7,10,15H2,1H3,(H,34,37)(H,30,31,32) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsinghua University
Curated by ChEMBL
| Assay Description
Inhibition of wild-type EGFR (unknown origin) assessed as remaining ATP level measured after 15 mins by luminescence analysis |
Bioorg Med Chem 25: 27-37 (2017)
Article DOI: 10.1016/j.bmc.2016.10.006
BindingDB Entry DOI: 10.7270/Q22N548X |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50205259

(CHEMBL3930762)Show SMILES Cc1ccc(Oc2ccc(Nc3ncnc4ccc(cc34)-c3cn(CCCCCC(=O)NO)nn3)cc2C)cn1 Show InChI InChI=1S/C29H30N8O3/c1-19-14-22(9-12-27(19)40-23-10-7-20(2)30-16-23)33-29-24-15-21(8-11-25(24)31-18-32-29)26-17-37(36-34-26)13-5-3-4-6-28(38)35-39/h7-12,14-18,39H,3-6,13H2,1-2H3,(H,35,38)(H,31,32,33) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsinghua University
Curated by ChEMBL
| Assay Description
Inhibition of wild-type EGFR (unknown origin) assessed as remaining ATP level measured after 15 mins by luminescence analysis |
Bioorg Med Chem 25: 27-37 (2017)
Article DOI: 10.1016/j.bmc.2016.10.006
BindingDB Entry DOI: 10.7270/Q22N548X |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50205267
(CHEMBL3939771)Show SMILES ONC(=O)CCCCCn1cc(nn1)-c1ccc2ncnc(Nc3ccc(OCc4nccs4)c(Cl)c3)c2c1 Show InChI InChI=1S/C26H25ClN8O3S/c27-20-13-18(6-8-23(20)38-15-25-28-9-11-39-25)31-26-19-12-17(5-7-21(19)29-16-30-26)22-14-35(34-32-22)10-3-1-2-4-24(36)33-37/h5-9,11-14,16,37H,1-4,10,15H2,(H,33,36)(H,29,30,31) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsinghua University
Curated by ChEMBL
| Assay Description
Inhibition of wild-type EGFR (unknown origin) assessed as remaining ATP level measured after 15 mins by luminescence analysis |
Bioorg Med Chem 25: 27-37 (2017)
Article DOI: 10.1016/j.bmc.2016.10.006
BindingDB Entry DOI: 10.7270/Q22N548X |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50205259

(CHEMBL3930762)Show SMILES Cc1ccc(Oc2ccc(Nc3ncnc4ccc(cc34)-c3cn(CCCCCC(=O)NO)nn3)cc2C)cn1 Show InChI InChI=1S/C29H30N8O3/c1-19-14-22(9-12-27(19)40-23-10-7-20(2)30-16-23)33-29-24-15-21(8-11-25(24)31-18-32-29)26-17-37(36-34-26)13-5-3-4-6-28(38)35-39/h7-12,14-18,39H,3-6,13H2,1-2H3,(H,35,38)(H,31,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsinghua University
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 (unknown origin) using acetylated peptide substrate preincubated for 15 mins followed by substrate addition measured after 60 min... |
Bioorg Med Chem 25: 27-37 (2017)
Article DOI: 10.1016/j.bmc.2016.10.006
BindingDB Entry DOI: 10.7270/Q22N548X |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50205268

(ARRY-334543 | ARRY-543 | ASLAN-001 | Varlitinib)Show SMILES C[C@@H]1COC(Nc2ccc3ncnc(Nc4ccc(OCc5nccs5)c(Cl)c4)c3c2)=N1 |r,c:34| Show InChI InChI=1S/C22H19ClN6O2S/c1-13-10-31-22(27-13)29-14-2-4-18-16(8-14)21(26-12-25-18)28-15-3-5-19(17(23)9-15)30-11-20-24-6-7-32-20/h2-9,12-13H,10-11H2,1H3,(H,27,29)(H,25,26,28)/t13-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsinghua University
Curated by ChEMBL
| Assay Description
Inhibition of EGFR (unknown origin) |
Bioorg Med Chem 25: 27-37 (2017)
Article DOI: 10.1016/j.bmc.2016.10.006
BindingDB Entry DOI: 10.7270/Q22N548X |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50205266

(CHEMBL3920583)Show SMILES ONC(=O)CCCCCn1cc(nn1)-c1ccc2ncnc(Nc3ccc(F)c(Cl)c3)c2c1 Show InChI InChI=1S/C22H21ClFN7O2/c23-17-11-15(6-7-18(17)24)27-22-16-10-14(5-8-19(16)25-13-26-22)20-12-31(30-28-20)9-3-1-2-4-21(32)29-33/h5-8,10-13,33H,1-4,9H2,(H,29,32)(H,25,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsinghua University
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 (unknown origin) using acetylated peptide substrate preincubated for 15 mins followed by substrate addition measured after 60 min... |
Bioorg Med Chem 25: 27-37 (2017)
Article DOI: 10.1016/j.bmc.2016.10.006
BindingDB Entry DOI: 10.7270/Q22N548X |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50205267

(CHEMBL3939771)Show SMILES ONC(=O)CCCCCn1cc(nn1)-c1ccc2ncnc(Nc3ccc(OCc4nccs4)c(Cl)c3)c2c1 Show InChI InChI=1S/C26H25ClN8O3S/c27-20-13-18(6-8-23(20)38-15-25-28-9-11-39-25)31-26-19-12-17(5-7-21(19)29-16-30-26)22-14-35(34-32-22)10-3-1-2-4-24(36)33-37/h5-9,11-14,16,37H,1-4,10,15H2,(H,33,36)(H,29,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsinghua University
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 (unknown origin) using acetylated peptide substrate preincubated for 15 mins followed by substrate addition measured after 60 min... |
Bioorg Med Chem 25: 27-37 (2017)
Article DOI: 10.1016/j.bmc.2016.10.006
BindingDB Entry DOI: 10.7270/Q22N548X |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50205256

