 Found 95 hits of Enzyme Inhibition Constant Data
Found 95 hits of Enzyme Inhibition Constant Data Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Sodium channel protein type 5 subunit alpha
(Homo sapiens (Human)) | BDBM50207061
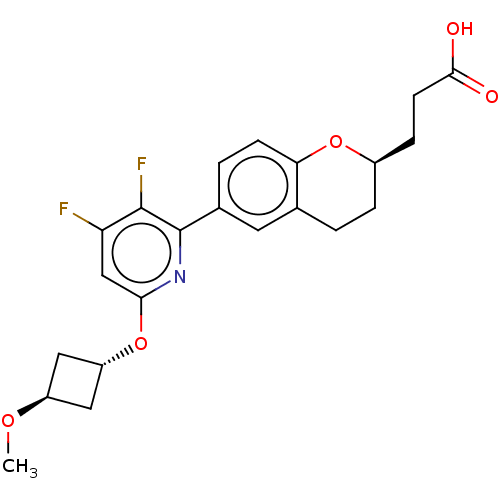
(CHEMBL3980898)Show SMILES CO[C@H]1C[C@@H](C1)Oc1cc(F)c(F)c(n1)-c1ccc2O[C@@H](CCC(O)=O)CCc2c1 |r,wU:4.6,wD:20.22,2.1,(19.4,-3.02,;19.4,-4.56,;20.74,-5.33,;21.14,-6.82,;22.62,-6.41,;22.23,-4.93,;23.96,-7.18,;25.29,-6.4,;25.28,-4.85,;26.62,-4.08,;26.62,-2.54,;27.95,-4.85,;29.29,-4.08,;27.95,-6.39,;26.62,-7.16,;29.28,-7.16,;29.28,-8.71,;30.61,-9.48,;31.94,-8.71,;33.28,-9.49,;34.63,-8.72,;35.96,-9.49,;37.3,-8.72,;38.63,-9.5,;38.63,-11.04,;39.97,-8.73,;34.63,-7.16,;33.28,-6.38,;31.94,-7.16,;30.61,-6.39,)| Show InChI InChI=1S/C22H23F2NO5/c1-28-15-9-16(10-15)30-19-11-17(23)21(24)22(25-19)13-3-6-18-12(8-13)2-4-14(29-18)5-7-20(26)27/h3,6,8,11,14-16H,2,4-5,7,9-10H2,1H3,(H,26,27)/t14-,15-,16-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Inhibition of Nav1.5 (unknown origin) |
ACS Med Chem Lett 8: 96-101 (2017)
Article DOI: 10.1021/acsmedchemlett.6b00394
BindingDB Entry DOI: 10.7270/Q2R213CP |
More data for this
Ligand-Target Pair | |
Voltage-dependent L-type calcium channel subunit alpha-1C
(Homo sapiens (Human)) | BDBM50207061

(CHEMBL3980898)Show SMILES CO[C@H]1C[C@@H](C1)Oc1cc(F)c(F)c(n1)-c1ccc2O[C@@H](CCC(O)=O)CCc2c1 |r,wU:4.6,wD:20.22,2.1,(19.4,-3.02,;19.4,-4.56,;20.74,-5.33,;21.14,-6.82,;22.62,-6.41,;22.23,-4.93,;23.96,-7.18,;25.29,-6.4,;25.28,-4.85,;26.62,-4.08,;26.62,-2.54,;27.95,-4.85,;29.29,-4.08,;27.95,-6.39,;26.62,-7.16,;29.28,-7.16,;29.28,-8.71,;30.61,-9.48,;31.94,-8.71,;33.28,-9.49,;34.63,-8.72,;35.96,-9.49,;37.3,-8.72,;38.63,-9.5,;38.63,-11.04,;39.97,-8.73,;34.63,-7.16,;33.28,-6.38,;31.94,-7.16,;30.61,-6.39,)| Show InChI InChI=1S/C22H23F2NO5/c1-28-15-9-16(10-15)30-19-11-17(23)21(24)22(25-19)13-3-6-18-12(8-13)2-4-14(29-18)5-7-20(26)27/h3,6,8,11,14-16H,2,4-5,7,9-10H2,1H3,(H,26,27)/t14-,15-,16-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Inhibition of Cav1.2 (unknown origin) |
ACS Med Chem Lett 8: 96-101 (2017)
Article DOI: 10.1021/acsmedchemlett.6b00394
BindingDB Entry DOI: 10.7270/Q2R213CP |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50207061

(CHEMBL3980898)Show SMILES CO[C@H]1C[C@@H](C1)Oc1cc(F)c(F)c(n1)-c1ccc2O[C@@H](CCC(O)=O)CCc2c1 |r,wU:4.6,wD:20.22,2.1,(19.4,-3.02,;19.4,-4.56,;20.74,-5.33,;21.14,-6.82,;22.62,-6.41,;22.23,-4.93,;23.96,-7.18,;25.29,-6.4,;25.28,-4.85,;26.62,-4.08,;26.62,-2.54,;27.95,-4.85,;29.29,-4.08,;27.95,-6.39,;26.62,-7.16,;29.28,-7.16,;29.28,-8.71,;30.61,-9.48,;31.94,-8.71,;33.28,-9.49,;34.63,-8.72,;35.96,-9.49,;37.3,-8.72,;38.63,-9.5,;38.63,-11.04,;39.97,-8.73,;34.63,-7.16,;33.28,-6.38,;31.94,-7.16,;30.61,-6.39,)| Show InChI InChI=1S/C22H23F2NO5/c1-28-15-9-16(10-15)30-19-11-17(23)21(24)22(25-19)13-3-6-18-12(8-13)2-4-14(29-18)5-7-20(26)27/h3,6,8,11,14-16H,2,4-5,7,9-10H2,1H3,(H,26,27)/t14-,15-,16-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Inhibition of human CYP3A4 |
ACS Med Chem Lett 8: 96-101 (2017)
Article DOI: 10.1021/acsmedchemlett.6b00394
BindingDB Entry DOI: 10.7270/Q2R213CP |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50207061

(CHEMBL3980898)Show SMILES CO[C@H]1C[C@@H](C1)Oc1cc(F)c(F)c(n1)-c1ccc2O[C@@H](CCC(O)=O)CCc2c1 |r,wU:4.6,wD:20.22,2.1,(19.4,-3.02,;19.4,-4.56,;20.74,-5.33,;21.14,-6.82,;22.62,-6.41,;22.23,-4.93,;23.96,-7.18,;25.29,-6.4,;25.28,-4.85,;26.62,-4.08,;26.62,-2.54,;27.95,-4.85,;29.29,-4.08,;27.95,-6.39,;26.62,-7.16,;29.28,-7.16,;29.28,-8.71,;30.61,-9.48,;31.94,-8.71,;33.28,-9.49,;34.63,-8.72,;35.96,-9.49,;37.3,-8.72,;38.63,-9.5,;38.63,-11.04,;39.97,-8.73,;34.63,-7.16,;33.28,-6.38,;31.94,-7.16,;30.61,-6.39,)| Show InChI InChI=1S/C22H23F2NO5/c1-28-15-9-16(10-15)30-19-11-17(23)21(24)22(25-19)13-3-6-18-12(8-13)2-4-14(29-18)5-7-20(26)27/h3,6,8,11,14-16H,2,4-5,7,9-10H2,1H3,(H,26,27)/t14-,15-,16-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2C9 |
ACS Med Chem Lett 8: 96-101 (2017)
Article DOI: 10.1021/acsmedchemlett.6b00394
BindingDB Entry DOI: 10.7270/Q2R213CP |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50207061

(CHEMBL3980898)Show SMILES CO[C@H]1C[C@@H](C1)Oc1cc(F)c(F)c(n1)-c1ccc2O[C@@H](CCC(O)=O)CCc2c1 |r,wU:4.6,wD:20.22,2.1,(19.4,-3.02,;19.4,-4.56,;20.74,-5.33,;21.14,-6.82,;22.62,-6.41,;22.23,-4.93,;23.96,-7.18,;25.29,-6.4,;25.28,-4.85,;26.62,-4.08,;26.62,-2.54,;27.95,-4.85,;29.29,-4.08,;27.95,-6.39,;26.62,-7.16,;29.28,-7.16,;29.28,-8.71,;30.61,-9.48,;31.94,-8.71,;33.28,-9.49,;34.63,-8.72,;35.96,-9.49,;37.3,-8.72,;38.63,-9.5,;38.63,-11.04,;39.97,-8.73,;34.63,-7.16,;33.28,-6.38,;31.94,-7.16,;30.61,-6.39,)| Show InChI InChI=1S/C22H23F2NO5/c1-28-15-9-16(10-15)30-19-11-17(23)21(24)22(25-19)13-3-6-18-12(8-13)2-4-14(29-18)5-7-20(26)27/h3,6,8,11,14-16H,2,4-5,7,9-10H2,1H3,(H,26,27)/t14-,15-,16-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2D6 |
ACS Med Chem Lett 8: 96-101 (2017)
Article DOI: 10.1021/acsmedchemlett.6b00394
BindingDB Entry DOI: 10.7270/Q2R213CP |
More data for this
Ligand-Target Pair | |
Free fatty acid receptor 1
(Homo sapiens (Human)) | BDBM50207058
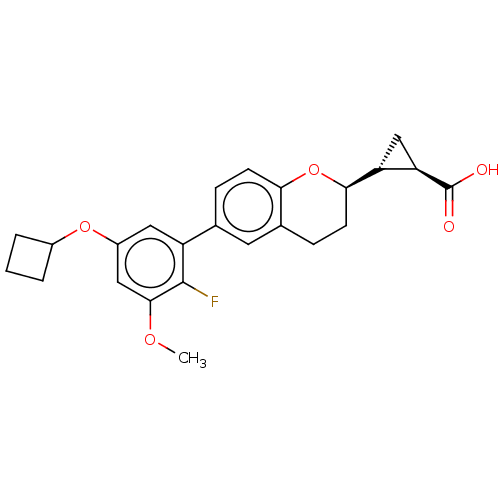
(CHEMBL3970166)Show SMILES [H][C@]1(C[C@@]1([H])[C@@]1([H])CCc2cc(ccc2O1)-c1cc(OC2CCC2)cc(OC)c1F)C(O)=O |r| Show InChI InChI=1S/C24H25FO5/c1-28-22-11-16(29-15-3-2-4-15)10-17(23(22)25)13-5-7-20-14(9-13)6-8-21(30-20)18-12-19(18)24(26)27/h5,7,9-11,15,18-19,21H,2-4,6,8,12H2,1H3,(H,26,27)/t18-,19-,21-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR40 expressed in HEK293 cells assessed as increase in IP1 accumulation after 60 mins by HTRF assay |
ACS Med Chem Lett 8: 96-101 (2017)
Article DOI: 10.1021/acsmedchemlett.6b00394
BindingDB Entry DOI: 10.7270/Q2R213CP |
More data for this
Ligand-Target Pair | |
Free fatty acid receptor 1
(Homo sapiens (Human)) | BDBM50207059

(CHEMBL3932664)Show SMILES COc1cc(OC2CC2)cc(c1F)-c1ccc2O[C@@H](CCc3nnn[nH]3)CCc2c1 |r| Show InChI InChI=1S/C22H23FN4O3/c1-28-20-12-17(29-15-5-6-15)11-18(22(20)23)13-3-8-19-14(10-13)2-4-16(30-19)7-9-21-24-26-27-25-21/h3,8,10-12,15-16H,2,4-7,9H2,1H3,(H,24,25,26,27)/t16-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR40 expressed in HEK293 cells assessed as increase in IP1 accumulation after 60 mins by HTRF assay |
ACS Med Chem Lett 8: 96-101 (2017)
Article DOI: 10.1021/acsmedchemlett.6b00394
BindingDB Entry DOI: 10.7270/Q2R213CP |
More data for this
Ligand-Target Pair | |
Free fatty acid receptor 4
(Homo sapiens (Human)) | BDBM50207063

(CHEMBL3971857)Show SMILES OC(=O)CC[C@H]1CCc2cc(ccc2O1)-c1cc(OC2CCC2)ncc1F |r| Show InChI InChI=1S/C21H22FNO4/c22-18-12-23-20(27-15-2-1-3-15)11-17(18)13-5-8-19-14(10-13)4-6-16(26-19)7-9-21(24)25/h5,8,10-12,15-16H,1-4,6-7,9H2,(H,24,25)/t16-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 230 | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR120 short isoform expressed in CHOK1 cells assessed as beta-arrestin recruitment after 90 mins by luminescence assay |
ACS Med Chem Lett 8: 96-101 (2017)
Article DOI: 10.1021/acsmedchemlett.6b00394
BindingDB Entry DOI: 10.7270/Q2R213CP |
More data for this
Ligand-Target Pair | |
Free fatty acid receptor 1
(Homo sapiens (Human)) | BDBM50207069

(CHEMBL3959068)Show SMILES [H][C@]1(C[C@@]1([H])[C@@]1([H])CCc2cc(ccc2O1)-c1cc(O[C@H]2C[C@@H](C2)OC)cc(F)c1F)C(O)=O |r,wU:5.6,1.0,20.22,wD:3.4,22.27,(25.35,-14.1,;25.76,-15.6,;25.75,-17.14,;24.43,-16.36,;24.41,-17.9,;23.08,-15.59,;22.96,-17.12,;23.09,-14.03,;21.74,-13.25,;20.4,-14.03,;19.07,-13.27,;17.74,-14.04,;17.73,-15.59,;19.06,-16.36,;20.39,-15.59,;21.74,-16.36,;16.4,-13.27,;15.07,-14.04,;13.74,-13.27,;12.4,-14.05,;11.07,-13.29,;9.59,-13.69,;9.19,-12.21,;10.67,-11.8,;7.86,-11.45,;7.86,-9.92,;13.73,-11.73,;15.07,-10.95,;15.07,-9.43,;16.41,-11.72,;17.74,-10.95,;27.1,-16.37,;27.09,-17.91,;28.43,-15.6,)| Show InChI InChI=1S/C24H24F2O5/c1-29-14-7-15(8-14)30-16-9-17(23(26)20(25)10-16)12-2-4-21-13(6-12)3-5-22(31-21)18-11-19(18)24(27)28/h2,4,6,9-10,14-15,18-19,22H,3,5,7-8,11H2,1H3,(H,27,28)/t14-,15-,18-,19-,22-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR40 expressed in HEK293 cells assessed as increase in IP1 accumulation after 60 mins by HTRF assay |
ACS Med Chem Lett 8: 96-101 (2017)
Article DOI: 10.1021/acsmedchemlett.6b00394
BindingDB Entry DOI: 10.7270/Q2R213CP |
More data for this
Ligand-Target Pair | |
Free fatty acid receptor 4
(Homo sapiens (Human)) | BDBM50207071
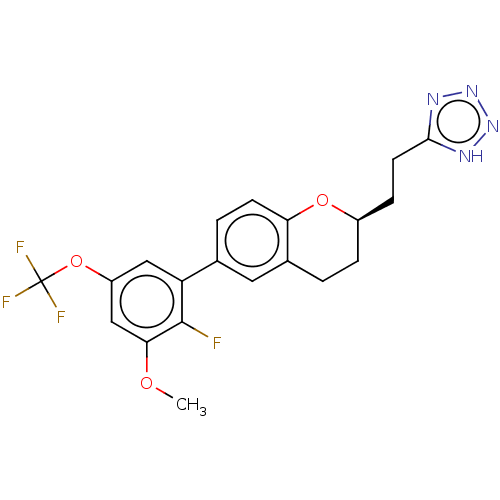
(CHEMBL3978465)Show SMILES COc1cc(OC(F)(F)F)cc(c1F)-c1ccc2O[C@@H](CCc3nnn[nH]3)CCc2c1 |r| Show InChI InChI=1S/C20H18F4N4O3/c1-29-17-10-14(31-20(22,23)24)9-15(19(17)21)11-3-6-16-12(8-11)2-4-13(30-16)5-7-18-25-27-28-26-18/h3,6,8-10,13H,2,4-5,7H2,1H3,(H,25,26,27,28)/t13-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 150 | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR120 short isoform expressed in CHOK1 cells assessed as beta-arrestin recruitment after 90 mins by luminescence assay |
ACS Med Chem Lett 8: 96-101 (2017)
Article DOI: 10.1021/acsmedchemlett.6b00394
BindingDB Entry DOI: 10.7270/Q2R213CP |
More data for this
Ligand-Target Pair | |
Free fatty acid receptor 4
(Homo sapiens (Human)) | BDBM50207073
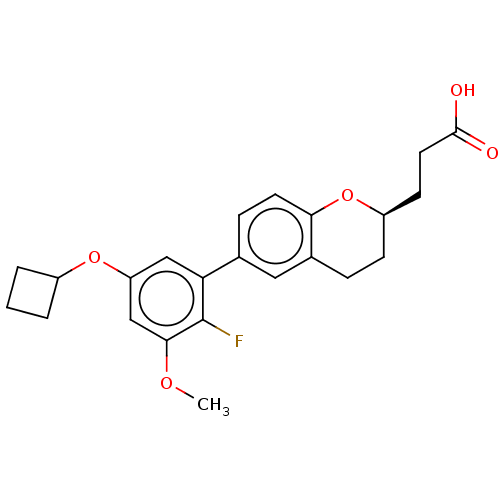
(CHEMBL3940429)Show SMILES COc1cc(OC2CCC2)cc(c1F)-c1ccc2O[C@@H](CCC(O)=O)CCc2c1 |r| Show InChI InChI=1S/C23H25FO5/c1-27-21-13-18(28-16-3-2-4-16)12-19(23(21)24)14-6-9-20-15(11-14)5-7-17(29-20)8-10-22(25)26/h6,9,11-13,16-17H,2-5,7-8,10H2,1H3,(H,25,26)/t17-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 9 | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR120 short isoform expressed in CHOK1 cells assessed as beta-arrestin recruitment after 90 mins by luminescence assay |
ACS Med Chem Lett 8: 96-101 (2017)
Article DOI: 10.1021/acsmedchemlett.6b00394
BindingDB Entry DOI: 10.7270/Q2R213CP |
More data for this
Ligand-Target Pair | |
Free fatty acid receptor 4
(Homo sapiens (Human)) | BDBM50207075

