 Found 51 hits of Enzyme Inhibition Constant Data
Found 51 hits of Enzyme Inhibition Constant Data Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50236320
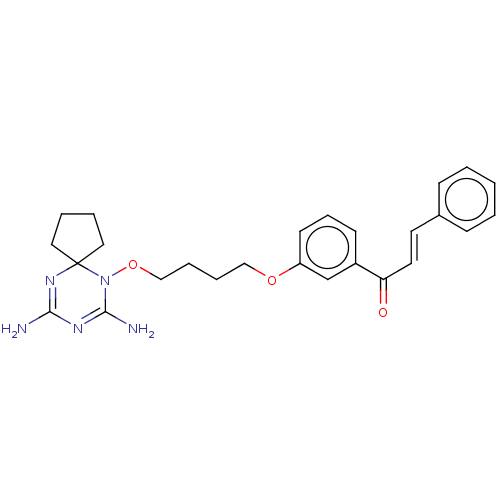
(CHEMBL4091106)Show SMILES Cl.NC1=NC2(CCCC2)N(OCCCCOc2cccc(c2)C(=O)\C=C\c2ccccc2)C(N)=N1 |c:35,t:1| Show InChI InChI=1S/C26H31N5O3/c27-24-29-25(28)31(26(30-24)15-4-5-16-26)34-18-7-6-17-33-22-12-8-11-21(19-22)23(32)14-13-20-9-2-1-3-10-20/h1-3,8-14,19H,4-7,15-18H2,(H4,27,28,29,30)/b14-13+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
National University of Singapore
Curated by ChEMBL
| Assay Description
Inhibitory concentration was evaluated against Farnesyltransferase in the farnesylation of H-ras protein |
J Med Chem 60: 1734-1745 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01253
BindingDB Entry DOI: 10.7270/Q2FJ2K2V |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50236287

(CHEMBL4071967)Show SMILES Cl.CC1(C)N=C(N)N=C(N)N1OCCCOc1cccc(\C=C\C(=O)c2ccccc2)c1 |t:3,6| Show InChI InChI=1S/C23H27N5O3/c1-23(2)27-21(24)26-22(25)28(23)31-15-7-14-30-19-11-6-8-17(16-19)12-13-20(29)18-9-4-3-5-10-18/h3-6,8-13,16H,7,14-15H2,1-2H3,(H4,24,25,26,27)/b13-12+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
National University of Singapore
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human DHFR assessed as reduction in conversion of DHF to THF measured every 30 secs for 6 mins by UV-Vis spectrophotometric... |
J Med Chem 60: 1734-1745 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01253
BindingDB Entry DOI: 10.7270/Q2FJ2K2V |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50236288
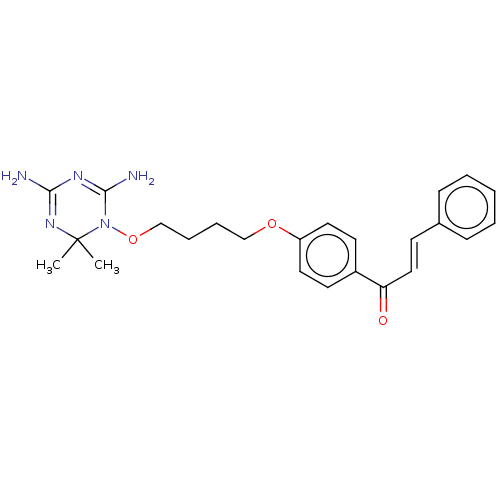
(CHEMBL4098945)Show SMILES Cl.CC1(C)N=C(N)N=C(N)N1OCCCCOc1ccc(cc1)C(=O)\C=C\c1ccccc1 |t:3,6| Show InChI InChI=1S/C24H29N5O3/c1-24(2)28-22(25)27-23(26)29(24)32-17-7-6-16-31-20-13-11-19(12-14-20)21(30)15-10-18-8-4-3-5-9-18/h3-5,8-15H,6-7,16-17H2,1-2H3,(H4,25,26,27,28)/b15-10+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
National University of Singapore
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human DHFR assessed as reduction in conversion of DHF to THF measured every 30 secs for 6 mins by UV-Vis spectrophotometric... |
J Med Chem 60: 1734-1745 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01253
BindingDB Entry DOI: 10.7270/Q2FJ2K2V |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50236325

(CHEMBL4099755)Show SMILES Cl.CC1(C)N=C(N)N=C(N)N1OCCCOc1cccc(c1)C(=O)CCc1ccccc1 |t:3,6| Show InChI InChI=1S/C23H29N5O3/c1-23(2)27-21(24)26-22(25)28(23)31-15-7-14-30-19-11-6-10-18(16-19)20(29)13-12-17-8-4-3-5-9-17/h3-6,8-11,16H,7,12-15H2,1-2H3,(H4,24,25,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
National University of Singapore
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human DHFR assessed as reduction in conversion of DHF to THF measured every 30 secs for 6 mins by UV-Vis spectrophotometric... |
J Med Chem 60: 1734-1745 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01253
BindingDB Entry DOI: 10.7270/Q2FJ2K2V |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50236291