(CHEMBL3911796)Show SMILES ONC(=O)CCCCCCn1cc(nn1)-c1ccc2ncnc(Nc3ccc(OCc4cccc(F)c4)c(Cl)c3)c2c1 Show InChI InChI=1S/C30H29ClFN7O3/c31-25-16-23(10-12-28(25)42-18-20-6-5-7-22(32)14-20)35-30-24-15-21(9-11-26(24)33-19-34-30)27-17-39(38-36-27)13-4-2-1-3-8-29(40)37-41/h5-7,9-12,14-17,19,41H,1-4,8,13,18H2,(H,37,40)(H,33,34,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 8.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsinghua University
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 (unknown origin) using acetylated peptide substrate preincubated for 15 mins followed by substrate addition measured after 60 min... |
Bioorg Med Chem 25: 27-37 (2017)
Article DOI: 10.1016/j.bmc.2016.10.006
BindingDB Entry DOI: 10.7270/Q22N548X |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50205265

(CHEMBL3957055)Show SMILES ONC(=O)CCCCCCn1cc(nn1)-c1ccc2ncnc(Nc3ccc(F)c(Cl)c3)c2c1 Show InChI InChI=1S/C23H23ClFN7O2/c24-18-12-16(7-8-19(18)25)28-23-17-11-15(6-9-20(17)26-14-27-23)21-13-32(31-29-21)10-4-2-1-3-5-22(33)30-34/h6-9,11-14,34H,1-5,10H2,(H,30,33)(H,26,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsinghua University
Curated by ChEMBL
| Assay Description
Inhibition of HDAC6 (unknown origin) using acetylated peptide substrate preincubated for 15 mins followed by substrate addition measured after 60 min... |
Bioorg Med Chem 25: 27-37 (2017)
Article DOI: 10.1016/j.bmc.2016.10.006
BindingDB Entry DOI: 10.7270/Q22N548X |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM5445

(CHEMBL554 | GW572016 | LAPATINIB DITOSYLATE | Lapa...)Show SMILES CS(=O)(=O)CCNCc1ccc(o1)-c1ccc2ncnc(Nc3ccc(OCc4cccc(F)c4)c(Cl)c3)c2c1 Show InChI InChI=1S/C29H26ClFN4O4S/c1-40(36,37)12-11-32-16-23-7-10-27(39-23)20-5-8-26-24(14-20)29(34-18-33-26)35-22-6-9-28(25(30)15-22)38-17-19-3-2-4-21(31)13-19/h2-10,13-15,18,32H,11-12,16-17H2,1H3,(H,33,34,35) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 9.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsinghua University
Curated by ChEMBL
| Assay Description
Inhibition of HER2 (unknown origin) expressed in baculovirus infected insect cells after 20 mins in presence of ATP by ELISA |
Bioorg Med Chem 25: 27-37 (2017)
Article DOI: 10.1016/j.bmc.2016.10.006
BindingDB Entry DOI: 10.7270/Q22N548X |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50205266

(CHEMBL3920583)Show SMILES ONC(=O)CCCCCn1cc(nn1)-c1ccc2ncnc(Nc3ccc(F)c(Cl)c3)c2c1 Show InChI InChI=1S/C22H21ClFN7O2/c23-17-11-15(6-7-18(17)24)27-22-16-10-14(5-8-19(16)25-13-26-22)20-12-31(30-28-20)9-3-1-2-4-21(32)29-33/h5-8,10-13,33H,1-4,9H2,(H,29,32)(H,25,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsinghua University
Curated by ChEMBL
| Assay Description
Inhibition of HDAC6 (unknown origin) using acetylated peptide substrate preincubated for 15 mins followed by substrate addition measured after 60 min... |
Bioorg Med Chem 25: 27-37 (2017)
Article DOI: 10.1016/j.bmc.2016.10.006
BindingDB Entry DOI: 10.7270/Q22N548X |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM5447

(CHEMBL939 | GEFITINIB | Iressa | N-(3-Chloro-4-flu...)Show SMILES COc1cc2ncnc(Nc3ccc(F)c(Cl)c3)c2cc1OCCCN1CCOCC1 Show InChI InChI=1S/C22H24ClFN4O3/c1-29-20-13-19-16(12-21(20)31-8-2-5-28-6-9-30-10-7-28)22(26-14-25-19)27-15-3-4-18(24)17(23)11-15/h3-4,11-14H,2,5-10H2,1H3,(H,25,26,27) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | 9.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsinghua University
Curated by ChEMBL
| Assay Description
Inhibition of wild-type EGFR (unknown origin) assessed as remaining ATP level measured after 15 mins by luminescence analysis |
Bioorg Med Chem 25: 27-37 (2017)
Article DOI: 10.1016/j.bmc.2016.10.006
BindingDB Entry DOI: 10.7270/Q22N548X |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50205258

(CHEMBL3893822)Show SMILES Cc1ccc(Oc2ccc(Nc3ncnc4ccc(cc34)-c3cn(CCCCCCC(=O)NO)nn3)cc2C)cn1 Show InChI InChI=1S/C30H32N8O3/c1-20-15-23(10-13-28(20)41-24-11-8-21(2)31-17-24)34-30-25-16-22(9-12-26(25)32-19-33-30)27-18-38(37-35-27)14-6-4-3-5-7-29(39)36-40/h8-13,15-19,40H,3-7,14H2,1-2H3,(H,36,39)(H,32,33,34) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsinghua University
Curated by ChEMBL
| Assay Description
Inhibition of wild-type EGFR (unknown origin) assessed as remaining ATP level measured after 15 mins by luminescence analysis |
Bioorg Med Chem 25: 27-37 (2017)
Article DOI: 10.1016/j.bmc.2016.10.006
BindingDB Entry DOI: 10.7270/Q22N548X |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM31340
(2-methoxy-N-[(E)-3-[4-[3-methyl-4-(6-methylpyridin...)Show SMILES COCC(=O)NC\C=C\c1ccc2ncnc(Nc3ccc(Oc4ccc(C)nc4)c(C)c3)c2c1 Show InChI InChI=1S/C27H27N5O3/c1-18-13-21(8-11-25(18)35-22-9-6-19(2)29-15-22)32-27-23-14-20(7-10-24(23)30-17-31-27)5-4-12-28-26(33)16-34-3/h4-11,13-15,17H,12,16H2,1-3H3,(H,28,33)(H,30,31,32)/b5-4+ | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsinghua University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human GST-fused HER2 (675 to 1255 residues) assessed as phosphotyrosine formation using poly(Glu:Tyr, 4:1) as substrate and... |
Bioorg Med Chem 25: 27-37 (2017)
Article DOI: 10.1016/j.bmc.2016.10.006
BindingDB Entry DOI: 10.7270/Q22N548X |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM50205255