(CHEMBL3898962)Show SMILES [H][C@]1(C[C@@]1([H])[C@@]1([H])CCc2cc(ccc2O1)-c1nc(OC2CCC2)cc(OC)c1F)C(O)=O |r| Show InChI InChI=1S/C23H24FNO5/c1-28-19-11-20(29-14-3-2-4-14)25-22(21(19)24)13-6-7-17-12(9-13)5-8-18(30-17)15-10-16(15)23(26)27/h6-7,9,11,14-16,18H,2-5,8,10H2,1H3,(H,26,27)/t15-,16-,18-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 18 | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR120 short isoform expressed in CHOK1 cells assessed as increase in IP1 accumulation after 60 mins by HTRF assay |
ACS Med Chem Lett 8: 96-101 (2017)
Article DOI: 10.1021/acsmedchemlett.6b00394
BindingDB Entry DOI: 10.7270/Q2R213CP |
More data for this
Ligand-Target Pair | |
Free fatty acid receptor 4
(Homo sapiens (Human)) | BDBM50207055

(CHEMBL3960231)Show SMILES OC(=O)CC[C@H]1CCc2cc(ccc2O1)-c1cc(OC(F)(F)F)ccc1F |r| Show InChI InChI=1S/C19H16F4O4/c20-16-6-4-14(27-19(21,22)23)10-15(16)11-2-7-17-12(9-11)1-3-13(26-17)5-8-18(24)25/h2,4,6-7,9-10,13H,1,3,5,8H2,(H,24,25)/t13-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 170 | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR120 short isoform expressed in CHOK1 cells assessed as increase in IP1 accumulation after 60 mins by HTRF assay |
ACS Med Chem Lett 8: 96-101 (2017)
Article DOI: 10.1021/acsmedchemlett.6b00394
BindingDB Entry DOI: 10.7270/Q2R213CP |
More data for this
Ligand-Target Pair | |
Free fatty acid receptor 4
(Homo sapiens (Human)) | BDBM50207077
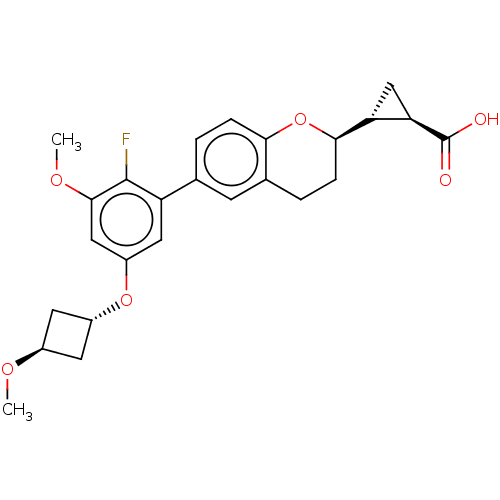
(CHEMBL3890035)Show SMILES [H][C@]1(C[C@@]1([H])[C@@]1([H])CCc2cc(ccc2O1)-c1cc(O[C@H]2C[C@@H](C2)OC)cc(OC)c1F)C(O)=O |r,wU:5.6,1.0,20.22,wD:3.4,22.27,(25.35,-14.1,;25.76,-15.6,;25.75,-17.14,;24.43,-16.36,;24.41,-17.9,;23.08,-15.59,;22.96,-17.12,;23.09,-14.03,;21.74,-13.25,;20.4,-14.03,;19.07,-13.27,;17.74,-14.04,;17.73,-15.59,;19.06,-16.36,;20.39,-15.59,;21.74,-16.36,;16.4,-13.27,;15.07,-14.04,;13.74,-13.27,;12.4,-14.05,;11.07,-13.29,;9.59,-13.69,;9.19,-12.21,;10.67,-11.8,;7.86,-11.45,;7.86,-9.92,;13.73,-11.73,;15.07,-10.95,;15.07,-9.44,;13.75,-8.67,;16.41,-11.72,;17.74,-10.95,;27.1,-16.37,;27.09,-17.91,;28.43,-15.6,)| Show InChI InChI=1S/C25H27FO6/c1-29-15-8-16(9-15)31-17-10-18(24(26)23(11-17)30-2)13-3-5-21-14(7-13)4-6-22(32-21)19-12-20(19)25(27)28/h3,5,7,10-11,15-16,19-20,22H,4,6,8-9,12H2,1-2H3,(H,27,28)/t15-,16-,19-,20-,22-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 55 | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR120 short isoform expressed in CHOK1 cells assessed as increase in IP1 accumulation after 60 mins by HTRF assay |
ACS Med Chem Lett 8: 96-101 (2017)
Article DOI: 10.1021/acsmedchemlett.6b00394
BindingDB Entry DOI: 10.7270/Q2R213CP |
More data for this
Ligand-Target Pair | |
Free fatty acid receptor 4
(Homo sapiens (Human)) | BDBM50207068
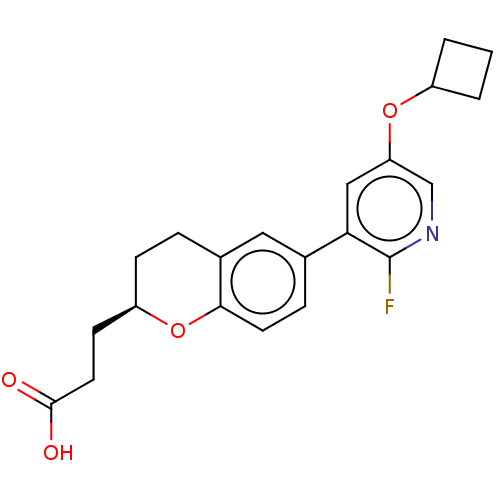
(CHEMBL3900705)Show SMILES OC(=O)CC[C@H]1CCc2cc(ccc2O1)-c1cc(OC2CCC2)cnc1F |r| Show InChI InChI=1S/C21H22FNO4/c22-21-18(11-17(12-23-21)26-15-2-1-3-15)13-5-8-19-14(10-13)4-6-16(27-19)7-9-20(24)25/h5,8,10-12,15-16H,1-4,6-7,9H2,(H,24,25)/t16-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 79 | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR120 short isoform expressed in CHOK1 cells assessed as increase in IP1 accumulation after 60 mins by HTRF assay |
ACS Med Chem Lett 8: 96-101 (2017)
Article DOI: 10.1021/acsmedchemlett.6b00394
BindingDB Entry DOI: 10.7270/Q2R213CP |
More data for this
Ligand-Target Pair | |
Free fatty acid receptor 1
(Homo sapiens (Human)) | BDBM50207056
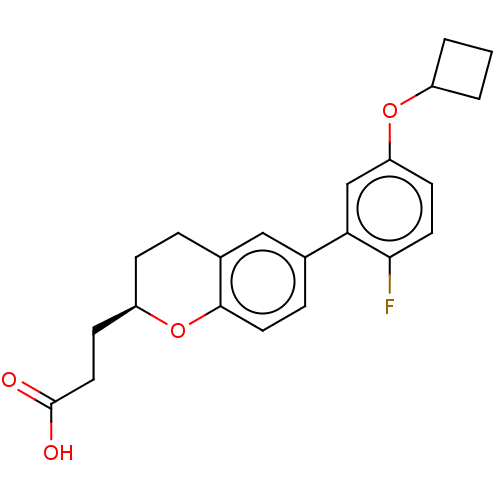
(CHEMBL3970238)Show SMILES OC(=O)CC[C@H]1CCc2cc(ccc2O1)-c1cc(OC2CCC2)ccc1F |r| Show InChI InChI=1S/C22H23FO4/c23-20-9-7-18(26-16-2-1-3-16)13-19(20)14-5-10-21-15(12-14)4-6-17(27-21)8-11-22(24)25/h5,7,9-10,12-13,16-17H,1-4,6,8,11H2,(H,24,25)/t17-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 3.60E+3 | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR40 expressed in HEK293 cells assessed as increase in IP1 accumulation after 60 mins by HTRF assay |
ACS Med Chem Lett 8: 96-101 (2017)
Article DOI: 10.1021/acsmedchemlett.6b00394
BindingDB Entry DOI: 10.7270/Q2R213CP |
More data for this
Ligand-Target Pair | |
Free fatty acid receptor 1
(Homo sapiens (Human)) | BDBM50207057
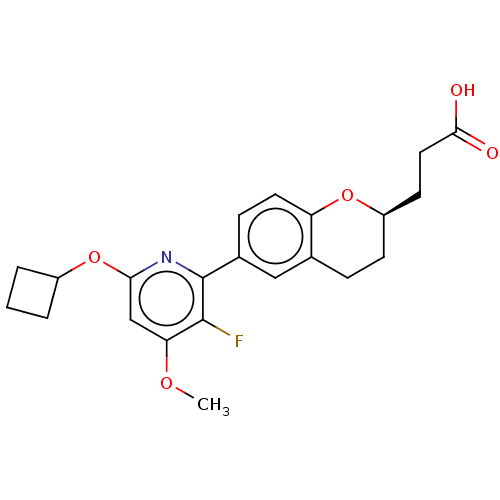
(CHEMBL3960738)Show SMILES COc1cc(OC2CCC2)nc(c1F)-c1ccc2O[C@@H](CCC(O)=O)CCc2c1 |r| Show InChI InChI=1S/C22H24FNO5/c1-27-18-12-19(29-15-3-2-4-15)24-22(21(18)23)14-6-9-17-13(11-14)5-7-16(28-17)8-10-20(25)26/h6,9,11-12,15-16H,2-5,7-8,10H2,1H3,(H,25,26)/t16-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR40 expressed in HEK293 cells assessed as increase in IP1 accumulation after 60 mins by HTRF assay |
ACS Med Chem Lett 8: 96-101 (2017)
Article DOI: 10.1021/acsmedchemlett.6b00394
BindingDB Entry DOI: 10.7270/Q2R213CP |
More data for this
Ligand-Target Pair | |
Free fatty acid receptor 4
(Homo sapiens (Human)) | BDBM50207062
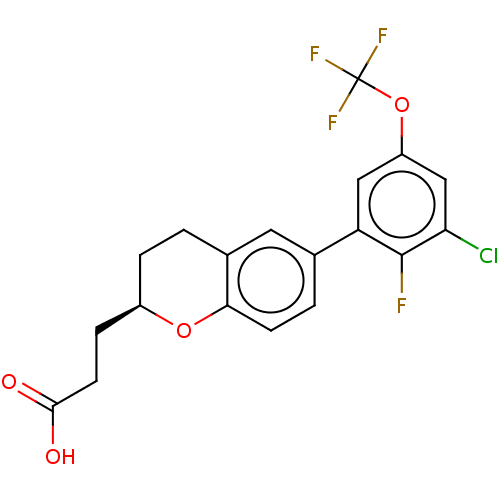
(CHEMBL3959144)Show SMILES OC(=O)CC[C@H]1CCc2cc(ccc2O1)-c1cc(OC(F)(F)F)cc(Cl)c1F |r| Show InChI InChI=1S/C19H15ClF4O4/c20-15-9-13(28-19(22,23)24)8-14(18(15)21)10-2-5-16-11(7-10)1-3-12(27-16)4-6-17(25)26/h2,5,7-9,12H,1,3-4,6H2,(H,25,26)/t12-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 72 | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR120 short isoform expressed in CHOK1 cells assessed as increase in IP1 accumulation after 60 mins by HTRF assay |
ACS Med Chem Lett 8: 96-101 (2017)
Article DOI: 10.1021/acsmedchemlett.6b00394
BindingDB Entry DOI: 10.7270/Q2R213CP |
More data for this
Ligand-Target Pair | |
Free fatty acid receptor 4
(Homo sapiens (Human)) | BDBM50207064
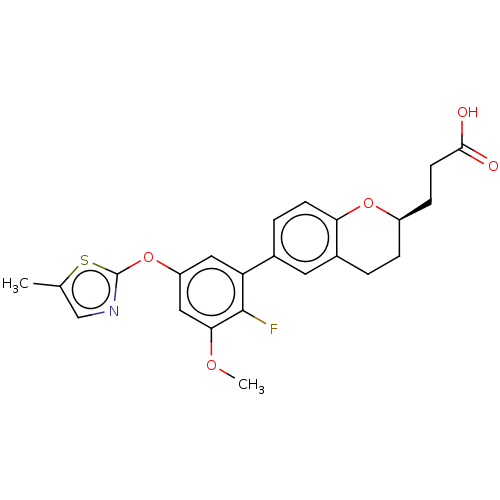
(CHEMBL3943315)Show SMILES COc1cc(Oc2ncc(C)s2)cc(c1F)-c1ccc2O[C@@H](CCC(O)=O)CCc2c1 |r| Show InChI InChI=1S/C23H22FNO5S/c1-13-12-25-23(31-13)30-17-10-18(22(24)20(11-17)28-2)14-4-7-19-15(9-14)3-5-16(29-19)6-8-21(26)27/h4,7,9-12,16H,3,5-6,8H2,1-2H3,(H,26,27)/t16-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 37 | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR120 short isoform expressed in CHOK1 cells assessed as increase in IP1 accumulation after 60 mins by HTRF assay |
ACS Med Chem Lett 8: 96-101 (2017)
Article DOI: 10.1021/acsmedchemlett.6b00394
BindingDB Entry DOI: 10.7270/Q2R213CP |
More data for this
Ligand-Target Pair | |
Free fatty acid receptor 4
(Homo sapiens (Human)) | BDBM50207065