(CHEMBL4060570)Show SMILES Cl.CC1(C)N=C(N)N=C(N)N1OCCCCOc1ccc(\C=C\C(=O)c2ccccc2)cc1 |t:3,6| Show InChI InChI=1S/C24H29N5O3/c1-24(2)28-22(25)27-23(26)29(24)32-17-7-6-16-31-20-13-10-18(11-14-20)12-15-21(30)19-8-4-3-5-9-19/h3-5,8-15H,6-7,16-17H2,1-2H3,(H4,25,26,27,28)/b15-12+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
National University of Singapore
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human DHFR assessed as reduction in conversion of DHF to THF measured every 30 secs for 6 mins by UV-Vis spectrophotometric... |
J Med Chem 60: 1734-1745 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01253
BindingDB Entry DOI: 10.7270/Q2FJ2K2V |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50236289
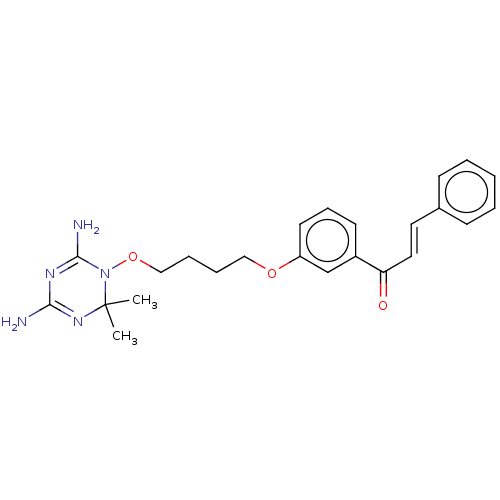
(CHEMBL4082453)Show SMILES Cl.CC1(C)N=C(N)N=C(N)N1OCCCCOc1cccc(c1)C(=O)\C=C\c1ccccc1 |t:3,6| Show InChI InChI=1S/C24H29N5O3/c1-24(2)28-22(25)27-23(26)29(24)32-16-7-6-15-31-20-12-8-11-19(17-20)21(30)14-13-18-9-4-3-5-10-18/h3-5,8-14,17H,6-7,15-16H2,1-2H3,(H4,25,26,27,28)/b14-13+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
National University of Singapore
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human DHFR assessed as reduction in conversion of DHF to THF measured every 30 secs for 6 mins by UV-Vis spectrophotometric... |
J Med Chem 60: 1734-1745 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01253
BindingDB Entry DOI: 10.7270/Q2FJ2K2V |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50236319
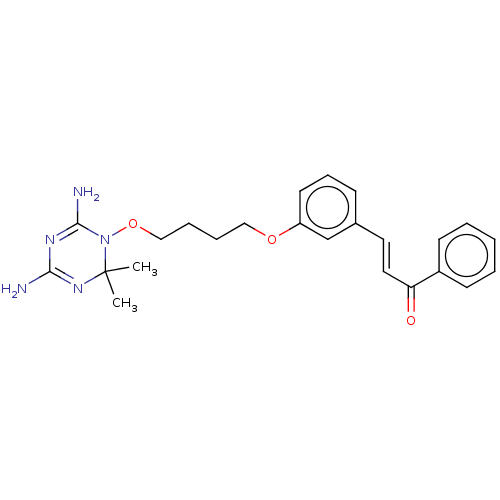
(CHEMBL4101407)Show SMILES Cl.CC1(C)N=C(N)N=C(N)N1OCCCCOc1cccc(\C=C\C(=O)c2ccccc2)c1 |t:3,6| Show InChI InChI=1S/C24H29N5O3/c1-24(2)28-22(25)27-23(26)29(24)32-16-7-6-15-31-20-12-8-9-18(17-20)13-14-21(30)19-10-4-3-5-11-19/h3-5,8-14,17H,6-7,15-16H2,1-2H3,(H4,25,26,27,28)/b14-13+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
National University of Singapore
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human DHFR assessed as reduction in conversion of DHF to THF measured every 30 secs for 6 mins by UV-Vis spectrophotometric... |
J Med Chem 60: 1734-1745 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01253
BindingDB Entry DOI: 10.7270/Q2FJ2K2V |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50236324

(CHEMBL4080801)Show SMILES Cl.CC1(C)N=C(N)N=C(N)N1OCCCOc1ccccc1 |t:3,6| Show InChI InChI=1S/C14H21N5O2/c1-14(2)18-12(15)17-13(16)19(14)21-10-6-9-20-11-7-4-3-5-8-11/h3-5,7-8H,6,9-10H2,1-2H3,(H4,15,16,17,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a |
National University of Singapore
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Farnesyltransferase for Farnesylation of H-ras protein InNIH 3T3 cells transformed with activated H-ras |
J Med Chem 60: 1734-1745 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01253
BindingDB Entry DOI: 10.7270/Q2FJ2K2V |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50236323
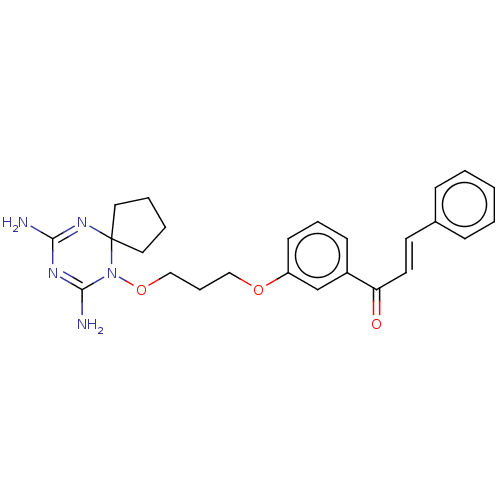
(CHEMBL4070144)Show SMILES Cl.NC1=NC2(CCCC2)N(OCCCOc2cccc(c2)C(=O)\C=C\c2ccccc2)C(N)=N1 |c:34,t:1| Show InChI InChI=1S/C25H29N5O3/c26-23-28-24(27)30(25(29-23)14-4-5-15-25)33-17-7-16-32-21-11-6-10-20(18-21)22(31)13-12-19-8-2-1-3-9-19/h1-3,6,8-13,18H,4-5,7,14-17H2,(H4,26,27,28,29)/b13-12+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.10 | n/a | n/a | n/a | n/a | n/a | n/a |
National University of Singapore
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Farnesyltransferase for Farnesylation of H-ras protein InNIH 3T3 cells transformed with activated H-ras |
J Med Chem 60: 1734-1745 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01253
BindingDB Entry DOI: 10.7270/Q2FJ2K2V |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50236286
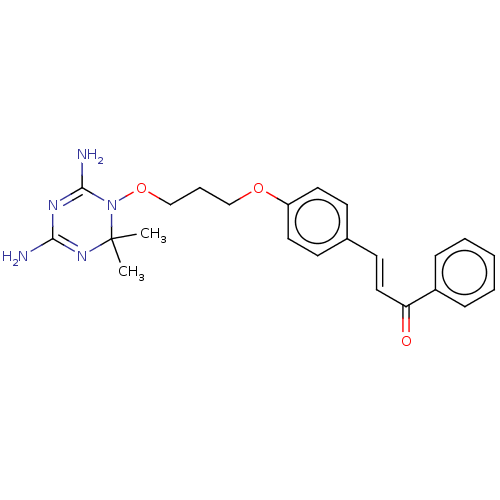
(CHEMBL4079985)Show SMILES Cl.CC1(C)N=C(N)N=C(N)N1OCCCOc1ccc(\C=C\C(=O)c2ccccc2)cc1 |t:3,6| Show InChI InChI=1S/C23H27N5O3/c1-23(2)27-21(24)26-22(25)28(23)31-16-6-15-30-19-12-9-17(10-13-19)11-14-20(29)18-7-4-3-5-8-18/h3-5,7-14H,6,15-16H2,1-2H3,(H4,24,25,26,27)/b14-11+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.40 | n/a | n/a | n/a | n/a | n/a | n/a |
National University of Singapore
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human DHFR assessed as reduction in conversion of DHF to THF measured every 30 secs for 6 mins by UV-Vis spectrophotometric... |
J Med Chem 60: 1734-1745 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01253
BindingDB Entry DOI: 10.7270/Q2FJ2K2V |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM66082