(CHEMBL3890526)Show SMILES [H][C@@]12CN(Cc3ccc(cc3)-c3ccc4ncnc(Nc5ccc(Oc6ccccc6)c(OC)c5)c4c3)C[C@]1([H])[C@H]2CO |r| Show InChI InChI=1S/C34H32N4O3/c1-40-33-16-25(12-14-32(33)41-26-5-3-2-4-6-26)37-34-27-15-24(11-13-31(27)35-21-36-34)23-9-7-22(8-10-23)17-38-18-28-29(19-38)30(28)20-39/h2-16,21,28-30,39H,17-20H2,1H3,(H,35,36,37)/t28-,29+,30+ | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsinghua University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human GST-fused HER2 (675 to 1255 residues) assessed as phosphotyrosine formation using poly(Glu:Tyr, 4:1) as substrate and... |
Bioorg Med Chem 25: 27-37 (2017)
Article DOI: 10.1016/j.bmc.2016.10.006
BindingDB Entry DOI: 10.7270/Q22N548X |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM19149

(CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...)Show InChI InChI=1S/C14H20N2O3/c17-13(15-12-8-4-3-5-9-12)10-6-1-2-7-11-14(18)16-19/h3-5,8-9,19H,1-2,6-7,10-11H2,(H,15,17)(H,16,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsinghua University
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 (unknown origin) using acetylated peptide substrate preincubated for 15 mins followed by substrate addition measured after 60 min... |
Bioorg Med Chem 25: 27-37 (2017)
Article DOI: 10.1016/j.bmc.2016.10.006
BindingDB Entry DOI: 10.7270/Q22N548X |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM5445

(CHEMBL554 | GW572016 | LAPATINIB DITOSYLATE | Lapa...)Show SMILES CS(=O)(=O)CCNCc1ccc(o1)-c1ccc2ncnc(Nc3ccc(OCc4cccc(F)c4)c(Cl)c3)c2c1 Show InChI InChI=1S/C29H26ClFN4O4S/c1-40(36,37)12-11-32-16-23-7-10-27(39-23)20-5-8-26-24(14-20)29(34-18-33-26)35-22-6-9-28(25(30)15-22)38-17-19-3-2-4-21(31)13-19/h2-10,13-15,18,32H,11-12,16-17H2,1H3,(H,33,34,35) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsinghua University
Curated by ChEMBL
| Assay Description
Inhibition of EGFR (unknown origin) expressed in baculovirus expression system using Biotin-(amino hexonoic acid)-EEEEYFELVAKKKCONH2 as substrate and... |
Bioorg Med Chem 25: 27-37 (2017)
Article DOI: 10.1016/j.bmc.2016.10.006
BindingDB Entry DOI: 10.7270/Q22N548X |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM19149

(CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...)Show InChI InChI=1S/C14H20N2O3/c17-13(15-12-8-4-3-5-9-12)10-6-1-2-7-11-14(18)16-19/h3-5,8-9,19H,1-2,6-7,10-11H2,(H,15,17)(H,16,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsinghua University
Curated by ChEMBL
| Assay Description
Inhibition of HDAC6 (unknown origin) using acetylated peptide substrate preincubated for 15 mins followed by substrate addition measured after 60 min... |
Bioorg Med Chem 25: 27-37 (2017)
Article DOI: 10.1016/j.bmc.2016.10.006
BindingDB Entry DOI: 10.7270/Q22N548X |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50205261

(CHEMBL3982710)Show SMILES COc1cc(Nc2ncnc3ccc(cc23)-c2cn(CCCCCCC(=O)NO)nn2)ccc1Oc1ccccc1 Show InChI InChI=1S/C30H31N7O4/c1-40-28-18-22(13-15-27(28)41-23-9-5-4-6-10-23)33-30-24-17-21(12-14-25(24)31-20-32-30)26-19-37(36-34-26)16-8-3-2-7-11-29(38)35-39/h4-6,9-10,12-15,17-20,39H,2-3,7-8,11,16H2,1H3,(H,35,38)(H,31,32,33) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsinghua University
Curated by ChEMBL
| Assay Description
Inhibition of wild-type EGFR (unknown origin) assessed as remaining ATP level measured after 15 mins by luminescence analysis |
Bioorg Med Chem 25: 27-37 (2017)
Article DOI: 10.1016/j.bmc.2016.10.006
BindingDB Entry DOI: 10.7270/Q22N548X |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50437353
(CHEMBL2408045)Show SMILES CNC(=O)CN1CCC(CC1)Oc1cc2c(Nc3cccc(Cl)c3F)ncnc2cc1OC Show InChI InChI=1S/C23H25ClFN5O3/c1-26-21(31)12-30-8-6-14(7-9-30)33-20-10-15-18(11-19(20)32-2)27-13-28-23(15)29-17-5-3-4-16(24)22(17)25/h3-5,10-11,13-14H,6-9,12H2,1-2H3,(H,26,31)(H,27,28,29) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsinghua University
Curated by ChEMBL
| Assay Description
Inhibition of human EGFR expressed in baculovirus/Sf21 system in presence of ATP by ELISA |
Bioorg Med Chem 25: 27-37 (2017)
Article DOI: 10.1016/j.bmc.2016.10.006
BindingDB Entry DOI: 10.7270/Q22N548X |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50205261