(CHEMBL3971308)Show SMILES [H][C@]1(C[C@@]1([H])[C@@]1([H])CCc2cc(ccc2O1)-c1cc(OC(F)(F)F)ccc1F)C(O)=O |r| Show InChI InChI=1S/C20H16F4O4/c21-16-4-3-12(28-20(22,23)24)8-13(16)10-1-5-17-11(7-10)2-6-18(27-17)14-9-15(14)19(25)26/h1,3-5,7-8,14-15,18H,2,6,9H2,(H,25,26)/t14-,15-,18-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 69 | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR120 short isoform expressed in CHOK1 cells assessed as increase in IP1 accumulation after 60 mins by HTRF assay |
ACS Med Chem Lett 8: 96-101 (2017)
Article DOI: 10.1021/acsmedchemlett.6b00394
BindingDB Entry DOI: 10.7270/Q2R213CP |
More data for this
Ligand-Target Pair | |
Free fatty acid receptor 4
(Homo sapiens (Human)) | BDBM50207055

(CHEMBL3960231)Show SMILES OC(=O)CC[C@H]1CCc2cc(ccc2O1)-c1cc(OC(F)(F)F)ccc1F |r| Show InChI InChI=1S/C19H16F4O4/c20-16-6-4-14(27-19(21,22)23)10-15(16)11-2-7-17-12(9-11)1-3-13(26-17)5-8-18(24)25/h2,4,6-7,9-10,13H,1,3,5,8H2,(H,24,25)/t13-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 17 | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR120 short isoform expressed in CHOK1 cells assessed as beta-arrestin recruitment after 90 mins by luminescence assay |
ACS Med Chem Lett 8: 96-101 (2017)
Article DOI: 10.1021/acsmedchemlett.6b00394
BindingDB Entry DOI: 10.7270/Q2R213CP |
More data for this
Ligand-Target Pair | |
Free fatty acid receptor 4
(Homo sapiens (Human)) | BDBM50207060

(CHEMBL3952043)Show SMILES CO[C@H]1C[C@@H](C1)Oc1cc(OC)c(F)c(n1)-c1ccc2O[C@@H](CCC(O)=O)CCc2c1 |r,wU:4.6,wD:21.23,2.1,(37.05,-1.75,;37.06,-3.29,;38.39,-4.05,;38.8,-5.54,;40.28,-5.13,;39.88,-3.65,;41.61,-5.9,;42.94,-5.12,;42.94,-3.58,;44.27,-2.81,;44.28,-1.27,;42.95,-.5,;45.61,-3.58,;46.94,-2.81,;45.6,-5.12,;44.28,-5.89,;46.93,-5.89,;46.93,-7.43,;48.27,-8.2,;49.6,-7.44,;50.93,-8.21,;52.28,-7.44,;53.61,-8.21,;54.95,-7.45,;56.28,-8.22,;56.28,-9.76,;57.62,-7.45,;52.29,-5.88,;50.94,-5.1,;49.6,-5.88,;48.26,-5.12,)| Show InChI InChI=1S/C23H26FNO6/c1-28-16-10-17(11-16)31-20-12-19(29-2)22(24)23(25-20)14-4-7-18-13(9-14)3-5-15(30-18)6-8-21(26)27/h4,7,9,12,15-17H,3,5-6,8,10-11H2,1-2H3,(H,26,27)/t15-,16-,17-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 5 | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR120 short isoform expressed in CHOK1 cells assessed as beta-arrestin recruitment after 90 mins by luminescence assay |
ACS Med Chem Lett 8: 96-101 (2017)
Article DOI: 10.1021/acsmedchemlett.6b00394
BindingDB Entry DOI: 10.7270/Q2R213CP |
More data for this
Ligand-Target Pair | |
Free fatty acid receptor 4
(Homo sapiens (Human)) | BDBM50207050

(CHEMBL3972870)Show SMILES Cc1cnc(Oc2cc(Cl)c(F)c(c2)-c2ccc3O[C@@H](CCC(O)=O)CCc3c2)s1 |r| Show InChI InChI=1S/C22H19ClFNO4S/c1-12-11-25-22(30-12)29-16-9-17(21(24)18(23)10-16)13-3-6-19-14(8-13)2-4-15(28-19)5-7-20(26)27/h3,6,8-11,15H,2,4-5,7H2,1H3,(H,26,27)/t15-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 14 | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR120 short isoform expressed in CHOK1 cells assessed as increase in IP1 accumulation after 60 mins by HTRF assay |
ACS Med Chem Lett 8: 96-101 (2017)
Article DOI: 10.1021/acsmedchemlett.6b00394
BindingDB Entry DOI: 10.7270/Q2R213CP |
More data for this
Ligand-Target Pair | |
Free fatty acid receptor 4
(Homo sapiens (Human)) | BDBM50207051

(CHEMBL3947070)Show SMILES OC(=O)CC[C@H]1CCc2cc(ccc2O1)-c1cc(OC2CCC2)cc(F)c1F |r| Show InChI InChI=1S/C22H22F2O4/c23-19-12-17(27-15-2-1-3-15)11-18(22(19)24)13-5-8-20-14(10-13)4-6-16(28-20)7-9-21(25)26/h5,8,10-12,15-16H,1-4,6-7,9H2,(H,25,26)/t16-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 21 | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR120 short isoform expressed in CHOK1 cells assessed as increase in IP1 accumulation after 60 mins by HTRF assay |
ACS Med Chem Lett 8: 96-101 (2017)
Article DOI: 10.1021/acsmedchemlett.6b00394
BindingDB Entry DOI: 10.7270/Q2R213CP |
More data for this
Ligand-Target Pair | |
Free fatty acid receptor 1
(Homo sapiens (Human)) | BDBM50207076

(CHEMBL3979448)Show SMILES Fc1ccc(OC(F)(F)F)cc1-c1ccc2O[C@@H](CCc3nnn[nH]3)CCc2c1 |r| Show InChI InChI=1S/C19H16F4N4O2/c20-16-6-4-14(29-19(21,22)23)10-15(16)11-2-7-17-12(9-11)1-3-13(28-17)5-8-18-24-26-27-25-18/h2,4,6-7,9-10,13H,1,3,5,8H2,(H,24,25,26,27)/t13-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR40 expressed in HEK293 cells assessed as increase in IP1 accumulation after 60 mins by HTRF assay |
ACS Med Chem Lett 8: 96-101 (2017)
Article DOI: 10.1021/acsmedchemlett.6b00394
BindingDB Entry DOI: 10.7270/Q2R213CP |
More data for this
Ligand-Target Pair | |
Free fatty acid receptor 1
(Homo sapiens (Human)) | BDBM50207061

(CHEMBL3980898)Show SMILES CO[C@H]1C[C@@H](C1)Oc1cc(F)c(F)c(n1)-c1ccc2O[C@@H](CCC(O)=O)CCc2c1 |r,wU:4.6,wD:20.22,2.1,(19.4,-3.02,;19.4,-4.56,;20.74,-5.33,;21.14,-6.82,;22.62,-6.41,;22.23,-4.93,;23.96,-7.18,;25.29,-6.4,;25.28,-4.85,;26.62,-4.08,;26.62,-2.54,;27.95,-4.85,;29.29,-4.08,;27.95,-6.39,;26.62,-7.16,;29.28,-7.16,;29.28,-8.71,;30.61,-9.48,;31.94,-8.71,;33.28,-9.49,;34.63,-8.72,;35.96,-9.49,;37.3,-8.72,;38.63,-9.5,;38.63,-11.04,;39.97,-8.73,;34.63,-7.16,;33.28,-6.38,;31.94,-7.16,;30.61,-6.39,)| Show InChI InChI=1S/C22H23F2NO5/c1-28-15-9-16(10-15)30-19-11-17(23)21(24)22(25-19)13-3-6-18-12(8-13)2-4-14(29-18)5-7-20(26)27/h3,6,8,11,14-16H,2,4-5,7,9-10H2,1H3,(H,26,27)/t14-,15-,16-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR40 expressed in HEK293 cells assessed as increase in IP1 accumulation after 60 mins by HTRF assay |
ACS Med Chem Lett 8: 96-101 (2017)
Article DOI: 10.1021/acsmedchemlett.6b00394
BindingDB Entry DOI: 10.7270/Q2R213CP |
More data for this
Ligand-Target Pair | |
Free fatty acid receptor 4
(Homo sapiens (Human)) | BDBM50207067

(CHEMBL3941726)Show SMILES OC(=O)CC[C@@H]1CCc2cc(ccc2O1)-c1cc(OC(F)(F)F)ccc1F |r| Show InChI InChI=1S/C19H16F4O4/c20-16-6-4-14(27-19(21,22)23)10-15(16)11-2-7-17-12(9-11)1-3-13(26-17)5-8-18(24)25/h2,4,6-7,9-10,13H,1,3,5,8H2,(H,24,25)/t13-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 180 | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR120 short isoform expressed in CHOK1 cells assessed as increase in IP1 accumulation after 60 mins by HTRF assay |
ACS Med Chem Lett 8: 96-101 (2017)
Article DOI: 10.1021/acsmedchemlett.6b00394
BindingDB Entry DOI: 10.7270/Q2R213CP |
More data for this
Ligand-Target Pair | |
Free fatty acid receptor 1
(Homo sapiens (Human)) | BDBM50207073

(CHEMBL3940429)Show SMILES COc1cc(OC2CCC2)cc(c1F)-c1ccc2O[C@@H](CCC(O)=O)CCc2c1 |r| Show InChI InChI=1S/C23H25FO5/c1-27-21-13-18(28-16-3-2-4-16)12-19(23(21)24)14-6-9-20-15(11-14)5-7-17(29-20)8-10-22(25)26/h6,9,11-13,16-17H,2-5,7-8,10H2,1H3,(H,25,26)/t17-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR40 expressed in HEK293 cells assessed as increase in IP1 accumulation after 60 mins by HTRF assay |
ACS Med Chem Lett 8: 96-101 (2017)
Article DOI: 10.1021/acsmedchemlett.6b00394
BindingDB Entry DOI: 10.7270/Q2R213CP |
More data for this
Ligand-Target Pair | |
Free fatty acid receptor 1
(Homo sapiens (Human)) | BDBM50207050

(CHEMBL3972870)Show SMILES Cc1cnc(Oc2cc(Cl)c(F)c(c2)-c2ccc3O[C@@H](CCC(O)=O)CCc3c2)s1 |r| Show InChI InChI=1S/C22H19ClFNO4S/c1-12-11-25-22(30-12)29-16-9-17(21(24)18(23)10-16)13-3-6-19-14(8-13)2-4-15(28-19)5-7-20(26)27/h3,6,8-11,15H,2,4-5,7H2,1H3,(H,26,27)/t15-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR40 expressed in HEK293 cells assessed as increase in IP1 accumulation after 60 mins by HTRF assay |
ACS Med Chem Lett 8: 96-101 (2017)
Article DOI: 10.1021/acsmedchemlett.6b00394
BindingDB Entry DOI: 10.7270/Q2R213CP |
More data for this
Ligand-Target Pair | |
Free fatty acid receptor 4
(Homo sapiens (Human)) | BDBM50207075

(CHEMBL3898962)Show SMILES [H][C@]1(C[C@@]1([H])[C@@]1([H])CCc2cc(ccc2O1)-c1nc(OC2CCC2)cc(OC)c1F)C(O)=O |r| Show InChI InChI=1S/C23H24FNO5/c1-28-19-11-20(29-14-3-2-4-14)25-22(21(19)24)13-6-7-17-12(9-13)5-8-18(30-17)15-10-16(15)23(26)27/h6-7,9,11,14-16,18H,2-5,8,10H2,1H3,(H,26,27)/t15-,16-,18-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 7.80 | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR120 short isoform expressed in CHOK1 cells assessed as beta-arrestin recruitment after 90 mins by luminescence assay |
ACS Med Chem Lett 8: 96-101 (2017)
Article DOI: 10.1021/acsmedchemlett.6b00394
BindingDB Entry DOI: 10.7270/Q2R213CP |
More data for this
Ligand-Target Pair | |
Free fatty acid receptor 4
(Homo sapiens (Human)) | BDBM50207050

(CHEMBL3972870)Show SMILES Cc1cnc(Oc2cc(Cl)c(F)c(c2)-c2ccc3O[C@@H](CCC(O)=O)CCc3c2)s1 |r| Show InChI InChI=1S/C22H19ClFNO4S/c1-12-11-25-22(30-12)29-16-9-17(21(24)18(23)10-16)13-3-6-19-14(8-13)2-4-15(28-19)5-7-20(26)27/h3,6,8-11,15H,2,4-5,7H2,1H3,(H,26,27)/t15-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 5.60 | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR120 short isoform expressed in CHOK1 cells assessed as beta-arrestin recruitment after 90 mins by luminescence assay |
ACS Med Chem Lett 8: 96-101 (2017)
Article DOI: 10.1021/acsmedchemlett.6b00394
BindingDB Entry DOI: 10.7270/Q2R213CP |
More data for this
Ligand-Target Pair | |
Free fatty acid receptor 1
(Homo sapiens (Human)) | BDBM50207066

(CHEMBL3890089)Show SMILES COc1cc(OC(F)(F)F)cc(c1F)-c1ccc2O[C@@H](CCC(O)=O)CCc2c1 |r| Show InChI InChI=1S/C20H18F4O5/c1-27-17-10-14(29-20(22,23)24)9-15(19(17)21)11-3-6-16-12(8-11)2-4-13(28-16)5-7-18(25)26/h3,6,8-10,13H,2,4-5,7H2,1H3,(H,25,26)/t13-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 4.40E+3 | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR40 expressed in HEK293 cells assessed as increase in IP1 accumulation after 60 mins by HTRF assay |
ACS Med Chem Lett 8: 96-101 (2017)
Article DOI: 10.1021/acsmedchemlett.6b00394
BindingDB Entry DOI: 10.7270/Q2R213CP |
More data for this
Ligand-Target Pair | |
Free fatty acid receptor 1
(Homo sapiens (Human)) | BDBM50207053
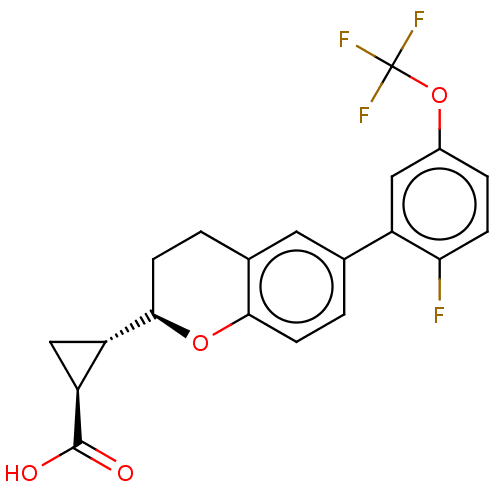
(CHEMBL3950444)Show SMILES [H][C@@]1(C[C@]1([H])[C@@]1([H])CCc2cc(ccc2O1)-c1cc(OC(F)(F)F)ccc1F)C(O)=O |r| Show InChI InChI=1S/C20H16F4O4/c21-16-4-3-12(28-20(22,23)24)8-13(16)10-1-5-17-11(7-10)2-6-18(27-17)14-9-15(14)19(25)26/h1,3-5,7-8,14-15,18H,2,6,9H2,(H,25,26)/t14-,15-,18+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR40 expressed in HEK293 cells assessed as increase in IP1 accumulation after 60 mins by HTRF assay |
ACS Med Chem Lett 8: 96-101 (2017)
Article DOI: 10.1021/acsmedchemlett.6b00394
BindingDB Entry DOI: 10.7270/Q2R213CP |
More data for this
Ligand-Target Pair | |
Free fatty acid receptor 4
(Homo sapiens (Human)) | BDBM50207064