((2S)-2-[[4-[(2,4-diaminopteridin-6-yl)methyl-methy...)Show SMILES CN(Cc1cnc2nc(N)nc(N)c2n1)c1ccc(cc1)C(=O)N[C@@H](CCC(O)=O)C(O)=O Show InChI InChI=1S/C20H22N8O5/c1-28(9-11-8-23-17-15(24-11)16(21)26-20(22)27-17)12-4-2-10(3-5-12)18(31)25-13(19(32)33)6-7-14(29)30/h2-5,8,13H,6-7,9H2,1H3,(H,25,31)(H,29,30)(H,32,33)(H4,21,22,23,26,27)/t13-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a |
National University of Singapore
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human DHFR assessed as reduction in conversion of DHF to THF measured every 30 secs for 6 mins by UV-Vis spectrophotometric... |
J Med Chem 60: 1734-1745 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01253
BindingDB Entry DOI: 10.7270/Q2FJ2K2V |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50236285
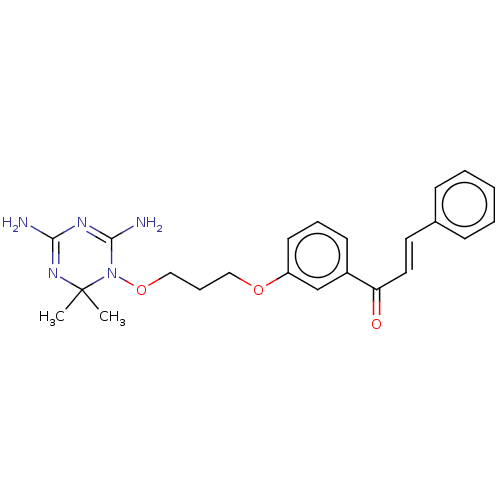
(CHEMBL4098009)Show SMILES Cl.CC1(C)N=C(N)N=C(N)N1OCCCOc1cccc(c1)C(=O)\C=C\c1ccccc1 |t:3,6| Show InChI InChI=1S/C23H27N5O3/c1-23(2)27-21(24)26-22(25)28(23)31-15-7-14-30-19-11-6-10-18(16-19)20(29)13-12-17-8-4-3-5-9-17/h3-6,8-13,16H,7,14-15H2,1-2H3,(H4,24,25,26,27)/b13-12+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
National University of Singapore
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human DHFR assessed as reduction in conversion of DHF to THF measured every 30 secs for 6 mins by UV-Vis spectrophotometric... |
J Med Chem 60: 1734-1745 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01253
BindingDB Entry DOI: 10.7270/Q2FJ2K2V |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50236284

(CHEMBL4069255)Show SMILES Cl.CC1(C)N=C(N)N=C(N)N1OCCCOc1ccc(cc1)C(=O)\C=C\c1ccccc1 |t:3,6| Show InChI InChI=1S/C23H27N5O3/c1-23(2)27-21(24)26-22(25)28(23)31-16-6-15-30-19-12-10-18(11-13-19)20(29)14-9-17-7-4-3-5-8-17/h3-5,7-14H,6,15-16H2,1-2H3,(H4,24,25,26,27)/b14-9+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 9.20 | n/a | n/a | n/a | n/a | n/a | n/a |
National University of Singapore
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human DHFR assessed as reduction in conversion of DHF to THF measured every 30 secs for 6 mins by UV-Vis spectrophotometric... |
J Med Chem 60: 1734-1745 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01253
BindingDB Entry DOI: 10.7270/Q2FJ2K2V |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50236290

(CHEMBL4083419)Show SMILES Cl.NC1=NC2(CCCC2)N(OCCOc2cccc(c2)C(=O)\C=C\c2ccccc2)C(N)=N1 |c:33,t:1| Show InChI InChI=1S/C24H27N5O3/c25-22-27-23(26)29(24(28-22)13-4-5-14-24)32-16-15-31-20-10-6-9-19(17-20)21(30)12-11-18-7-2-1-3-8-18/h1-3,6-12,17H,4-5,13-16H2,(H4,25,26,27,28)/b12-11+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
National University of Singapore
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human DHFR assessed as reduction in conversion of DHF to THF measured every 30 secs for 6 mins by UV-Vis spectrophotometric... |
J Med Chem 60: 1734-1745 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01253
BindingDB Entry DOI: 10.7270/Q2FJ2K2V |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50236283

(CHEMBL4074887)Show SMILES Cl.CC1(C)N=C(N)N=C(N)N1OCCOc1ccc(cc1)C(=O)\C=C\c1ccccc1 |t:3,6| Show InChI InChI=1S/C22H25N5O3/c1-22(2)26-20(23)25-21(24)27(22)30-15-14-29-18-11-9-17(10-12-18)19(28)13-8-16-6-4-3-5-7-16/h3-13H,14-15H2,1-2H3,(H4,23,24,25,26)/b13-8+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
National University of Singapore
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human DHFR assessed as reduction in conversion of DHF to THF measured every 30 secs for 6 mins by UV-Vis spectrophotometric... |
J Med Chem 60: 1734-1745 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01253
BindingDB Entry DOI: 10.7270/Q2FJ2K2V |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM82109