(CHEMBL3982710)Show SMILES COc1cc(Nc2ncnc3ccc(cc23)-c2cn(CCCCCCC(=O)NO)nn2)ccc1Oc1ccccc1 Show InChI InChI=1S/C30H31N7O4/c1-40-28-18-22(13-15-27(28)41-23-9-5-4-6-10-23)33-30-24-17-21(12-14-25(24)31-20-32-30)26-19-37(36-34-26)16-8-3-2-7-11-29(38)35-39/h4-6,9-10,12-15,17-20,39H,2-3,7-8,11,16H2,1H3,(H,35,38)(H,31,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsinghua University
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 (unknown origin) using acetylated peptide substrate preincubated for 15 mins followed by substrate addition measured after 60 min... |
Bioorg Med Chem 25: 27-37 (2017)
Article DOI: 10.1016/j.bmc.2016.10.006
BindingDB Entry DOI: 10.7270/Q22N548X |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50205259

(CHEMBL3930762)Show SMILES Cc1ccc(Oc2ccc(Nc3ncnc4ccc(cc34)-c3cn(CCCCCC(=O)NO)nn3)cc2C)cn1 Show InChI InChI=1S/C29H30N8O3/c1-19-14-22(9-12-27(19)40-23-10-7-20(2)30-16-23)33-29-24-15-21(8-11-25(24)31-18-32-29)26-17-37(36-34-26)13-5-3-4-6-28(38)35-39/h7-12,14-18,39H,3-6,13H2,1-2H3,(H,35,38)(H,31,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsinghua University
Curated by ChEMBL
| Assay Description
Inhibition of HDAC6 (unknown origin) using acetylated peptide substrate preincubated for 15 mins followed by substrate addition measured after 60 min... |
Bioorg Med Chem 25: 27-37 (2017)
Article DOI: 10.1016/j.bmc.2016.10.006
BindingDB Entry DOI: 10.7270/Q22N548X |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50205258

(CHEMBL3893822)Show SMILES Cc1ccc(Oc2ccc(Nc3ncnc4ccc(cc34)-c3cn(CCCCCCC(=O)NO)nn3)cc2C)cn1 Show InChI InChI=1S/C30H32N8O3/c1-20-15-23(10-13-28(20)41-24-11-8-21(2)31-17-24)34-30-25-16-22(9-12-26(25)32-19-33-30)27-18-38(37-35-27)14-6-4-3-5-7-29(39)36-40/h8-13,15-19,40H,3-7,14H2,1-2H3,(H,36,39)(H,32,33,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsinghua University
Curated by ChEMBL
| Assay Description
Inhibition of HDAC6 (unknown origin) using acetylated peptide substrate preincubated for 15 mins followed by substrate addition measured after 60 min... |
Bioorg Med Chem 25: 27-37 (2017)
Article DOI: 10.1016/j.bmc.2016.10.006
BindingDB Entry DOI: 10.7270/Q22N548X |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50205267

(CHEMBL3939771)Show SMILES ONC(=O)CCCCCn1cc(nn1)-c1ccc2ncnc(Nc3ccc(OCc4nccs4)c(Cl)c3)c2c1 Show InChI InChI=1S/C26H25ClN8O3S/c27-20-13-18(6-8-23(20)38-15-25-28-9-11-39-25)31-26-19-12-17(5-7-21(19)29-16-30-26)22-14-35(34-32-22)10-3-1-2-4-24(36)33-37/h5-9,11-14,16,37H,1-4,10,15H2,(H,33,36)(H,29,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsinghua University
Curated by ChEMBL
| Assay Description
Inhibition of HDAC6 (unknown origin) using acetylated peptide substrate preincubated for 15 mins followed by substrate addition measured after 60 min... |
Bioorg Med Chem 25: 27-37 (2017)
Article DOI: 10.1016/j.bmc.2016.10.006
BindingDB Entry DOI: 10.7270/Q22N548X |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM50437353

(CHEMBL2408045)Show SMILES CNC(=O)CN1CCC(CC1)Oc1cc2c(Nc3cccc(Cl)c3F)ncnc2cc1OC Show InChI InChI=1S/C23H25ClFN5O3/c1-26-21(31)12-30-8-6-14(7-9-30)33-20-10-15-18(11-19(20)32-2)27-13-28-23(15)29-17-5-3-4-16(24)22(17)25/h3-5,10-11,13-14H,6-9,12H2,1-2H3,(H,26,31)(H,27,28,29) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsinghua University
Curated by ChEMBL
| Assay Description
Inhibition of human HER2 expressed in baculovirus/Sf21 system in presence of ATP by ELISA |
Bioorg Med Chem 25: 27-37 (2017)
Article DOI: 10.1016/j.bmc.2016.10.006
BindingDB Entry DOI: 10.7270/Q22N548X |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50205260

(CHEMBL3902778)Show SMILES ONC(=O)CCCCCCn1cc(nn1)-c1ccc2ncnc(Nc3ccc(OCc4nccs4)c(Cl)c3)c2c1 Show InChI InChI=1S/C27H27ClN8O3S/c28-21-14-19(7-9-24(21)39-16-26-29-10-12-40-26)32-27-20-13-18(6-8-22(20)30-17-31-27)23-15-36(35-33-23)11-4-2-1-3-5-25(37)34-38/h6-10,12-15,17,38H,1-5,11,16H2,(H,34,37)(H,30,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsinghua University
Curated by ChEMBL
| Assay Description
Inhibition of HDAC6 (unknown origin) using acetylated peptide substrate preincubated for 15 mins followed by substrate addition measured after 60 min... |
Bioorg Med Chem 25: 27-37 (2017)
Article DOI: 10.1016/j.bmc.2016.10.006
BindingDB Entry DOI: 10.7270/Q22N548X |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM50205256