(CHEMBL3943315)Show SMILES COc1cc(Oc2ncc(C)s2)cc(c1F)-c1ccc2O[C@@H](CCC(O)=O)CCc2c1 |r| Show InChI InChI=1S/C23H22FNO5S/c1-13-12-25-23(31-13)30-17-10-18(22(24)20(11-17)28-2)14-4-7-19-15(9-14)3-5-16(29-19)6-8-21(26)27/h4,7,9-12,16H,3,5-6,8H2,1-2H3,(H,26,27)/t16-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 13 | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR120 short isoform expressed in CHOK1 cells assessed as beta-arrestin recruitment after 90 mins by luminescence assay |
ACS Med Chem Lett 8: 96-101 (2017)
Article DOI: 10.1021/acsmedchemlett.6b00394
BindingDB Entry DOI: 10.7270/Q2R213CP |
More data for this
Ligand-Target Pair | |
Free fatty acid receptor 4
(Homo sapiens (Human)) | BDBM50207071

(CHEMBL3978465)Show SMILES COc1cc(OC(F)(F)F)cc(c1F)-c1ccc2O[C@@H](CCc3nnn[nH]3)CCc2c1 |r| Show InChI InChI=1S/C20H18F4N4O3/c1-29-17-10-14(31-20(22,23)24)9-15(19(17)21)11-3-6-16-12(8-11)2-4-13(30-16)5-7-18-25-27-28-26-18/h3,6,8-10,13H,2,4-5,7H2,1H3,(H,25,26,27,28)/t13-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 130 | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR120 short isoform expressed in CHOK1 cells assessed as increase in IP1 accumulation after 60 mins by HTRF assay |
ACS Med Chem Lett 8: 96-101 (2017)
Article DOI: 10.1021/acsmedchemlett.6b00394
BindingDB Entry DOI: 10.7270/Q2R213CP |
More data for this
Ligand-Target Pair | |
Free fatty acid receptor 4
(Homo sapiens (Human)) | BDBM50207077

(CHEMBL3890035)Show SMILES [H][C@]1(C[C@@]1([H])[C@@]1([H])CCc2cc(ccc2O1)-c1cc(O[C@H]2C[C@@H](C2)OC)cc(OC)c1F)C(O)=O |r,wU:5.6,1.0,20.22,wD:3.4,22.27,(25.35,-14.1,;25.76,-15.6,;25.75,-17.14,;24.43,-16.36,;24.41,-17.9,;23.08,-15.59,;22.96,-17.12,;23.09,-14.03,;21.74,-13.25,;20.4,-14.03,;19.07,-13.27,;17.74,-14.04,;17.73,-15.59,;19.06,-16.36,;20.39,-15.59,;21.74,-16.36,;16.4,-13.27,;15.07,-14.04,;13.74,-13.27,;12.4,-14.05,;11.07,-13.29,;9.59,-13.69,;9.19,-12.21,;10.67,-11.8,;7.86,-11.45,;7.86,-9.92,;13.73,-11.73,;15.07,-10.95,;15.07,-9.44,;13.75,-8.67,;16.41,-11.72,;17.74,-10.95,;27.1,-16.37,;27.09,-17.91,;28.43,-15.6,)| Show InChI InChI=1S/C25H27FO6/c1-29-15-8-16(9-15)31-17-10-18(24(26)23(11-17)30-2)13-3-5-21-14(7-13)4-6-22(32-21)19-12-20(19)25(27)28/h3,5,7,10-11,15-16,19-20,22H,4,6,8-9,12H2,1-2H3,(H,27,28)/t15-,16-,19-,20-,22-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 78 | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR120 short isoform expressed in CHOK1 cells assessed as beta-arrestin recruitment after 90 mins by luminescence assay |
ACS Med Chem Lett 8: 96-101 (2017)
Article DOI: 10.1021/acsmedchemlett.6b00394
BindingDB Entry DOI: 10.7270/Q2R213CP |
More data for this
Ligand-Target Pair | |
Free fatty acid receptor 1
(Homo sapiens (Human)) | BDBM50207071

(CHEMBL3978465)Show SMILES COc1cc(OC(F)(F)F)cc(c1F)-c1ccc2O[C@@H](CCc3nnn[nH]3)CCc2c1 |r| Show InChI InChI=1S/C20H18F4N4O3/c1-29-17-10-14(31-20(22,23)24)9-15(19(17)21)11-3-6-16-12(8-11)2-4-13(30-16)5-7-18-25-27-28-26-18/h3,6,8-10,13H,2,4-5,7H2,1H3,(H,25,26,27,28)/t13-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR40 expressed in HEK293 cells assessed as increase in IP1 accumulation after 60 mins by HTRF assay |
ACS Med Chem Lett 8: 96-101 (2017)
Article DOI: 10.1021/acsmedchemlett.6b00394
BindingDB Entry DOI: 10.7270/Q2R213CP |
More data for this
Ligand-Target Pair | |
Free fatty acid receptor 4
(Homo sapiens (Human)) | BDBM50207052
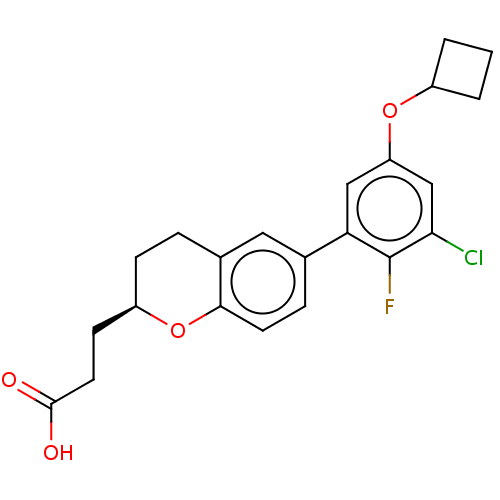
(CHEMBL3909773)Show SMILES OC(=O)CC[C@H]1CCc2cc(ccc2O1)-c1cc(OC2CCC2)cc(Cl)c1F |r| Show InChI InChI=1S/C22H22ClFO4/c23-19-12-17(27-15-2-1-3-15)11-18(22(19)24)13-5-8-20-14(10-13)4-6-16(28-20)7-9-21(25)26/h5,8,10-12,15-16H,1-4,6-7,9H2,(H,25,26)/t16-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 8.30 | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR120 short isoform expressed in CHOK1 cells assessed as beta-arrestin recruitment after 90 mins by luminescence assay |
ACS Med Chem Lett 8: 96-101 (2017)
Article DOI: 10.1021/acsmedchemlett.6b00394
BindingDB Entry DOI: 10.7270/Q2R213CP |
More data for this
Ligand-Target Pair | |
Free fatty acid receptor 4
(Homo sapiens (Human)) | BDBM50207057

(CHEMBL3960738)Show SMILES COc1cc(OC2CCC2)nc(c1F)-c1ccc2O[C@@H](CCC(O)=O)CCc2c1 |r| Show InChI InChI=1S/C22H24FNO5/c1-27-18-12-19(29-15-3-2-4-15)24-22(21(18)23)14-6-9-17-13(11-14)5-7-16(28-17)8-10-20(25)26/h6,9,11-12,15-16H,2-5,7-8,10H2,1H3,(H,25,26)/t16-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 15 | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR120 short isoform expressed in CHOK1 cells assessed as increase in IP1 accumulation after 60 mins by HTRF assay |
ACS Med Chem Lett 8: 96-101 (2017)
Article DOI: 10.1021/acsmedchemlett.6b00394
BindingDB Entry DOI: 10.7270/Q2R213CP |
More data for this
Ligand-Target Pair | |
Free fatty acid receptor 4
(Mus musculus) | BDBM50207060

(CHEMBL3952043)Show SMILES CO[C@H]1C[C@@H](C1)Oc1cc(OC)c(F)c(n1)-c1ccc2O[C@@H](CCC(O)=O)CCc2c1 |r,wU:4.6,wD:21.23,2.1,(37.05,-1.75,;37.06,-3.29,;38.39,-4.05,;38.8,-5.54,;40.28,-5.13,;39.88,-3.65,;41.61,-5.9,;42.94,-5.12,;42.94,-3.58,;44.27,-2.81,;44.28,-1.27,;42.95,-.5,;45.61,-3.58,;46.94,-2.81,;45.6,-5.12,;44.28,-5.89,;46.93,-5.89,;46.93,-7.43,;48.27,-8.2,;49.6,-7.44,;50.93,-8.21,;52.28,-7.44,;53.61,-8.21,;54.95,-7.45,;56.28,-8.22,;56.28,-9.76,;57.62,-7.45,;52.29,-5.88,;50.94,-5.1,;49.6,-5.88,;48.26,-5.12,)| Show InChI InChI=1S/C23H26FNO6/c1-28-16-10-17(11-16)31-20-12-19(29-2)22(24)23(25-20)14-4-7-18-13(9-14)3-5-15(30-18)6-8-21(26)27/h4,7,9,12,15-17H,3,5-6,8,10-11H2,1-2H3,(H,26,27)/t15-,16-,17-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 4.60 | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Agonist activity at mouse GPR120 expressed in human U2OS cells assessed as beta-arrestin recruitment after 90 mins by luminescence assay |
ACS Med Chem Lett 8: 96-101 (2017)
Article DOI: 10.1021/acsmedchemlett.6b00394
BindingDB Entry DOI: 10.7270/Q2R213CP |
More data for this
Ligand-Target Pair | |
Free fatty acid receptor 4
(Homo sapiens (Human)) | BDBM50207061

(CHEMBL3980898)Show SMILES CO[C@H]1C[C@@H](C1)Oc1cc(F)c(F)c(n1)-c1ccc2O[C@@H](CCC(O)=O)CCc2c1 |r,wU:4.6,wD:20.22,2.1,(19.4,-3.02,;19.4,-4.56,;20.74,-5.33,;21.14,-6.82,;22.62,-6.41,;22.23,-4.93,;23.96,-7.18,;25.29,-6.4,;25.28,-4.85,;26.62,-4.08,;26.62,-2.54,;27.95,-4.85,;29.29,-4.08,;27.95,-6.39,;26.62,-7.16,;29.28,-7.16,;29.28,-8.71,;30.61,-9.48,;31.94,-8.71,;33.28,-9.49,;34.63,-8.72,;35.96,-9.49,;37.3,-8.72,;38.63,-9.5,;38.63,-11.04,;39.97,-8.73,;34.63,-7.16,;33.28,-6.38,;31.94,-7.16,;30.61,-6.39,)| Show InChI InChI=1S/C22H23F2NO5/c1-28-15-9-16(10-15)30-19-11-17(23)21(24)22(25-19)13-3-6-18-12(8-13)2-4-14(29-18)5-7-20(26)27/h3,6,8,11,14-16H,2,4-5,7,9-10H2,1H3,(H,26,27)/t14-,15-,16-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 24 | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR120 short isoform expressed in CHOK1 cells assessed as beta-arrestin recruitment after 90 mins by luminescence assay |
ACS Med Chem Lett 8: 96-101 (2017)
Article DOI: 10.1021/acsmedchemlett.6b00394
BindingDB Entry DOI: 10.7270/Q2R213CP |
More data for this
Ligand-Target Pair | |
Free fatty acid receptor 4
(Homo sapiens (Human)) | BDBM50207061

(CHEMBL3980898)Show SMILES CO[C@H]1C[C@@H](C1)Oc1cc(F)c(F)c(n1)-c1ccc2O[C@@H](CCC(O)=O)CCc2c1 |r,wU:4.6,wD:20.22,2.1,(19.4,-3.02,;19.4,-4.56,;20.74,-5.33,;21.14,-6.82,;22.62,-6.41,;22.23,-4.93,;23.96,-7.18,;25.29,-6.4,;25.28,-4.85,;26.62,-4.08,;26.62,-2.54,;27.95,-4.85,;29.29,-4.08,;27.95,-6.39,;26.62,-7.16,;29.28,-7.16,;29.28,-8.71,;30.61,-9.48,;31.94,-8.71,;33.28,-9.49,;34.63,-8.72,;35.96,-9.49,;37.3,-8.72,;38.63,-9.5,;38.63,-11.04,;39.97,-8.73,;34.63,-7.16,;33.28,-6.38,;31.94,-7.16,;30.61,-6.39,)| Show InChI InChI=1S/C22H23F2NO5/c1-28-15-9-16(10-15)30-19-11-17(23)21(24)22(25-19)13-3-6-18-12(8-13)2-4-14(29-18)5-7-20(26)27/h3,6,8,11,14-16H,2,4-5,7,9-10H2,1H3,(H,26,27)/t14-,15-,16-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 35 | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR120 short isoform expressed in CHOK1 cells assessed as increase in IP1 accumulation after 60 mins by HTRF assay |
ACS Med Chem Lett 8: 96-101 (2017)
Article DOI: 10.1021/acsmedchemlett.6b00394
BindingDB Entry DOI: 10.7270/Q2R213CP |
More data for this
Ligand-Target Pair | |
Free fatty acid receptor 4
(Homo sapiens (Human)) | BDBM50207069

(CHEMBL3959068)Show SMILES [H][C@]1(C[C@@]1([H])[C@@]1([H])CCc2cc(ccc2O1)-c1cc(O[C@H]2C[C@@H](C2)OC)cc(F)c1F)C(O)=O |r,wU:5.6,1.0,20.22,wD:3.4,22.27,(25.35,-14.1,;25.76,-15.6,;25.75,-17.14,;24.43,-16.36,;24.41,-17.9,;23.08,-15.59,;22.96,-17.12,;23.09,-14.03,;21.74,-13.25,;20.4,-14.03,;19.07,-13.27,;17.74,-14.04,;17.73,-15.59,;19.06,-16.36,;20.39,-15.59,;21.74,-16.36,;16.4,-13.27,;15.07,-14.04,;13.74,-13.27,;12.4,-14.05,;11.07,-13.29,;9.59,-13.69,;9.19,-12.21,;10.67,-11.8,;7.86,-11.45,;7.86,-9.92,;13.73,-11.73,;15.07,-10.95,;15.07,-9.43,;16.41,-11.72,;17.74,-10.95,;27.1,-16.37,;27.09,-17.91,;28.43,-15.6,)| Show InChI InChI=1S/C24H24F2O5/c1-29-14-7-15(8-14)30-16-9-17(23(26)20(25)10-16)12-2-4-21-13(6-12)3-5-22(31-21)18-11-19(18)24(27)28/h2,4,6,9-10,14-15,18-19,22H,3,5,7-8,11H2,1H3,(H,27,28)/t14-,15-,18-,19-,22-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 17 | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR120 short isoform expressed in CHOK1 cells assessed as beta-arrestin recruitment after 90 mins by luminescence assay |
ACS Med Chem Lett 8: 96-101 (2017)
Article DOI: 10.1021/acsmedchemlett.6b00394
BindingDB Entry DOI: 10.7270/Q2R213CP |
More data for this
Ligand-Target Pair | |
Free fatty acid receptor 4
(Homo sapiens (Human)) | BDBM50207072
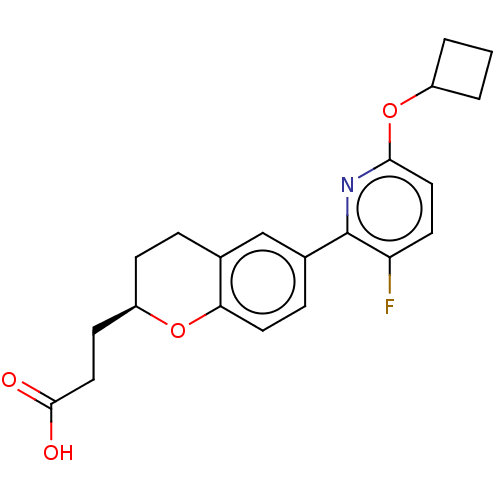
(CHEMBL3946820)Show SMILES OC(=O)CC[C@H]1CCc2cc(ccc2O1)-c1nc(OC2CCC2)ccc1F |r| Show InChI InChI=1S/C21H22FNO4/c22-17-8-10-19(27-15-2-1-3-15)23-21(17)14-5-9-18-13(12-14)4-6-16(26-18)7-11-20(24)25/h5,8-10,12,15-16H,1-4,6-7,11H2,(H,24,25)/t16-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 21 | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR120 short isoform expressed in CHOK1 cells assessed as beta-arrestin recruitment after 90 mins by luminescence assay |
ACS Med Chem Lett 8: 96-101 (2017)
Article DOI: 10.1021/acsmedchemlett.6b00394
BindingDB Entry DOI: 10.7270/Q2R213CP |
More data for this
Ligand-Target Pair | |
Free fatty acid receptor 4
(Homo sapiens (Human)) | BDBM50207059