(6,8,10-Traiazaspiro[4.5]deca-6,8-diene-7,9-diamine...)Show SMILES NC1=NC2(CCCC2)N(OCCCOc2ccccc2)C(N)=N1 |c:23,t:1| Show InChI InChI=1S/C16H23N5O2/c17-14-19-15(18)21(16(20-14)9-4-5-10-16)23-12-6-11-22-13-7-2-1-3-8-13/h1-3,7-8H,4-6,9-12H2,(H4,17,18,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
National University of Singapore
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Geranylgeranyl transferase in the geranylgeranylation of H-ras-CAIL protein |
J Med Chem 60: 1734-1745 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01253
BindingDB Entry DOI: 10.7270/Q2FJ2K2V |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50236282
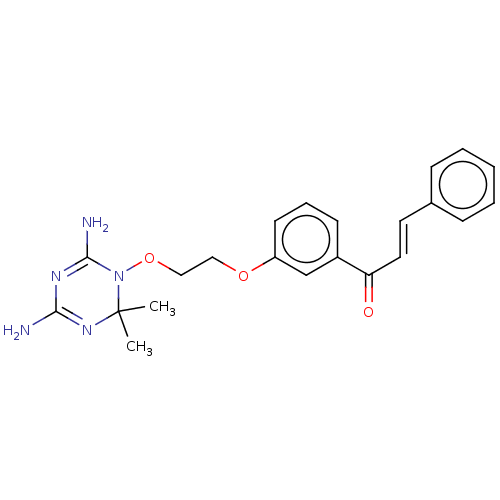
(CHEMBL4104895)Show SMILES Cl.CC1(C)N=C(N)N=C(N)N1OCCOc1cccc(c1)C(=O)\C=C\c1ccccc1 |t:3,6| Show InChI InChI=1S/C22H25N5O3/c1-22(2)26-20(23)25-21(24)27(22)30-14-13-29-18-10-6-9-17(15-18)19(28)12-11-16-7-4-3-5-8-16/h3-12,15H,13-14H2,1-2H3,(H4,23,24,25,26)/b12-11+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
National University of Singapore
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human DHFR assessed as reduction in conversion of DHF to THF measured every 30 secs for 6 mins by UV-Vis spectrophotometric... |
J Med Chem 60: 1734-1745 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01253
BindingDB Entry DOI: 10.7270/Q2FJ2K2V |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50236322

(CHEMBL4080662)Show SMILES Cl.CC1(C)N=C(N)N=C(N)N1OCCOc1ccc(\C=C\C(=O)c2ccccc2)cc1 |t:3,6| Show InChI InChI=1S/C22H25N5O3/c1-22(2)26-20(23)25-21(24)27(22)30-15-14-29-18-11-8-16(9-12-18)10-13-19(28)17-6-4-3-5-7-17/h3-13H,14-15H2,1-2H3,(H4,23,24,25,26)/b13-10+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 53 | n/a | n/a | n/a | n/a | n/a | n/a |
National University of Singapore
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human DHFR assessed as reduction in conversion of DHF to THF measured every 30 secs for 6 mins by UV-Vis spectrophotometric... |
J Med Chem 60: 1734-1745 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01253
BindingDB Entry DOI: 10.7270/Q2FJ2K2V |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50236321

(CHEMBL4097389)Show SMILES Cl.CC1(C)N=C(N)N=C(N)N1OCCOc1cccc(\C=C\C(=O)c2ccccc2)c1 |t:3,6| Show InChI InChI=1S/C22H25N5O3/c1-22(2)26-20(23)25-21(24)27(22)30-14-13-29-18-10-6-7-16(15-18)11-12-19(28)17-8-4-3-5-9-17/h3-12,15H,13-14H2,1-2H3,(H4,23,24,25,26)/b12-11+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 59 | n/a | n/a | n/a | n/a | n/a | n/a |
National University of Singapore
Curated by ChEMBL
| Assay Description
Binding affinity towards human muscarinic M3 receptor |
J Med Chem 60: 1734-1745 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01253
BindingDB Entry DOI: 10.7270/Q2FJ2K2V |
More data for this
Ligand-Target Pair | |
Thioredoxin reductase 1, cytoplasmic
(Rattus norvegicus) | BDBM50236287

(CHEMBL4071967)Show SMILES Cl.CC1(C)N=C(N)N=C(N)N1OCCCOc1cccc(\C=C\C(=O)c2ccccc2)c1 |t:3,6| Show InChI InChI=1S/C23H27N5O3/c1-23(2)27-21(24)26-22(25)28(23)31-15-7-14-30-19-11-6-8-17(16-19)12-13-20(29)18-9-4-3-5-10-18/h3-6,8-13,16H,7,14-15H2,1-2H3,(H4,24,25,26,27)/b13-12+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National University of Singapore
Curated by ChEMBL
| Assay Description
Inhibitory concentration was evaluated against Farnesyltransferase in the farnesylation of H-ras protein |
J Med Chem 60: 1734-1745 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01253
BindingDB Entry DOI: 10.7270/Q2FJ2K2V |
More data for this
Ligand-Target Pair | |
Thioredoxin reductase 1, cytoplasmic
(Rattus norvegicus) | BDBM50236319

(CHEMBL4101407)Show SMILES Cl.CC1(C)N=C(N)N=C(N)N1OCCCCOc1cccc(\C=C\C(=O)c2ccccc2)c1 |t:3,6| Show InChI InChI=1S/C24H29N5O3/c1-24(2)28-22(25)27-23(26)29(24)32-16-7-6-15-31-20-12-8-9-18(17-20)13-14-21(30)19-10-4-3-5-11-19/h3-5,8-14,17H,6-7,15-16H2,1-2H3,(H4,25,26,27,28)/b14-13+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National University of Singapore
Curated by ChEMBL
| Assay Description
Inhibitory concentration was evaluated against Farnesyltransferase in the farnesylation of H-ras protein |
J Med Chem 60: 1734-1745 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01253
BindingDB Entry DOI: 10.7270/Q2FJ2K2V |
More data for this
Ligand-Target Pair | |
Thioredoxin reductase 1, cytoplasmic
(Rattus norvegicus) | BDBM50236321