(CHEMBL3911796)Show SMILES ONC(=O)CCCCCCn1cc(nn1)-c1ccc2ncnc(Nc3ccc(OCc4cccc(F)c4)c(Cl)c3)c2c1 Show InChI InChI=1S/C30H29ClFN7O3/c31-25-16-23(10-12-28(25)42-18-20-6-5-7-22(32)14-20)35-30-24-15-21(9-11-26(24)33-19-34-30)27-17-39(38-36-27)13-4-2-1-3-8-29(40)37-41/h5-7,9-12,14-17,19,41H,1-4,8,13,18H2,(H,37,40)(H,33,34,35) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsinghua University
Curated by ChEMBL
| Assay Description
Inhibition of HER2 (unknown origin) assessed as remaining ATP level measured after 15 mins by luminescence analysis |
Bioorg Med Chem 25: 27-37 (2017)
Article DOI: 10.1016/j.bmc.2016.10.006
BindingDB Entry DOI: 10.7270/Q22N548X |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM50307768

(7-(4-(3-Ethynylphenylamino)-7-methoxyquinazolin-6-...)Show SMILES COc1cc2ncnc(Nc3cccc(c3)C#C)c2cc1OCCCCCCC(=O)NO Show InChI InChI=1S/C24H26N4O4/c1-3-17-9-8-10-18(13-17)27-24-19-14-22(21(31-2)15-20(19)25-16-26-24)32-12-7-5-4-6-11-23(29)28-30/h1,8-10,13-16,30H,4-7,11-12H2,2H3,(H,28,29)(H,25,26,27) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsinghua University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human amino-terminal GST-fused HER2 (Lys676-Val1255) expressed in baculovirus expression system using Biotin-FLT3 (Tyr589) ... |
Bioorg Med Chem 25: 27-37 (2017)
Article DOI: 10.1016/j.bmc.2016.10.006
BindingDB Entry DOI: 10.7270/Q22N548X |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50205261

(CHEMBL3982710)Show SMILES COc1cc(Nc2ncnc3ccc(cc23)-c2cn(CCCCCCC(=O)NO)nn2)ccc1Oc1ccccc1 Show InChI InChI=1S/C30H31N7O4/c1-40-28-18-22(13-15-27(28)41-23-9-5-4-6-10-23)33-30-24-17-21(12-14-25(24)31-20-32-30)26-19-37(36-34-26)16-8-3-2-7-11-29(38)35-39/h4-6,9-10,12-15,17-20,39H,2-3,7-8,11,16H2,1H3,(H,35,38)(H,31,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsinghua University
Curated by ChEMBL
| Assay Description
Inhibition of HDAC6 (unknown origin) using acetylated peptide substrate preincubated for 15 mins followed by substrate addition measured after 60 min... |
Bioorg Med Chem 25: 27-37 (2017)
Article DOI: 10.1016/j.bmc.2016.10.006
BindingDB Entry DOI: 10.7270/Q22N548X |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50205257

(CHEMBL3921816)Show SMILES COc1cc(Nc2ncnc3ccc(cc23)-c2cn(CCCCCC(=O)NO)nn2)ccc1Oc1ccccc1 Show InChI InChI=1S/C29H29N7O4/c1-39-27-17-21(12-14-26(27)40-22-8-4-2-5-9-22)32-29-23-16-20(11-13-24(23)30-19-31-29)25-18-36(35-33-25)15-7-3-6-10-28(37)34-38/h2,4-5,8-9,11-14,16-19,38H,3,6-7,10,15H2,1H3,(H,34,37)(H,30,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsinghua University
Curated by ChEMBL
| Assay Description
Inhibition of HDAC6 (unknown origin) using acetylated peptide substrate preincubated for 15 mins followed by substrate addition measured after 60 min... |
Bioorg Med Chem 25: 27-37 (2017)
Article DOI: 10.1016/j.bmc.2016.10.006
BindingDB Entry DOI: 10.7270/Q22N548X |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50205256

(CHEMBL3911796)Show SMILES ONC(=O)CCCCCCn1cc(nn1)-c1ccc2ncnc(Nc3ccc(OCc4cccc(F)c4)c(Cl)c3)c2c1 Show InChI InChI=1S/C30H29ClFN7O3/c31-25-16-23(10-12-28(25)42-18-20-6-5-7-22(32)14-20)35-30-24-15-21(9-11-26(24)33-19-34-30)27-17-39(38-36-27)13-4-2-1-3-8-29(40)37-41/h5-7,9-12,14-17,19,41H,1-4,8,13,18H2,(H,37,40)(H,33,34,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsinghua University
Curated by ChEMBL
| Assay Description
Inhibition of HDAC6 (unknown origin) using acetylated peptide substrate preincubated for 15 mins followed by substrate addition measured after 60 min... |
Bioorg Med Chem 25: 27-37 (2017)
Article DOI: 10.1016/j.bmc.2016.10.006
BindingDB Entry DOI: 10.7270/Q22N548X |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM5445

(CHEMBL554 | GW572016 | LAPATINIB DITOSYLATE | Lapa...)Show SMILES CS(=O)(=O)CCNCc1ccc(o1)-c1ccc2ncnc(Nc3ccc(OCc4cccc(F)c4)c(Cl)c3)c2c1 Show InChI InChI=1S/C29H26ClFN4O4S/c1-40(36,37)12-11-32-16-23-7-10-27(39-23)20-5-8-26-24(14-20)29(34-18-33-26)35-22-6-9-28(25(30)15-22)38-17-19-3-2-4-21(31)13-19/h2-10,13-15,18,32H,11-12,16-17H2,1H3,(H,33,34,35) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsinghua University
Curated by ChEMBL
| Assay Description
Inhibition of HER2 (unknown origin) assessed as remaining ATP level measured after 15 mins by luminescence analysis |
Bioorg Med Chem 25: 27-37 (2017)
Article DOI: 10.1016/j.bmc.2016.10.006
BindingDB Entry DOI: 10.7270/Q22N548X |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50205257