(CHEMBL3932664)Show SMILES COc1cc(OC2CC2)cc(c1F)-c1ccc2O[C@@H](CCc3nnn[nH]3)CCc2c1 |r| Show InChI InChI=1S/C22H23FN4O3/c1-28-20-12-17(29-15-5-6-15)11-18(22(20)23)13-3-8-19-14(10-13)2-4-16(30-19)7-9-21-24-26-27-25-21/h3,8,10-12,15-16H,2,4-7,9H2,1H3,(H,24,25,26,27)/t16-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 32 | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR120 short isoform expressed in CHOK1 cells assessed as beta-arrestin recruitment after 90 mins by luminescence assay |
ACS Med Chem Lett 8: 96-101 (2017)
Article DOI: 10.1021/acsmedchemlett.6b00394
BindingDB Entry DOI: 10.7270/Q2R213CP |
More data for this
Ligand-Target Pair | |
Free fatty acid receptor 1
(Homo sapiens (Human)) | BDBM50207063

(CHEMBL3971857)Show SMILES OC(=O)CC[C@H]1CCc2cc(ccc2O1)-c1cc(OC2CCC2)ncc1F |r| Show InChI InChI=1S/C21H22FNO4/c22-18-12-23-20(27-15-2-1-3-15)11-17(18)13-5-8-19-14(10-13)4-6-16(26-19)7-9-21(24)25/h5,8,10-12,15-16H,1-4,6-7,9H2,(H,24,25)/t16-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR40 expressed in HEK293 cells assessed as increase in IP1 accumulation after 60 mins by HTRF assay |
ACS Med Chem Lett 8: 96-101 (2017)
Article DOI: 10.1021/acsmedchemlett.6b00394
BindingDB Entry DOI: 10.7270/Q2R213CP |
More data for this
Ligand-Target Pair | |
Free fatty acid receptor 4
(Homo sapiens (Human)) | BDBM50207076

(CHEMBL3979448)Show SMILES Fc1ccc(OC(F)(F)F)cc1-c1ccc2O[C@@H](CCc3nnn[nH]3)CCc2c1 |r| Show InChI InChI=1S/C19H16F4N4O2/c20-16-6-4-14(29-19(21,22)23)10-15(16)11-2-7-17-12(9-11)1-3-13(28-17)5-8-18-24-26-27-25-18/h2,4,6-7,9-10,13H,1,3,5,8H2,(H,24,25,26,27)/t13-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 890 | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR120 short isoform expressed in CHOK1 cells assessed as beta-arrestin recruitment after 90 mins by luminescence assay |
ACS Med Chem Lett 8: 96-101 (2017)
Article DOI: 10.1021/acsmedchemlett.6b00394
BindingDB Entry DOI: 10.7270/Q2R213CP |
More data for this
Ligand-Target Pair | |
Free fatty acid receptor 1
(Homo sapiens (Human)) | BDBM50207054
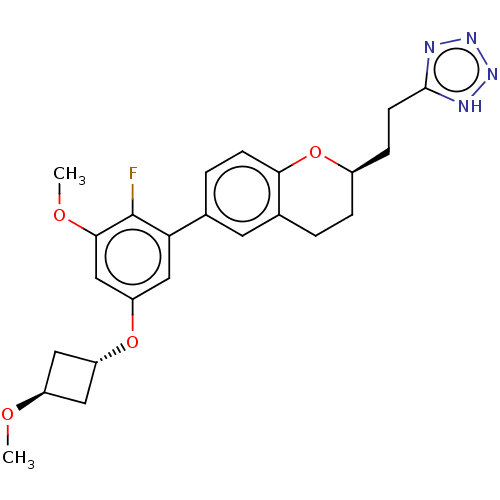
(CHEMBL3907996)Show SMILES CO[C@H]1C[C@@H](C1)Oc1cc(OC)c(F)c(c1)-c1ccc2O[C@@H](CCc3nnn[nH]3)CCc2c1 |r,wU:4.6,wD:21.23,2.1,(3.22,-26.55,;3.22,-28.09,;4.56,-28.86,;4.97,-30.34,;6.45,-29.94,;6.05,-28.45,;7.78,-30.7,;9.11,-29.93,;9.11,-28.38,;10.44,-27.61,;10.44,-26.07,;9.1,-25.3,;11.77,-28.38,;13.11,-27.61,;11.77,-29.92,;10.45,-30.69,;13.1,-30.69,;13.1,-32.23,;14.43,-33,;15.76,-32.24,;17.1,-33.01,;18.45,-32.24,;19.78,-33.02,;21.12,-32.25,;22.45,-33.02,;23.87,-32.4,;24.89,-33.54,;24.12,-34.87,;22.61,-34.55,;18.45,-30.69,;17.11,-29.9,;15.77,-30.68,;14.43,-29.92,)| Show InChI InChI=1S/C24H27FN4O4/c1-30-17-10-18(11-17)32-19-12-20(24(25)22(13-19)31-2)14-4-7-21-15(9-14)3-5-16(33-21)6-8-23-26-28-29-27-23/h4,7,9,12-13,16-18H,3,5-6,8,10-11H2,1-2H3,(H,26,27,28,29)/t16-,17-,18-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR40 expressed in HEK293 cells assessed as increase in IP1 accumulation after 60 mins by HTRF assay |
ACS Med Chem Lett 8: 96-101 (2017)
Article DOI: 10.1021/acsmedchemlett.6b00394
BindingDB Entry DOI: 10.7270/Q2R213CP |
More data for this
Ligand-Target Pair | |
Free fatty acid receptor 4
(Homo sapiens (Human)) | BDBM50207053

(CHEMBL3950444)Show SMILES [H][C@@]1(C[C@]1([H])[C@@]1([H])CCc2cc(ccc2O1)-c1cc(OC(F)(F)F)ccc1F)C(O)=O |r| Show InChI InChI=1S/C20H16F4O4/c21-16-4-3-12(28-20(22,23)24)8-13(16)10-1-5-17-11(7-10)2-6-18(27-17)14-9-15(14)19(25)26/h1,3-5,7-8,14-15,18H,2,6,9H2,(H,25,26)/t14-,15-,18+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 130 | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR120 short isoform expressed in CHOK1 cells assessed as beta-arrestin recruitment after 90 mins by luminescence assay |
ACS Med Chem Lett 8: 96-101 (2017)
Article DOI: 10.1021/acsmedchemlett.6b00394
BindingDB Entry DOI: 10.7270/Q2R213CP |
More data for this
Ligand-Target Pair | |
Free fatty acid receptor 4
(Homo sapiens (Human)) | BDBM50207059

(CHEMBL3932664)Show SMILES COc1cc(OC2CC2)cc(c1F)-c1ccc2O[C@@H](CCc3nnn[nH]3)CCc2c1 |r| Show InChI InChI=1S/C22H23FN4O3/c1-28-20-12-17(29-15-5-6-15)11-18(22(20)23)13-3-8-19-14(10-13)2-4-16(30-19)7-9-21-24-26-27-25-21/h3,8,10-12,15-16H,2,4-7,9H2,1H3,(H,24,25,26,27)/t16-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 51 | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR120 short isoform expressed in CHOK1 cells assessed as increase in IP1 accumulation after 60 mins by HTRF assay |
ACS Med Chem Lett 8: 96-101 (2017)
Article DOI: 10.1021/acsmedchemlett.6b00394
BindingDB Entry DOI: 10.7270/Q2R213CP |
More data for this
Ligand-Target Pair | |
Free fatty acid receptor 4
(Homo sapiens (Human)) | BDBM50207052

(CHEMBL3909773)Show SMILES OC(=O)CC[C@H]1CCc2cc(ccc2O1)-c1cc(OC2CCC2)cc(Cl)c1F |r| Show InChI InChI=1S/C22H22ClFO4/c23-19-12-17(27-15-2-1-3-15)11-18(22(19)24)13-5-8-20-14(10-13)4-6-16(28-20)7-9-21(25)26/h5,8,10-12,15-16H,1-4,6-7,9H2,(H,25,26)/t16-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 40 | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR120 short isoform expressed in CHOK1 cells assessed as increase in IP1 accumulation after 60 mins by HTRF assay |
ACS Med Chem Lett 8: 96-101 (2017)
Article DOI: 10.1021/acsmedchemlett.6b00394
BindingDB Entry DOI: 10.7270/Q2R213CP |
More data for this
Ligand-Target Pair | |
Free fatty acid receptor 1
(Homo sapiens (Human)) | BDBM50207068

(CHEMBL3900705)Show SMILES OC(=O)CC[C@H]1CCc2cc(ccc2O1)-c1cc(OC2CCC2)cnc1F |r| Show InChI InChI=1S/C21H22FNO4/c22-21-18(11-17(12-23-21)26-15-2-1-3-15)13-5-8-19-14(10-13)4-6-16(27-19)7-9-20(24)25/h5,8,10-12,15-16H,1-4,6-7,9H2,(H,24,25)/t16-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR40 expressed in HEK293 cells assessed as increase in IP1 accumulation after 60 mins by HTRF assay |
ACS Med Chem Lett 8: 96-101 (2017)
Article DOI: 10.1021/acsmedchemlett.6b00394
BindingDB Entry DOI: 10.7270/Q2R213CP |
More data for this
Ligand-Target Pair | |
Free fatty acid receptor 4
(Homo sapiens (Human)) | BDBM50207058

(CHEMBL3970166)Show SMILES [H][C@]1(C[C@@]1([H])[C@@]1([H])CCc2cc(ccc2O1)-c1cc(OC2CCC2)cc(OC)c1F)C(O)=O |r| Show InChI InChI=1S/C24H25FO5/c1-28-22-11-16(29-15-3-2-4-15)10-17(23(22)25)13-5-7-20-14(9-13)6-8-21(30-20)18-12-19(18)24(26)27/h5,7,9-11,15,18-19,21H,2-4,6,8,12H2,1H3,(H,26,27)/t18-,19-,21-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 25 | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR120 short isoform expressed in CHOK1 cells assessed as increase in IP1 accumulation after 60 mins by HTRF assay |
ACS Med Chem Lett 8: 96-101 (2017)
Article DOI: 10.1021/acsmedchemlett.6b00394
BindingDB Entry DOI: 10.7270/Q2R213CP |
More data for this
Ligand-Target Pair | |
Free fatty acid receptor 4
(Homo sapiens (Human)) | BDBM50207062

(CHEMBL3959144)Show SMILES OC(=O)CC[C@H]1CCc2cc(ccc2O1)-c1cc(OC(F)(F)F)cc(Cl)c1F |r| Show InChI InChI=1S/C19H15ClF4O4/c20-15-9-13(28-19(22,23)24)8-14(18(15)21)10-2-5-16-11(7-10)1-3-12(27-16)4-6-17(25)26/h2,5,7-9,12H,1,3-4,6H2,(H,25,26)/t12-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 17 | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR120 short isoform expressed in CHOK1 cells assessed as beta-arrestin recruitment after 90 mins by luminescence assay |
ACS Med Chem Lett 8: 96-101 (2017)
Article DOI: 10.1021/acsmedchemlett.6b00394
BindingDB Entry DOI: 10.7270/Q2R213CP |
More data for this
Ligand-Target Pair | |
Free fatty acid receptor 4
(Homo sapiens (Human)) | BDBM50207065

(CHEMBL3971308)Show SMILES [H][C@]1(C[C@@]1([H])[C@@]1([H])CCc2cc(ccc2O1)-c1cc(OC(F)(F)F)ccc1F)C(O)=O |r| Show InChI InChI=1S/C20H16F4O4/c21-16-4-3-12(28-20(22,23)24)8-13(16)10-1-5-17-11(7-10)2-6-18(27-17)14-9-15(14)19(25)26/h1,3-5,7-8,14-15,18H,2,6,9H2,(H,25,26)/t14-,15-,18-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 33 | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR120 short isoform expressed in CHOK1 cells assessed as beta-arrestin recruitment after 90 mins by luminescence assay |
ACS Med Chem Lett 8: 96-101 (2017)
Article DOI: 10.1021/acsmedchemlett.6b00394
BindingDB Entry DOI: 10.7270/Q2R213CP |
More data for this
Ligand-Target Pair | |
Free fatty acid receptor 1
(Homo sapiens (Human)) | BDBM50207062

(CHEMBL3959144)Show SMILES OC(=O)CC[C@H]1CCc2cc(ccc2O1)-c1cc(OC(F)(F)F)cc(Cl)c1F |r| Show InChI InChI=1S/C19H15ClF4O4/c20-15-9-13(28-19(22,23)24)8-14(18(15)21)10-2-5-16-11(7-10)1-3-12(27-16)4-6-17(25)26/h2,5,7-9,12H,1,3-4,6H2,(H,25,26)/t12-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR40 expressed in HEK293 cells assessed as increase in IP1 accumulation after 60 mins by HTRF assay |
ACS Med Chem Lett 8: 96-101 (2017)
Article DOI: 10.1021/acsmedchemlett.6b00394
BindingDB Entry DOI: 10.7270/Q2R213CP |
More data for this
Ligand-Target Pair | |
Free fatty acid receptor 1
(Homo sapiens (Human)) | BDBM50207070
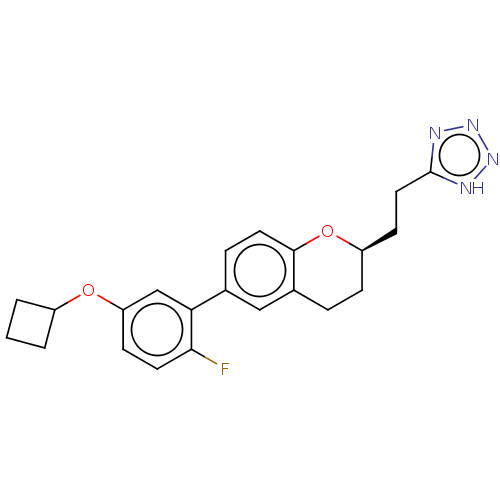
(CHEMBL3986721)Show SMILES Fc1ccc(OC2CCC2)cc1-c1ccc2O[C@@H](CCc3nnn[nH]3)CCc2c1 |r| Show InChI InChI=1S/C22H23FN4O2/c23-20-9-7-18(28-16-2-1-3-16)13-19(20)14-5-10-21-15(12-14)4-6-17(29-21)8-11-22-24-26-27-25-22/h5,7,9-10,12-13,16-17H,1-4,6,8,11H2,(H,24,25,26,27)/t17-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR40 expressed in HEK293 cells assessed as increase in IP1 accumulation after 60 mins by HTRF assay |
ACS Med Chem Lett 8: 96-101 (2017)
Article DOI: 10.1021/acsmedchemlett.6b00394
BindingDB Entry DOI: 10.7270/Q2R213CP |
More data for this
Ligand-Target Pair | |
Free fatty acid receptor 4
(Homo sapiens (Human)) | BDBM50207073

(CHEMBL3940429)Show SMILES COc1cc(OC2CCC2)cc(c1F)-c1ccc2O[C@@H](CCC(O)=O)CCc2c1 |r| Show InChI InChI=1S/C23H25FO5/c1-27-21-13-18(28-16-3-2-4-16)12-19(23(21)24)14-6-9-20-15(11-14)5-7-17(29-20)8-10-22(25)26/h6,9,11-13,16-17H,2-5,7-8,10H2,1H3,(H,25,26)/t17-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 15 | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR120 short isoform expressed in CHOK1 cells assessed as increase in IP1 accumulation after 60 mins by HTRF assay |
ACS Med Chem Lett 8: 96-101 (2017)
Article DOI: 10.1021/acsmedchemlett.6b00394
BindingDB Entry DOI: 10.7270/Q2R213CP |
More data for this
Ligand-Target Pair | |
Free fatty acid receptor 4
(Homo sapiens (Human)) | BDBM50207056