(CHEMBL4097389)Show SMILES Cl.CC1(C)N=C(N)N=C(N)N1OCCOc1cccc(\C=C\C(=O)c2ccccc2)c1 |t:3,6| Show InChI InChI=1S/C22H25N5O3/c1-22(2)26-20(23)25-21(24)27(22)30-14-13-29-18-10-6-7-16(15-18)11-12-19(28)17-8-4-3-5-9-17/h3-12,15H,13-14H2,1-2H3,(H4,23,24,25,26)/b12-11+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National University of Singapore
Curated by ChEMBL
| Assay Description
Inhibition of high affinity uptake of [3H]dopamine into striatal nerve endings (synaptosomes) |
J Med Chem 60: 1734-1745 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01253
BindingDB Entry DOI: 10.7270/Q2FJ2K2V |
More data for this
Ligand-Target Pair | |
Thioredoxin reductase 1, cytoplasmic
(Rattus norvegicus) | BDBM50236287

(CHEMBL4071967)Show SMILES Cl.CC1(C)N=C(N)N=C(N)N1OCCCOc1cccc(\C=C\C(=O)c2ccccc2)c1 |t:3,6| Show InChI InChI=1S/C23H27N5O3/c1-23(2)27-21(24)26-22(25)28(23)31-15-7-14-30-19-11-6-8-17(16-19)12-13-20(29)18-9-4-3-5-10-18/h3-6,8-13,16H,7,14-15H2,1-2H3,(H4,24,25,26,27)/b13-12+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National University of Singapore
Curated by ChEMBL
| Assay Description
Binding affinity towards dopamine transporter using [3H]- mazindol as radioligand in rat striatal membranes |
J Med Chem 60: 1734-1745 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01253
BindingDB Entry DOI: 10.7270/Q2FJ2K2V |
More data for this
Ligand-Target Pair | |
Thioredoxin reductase 1, cytoplasmic
(Rattus norvegicus) | BDBM50236290

(CHEMBL4083419)Show SMILES Cl.NC1=NC2(CCCC2)N(OCCOc2cccc(c2)C(=O)\C=C\c2ccccc2)C(N)=N1 |c:33,t:1| Show InChI InChI=1S/C24H27N5O3/c25-22-27-23(26)29(24(28-22)13-4-5-14-24)32-16-15-31-20-10-6-9-19(17-20)21(30)12-11-18-7-2-1-3-8-18/h1-3,6-12,17H,4-5,13-16H2,(H4,25,26,27,28)/b12-11+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National University of Singapore
Curated by ChEMBL
| Assay Description
Inhibitory concentration was evaluated against Farnesyltransferase in the farnesylation of H-ras protein |
J Med Chem 60: 1734-1745 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01253
BindingDB Entry DOI: 10.7270/Q2FJ2K2V |
More data for this
Ligand-Target Pair | |
Thioredoxin reductase 1, cytoplasmic
(Rattus norvegicus) | BDBM50236323

(CHEMBL4070144)Show SMILES Cl.NC1=NC2(CCCC2)N(OCCCOc2cccc(c2)C(=O)\C=C\c2ccccc2)C(N)=N1 |c:34,t:1| Show InChI InChI=1S/C25H29N5O3/c26-23-28-24(27)30(25(29-23)14-4-5-15-25)33-17-7-16-32-21-11-6-10-20(18-21)22(31)13-12-19-8-2-1-3-9-19/h1-3,6,8-13,18H,4-5,7,14-17H2,(H4,26,27,28,29)/b13-12+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National University of Singapore
Curated by ChEMBL
| Assay Description
Inhibition of recombinant rat liver thioredoxin reductase after 60 mins by DTNB reduction assay |
J Med Chem 60: 1734-1745 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01253
BindingDB Entry DOI: 10.7270/Q2FJ2K2V |
More data for this
Ligand-Target Pair | |
Thioredoxin reductase 1, cytoplasmic
(Rattus norvegicus) | BDBM50236321

(CHEMBL4097389)Show SMILES Cl.CC1(C)N=C(N)N=C(N)N1OCCOc1cccc(\C=C\C(=O)c2ccccc2)c1 |t:3,6| Show InChI InChI=1S/C22H25N5O3/c1-22(2)26-20(23)25-21(24)27(22)30-14-13-29-18-10-6-7-16(15-18)11-12-19(28)17-8-4-3-5-9-17/h3-12,15H,13-14H2,1-2H3,(H4,23,24,25,26)/b12-11+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National University of Singapore
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Farnesyltransferase for Farnesylation of H-ras protein InNIH 3T3 cells transformed with activated H-ras |
J Med Chem 60: 1734-1745 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01253
BindingDB Entry DOI: 10.7270/Q2FJ2K2V |
More data for this
Ligand-Target Pair | |
Thioredoxin reductase 1, cytoplasmic
(Rattus norvegicus) | BDBM50236320

(CHEMBL4091106)Show SMILES Cl.NC1=NC2(CCCC2)N(OCCCCOc2cccc(c2)C(=O)\C=C\c2ccccc2)C(N)=N1 |c:35,t:1| Show InChI InChI=1S/C26H31N5O3/c27-24-29-25(28)31(26(30-24)15-4-5-16-26)34-18-7-6-17-33-22-12-8-11-21(19-22)23(32)14-13-20-9-2-1-3-10-20/h1-3,8-14,19H,4-7,15-18H2,(H4,27,28,29,30)/b14-13+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National University of Singapore
Curated by ChEMBL
| Assay Description
Inhibition of recombinant rat liver thioredoxin reductase after 60 mins by DTNB reduction assay |
J Med Chem 60: 1734-1745 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01253
BindingDB Entry DOI: 10.7270/Q2FJ2K2V |
More data for this
Ligand-Target Pair | |
Thioredoxin reductase 1, cytoplasmic
(Rattus norvegicus) | BDBM50236319

(CHEMBL4101407)Show SMILES Cl.CC1(C)N=C(N)N=C(N)N1OCCCCOc1cccc(\C=C\C(=O)c2ccccc2)c1 |t:3,6| Show InChI InChI=1S/C24H29N5O3/c1-24(2)28-22(25)27-23(26)29(24)32-16-7-6-15-31-20-12-8-9-18(17-20)13-14-21(30)19-10-4-3-5-11-19/h3-5,8-14,17H,6-7,15-16H2,1-2H3,(H4,25,26,27,28)/b14-13+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National University of Singapore
Curated by ChEMBL
| Assay Description
Inhibition of recombinant rat liver thioredoxin reductase after 30 mins by DTNB reduction assay |
J Med Chem 60: 1734-1745 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01253
BindingDB Entry DOI: 10.7270/Q2FJ2K2V |
More data for this
Ligand-Target Pair | |
Thioredoxin reductase 1, cytoplasmic
(Rattus norvegicus) | BDBM50236289