(CHEMBL3921816)Show SMILES COc1cc(Nc2ncnc3ccc(cc23)-c2cn(CCCCCC(=O)NO)nn2)ccc1Oc1ccccc1 Show InChI InChI=1S/C29H29N7O4/c1-39-27-17-21(12-14-26(27)40-22-8-4-2-5-9-22)32-29-23-16-20(11-13-24(23)30-19-31-29)25-18-36(35-33-25)15-7-3-6-10-28(37)34-38/h2,4-5,8-9,11-14,16-19,38H,3,6-7,10,15H2,1H3,(H,34,37)(H,30,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsinghua University
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 (unknown origin) using acetylated peptide substrate preincubated for 15 mins followed by substrate addition measured after 60 min... |
Bioorg Med Chem 25: 27-37 (2017)
Article DOI: 10.1016/j.bmc.2016.10.006
BindingDB Entry DOI: 10.7270/Q22N548X |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM5447

(CHEMBL939 | GEFITINIB | Iressa | N-(3-Chloro-4-flu...)Show SMILES COc1cc2ncnc(Nc3ccc(F)c(Cl)c3)c2cc1OCCCN1CCOCC1 Show InChI InChI=1S/C22H24ClFN4O3/c1-29-20-13-19-16(12-21(20)31-8-2-5-28-6-9-30-10-7-28)22(26-14-25-19)27-15-3-4-18(24)17(23)11-15/h3-4,11-14H,2,5-10H2,1H3,(H,25,26,27) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsinghua University
Curated by ChEMBL
| Assay Description
Inhibition of EGFR in human A431 cell membranes preincubated for 30 mins prior to addition of peptide substrate and [gamma32]-ATP measured after 10 m... |
Bioorg Med Chem 25: 27-37 (2017)
Article DOI: 10.1016/j.bmc.2016.10.006
BindingDB Entry DOI: 10.7270/Q22N548X |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50205263

(CHEMBL3964033)Show SMILES ONC(=O)CCCCCn1cc(nn1)-c1ccc2ncnc(Nc3ccc(OCc4cccc(F)c4)c(Cl)c3)c2c1 Show InChI InChI=1S/C29H27ClFN7O3/c30-24-15-22(9-11-27(24)41-17-19-5-4-6-21(31)13-19)34-29-23-14-20(8-10-25(23)32-18-33-29)26-16-38(37-35-26)12-3-1-2-7-28(39)36-40/h4-6,8-11,13-16,18,40H,1-3,7,12,17H2,(H,36,39)(H,32,33,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsinghua University
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 (unknown origin) using acetylated peptide substrate preincubated for 15 mins followed by substrate addition measured after 60 min... |
Bioorg Med Chem 25: 27-37 (2017)
Article DOI: 10.1016/j.bmc.2016.10.006
BindingDB Entry DOI: 10.7270/Q22N548X |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50205263

(CHEMBL3964033)Show SMILES ONC(=O)CCCCCn1cc(nn1)-c1ccc2ncnc(Nc3ccc(OCc4cccc(F)c4)c(Cl)c3)c2c1 Show InChI InChI=1S/C29H27ClFN7O3/c30-24-15-22(9-11-27(24)41-17-19-5-4-6-21(31)13-19)34-29-23-14-20(8-10-25(23)32-18-33-29)26-16-38(37-35-26)12-3-1-2-7-28(39)36-40/h4-6,8-11,13-16,18,40H,1-3,7,12,17H2,(H,36,39)(H,32,33,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 94 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsinghua University
Curated by ChEMBL
| Assay Description
Inhibition of HDAC6 (unknown origin) using acetylated peptide substrate preincubated for 15 mins followed by substrate addition measured after 60 min... |
Bioorg Med Chem 25: 27-37 (2017)
Article DOI: 10.1016/j.bmc.2016.10.006
BindingDB Entry DOI: 10.7270/Q22N548X |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM50205266

(CHEMBL3920583)Show SMILES ONC(=O)CCCCCn1cc(nn1)-c1ccc2ncnc(Nc3ccc(F)c(Cl)c3)c2c1 Show InChI InChI=1S/C22H21ClFN7O2/c23-17-11-15(6-7-18(17)24)27-22-16-10-14(5-8-19(16)25-13-26-22)20-12-31(30-28-20)9-3-1-2-4-21(32)29-33/h5-8,10-13,33H,1-4,9H2,(H,29,32)(H,25,26,27) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 166 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsinghua University
Curated by ChEMBL
| Assay Description
Inhibition of HER2 (unknown origin) assessed as remaining ATP level measured after 15 mins by luminescence analysis |
Bioorg Med Chem 25: 27-37 (2017)
Article DOI: 10.1016/j.bmc.2016.10.006
BindingDB Entry DOI: 10.7270/Q22N548X |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM50205264

(CHEMBL3930620)Show SMILES ONC(=O)CCCCCCn1cc(nn1)-c1ccc2ncnc(Nc3cccc(Cl)c3F)c2c1 Show InChI InChI=1S/C23H23ClFN7O2/c24-17-6-5-7-19(22(17)25)28-23-16-12-15(9-10-18(16)26-14-27-23)20-13-32(31-29-20)11-4-2-1-3-8-21(33)30-34/h5-7,9-10,12-14,34H,1-4,8,11H2,(H,30,33)(H,26,27,28) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 175 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsinghua University
Curated by ChEMBL
| Assay Description
Inhibition of HER2 (unknown origin) assessed as remaining ATP level measured after 15 mins by luminescence analysis |
Bioorg Med Chem 25: 27-37 (2017)
Article DOI: 10.1016/j.bmc.2016.10.006
BindingDB Entry DOI: 10.7270/Q22N548X |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM50205265