(CHEMBL3970238)Show SMILES OC(=O)CC[C@H]1CCc2cc(ccc2O1)-c1cc(OC2CCC2)ccc1F |r| Show InChI InChI=1S/C22H23FO4/c23-20-9-7-18(26-16-2-1-3-16)13-19(20)14-5-10-21-15(12-14)4-6-17(27-21)8-11-22(24)25/h5,7,9-10,12-13,16-17H,1-4,6,8,11H2,(H,24,25)/t17-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 10 | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR120 short isoform expressed in CHOK1 cells assessed as beta-arrestin recruitment after 90 mins by luminescence assay |
ACS Med Chem Lett 8: 96-101 (2017)
Article DOI: 10.1021/acsmedchemlett.6b00394
BindingDB Entry DOI: 10.7270/Q2R213CP |
More data for this
Ligand-Target Pair | |
Free fatty acid receptor 4
(Homo sapiens (Human)) | BDBM50207051

(CHEMBL3947070)Show SMILES OC(=O)CC[C@H]1CCc2cc(ccc2O1)-c1cc(OC2CCC2)cc(F)c1F |r| Show InChI InChI=1S/C22H22F2O4/c23-19-12-17(27-15-2-1-3-15)11-18(22(19)24)13-5-8-20-14(10-13)4-6-16(28-20)7-9-21(25)26/h5,8,10-12,15-16H,1-4,6-7,9H2,(H,25,26)/t16-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 13 | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR120 short isoform expressed in CHOK1 cells assessed as beta-arrestin recruitment after 90 mins by luminescence assay |
ACS Med Chem Lett 8: 96-101 (2017)
Article DOI: 10.1021/acsmedchemlett.6b00394
BindingDB Entry DOI: 10.7270/Q2R213CP |
More data for this
Ligand-Target Pair | |
Free fatty acid receptor 4
(Homo sapiens (Human)) | BDBM50207054

(CHEMBL3907996)Show SMILES CO[C@H]1C[C@@H](C1)Oc1cc(OC)c(F)c(c1)-c1ccc2O[C@@H](CCc3nnn[nH]3)CCc2c1 |r,wU:4.6,wD:21.23,2.1,(3.22,-26.55,;3.22,-28.09,;4.56,-28.86,;4.97,-30.34,;6.45,-29.94,;6.05,-28.45,;7.78,-30.7,;9.11,-29.93,;9.11,-28.38,;10.44,-27.61,;10.44,-26.07,;9.1,-25.3,;11.77,-28.38,;13.11,-27.61,;11.77,-29.92,;10.45,-30.69,;13.1,-30.69,;13.1,-32.23,;14.43,-33,;15.76,-32.24,;17.1,-33.01,;18.45,-32.24,;19.78,-33.02,;21.12,-32.25,;22.45,-33.02,;23.87,-32.4,;24.89,-33.54,;24.12,-34.87,;22.61,-34.55,;18.45,-30.69,;17.11,-29.9,;15.77,-30.68,;14.43,-29.92,)| Show InChI InChI=1S/C24H27FN4O4/c1-30-17-10-18(11-17)32-19-12-20(24(25)22(13-19)31-2)14-4-7-21-15(9-14)3-5-16(33-21)6-8-23-26-28-29-27-23/h4,7,9,12-13,16-18H,3,5-6,8,10-11H2,1-2H3,(H,26,27,28,29)/t16-,17-,18-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 43 | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR120 short isoform expressed in CHOK1 cells assessed as beta-arrestin recruitment after 90 mins by luminescence assay |
ACS Med Chem Lett 8: 96-101 (2017)
Article DOI: 10.1021/acsmedchemlett.6b00394
BindingDB Entry DOI: 10.7270/Q2R213CP |
More data for this
Ligand-Target Pair | |
Free fatty acid receptor 4
(Mus musculus) | BDBM50207060

(CHEMBL3952043)Show SMILES CO[C@H]1C[C@@H](C1)Oc1cc(OC)c(F)c(n1)-c1ccc2O[C@@H](CCC(O)=O)CCc2c1 |r,wU:4.6,wD:21.23,2.1,(37.05,-1.75,;37.06,-3.29,;38.39,-4.05,;38.8,-5.54,;40.28,-5.13,;39.88,-3.65,;41.61,-5.9,;42.94,-5.12,;42.94,-3.58,;44.27,-2.81,;44.28,-1.27,;42.95,-.5,;45.61,-3.58,;46.94,-2.81,;45.6,-5.12,;44.28,-5.89,;46.93,-5.89,;46.93,-7.43,;48.27,-8.2,;49.6,-7.44,;50.93,-8.21,;52.28,-7.44,;53.61,-8.21,;54.95,-7.45,;56.28,-8.22,;56.28,-9.76,;57.62,-7.45,;52.29,-5.88,;50.94,-5.1,;49.6,-5.88,;48.26,-5.12,)| Show InChI InChI=1S/C23H26FNO6/c1-28-16-10-17(11-16)31-20-12-19(29-2)22(24)23(25-20)14-4-7-18-13(9-14)3-5-15(30-18)6-8-21(26)27/h4,7,9,12,15-17H,3,5-6,8,10-11H2,1-2H3,(H,26,27)/t15-,16-,17-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 5.60 | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Agonist activity at mouse GPR120 expressed in CHOK1 cells assessed as increase in IP1 accumulation after 60 mins by HTRF assay |
ACS Med Chem Lett 8: 96-101 (2017)
Article DOI: 10.1021/acsmedchemlett.6b00394
BindingDB Entry DOI: 10.7270/Q2R213CP |
More data for this
Ligand-Target Pair | |
Free fatty acid receptor 4
(Homo sapiens (Human)) | BDBM50207058

(CHEMBL3970166)Show SMILES [H][C@]1(C[C@@]1([H])[C@@]1([H])CCc2cc(ccc2O1)-c1cc(OC2CCC2)cc(OC)c1F)C(O)=O |r| Show InChI InChI=1S/C24H25FO5/c1-28-22-11-16(29-15-3-2-4-15)10-17(23(22)25)13-5-7-20-14(9-13)6-8-21(30-20)18-12-19(18)24(26)27/h5,7,9-11,15,18-19,21H,2-4,6,8,12H2,1H3,(H,26,27)/t18-,19-,21-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 8 | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR120 short isoform expressed in CHOK1 cells assessed as beta-arrestin recruitment after 90 mins by luminescence assay |
ACS Med Chem Lett 8: 96-101 (2017)
Article DOI: 10.1021/acsmedchemlett.6b00394
BindingDB Entry DOI: 10.7270/Q2R213CP |
More data for this
Ligand-Target Pair | |
Free fatty acid receptor 4
(Homo sapiens (Human)) | BDBM50207054

(CHEMBL3907996)Show SMILES CO[C@H]1C[C@@H](C1)Oc1cc(OC)c(F)c(c1)-c1ccc2O[C@@H](CCc3nnn[nH]3)CCc2c1 |r,wU:4.6,wD:21.23,2.1,(3.22,-26.55,;3.22,-28.09,;4.56,-28.86,;4.97,-30.34,;6.45,-29.94,;6.05,-28.45,;7.78,-30.7,;9.11,-29.93,;9.11,-28.38,;10.44,-27.61,;10.44,-26.07,;9.1,-25.3,;11.77,-28.38,;13.11,-27.61,;11.77,-29.92,;10.45,-30.69,;13.1,-30.69,;13.1,-32.23,;14.43,-33,;15.76,-32.24,;17.1,-33.01,;18.45,-32.24,;19.78,-33.02,;21.12,-32.25,;22.45,-33.02,;23.87,-32.4,;24.89,-33.54,;24.12,-34.87,;22.61,-34.55,;18.45,-30.69,;17.11,-29.9,;15.77,-30.68,;14.43,-29.92,)| Show InChI InChI=1S/C24H27FN4O4/c1-30-17-10-18(11-17)32-19-12-20(24(25)22(13-19)31-2)14-4-7-21-15(9-14)3-5-16(33-21)6-8-23-26-28-29-27-23/h4,7,9,12-13,16-18H,3,5-6,8,10-11H2,1-2H3,(H,26,27,28,29)/t16-,17-,18-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 53 | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR120 short isoform expressed in CHOK1 cells assessed as increase in IP1 accumulation after 60 mins by HTRF assay |
ACS Med Chem Lett 8: 96-101 (2017)
Article DOI: 10.1021/acsmedchemlett.6b00394
BindingDB Entry DOI: 10.7270/Q2R213CP |
More data for this
Ligand-Target Pair | |
Free fatty acid receptor 4
(Homo sapiens (Human)) | BDBM50207074

(CHEMBL3916986)Show SMILES CO[C@H]1C[C@@H](C1)Oc1cc(F)c(F)c(c1)-c1ccc2O[C@@H](CCc3nnn[nH]3)CCc2c1 |r,wU:4.6,wD:20.22,2.1,(24.37,-19.15,;24.37,-20.69,;25.71,-21.45,;26.11,-22.94,;27.59,-22.53,;27.19,-21.05,;28.93,-23.3,;30.26,-22.52,;30.25,-20.97,;31.59,-20.2,;31.59,-18.66,;32.92,-20.97,;34.26,-20.21,;32.92,-22.52,;31.59,-23.29,;34.25,-23.29,;34.25,-24.83,;35.58,-25.6,;36.91,-24.83,;38.25,-25.61,;39.6,-24.84,;40.93,-25.61,;42.27,-24.85,;43.6,-25.62,;45.01,-24.99,;46.04,-26.14,;45.27,-27.47,;43.76,-27.15,;39.6,-23.28,;38.25,-22.5,;36.91,-23.28,;35.58,-22.51,)| Show InChI InChI=1S/C23H24F2N4O3/c1-30-16-9-17(10-16)31-18-11-19(23(25)20(24)12-18)13-3-6-21-14(8-13)2-4-15(32-21)5-7-22-26-28-29-27-22/h3,6,8,11-12,15-17H,2,4-5,7,9-10H2,1H3,(H,26,27,28,29)/t15-,16-,17-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 240 | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR120 short isoform expressed in CHOK1 cells assessed as increase in IP1 accumulation after 60 mins by HTRF assay |
ACS Med Chem Lett 8: 96-101 (2017)
Article DOI: 10.1021/acsmedchemlett.6b00394
BindingDB Entry DOI: 10.7270/Q2R213CP |
More data for this
Ligand-Target Pair | |
Free fatty acid receptor 4
(Homo sapiens (Human)) | BDBM50207069

(CHEMBL3959068)Show SMILES [H][C@]1(C[C@@]1([H])[C@@]1([H])CCc2cc(ccc2O1)-c1cc(O[C@H]2C[C@@H](C2)OC)cc(F)c1F)C(O)=O |r,wU:5.6,1.0,20.22,wD:3.4,22.27,(25.35,-14.1,;25.76,-15.6,;25.75,-17.14,;24.43,-16.36,;24.41,-17.9,;23.08,-15.59,;22.96,-17.12,;23.09,-14.03,;21.74,-13.25,;20.4,-14.03,;19.07,-13.27,;17.74,-14.04,;17.73,-15.59,;19.06,-16.36,;20.39,-15.59,;21.74,-16.36,;16.4,-13.27,;15.07,-14.04,;13.74,-13.27,;12.4,-14.05,;11.07,-13.29,;9.59,-13.69,;9.19,-12.21,;10.67,-11.8,;7.86,-11.45,;7.86,-9.92,;13.73,-11.73,;15.07,-10.95,;15.07,-9.43,;16.41,-11.72,;17.74,-10.95,;27.1,-16.37,;27.09,-17.91,;28.43,-15.6,)| Show InChI InChI=1S/C24H24F2O5/c1-29-14-7-15(8-14)30-16-9-17(23(26)20(25)10-16)12-2-4-21-13(6-12)3-5-22(31-21)18-11-19(18)24(27)28/h2,4,6,9-10,14-15,18-19,22H,3,5,7-8,11H2,1H3,(H,27,28)/t14-,15-,18-,19-,22-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 45 | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR120 short isoform expressed in CHOK1 cells assessed as increase in IP1 accumulation after 60 mins by HTRF assay |
ACS Med Chem Lett 8: 96-101 (2017)
Article DOI: 10.1021/acsmedchemlett.6b00394
BindingDB Entry DOI: 10.7270/Q2R213CP |
More data for this
Ligand-Target Pair | |
Free fatty acid receptor 4
(Homo sapiens (Human)) | BDBM50207066

(CHEMBL3890089)Show SMILES COc1cc(OC(F)(F)F)cc(c1F)-c1ccc2O[C@@H](CCC(O)=O)CCc2c1 |r| Show InChI InChI=1S/C20H18F4O5/c1-27-17-10-14(29-20(22,23)24)9-15(19(17)21)11-3-6-16-12(8-11)2-4-13(28-16)5-7-18(25)26/h3,6,8-10,13H,2,4-5,7H2,1H3,(H,25,26)/t13-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 11 | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR120 short isoform expressed in CHOK1 cells assessed as beta-arrestin recruitment after 90 mins by luminescence assay |
ACS Med Chem Lett 8: 96-101 (2017)
Article DOI: 10.1021/acsmedchemlett.6b00394
BindingDB Entry DOI: 10.7270/Q2R213CP |
More data for this
Ligand-Target Pair | |
Free fatty acid receptor 4
(Homo sapiens (Human)) | BDBM50207063

(CHEMBL3971857)Show SMILES OC(=O)CC[C@H]1CCc2cc(ccc2O1)-c1cc(OC2CCC2)ncc1F |r| Show InChI InChI=1S/C21H22FNO4/c22-18-12-23-20(27-15-2-1-3-15)11-17(18)13-5-8-19-14(10-13)4-6-16(26-19)7-9-21(24)25/h5,8,10-12,15-16H,1-4,6-7,9H2,(H,24,25)/t16-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 250 | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR120 short isoform expressed in CHOK1 cells assessed as increase in IP1 accumulation after 60 mins by HTRF assay |
ACS Med Chem Lett 8: 96-101 (2017)
Article DOI: 10.1021/acsmedchemlett.6b00394
BindingDB Entry DOI: 10.7270/Q2R213CP |
More data for this
Ligand-Target Pair | |
Free fatty acid receptor 1
(Homo sapiens (Human)) | BDBM50207051

(CHEMBL3947070)Show SMILES OC(=O)CC[C@H]1CCc2cc(ccc2O1)-c1cc(OC2CCC2)cc(F)c1F |r| Show InChI InChI=1S/C22H22F2O4/c23-19-12-17(27-15-2-1-3-15)11-18(22(19)24)13-5-8-20-14(10-13)4-6-16(28-20)7-9-21(25)26/h5,8,10-12,15-16H,1-4,6-7,9H2,(H,25,26)/t16-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR40 expressed in HEK293 cells assessed as increase in IP1 accumulation after 60 mins by HTRF assay |
ACS Med Chem Lett 8: 96-101 (2017)
Article DOI: 10.1021/acsmedchemlett.6b00394
BindingDB Entry DOI: 10.7270/Q2R213CP |
More data for this
Ligand-Target Pair | |
Free fatty acid receptor 1
(Homo sapiens (Human)) | BDBM50207052

(CHEMBL3909773)Show SMILES OC(=O)CC[C@H]1CCc2cc(ccc2O1)-c1cc(OC2CCC2)cc(Cl)c1F |r| Show InChI InChI=1S/C22H22ClFO4/c23-19-12-17(27-15-2-1-3-15)11-18(22(19)24)13-5-8-20-14(10-13)4-6-16(28-20)7-9-21(25)26/h5,8,10-12,15-16H,1-4,6-7,9H2,(H,25,26)/t16-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR40 expressed in HEK293 cells assessed as increase in IP1 accumulation after 60 mins by HTRF assay |
ACS Med Chem Lett 8: 96-101 (2017)
Article DOI: 10.1021/acsmedchemlett.6b00394
BindingDB Entry DOI: 10.7270/Q2R213CP |
More data for this
Ligand-Target Pair | |
Free fatty acid receptor 4
(Mus musculus) | BDBM50207061