(CHEMBL4082453)Show SMILES Cl.CC1(C)N=C(N)N=C(N)N1OCCCCOc1cccc(c1)C(=O)\C=C\c1ccccc1 |t:3,6| Show InChI InChI=1S/C24H29N5O3/c1-24(2)28-22(25)27-23(26)29(24)32-16-7-6-15-31-20-12-8-11-19(17-20)21(30)14-13-18-9-4-3-5-10-18/h3-5,8-14,17H,6-7,15-16H2,1-2H3,(H4,25,26,27,28)/b14-13+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National University of Singapore
Curated by ChEMBL
| Assay Description
Inhibition of recombinant rat liver thioredoxin reductase after 60 mins by DTNB reduction assay |
J Med Chem 60: 1734-1745 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01253
BindingDB Entry DOI: 10.7270/Q2FJ2K2V |
More data for this
Ligand-Target Pair | |
Thioredoxin reductase 1, cytoplasmic
(Rattus norvegicus) | BDBM50236282

(CHEMBL4104895)Show SMILES Cl.CC1(C)N=C(N)N=C(N)N1OCCOc1cccc(c1)C(=O)\C=C\c1ccccc1 |t:3,6| Show InChI InChI=1S/C22H25N5O3/c1-22(2)26-20(23)25-21(24)27(22)30-14-13-29-18-10-6-9-17(15-18)19(28)12-11-16-7-4-3-5-8-16/h3-12,15H,13-14H2,1-2H3,(H4,23,24,25,26)/b12-11+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National University of Singapore
Curated by ChEMBL
| Assay Description
Inhibition of recombinant rat liver thioredoxin reductase after 60 mins by DTNB reduction assay |
J Med Chem 60: 1734-1745 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01253
BindingDB Entry DOI: 10.7270/Q2FJ2K2V |
More data for this
Ligand-Target Pair | |
Thioredoxin reductase 1, cytoplasmic
(Rattus norvegicus) | BDBM50236323

(CHEMBL4070144)Show SMILES Cl.NC1=NC2(CCCC2)N(OCCCOc2cccc(c2)C(=O)\C=C\c2ccccc2)C(N)=N1 |c:34,t:1| Show InChI InChI=1S/C25H29N5O3/c26-23-28-24(27)30(25(29-23)14-4-5-15-25)33-17-7-16-32-21-11-6-10-20(18-21)22(31)13-12-19-8-2-1-3-9-19/h1-3,6,8-13,18H,4-5,7,14-17H2,(H4,26,27,28,29)/b13-12+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National University of Singapore
Curated by ChEMBL
| Assay Description
Inhibition of recombinant rat liver thioredoxin reductase after 30 mins by DTNB reduction assay |
J Med Chem 60: 1734-1745 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01253
BindingDB Entry DOI: 10.7270/Q2FJ2K2V |
More data for this
Ligand-Target Pair | |
Thioredoxin reductase 1, cytoplasmic
(Rattus norvegicus) | BDBM50236290

(CHEMBL4083419)Show SMILES Cl.NC1=NC2(CCCC2)N(OCCOc2cccc(c2)C(=O)\C=C\c2ccccc2)C(N)=N1 |c:33,t:1| Show InChI InChI=1S/C24H27N5O3/c25-22-27-23(26)29(24(28-22)13-4-5-14-24)32-16-15-31-20-10-6-9-19(17-20)21(30)12-11-18-7-2-1-3-8-18/h1-3,6-12,17H,4-5,13-16H2,(H4,25,26,27,28)/b12-11+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National University of Singapore
Curated by ChEMBL
| Assay Description
Inhibition of recombinant rat liver thioredoxin reductase after 30 mins by DTNB reduction assay |
J Med Chem 60: 1734-1745 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01253
BindingDB Entry DOI: 10.7270/Q2FJ2K2V |
More data for this
Ligand-Target Pair | |
Thioredoxin reductase 1, cytoplasmic
(Rattus norvegicus) | BDBM50236285

(CHEMBL4098009)Show SMILES Cl.CC1(C)N=C(N)N=C(N)N1OCCCOc1cccc(c1)C(=O)\C=C\c1ccccc1 |t:3,6| Show InChI InChI=1S/C23H27N5O3/c1-23(2)27-21(24)26-22(25)28(23)31-15-7-14-30-19-11-6-10-18(16-19)20(29)13-12-17-8-4-3-5-9-17/h3-6,8-13,16H,7,14-15H2,1-2H3,(H4,24,25,26,27)/b13-12+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National University of Singapore
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Geranylgeranyl transferase in the geranylgeranylation of H-ras-CAIL protein |
J Med Chem 60: 1734-1745 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01253
BindingDB Entry DOI: 10.7270/Q2FJ2K2V |
More data for this
Ligand-Target Pair | |
Thioredoxin reductase 1, cytoplasmic
(Rattus norvegicus) | BDBM50236320

(CHEMBL4091106)Show SMILES Cl.NC1=NC2(CCCC2)N(OCCCCOc2cccc(c2)C(=O)\C=C\c2ccccc2)C(N)=N1 |c:35,t:1| Show InChI InChI=1S/C26H31N5O3/c27-24-29-25(28)31(26(30-24)15-4-5-16-26)34-18-7-6-17-33-22-12-8-11-21(19-22)23(32)14-13-20-9-2-1-3-10-20/h1-3,8-14,19H,4-7,15-18H2,(H4,27,28,29,30)/b14-13+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National University of Singapore
Curated by ChEMBL
| Assay Description
Affinity towards human M1 receptor expressed in CHO cells using [3H]QNB as radioligand |
J Med Chem 60: 1734-1745 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01253
BindingDB Entry DOI: 10.7270/Q2FJ2K2V |
More data for this
Ligand-Target Pair | |
Thioredoxin reductase 1, cytoplasmic
(Rattus norvegicus) | BDBM50236285