(CHEMBL3957055)Show SMILES ONC(=O)CCCCCCn1cc(nn1)-c1ccc2ncnc(Nc3ccc(F)c(Cl)c3)c2c1 Show InChI InChI=1S/C23H23ClFN7O2/c24-18-12-16(7-8-19(18)25)28-23-17-11-15(6-9-20(17)26-14-27-23)21-13-32(31-29-21)10-4-2-1-3-5-22(33)30-34/h6-9,11-14,34H,1-5,10H2,(H,30,33)(H,26,27,28) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 177 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsinghua University
Curated by ChEMBL
| Assay Description
Inhibition of HER2 (unknown origin) assessed as remaining ATP level measured after 15 mins by luminescence analysis |
Bioorg Med Chem 25: 27-37 (2017)
Article DOI: 10.1016/j.bmc.2016.10.006
BindingDB Entry DOI: 10.7270/Q22N548X |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM50205263

(CHEMBL3964033)Show SMILES ONC(=O)CCCCCn1cc(nn1)-c1ccc2ncnc(Nc3ccc(OCc4cccc(F)c4)c(Cl)c3)c2c1 Show InChI InChI=1S/C29H27ClFN7O3/c30-24-15-22(9-11-27(24)41-17-19-5-4-6-21(31)13-19)34-29-23-14-20(8-10-25(23)32-18-33-29)26-16-38(37-35-26)12-3-1-2-7-28(39)36-40/h4-6,8-11,13-16,18,40H,1-3,7,12,17H2,(H,36,39)(H,32,33,34) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 193 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsinghua University
Curated by ChEMBL
| Assay Description
Inhibition of HER2 (unknown origin) assessed as remaining ATP level measured after 15 mins by luminescence analysis |
Bioorg Med Chem 25: 27-37 (2017)
Article DOI: 10.1016/j.bmc.2016.10.006
BindingDB Entry DOI: 10.7270/Q22N548X |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM50205262

(CHEMBL3929484)Show SMILES ONC(=O)CCCCCn1cc(nn1)-c1ccc2ncnc(Nc3cccc(Cl)c3F)c2c1 Show InChI InChI=1S/C22H21ClFN7O2/c23-16-5-4-6-18(21(16)24)27-22-15-11-14(8-9-17(15)25-13-26-22)19-12-31(30-28-19)10-3-1-2-7-20(32)29-33/h4-6,8-9,11-13,33H,1-3,7,10H2,(H,29,32)(H,25,26,27) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 386 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsinghua University
Curated by ChEMBL
| Assay Description
Inhibition of HER2 (unknown origin) assessed as remaining ATP level measured after 15 mins by luminescence analysis |
Bioorg Med Chem 25: 27-37 (2017)
Article DOI: 10.1016/j.bmc.2016.10.006
BindingDB Entry DOI: 10.7270/Q22N548X |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM50205267

(CHEMBL3939771)Show SMILES ONC(=O)CCCCCn1cc(nn1)-c1ccc2ncnc(Nc3ccc(OCc4nccs4)c(Cl)c3)c2c1 Show InChI InChI=1S/C26H25ClN8O3S/c27-20-13-18(6-8-23(20)38-15-25-28-9-11-39-25)31-26-19-12-17(5-7-21(19)29-16-30-26)22-14-35(34-32-22)10-3-1-2-4-24(36)33-37/h5-9,11-14,16,37H,1-4,10,15H2,(H,33,36)(H,29,30,31) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 537 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsinghua University
Curated by ChEMBL
| Assay Description
Inhibition of HER2 (unknown origin) assessed as remaining ATP level measured after 15 mins by luminescence analysis |
Bioorg Med Chem 25: 27-37 (2017)
Article DOI: 10.1016/j.bmc.2016.10.006
BindingDB Entry DOI: 10.7270/Q22N548X |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50205255

(CHEMBL3890526)Show SMILES [H][C@@]12CN(Cc3ccc(cc3)-c3ccc4ncnc(Nc5ccc(Oc6ccccc6)c(OC)c5)c4c3)C[C@]1([H])[C@H]2CO |r| Show InChI InChI=1S/C34H32N4O3/c1-40-33-16-25(12-14-32(33)41-26-5-3-2-4-6-26)37-34-27-15-24(11-13-31(27)35-21-36-34)23-9-7-22(8-10-23)17-38-18-28-29(19-38)30(28)20-39/h2-16,21,28-30,39H,17-20H2,1H3,(H,35,36,37)/t28-,29+,30+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 670 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsinghua University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human GST-fused EGFR (668 to 1211 residues) assessed as phosphotyrosine formation using poly(Glu:Tyr, 4:1) as substrate and... |
Bioorg Med Chem 25: 27-37 (2017)
Article DOI: 10.1016/j.bmc.2016.10.006
BindingDB Entry DOI: 10.7270/Q22N548X |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM50205260

(CHEMBL3902778)Show SMILES ONC(=O)CCCCCCn1cc(nn1)-c1ccc2ncnc(Nc3ccc(OCc4nccs4)c(Cl)c3)c2c1 Show InChI InChI=1S/C27H27ClN8O3S/c28-21-14-19(7-9-24(21)39-16-26-29-10-12-40-26)32-27-20-13-18(6-8-22(20)30-17-31-27)23-15-36(35-33-23)11-4-2-1-3-5-25(37)34-38/h6-10,12-15,17,38H,1-5,11,16H2,(H,34,37)(H,30,31,32) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 707 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsinghua University
Curated by ChEMBL
| Assay Description
Inhibition of HER2 (unknown origin) assessed as remaining ATP level measured after 15 mins by luminescence analysis |
Bioorg Med Chem 25: 27-37 (2017)
Article DOI: 10.1016/j.bmc.2016.10.006
BindingDB Entry DOI: 10.7270/Q22N548X |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM50205259