(CHEMBL3980898)Show SMILES CO[C@H]1C[C@@H](C1)Oc1cc(F)c(F)c(n1)-c1ccc2O[C@@H](CCC(O)=O)CCc2c1 |r,wU:4.6,wD:20.22,2.1,(19.4,-3.02,;19.4,-4.56,;20.74,-5.33,;21.14,-6.82,;22.62,-6.41,;22.23,-4.93,;23.96,-7.18,;25.29,-6.4,;25.28,-4.85,;26.62,-4.08,;26.62,-2.54,;27.95,-4.85,;29.29,-4.08,;27.95,-6.39,;26.62,-7.16,;29.28,-7.16,;29.28,-8.71,;30.61,-9.48,;31.94,-8.71,;33.28,-9.49,;34.63,-8.72,;35.96,-9.49,;37.3,-8.72,;38.63,-9.5,;38.63,-11.04,;39.97,-8.73,;34.63,-7.16,;33.28,-6.38,;31.94,-7.16,;30.61,-6.39,)| Show InChI InChI=1S/C22H23F2NO5/c1-28-15-9-16(10-15)30-19-11-17(23)21(24)22(25-19)13-3-6-18-12(8-13)2-4-14(29-18)5-7-20(26)27/h3,6,8,11,14-16H,2,4-5,7,9-10H2,1H3,(H,26,27)/t14-,15-,16-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 6.80 | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Agonist activity at mouse GPR120 expressed in human U2OS cells assessed as beta-arrestin recruitment after 90 mins by luminescence assay |
ACS Med Chem Lett 8: 96-101 (2017)
Article DOI: 10.1021/acsmedchemlett.6b00394
BindingDB Entry DOI: 10.7270/Q2R213CP |
More data for this
Ligand-Target Pair | |
Free fatty acid receptor 4
(Homo sapiens (Human)) | BDBM50207053

(CHEMBL3950444)Show SMILES [H][C@@]1(C[C@]1([H])[C@@]1([H])CCc2cc(ccc2O1)-c1cc(OC(F)(F)F)ccc1F)C(O)=O |r| Show InChI InChI=1S/C20H16F4O4/c21-16-4-3-12(28-20(22,23)24)8-13(16)10-1-5-17-11(7-10)2-6-18(27-17)14-9-15(14)19(25)26/h1,3-5,7-8,14-15,18H,2,6,9H2,(H,25,26)/t14-,15-,18+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 320 | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR120 short isoform expressed in CHOK1 cells assessed as increase in IP1 accumulation after 60 mins by HTRF assay |
ACS Med Chem Lett 8: 96-101 (2017)
Article DOI: 10.1021/acsmedchemlett.6b00394
BindingDB Entry DOI: 10.7270/Q2R213CP |
More data for this
Ligand-Target Pair | |
Free fatty acid receptor 4
(Homo sapiens (Human)) | BDBM50207067

(CHEMBL3941726)Show SMILES OC(=O)CC[C@@H]1CCc2cc(ccc2O1)-c1cc(OC(F)(F)F)ccc1F |r| Show InChI InChI=1S/C19H16F4O4/c20-16-6-4-14(27-19(21,22)23)10-15(16)11-2-7-17-12(9-11)1-3-13(26-17)5-8-18(24)25/h2,4,6-7,9-10,13H,1,3,5,8H2,(H,24,25)/t13-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 42 | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR120 short isoform expressed in CHOK1 cells assessed as beta-arrestin recruitment after 90 mins by luminescence assay |
ACS Med Chem Lett 8: 96-101 (2017)
Article DOI: 10.1021/acsmedchemlett.6b00394
BindingDB Entry DOI: 10.7270/Q2R213CP |
More data for this
Ligand-Target Pair | |
Free fatty acid receptor 1
(Homo sapiens (Human)) | BDBM50207064

(CHEMBL3943315)Show SMILES COc1cc(Oc2ncc(C)s2)cc(c1F)-c1ccc2O[C@@H](CCC(O)=O)CCc2c1 |r| Show InChI InChI=1S/C23H22FNO5S/c1-13-12-25-23(31-13)30-17-10-18(22(24)20(11-17)28-2)14-4-7-19-15(9-14)3-5-16(29-19)6-8-21(26)27/h4,7,9-12,16H,3,5-6,8H2,1-2H3,(H,26,27)/t16-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR40 expressed in HEK293 cells assessed as increase in IP1 accumulation after 60 mins by HTRF assay |
ACS Med Chem Lett 8: 96-101 (2017)
Article DOI: 10.1021/acsmedchemlett.6b00394
BindingDB Entry DOI: 10.7270/Q2R213CP |
More data for this
Ligand-Target Pair | |
Free fatty acid receptor 4
(Homo sapiens (Human)) | BDBM50207060

(CHEMBL3952043)Show SMILES CO[C@H]1C[C@@H](C1)Oc1cc(OC)c(F)c(n1)-c1ccc2O[C@@H](CCC(O)=O)CCc2c1 |r,wU:4.6,wD:21.23,2.1,(37.05,-1.75,;37.06,-3.29,;38.39,-4.05,;38.8,-5.54,;40.28,-5.13,;39.88,-3.65,;41.61,-5.9,;42.94,-5.12,;42.94,-3.58,;44.27,-2.81,;44.28,-1.27,;42.95,-.5,;45.61,-3.58,;46.94,-2.81,;45.6,-5.12,;44.28,-5.89,;46.93,-5.89,;46.93,-7.43,;48.27,-8.2,;49.6,-7.44,;50.93,-8.21,;52.28,-7.44,;53.61,-8.21,;54.95,-7.45,;56.28,-8.22,;56.28,-9.76,;57.62,-7.45,;52.29,-5.88,;50.94,-5.1,;49.6,-5.88,;48.26,-5.12,)| Show InChI InChI=1S/C23H26FNO6/c1-28-16-10-17(11-16)31-20-12-19(29-2)22(24)23(25-20)14-4-7-18-13(9-14)3-5-15(30-18)6-8-21(26)27/h4,7,9,12,15-17H,3,5-6,8,10-11H2,1-2H3,(H,26,27)/t15-,16-,17-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 14 | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR120 short isoform expressed in CHOK1 cells assessed as increase in IP1 accumulation after 60 mins by HTRF assay |
ACS Med Chem Lett 8: 96-101 (2017)
Article DOI: 10.1021/acsmedchemlett.6b00394
BindingDB Entry DOI: 10.7270/Q2R213CP |
More data for this
Ligand-Target Pair | |
Free fatty acid receptor 4
(Homo sapiens (Human)) | BDBM50207072

(CHEMBL3946820)Show SMILES OC(=O)CC[C@H]1CCc2cc(ccc2O1)-c1nc(OC2CCC2)ccc1F |r| Show InChI InChI=1S/C21H22FNO4/c22-17-8-10-19(27-15-2-1-3-15)23-21(17)14-5-9-18-13(12-14)4-6-16(26-18)7-11-20(24)25/h5,8-10,12,15-16H,1-4,6-7,11H2,(H,24,25)/t16-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 30 | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR120 short isoform expressed in CHOK1 cells assessed as increase in IP1 accumulation after 60 mins by HTRF assay |
ACS Med Chem Lett 8: 96-101 (2017)
Article DOI: 10.1021/acsmedchemlett.6b00394
BindingDB Entry DOI: 10.7270/Q2R213CP |
More data for this
Ligand-Target Pair | |
Free fatty acid receptor 4
(Homo sapiens (Human)) | BDBM50207066

(CHEMBL3890089)Show SMILES COc1cc(OC(F)(F)F)cc(c1F)-c1ccc2O[C@@H](CCC(O)=O)CCc2c1 |r| Show InChI InChI=1S/C20H18F4O5/c1-27-17-10-14(29-20(22,23)24)9-15(19(17)21)11-3-6-16-12(8-11)2-4-13(28-16)5-7-18(25)26/h3,6,8-10,13H,2,4-5,7H2,1H3,(H,25,26)/t13-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 25 | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR120 short isoform expressed in CHOK1 cells assessed as increase in IP1 accumulation after 60 mins by HTRF assay |
ACS Med Chem Lett 8: 96-101 (2017)
Article DOI: 10.1021/acsmedchemlett.6b00394
BindingDB Entry DOI: 10.7270/Q2R213CP |
More data for this
Ligand-Target Pair | |
Free fatty acid receptor 1
(Homo sapiens (Human)) | BDBM50207060

(CHEMBL3952043)Show SMILES CO[C@H]1C[C@@H](C1)Oc1cc(OC)c(F)c(n1)-c1ccc2O[C@@H](CCC(O)=O)CCc2c1 |r,wU:4.6,wD:21.23,2.1,(37.05,-1.75,;37.06,-3.29,;38.39,-4.05,;38.8,-5.54,;40.28,-5.13,;39.88,-3.65,;41.61,-5.9,;42.94,-5.12,;42.94,-3.58,;44.27,-2.81,;44.28,-1.27,;42.95,-.5,;45.61,-3.58,;46.94,-2.81,;45.6,-5.12,;44.28,-5.89,;46.93,-5.89,;46.93,-7.43,;48.27,-8.2,;49.6,-7.44,;50.93,-8.21,;52.28,-7.44,;53.61,-8.21,;54.95,-7.45,;56.28,-8.22,;56.28,-9.76,;57.62,-7.45,;52.29,-5.88,;50.94,-5.1,;49.6,-5.88,;48.26,-5.12,)| Show InChI InChI=1S/C23H26FNO6/c1-28-16-10-17(11-16)31-20-12-19(29-2)22(24)23(25-20)14-4-7-18-13(9-14)3-5-15(30-18)6-8-21(26)27/h4,7,9,12,15-17H,3,5-6,8,10-11H2,1-2H3,(H,26,27)/t15-,16-,17-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR40 expressed in HEK293 cells assessed as increase in IP1 accumulation after 60 mins by HTRF assay |
ACS Med Chem Lett 8: 96-101 (2017)
Article DOI: 10.1021/acsmedchemlett.6b00394
BindingDB Entry DOI: 10.7270/Q2R213CP |
More data for this
Ligand-Target Pair | |
Free fatty acid receptor 1
(Homo sapiens (Human)) | BDBM50207077

(CHEMBL3890035)Show SMILES [H][C@]1(C[C@@]1([H])[C@@]1([H])CCc2cc(ccc2O1)-c1cc(O[C@H]2C[C@@H](C2)OC)cc(OC)c1F)C(O)=O |r,wU:5.6,1.0,20.22,wD:3.4,22.27,(25.35,-14.1,;25.76,-15.6,;25.75,-17.14,;24.43,-16.36,;24.41,-17.9,;23.08,-15.59,;22.96,-17.12,;23.09,-14.03,;21.74,-13.25,;20.4,-14.03,;19.07,-13.27,;17.74,-14.04,;17.73,-15.59,;19.06,-16.36,;20.39,-15.59,;21.74,-16.36,;16.4,-13.27,;15.07,-14.04,;13.74,-13.27,;12.4,-14.05,;11.07,-13.29,;9.59,-13.69,;9.19,-12.21,;10.67,-11.8,;7.86,-11.45,;7.86,-9.92,;13.73,-11.73,;15.07,-10.95,;15.07,-9.44,;13.75,-8.67,;16.41,-11.72,;17.74,-10.95,;27.1,-16.37,;27.09,-17.91,;28.43,-15.6,)| Show InChI InChI=1S/C25H27FO6/c1-29-15-8-16(9-15)31-17-10-18(24(26)23(11-17)30-2)13-3-5-21-14(7-13)4-6-22(32-21)19-12-20(19)25(27)28/h3,5,7,10-11,15-16,19-20,22H,4,6,8-9,12H2,1-2H3,(H,27,28)/t15-,16-,19-,20-,22-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR40 expressed in HEK293 cells assessed as increase in IP1 accumulation after 60 mins by HTRF assay |
ACS Med Chem Lett 8: 96-101 (2017)
Article DOI: 10.1021/acsmedchemlett.6b00394
BindingDB Entry DOI: 10.7270/Q2R213CP |
More data for this
Ligand-Target Pair | |
Free fatty acid receptor 1
(Homo sapiens (Human)) | BDBM50207074

(CHEMBL3916986)Show SMILES CO[C@H]1C[C@@H](C1)Oc1cc(F)c(F)c(c1)-c1ccc2O[C@@H](CCc3nnn[nH]3)CCc2c1 |r,wU:4.6,wD:20.22,2.1,(24.37,-19.15,;24.37,-20.69,;25.71,-21.45,;26.11,-22.94,;27.59,-22.53,;27.19,-21.05,;28.93,-23.3,;30.26,-22.52,;30.25,-20.97,;31.59,-20.2,;31.59,-18.66,;32.92,-20.97,;34.26,-20.21,;32.92,-22.52,;31.59,-23.29,;34.25,-23.29,;34.25,-24.83,;35.58,-25.6,;36.91,-24.83,;38.25,-25.61,;39.6,-24.84,;40.93,-25.61,;42.27,-24.85,;43.6,-25.62,;45.01,-24.99,;46.04,-26.14,;45.27,-27.47,;43.76,-27.15,;39.6,-23.28,;38.25,-22.5,;36.91,-23.28,;35.58,-22.51,)| Show InChI InChI=1S/C23H24F2N4O3/c1-30-16-9-17(10-16)31-18-11-19(23(25)20(24)12-18)13-3-6-21-14(8-13)2-4-15(32-21)5-7-22-26-28-29-27-22/h3,6,8,11-12,15-17H,2,4-5,7,9-10H2,1H3,(H,26,27,28,29)/t15-,16-,17-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR40 expressed in HEK293 cells assessed as increase in IP1 accumulation after 60 mins by HTRF assay |
ACS Med Chem Lett 8: 96-101 (2017)
Article DOI: 10.1021/acsmedchemlett.6b00394
BindingDB Entry DOI: 10.7270/Q2R213CP |
More data for this
Ligand-Target Pair | |
Free fatty acid receptor 4
(Homo sapiens (Human)) | BDBM50207070

(CHEMBL3986721)Show SMILES Fc1ccc(OC2CCC2)cc1-c1ccc2O[C@@H](CCc3nnn[nH]3)CCc2c1 |r| Show InChI InChI=1S/C22H23FN4O2/c23-20-9-7-18(28-16-2-1-3-16)13-19(20)14-5-10-21-15(12-14)4-6-17(29-21)8-11-22-24-26-27-25-22/h5,7,9-10,12-13,16-17H,1-4,6,8,11H2,(H,24,25,26,27)/t17-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | n/a | n/a | 84 | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR120 short isoform expressed in CHOK1 cells assessed as beta-arrestin recruitment after 90 mins by luminescence assay |
ACS Med Chem Lett 8: 96-101 (2017)
Article DOI: 10.1021/acsmedchemlett.6b00394
BindingDB Entry DOI: 10.7270/Q2R213CP |
More data for this
Ligand-Target Pair | |
Free fatty acid receptor 1
(Homo sapiens (Human)) | BDBM50207075

(CHEMBL3898962)Show SMILES [H][C@]1(C[C@@]1([H])[C@@]1([H])CCc2cc(ccc2O1)-c1nc(OC2CCC2)cc(OC)c1F)C(O)=O |r| Show InChI InChI=1S/C23H24FNO5/c1-28-19-11-20(29-14-3-2-4-14)25-22(21(19)24)13-6-7-17-12(9-13)5-8-18(30-17)15-10-16(15)23(26)27/h6-7,9,11,14-16,18H,2-5,8,10H2,1H3,(H,26,27)/t15-,16-,18-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR40 expressed in HEK293 cells assessed as increase in IP1 accumulation after 60 mins by HTRF assay |
ACS Med Chem Lett 8: 96-101 (2017)
Article DOI: 10.1021/acsmedchemlett.6b00394
BindingDB Entry DOI: 10.7270/Q2R213CP |
More data for this
Ligand-Target Pair | |
Free fatty acid receptor 1
(Homo sapiens (Human)) | BDBM50207072