(CHEMBL4098009)Show SMILES Cl.CC1(C)N=C(N)N=C(N)N1OCCCOc1cccc(c1)C(=O)\C=C\c1ccccc1 |t:3,6| Show InChI InChI=1S/C23H27N5O3/c1-23(2)27-21(24)26-22(25)28(23)31-15-7-14-30-19-11-6-10-18(16-19)20(29)13-12-17-8-4-3-5-9-17/h3-6,8-13,16H,7,14-15H2,1-2H3,(H4,24,25,26,27)/b13-12+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.08E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National University of Singapore
Curated by ChEMBL
| Assay Description
Inhibition of recombinant rat liver thioredoxin reductase after 30 mins by DTNB reduction assay |
J Med Chem 60: 1734-1745 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01253
BindingDB Entry DOI: 10.7270/Q2FJ2K2V |
More data for this
Ligand-Target Pair | |
Thioredoxin reductase 1, cytoplasmic
(Rattus norvegicus) | BDBM50236282

(CHEMBL4104895)Show SMILES Cl.CC1(C)N=C(N)N=C(N)N1OCCOc1cccc(c1)C(=O)\C=C\c1ccccc1 |t:3,6| Show InChI InChI=1S/C22H25N5O3/c1-22(2)26-20(23)25-21(24)27(22)30-14-13-29-18-10-6-9-17(15-18)19(28)12-11-16-7-4-3-5-8-16/h3-12,15H,13-14H2,1-2H3,(H4,23,24,25,26)/b12-11+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.08E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National University of Singapore
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Farnesyltransferase for Farnesylation of H-ras protein InNIH 3T3 cells transformed with activated H-ras |
J Med Chem 60: 1734-1745 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01253
BindingDB Entry DOI: 10.7270/Q2FJ2K2V |
More data for this
Ligand-Target Pair | |
Thioredoxin reductase 1, cytoplasmic
(Rattus norvegicus) | BDBM50236289

(CHEMBL4082453)Show SMILES Cl.CC1(C)N=C(N)N=C(N)N1OCCCCOc1cccc(c1)C(=O)\C=C\c1ccccc1 |t:3,6| Show InChI InChI=1S/C24H29N5O3/c1-24(2)28-22(25)27-23(26)29(24)32-16-7-6-15-31-20-12-8-11-19(17-20)21(30)14-13-18-9-4-3-5-10-18/h3-5,8-14,17H,6-7,15-16H2,1-2H3,(H4,25,26,27,28)/b14-13+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.12E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National University of Singapore
Curated by ChEMBL
| Assay Description
Inhibition of recombinant rat liver thioredoxin reductase after 30 mins by DTNB reduction assay |
J Med Chem 60: 1734-1745 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01253
BindingDB Entry DOI: 10.7270/Q2FJ2K2V |
More data for this
Ligand-Target Pair | |
Thioredoxin reductase 1, cytoplasmic
(Rattus norvegicus) | BDBM50236283

(CHEMBL4074887)Show SMILES Cl.CC1(C)N=C(N)N=C(N)N1OCCOc1ccc(cc1)C(=O)\C=C\c1ccccc1 |t:3,6| Show InChI InChI=1S/C22H25N5O3/c1-22(2)26-20(23)25-21(24)27(22)30-15-14-29-18-11-9-17(10-12-18)19(28)13-8-16-6-4-3-5-7-16/h3-13H,14-15H2,1-2H3,(H4,23,24,25,26)/b13-8+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.04E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National University of Singapore
Curated by ChEMBL
| Assay Description
In vitro functional agonism against M1 muscarinic receptor (PI) |
J Med Chem 60: 1734-1745 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01253
BindingDB Entry DOI: 10.7270/Q2FJ2K2V |
More data for this
Ligand-Target Pair | |
Thioredoxin reductase 1, cytoplasmic
(Rattus norvegicus) | BDBM50236291

(CHEMBL4060570)Show SMILES Cl.CC1(C)N=C(N)N=C(N)N1OCCCCOc1ccc(\C=C\C(=O)c2ccccc2)cc1 |t:3,6| Show InChI InChI=1S/C24H29N5O3/c1-24(2)28-22(25)27-23(26)29(24)32-17-7-6-16-31-20-13-10-18(11-14-20)12-15-21(30)19-8-4-3-5-9-19/h3-5,8-15H,6-7,16-17H2,1-2H3,(H4,25,26,27,28)/b15-12+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.11E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National University of Singapore
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Farnesyltransferase for Farnesylation of H-ras protein InNIH 3T3 cells transformed with activated H-ras |
J Med Chem 60: 1734-1745 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01253
BindingDB Entry DOI: 10.7270/Q2FJ2K2V |
More data for this
Ligand-Target Pair | |
Thioredoxin reductase 1, cytoplasmic
(Rattus norvegicus) | BDBM50236322

(CHEMBL4080662)Show SMILES Cl.CC1(C)N=C(N)N=C(N)N1OCCOc1ccc(\C=C\C(=O)c2ccccc2)cc1 |t:3,6| Show InChI InChI=1S/C22H25N5O3/c1-22(2)26-20(23)25-21(24)27(22)30-15-14-29-18-11-8-16(9-12-18)10-13-19(28)17-6-4-3-5-7-17/h3-13H,14-15H2,1-2H3,(H4,23,24,25,26)/b13-10+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.11E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National University of Singapore
Curated by ChEMBL
| Assay Description
Binding affinity towards human muscarinic M2 receptor |
J Med Chem 60: 1734-1745 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01253
BindingDB Entry DOI: 10.7270/Q2FJ2K2V |
More data for this
Ligand-Target Pair | |
Thioredoxin reductase 1, cytoplasmic
(Rattus norvegicus) | BDBM50236286

(CHEMBL4079985)Show SMILES Cl.CC1(C)N=C(N)N=C(N)N1OCCCOc1ccc(\C=C\C(=O)c2ccccc2)cc1 |t:3,6| Show InChI InChI=1S/C23H27N5O3/c1-23(2)27-21(24)26-22(25)28(23)31-16-6-15-30-19-12-9-17(10-13-19)11-14-20(29)18-7-4-3-5-8-18/h3-5,7-14H,6,15-16H2,1-2H3,(H4,24,25,26,27)/b14-11+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.57E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National University of Singapore
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Farnesyltransferase for Farnesylation of H-ras protein InNIH 3T3 cells transformed with activated H-ras |
J Med Chem 60: 1734-1745 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01253
BindingDB Entry DOI: 10.7270/Q2FJ2K2V |
More data for this
Ligand-Target Pair | |
Thioredoxin reductase 1, cytoplasmic
(Rattus norvegicus) | BDBM29143
(CHEMBL7976 | Chalcone 1 | Chalcone, 13 | cid_63776...)Show InChI InChI=1S/C15H12O/c16-15(14-9-5-2-6-10-14)12-11-13-7-3-1-4-8-13/h1-12H/b12-11+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.67E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National University of Singapore
Curated by ChEMBL
| Assay Description
Inhibition of recombinant rat liver thioredoxin reductase after 60 mins by DTNB reduction assay |
J Med Chem 60: 1734-1745 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01253
BindingDB Entry DOI: 10.7270/Q2FJ2K2V |
More data for this
Ligand-Target Pair | |
Thioredoxin reductase 1, cytoplasmic
(Rattus norvegicus) | BDBM50236288