(CHEMBL3930762)Show SMILES Cc1ccc(Oc2ccc(Nc3ncnc4ccc(cc34)-c3cn(CCCCCC(=O)NO)nn3)cc2C)cn1 Show InChI InChI=1S/C29H30N8O3/c1-19-14-22(9-12-27(19)40-23-10-7-20(2)30-16-23)33-29-24-15-21(8-11-25(24)31-18-32-29)26-17-37(36-34-26)13-5-3-4-6-28(38)35-39/h7-12,14-18,39H,3-6,13H2,1-2H3,(H,35,38)(H,31,32,33) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsinghua University
Curated by ChEMBL
| Assay Description
Inhibition of HER2 (unknown origin) assessed as remaining ATP level measured after 15 mins by luminescence analysis |
Bioorg Med Chem 25: 27-37 (2017)
Article DOI: 10.1016/j.bmc.2016.10.006
BindingDB Entry DOI: 10.7270/Q22N548X |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM50205258

(CHEMBL3893822)Show SMILES Cc1ccc(Oc2ccc(Nc3ncnc4ccc(cc34)-c3cn(CCCCCCC(=O)NO)nn3)cc2C)cn1 Show InChI InChI=1S/C30H32N8O3/c1-20-15-23(10-13-28(20)41-24-11-8-21(2)31-17-24)34-30-25-16-22(9-12-26(25)32-19-33-30)27-18-38(37-35-27)14-6-4-3-5-7-29(39)36-40/h8-13,15-19,40H,3-7,14H2,1-2H3,(H,36,39)(H,32,33,34) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsinghua University
Curated by ChEMBL
| Assay Description
Inhibition of HER2 (unknown origin) assessed as remaining ATP level measured after 15 mins by luminescence analysis |
Bioorg Med Chem 25: 27-37 (2017)
Article DOI: 10.1016/j.bmc.2016.10.006
BindingDB Entry DOI: 10.7270/Q22N548X |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM50205261

(CHEMBL3982710)Show SMILES COc1cc(Nc2ncnc3ccc(cc23)-c2cn(CCCCCCC(=O)NO)nn2)ccc1Oc1ccccc1 Show InChI InChI=1S/C30H31N7O4/c1-40-28-18-22(13-15-27(28)41-23-9-5-4-6-10-23)33-30-24-17-21(12-14-25(24)31-20-32-30)26-19-37(36-34-26)16-8-3-2-7-11-29(38)35-39/h4-6,9-10,12-15,17-20,39H,2-3,7-8,11,16H2,1H3,(H,35,38)(H,31,32,33) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsinghua University
Curated by ChEMBL
| Assay Description
Inhibition of HER2 (unknown origin) assessed as remaining ATP level measured after 15 mins by luminescence analysis |
Bioorg Med Chem 25: 27-37 (2017)
Article DOI: 10.1016/j.bmc.2016.10.006
BindingDB Entry DOI: 10.7270/Q22N548X |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM50205257

(CHEMBL3921816)Show SMILES COc1cc(Nc2ncnc3ccc(cc23)-c2cn(CCCCCC(=O)NO)nn2)ccc1Oc1ccccc1 Show InChI InChI=1S/C29H29N7O4/c1-39-27-17-21(12-14-26(27)40-22-8-4-2-5-9-22)32-29-23-16-20(11-13-24(23)30-19-31-29)25-18-36(35-33-25)15-7-3-6-10-28(37)34-38/h2,4-5,8-9,11-14,16-19,38H,3,6-7,10,15H2,1H3,(H,34,37)(H,30,31,32) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsinghua University
Curated by ChEMBL
| Assay Description
Inhibition of HER2 (unknown origin) assessed as remaining ATP level measured after 15 mins by luminescence analysis |
Bioorg Med Chem 25: 27-37 (2017)
Article DOI: 10.1016/j.bmc.2016.10.006
BindingDB Entry DOI: 10.7270/Q22N548X |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM5447

(CHEMBL939 | GEFITINIB | Iressa | N-(3-Chloro-4-flu...)Show SMILES COc1cc2ncnc(Nc3ccc(F)c(Cl)c3)c2cc1OCCCN1CCOCC1 Show InChI InChI=1S/C22H24ClFN4O3/c1-29-20-13-19-16(12-21(20)31-8-2-5-28-6-9-30-10-7-28)22(26-14-25-19)27-15-3-4-18(24)17(23)11-15/h3-4,11-14H,2,5-10H2,1H3,(H,25,26,27) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsinghua University
Curated by ChEMBL
| Assay Description
Inhibition of HER2 (unknown origin) expressed in baculovirus infected insect cells after 20 mins in presence of ATP by ELISA |
Bioorg Med Chem 25: 27-37 (2017)
Article DOI: 10.1016/j.bmc.2016.10.006
BindingDB Entry DOI: 10.7270/Q22N548X |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM31340

(2-methoxy-N-[(E)-3-[4-[3-methyl-4-(6-methylpyridin...)Show SMILES COCC(=O)NC\C=C\c1ccc2ncnc(Nc3ccc(Oc4ccc(C)nc4)c(C)c3)c2c1 Show InChI InChI=1S/C27H27N5O3/c1-18-13-21(8-11-25(18)35-22-9-6-19(2)29-15-22)32-27-23-14-20(7-10-24(23)30-17-31-27)5-4-12-28-26(33)16-34-3/h4-11,13-15,17H,12,16H2,1-3H3,(H,28,33)(H,30,31,32)/b5-4+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 6.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsinghua University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human GST-fused EGFR (668 to 1211 residues) assessed as phosphotyrosine formation using poly(Glu:Tyr, 4:1) as substrate and... |
Bioorg Med Chem 25: 27-37 (2017)
Article DOI: 10.1016/j.bmc.2016.10.006
BindingDB Entry DOI: 10.7270/Q22N548X |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data