(CHEMBL3946820)Show SMILES OC(=O)CC[C@H]1CCc2cc(ccc2O1)-c1nc(OC2CCC2)ccc1F |r| Show InChI InChI=1S/C21H22FNO4/c22-17-8-10-19(27-15-2-1-3-15)23-21(17)14-5-9-18-13(12-14)4-6-16(26-18)7-11-20(24)25/h5,8-10,12,15-16H,1-4,6-7,11H2,(H,24,25)/t16-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR40 expressed in HEK293 cells assessed as increase in IP1 accumulation after 60 mins by HTRF assay |
ACS Med Chem Lett 8: 96-101 (2017)
Article DOI: 10.1021/acsmedchemlett.6b00394
BindingDB Entry DOI: 10.7270/Q2R213CP |
More data for this
Ligand-Target Pair | |
Free fatty acid receptor 4
(Homo sapiens (Human)) | BDBM50207074

(CHEMBL3916986)Show SMILES CO[C@H]1C[C@@H](C1)Oc1cc(F)c(F)c(c1)-c1ccc2O[C@@H](CCc3nnn[nH]3)CCc2c1 |r,wU:4.6,wD:20.22,2.1,(24.37,-19.15,;24.37,-20.69,;25.71,-21.45,;26.11,-22.94,;27.59,-22.53,;27.19,-21.05,;28.93,-23.3,;30.26,-22.52,;30.25,-20.97,;31.59,-20.2,;31.59,-18.66,;32.92,-20.97,;34.26,-20.21,;32.92,-22.52,;31.59,-23.29,;34.25,-23.29,;34.25,-24.83,;35.58,-25.6,;36.91,-24.83,;38.25,-25.61,;39.6,-24.84,;40.93,-25.61,;42.27,-24.85,;43.6,-25.62,;45.01,-24.99,;46.04,-26.14,;45.27,-27.47,;43.76,-27.15,;39.6,-23.28,;38.25,-22.5,;36.91,-23.28,;35.58,-22.51,)| Show InChI InChI=1S/C23H24F2N4O3/c1-30-16-9-17(10-16)31-18-11-19(23(25)20(24)12-18)13-3-6-21-14(8-13)2-4-15(32-21)5-7-22-26-28-29-27-22/h3,6,8,11-12,15-17H,2,4-5,7,9-10H2,1H3,(H,26,27,28,29)/t15-,16-,17-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 170 | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR120 short isoform expressed in CHOK1 cells assessed as beta-arrestin recruitment after 90 mins by luminescence assay |
ACS Med Chem Lett 8: 96-101 (2017)
Article DOI: 10.1021/acsmedchemlett.6b00394
BindingDB Entry DOI: 10.7270/Q2R213CP |
More data for this
Ligand-Target Pair | |
Free fatty acid receptor 4
(Homo sapiens (Human)) | BDBM50207057

(CHEMBL3960738)Show SMILES COc1cc(OC2CCC2)nc(c1F)-c1ccc2O[C@@H](CCC(O)=O)CCc2c1 |r| Show InChI InChI=1S/C22H24FNO5/c1-27-18-12-19(29-15-3-2-4-15)24-22(21(18)23)14-6-9-17-13(11-14)5-7-16(28-17)8-10-20(25)26/h6,9,11-12,15-16H,2-5,7-8,10H2,1H3,(H,25,26)/t16-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 6.20 | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR120 short isoform expressed in CHOK1 cells assessed as beta-arrestin recruitment after 90 mins by luminescence assay |
ACS Med Chem Lett 8: 96-101 (2017)
Article DOI: 10.1021/acsmedchemlett.6b00394
BindingDB Entry DOI: 10.7270/Q2R213CP |
More data for this
Ligand-Target Pair | |
Free fatty acid receptor 1
(Homo sapiens (Human)) | BDBM50207055

(CHEMBL3960231)Show SMILES OC(=O)CC[C@H]1CCc2cc(ccc2O1)-c1cc(OC(F)(F)F)ccc1F |r| Show InChI InChI=1S/C19H16F4O4/c20-16-6-4-14(27-19(21,22)23)10-15(16)11-2-7-17-12(9-11)1-3-13(26-17)5-8-18(24)25/h2,4,6-7,9-10,13H,1,3,5,8H2,(H,24,25)/t13-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR40 expressed in HEK293 cells assessed as increase in IP1 accumulation after 60 mins by HTRF assay |
ACS Med Chem Lett 8: 96-101 (2017)
Article DOI: 10.1021/acsmedchemlett.6b00394
BindingDB Entry DOI: 10.7270/Q2R213CP |
More data for this
Ligand-Target Pair | |
Free fatty acid receptor 4
(Homo sapiens (Human)) | BDBM50207070

(CHEMBL3986721)Show SMILES Fc1ccc(OC2CCC2)cc1-c1ccc2O[C@@H](CCc3nnn[nH]3)CCc2c1 |r| Show InChI InChI=1S/C22H23FN4O2/c23-20-9-7-18(28-16-2-1-3-16)13-19(20)14-5-10-21-15(12-14)4-6-17(29-21)8-11-22-24-26-27-25-22/h5,7,9-10,12-13,16-17H,1-4,6,8,11H2,(H,24,25,26,27)/t17-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | n/a | n/a | 220 | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR120 short isoform expressed in CHOK1 cells assessed as increase in IP1 accumulation after 60 mins by HTRF assay |
ACS Med Chem Lett 8: 96-101 (2017)
Article DOI: 10.1021/acsmedchemlett.6b00394
BindingDB Entry DOI: 10.7270/Q2R213CP |
More data for this
Ligand-Target Pair | |
Free fatty acid receptor 4
(Homo sapiens (Human)) | BDBM50207076

(CHEMBL3979448)Show SMILES Fc1ccc(OC(F)(F)F)cc1-c1ccc2O[C@@H](CCc3nnn[nH]3)CCc2c1 |r| Show InChI InChI=1S/C19H16F4N4O2/c20-16-6-4-14(29-19(21,22)23)10-15(16)11-2-7-17-12(9-11)1-3-13(28-17)5-8-18-24-26-27-25-18/h2,4,6-7,9-10,13H,1,3,5,8H2,(H,24,25,26,27)/t13-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 330 | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR120 short isoform expressed in CHOK1 cells assessed as increase in IP1 accumulation after 60 mins by HTRF assay |
ACS Med Chem Lett 8: 96-101 (2017)
Article DOI: 10.1021/acsmedchemlett.6b00394
BindingDB Entry DOI: 10.7270/Q2R213CP |
More data for this
Ligand-Target Pair | |
Free fatty acid receptor 1
(Homo sapiens (Human)) | BDBM50207065

(CHEMBL3971308)Show SMILES [H][C@]1(C[C@@]1([H])[C@@]1([H])CCc2cc(ccc2O1)-c1cc(OC(F)(F)F)ccc1F)C(O)=O |r| Show InChI InChI=1S/C20H16F4O4/c21-16-4-3-12(28-20(22,23)24)8-13(16)10-1-5-17-11(7-10)2-6-18(27-17)14-9-15(14)19(25)26/h1,3-5,7-8,14-15,18H,2,6,9H2,(H,25,26)/t14-,15-,18-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR40 expressed in HEK293 cells assessed as increase in IP1 accumulation after 60 mins by HTRF assay |
ACS Med Chem Lett 8: 96-101 (2017)
Article DOI: 10.1021/acsmedchemlett.6b00394
BindingDB Entry DOI: 10.7270/Q2R213CP |
More data for this
Ligand-Target Pair | |
Free fatty acid receptor 4
(Mus musculus) | BDBM50207061

(CHEMBL3980898)Show SMILES CO[C@H]1C[C@@H](C1)Oc1cc(F)c(F)c(n1)-c1ccc2O[C@@H](CCC(O)=O)CCc2c1 |r,wU:4.6,wD:20.22,2.1,(19.4,-3.02,;19.4,-4.56,;20.74,-5.33,;21.14,-6.82,;22.62,-6.41,;22.23,-4.93,;23.96,-7.18,;25.29,-6.4,;25.28,-4.85,;26.62,-4.08,;26.62,-2.54,;27.95,-4.85,;29.29,-4.08,;27.95,-6.39,;26.62,-7.16,;29.28,-7.16,;29.28,-8.71,;30.61,-9.48,;31.94,-8.71,;33.28,-9.49,;34.63,-8.72,;35.96,-9.49,;37.3,-8.72,;38.63,-9.5,;38.63,-11.04,;39.97,-8.73,;34.63,-7.16,;33.28,-6.38,;31.94,-7.16,;30.61,-6.39,)| Show InChI InChI=1S/C22H23F2NO5/c1-28-15-9-16(10-15)30-19-11-17(23)21(24)22(25-19)13-3-6-18-12(8-13)2-4-14(29-18)5-7-20(26)27/h3,6,8,11,14-16H,2,4-5,7,9-10H2,1H3,(H,26,27)/t14-,15-,16-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 6.40 | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Agonist activity at mouse GPR120 expressed in CHOK1 cells assessed as increase in IP1 accumulation after 60 mins by HTRF assay |
ACS Med Chem Lett 8: 96-101 (2017)
Article DOI: 10.1021/acsmedchemlett.6b00394
BindingDB Entry DOI: 10.7270/Q2R213CP |
More data for this
Ligand-Target Pair | |
Free fatty acid receptor 1
(Mus musculus) | BDBM50207060

(CHEMBL3952043)Show SMILES CO[C@H]1C[C@@H](C1)Oc1cc(OC)c(F)c(n1)-c1ccc2O[C@@H](CCC(O)=O)CCc2c1 |r,wU:4.6,wD:21.23,2.1,(37.05,-1.75,;37.06,-3.29,;38.39,-4.05,;38.8,-5.54,;40.28,-5.13,;39.88,-3.65,;41.61,-5.9,;42.94,-5.12,;42.94,-3.58,;44.27,-2.81,;44.28,-1.27,;42.95,-.5,;45.61,-3.58,;46.94,-2.81,;45.6,-5.12,;44.28,-5.89,;46.93,-5.89,;46.93,-7.43,;48.27,-8.2,;49.6,-7.44,;50.93,-8.21,;52.28,-7.44,;53.61,-8.21,;54.95,-7.45,;56.28,-8.22,;56.28,-9.76,;57.62,-7.45,;52.29,-5.88,;50.94,-5.1,;49.6,-5.88,;48.26,-5.12,)| Show InChI InChI=1S/C23H26FNO6/c1-28-16-10-17(11-16)31-20-12-19(29-2)22(24)23(25-20)14-4-7-18-13(9-14)3-5-15(30-18)6-8-21(26)27/h4,7,9,12,15-17H,3,5-6,8,10-11H2,1-2H3,(H,26,27)/t15-,16-,17-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Agonist activity at mouse GPR40 assessed as increase in IP1 accumulation after 60 mins by HTRF assay |
ACS Med Chem Lett 8: 96-101 (2017)
Article DOI: 10.1021/acsmedchemlett.6b00394
BindingDB Entry DOI: 10.7270/Q2R213CP |
More data for this
Ligand-Target Pair | |
Free fatty acid receptor 4
(Homo sapiens (Human)) | BDBM50207056

(CHEMBL3970238)Show SMILES OC(=O)CC[C@H]1CCc2cc(ccc2O1)-c1cc(OC2CCC2)ccc1F |r| Show InChI InChI=1S/C22H23FO4/c23-20-9-7-18(26-16-2-1-3-16)13-19(20)14-5-10-21-15(12-14)4-6-17(27-21)8-11-22(24)25/h5,7,9-10,12-13,16-17H,1-4,6,8,11H2,(H,24,25)/t17-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 28 | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR120 short isoform expressed in CHOK1 cells assessed as increase in IP1 accumulation after 60 mins by HTRF assay |
ACS Med Chem Lett 8: 96-101 (2017)
Article DOI: 10.1021/acsmedchemlett.6b00394
BindingDB Entry DOI: 10.7270/Q2R213CP |
More data for this
Ligand-Target Pair | |
Free fatty acid receptor 1
(Homo sapiens (Human)) | BDBM50207067

(CHEMBL3941726)Show SMILES OC(=O)CC[C@@H]1CCc2cc(ccc2O1)-c1cc(OC(F)(F)F)ccc1F |r| Show InChI InChI=1S/C19H16F4O4/c20-16-6-4-14(27-19(21,22)23)10-15(16)11-2-7-17-12(9-11)1-3-13(26-17)5-8-18(24)25/h2,4,6-7,9-10,13H,1,3,5,8H2,(H,24,25)/t13-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR40 expressed in HEK293 cells assessed as increase in IP1 accumulation after 60 mins by HTRF assay |
ACS Med Chem Lett 8: 96-101 (2017)
Article DOI: 10.1021/acsmedchemlett.6b00394
BindingDB Entry DOI: 10.7270/Q2R213CP |
More data for this
Ligand-Target Pair | |
Free fatty acid receptor 1
(Mus musculus) | BDBM50207061

(CHEMBL3980898)Show SMILES CO[C@H]1C[C@@H](C1)Oc1cc(F)c(F)c(n1)-c1ccc2O[C@@H](CCC(O)=O)CCc2c1 |r,wU:4.6,wD:20.22,2.1,(19.4,-3.02,;19.4,-4.56,;20.74,-5.33,;21.14,-6.82,;22.62,-6.41,;22.23,-4.93,;23.96,-7.18,;25.29,-6.4,;25.28,-4.85,;26.62,-4.08,;26.62,-2.54,;27.95,-4.85,;29.29,-4.08,;27.95,-6.39,;26.62,-7.16,;29.28,-7.16,;29.28,-8.71,;30.61,-9.48,;31.94,-8.71,;33.28,-9.49,;34.63,-8.72,;35.96,-9.49,;37.3,-8.72,;38.63,-9.5,;38.63,-11.04,;39.97,-8.73,;34.63,-7.16,;33.28,-6.38,;31.94,-7.16,;30.61,-6.39,)| Show InChI InChI=1S/C22H23F2NO5/c1-28-15-9-16(10-15)30-19-11-17(23)21(24)22(25-19)13-3-6-18-12(8-13)2-4-14(29-18)5-7-20(26)27/h3,6,8,11,14-16H,2,4-5,7,9-10H2,1H3,(H,26,27)/t14-,15-,16-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Agonist activity at mouse GPR40 assessed as increase in IP1 accumulation after 60 mins by HTRF assay |
ACS Med Chem Lett 8: 96-101 (2017)
Article DOI: 10.1021/acsmedchemlett.6b00394
BindingDB Entry DOI: 10.7270/Q2R213CP |
More data for this
Ligand-Target Pair | |
Free fatty acid receptor 4
(Homo sapiens (Human)) | BDBM50207068

(CHEMBL3900705)Show SMILES OC(=O)CC[C@H]1CCc2cc(ccc2O1)-c1cc(OC2CCC2)cnc1F |r| Show InChI InChI=1S/C21H22FNO4/c22-21-18(11-17(12-23-21)26-15-2-1-3-15)13-5-8-19-14(10-13)4-6-16(27-19)7-9-20(24)25/h5,8,10-12,15-16H,1-4,6-7,9H2,(H,24,25)/t16-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 140 | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR120 short isoform expressed in CHOK1 cells assessed as beta-arrestin recruitment after 90 mins by luminescence assay |
ACS Med Chem Lett 8: 96-101 (2017)
Article DOI: 10.1021/acsmedchemlett.6b00394
BindingDB Entry DOI: 10.7270/Q2R213CP |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data