(CHEMBL4098945)Show SMILES Cl.CC1(C)N=C(N)N=C(N)N1OCCCCOc1ccc(cc1)C(=O)\C=C\c1ccccc1 |t:3,6| Show InChI InChI=1S/C24H29N5O3/c1-24(2)28-22(25)27-23(26)29(24)32-17-7-6-16-31-20-13-11-19(12-14-20)21(30)15-10-18-8-4-3-5-9-18/h3-5,8-15H,6-7,16-17H2,1-2H3,(H4,25,26,27,28)/b15-10+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National University of Singapore
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Geranylgeranyl transferase in the geranylgeranylation of H-ras-CAIL protein |
J Med Chem 60: 1734-1745 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01253
BindingDB Entry DOI: 10.7270/Q2FJ2K2V |
More data for this
Ligand-Target Pair | |
Thioredoxin reductase 1, cytoplasmic
(Rattus norvegicus) | BDBM50236322

(CHEMBL4080662)Show SMILES Cl.CC1(C)N=C(N)N=C(N)N1OCCOc1ccc(\C=C\C(=O)c2ccccc2)cc1 |t:3,6| Show InChI InChI=1S/C22H25N5O3/c1-22(2)26-20(23)25-21(24)27(22)30-15-14-29-18-11-8-16(9-12-18)10-13-19(28)17-6-4-3-5-7-17/h3-13H,14-15H2,1-2H3,(H4,23,24,25,26)/b13-10+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.98E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National University of Singapore
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Farnesyltransferase for Farnesylation of H-ras protein InNIH 3T3 cells transformed with activated H-ras |
J Med Chem 60: 1734-1745 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01253
BindingDB Entry DOI: 10.7270/Q2FJ2K2V |
More data for this
Ligand-Target Pair | |
Thioredoxin reductase 1, cytoplasmic
(Rattus norvegicus) | BDBM50236291

(CHEMBL4060570)Show SMILES Cl.CC1(C)N=C(N)N=C(N)N1OCCCCOc1ccc(\C=C\C(=O)c2ccccc2)cc1 |t:3,6| Show InChI InChI=1S/C24H29N5O3/c1-24(2)28-22(25)27-23(26)29(24)32-17-7-6-16-31-20-13-10-18(11-14-20)12-15-21(30)19-8-4-3-5-9-19/h3-5,8-15H,6-7,16-17H2,1-2H3,(H4,25,26,27,28)/b15-12+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.16E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National University of Singapore
Curated by ChEMBL
| Assay Description
Inhibition of high affinity uptake of [3H]dopamine into striatal nerve endings (synaptosomes) |
J Med Chem 60: 1734-1745 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01253
BindingDB Entry DOI: 10.7270/Q2FJ2K2V |
More data for this
Ligand-Target Pair | |
Thioredoxin reductase 1, cytoplasmic
(Rattus norvegicus) | BDBM50236284

(CHEMBL4069255)Show SMILES Cl.CC1(C)N=C(N)N=C(N)N1OCCCOc1ccc(cc1)C(=O)\C=C\c1ccccc1 |t:3,6| Show InChI InChI=1S/C23H27N5O3/c1-23(2)27-21(24)26-22(25)28(23)31-16-6-15-30-19-12-10-18(11-13-19)20(29)14-9-17-7-4-3-5-8-17/h3-5,7-14H,6,15-16H2,1-2H3,(H4,24,25,26,27)/b14-9+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 3.24E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National University of Singapore
Curated by ChEMBL
| Assay Description
Inhibition of recombinant rat liver thioredoxin reductase after 60 mins by DTNB reduction assay |
J Med Chem 60: 1734-1745 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01253
BindingDB Entry DOI: 10.7270/Q2FJ2K2V |
More data for this
Ligand-Target Pair | |
Thioredoxin reductase 1, cytoplasmic
(Rattus norvegicus) | BDBM50236286

(CHEMBL4079985)Show SMILES Cl.CC1(C)N=C(N)N=C(N)N1OCCCOc1ccc(\C=C\C(=O)c2ccccc2)cc1 |t:3,6| Show InChI InChI=1S/C23H27N5O3/c1-23(2)27-21(24)26-22(25)28(23)31-16-6-15-30-19-12-9-17(10-13-19)11-14-20(29)18-7-4-3-5-8-18/h3-5,7-14H,6,15-16H2,1-2H3,(H4,24,25,26,27)/b14-11+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.59E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National University of Singapore
Curated by ChEMBL
| Assay Description
Inhibition of recombinant rat liver thioredoxin reductase after 30 mins by DTNB reduction assay |
J Med Chem 60: 1734-1745 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01253
BindingDB Entry DOI: 10.7270/Q2FJ2K2V |
More data for this
Ligand-Target Pair | |
Thioredoxin reductase 1, cytoplasmic
(Rattus norvegicus) | BDBM50236283

(CHEMBL4074887)Show SMILES Cl.CC1(C)N=C(N)N=C(N)N1OCCOc1ccc(cc1)C(=O)\C=C\c1ccccc1 |t:3,6| Show InChI InChI=1S/C22H25N5O3/c1-22(2)26-20(23)25-21(24)27(22)30-15-14-29-18-11-9-17(10-12-18)19(28)13-8-16-6-4-3-5-7-16/h3-13H,14-15H2,1-2H3,(H4,23,24,25,26)/b13-8+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.33E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National University of Singapore
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Geranylgeranyl transferase in the geranylgeranylation of H-ras-CAIL protein |
J Med Chem 60: 1734-1745 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01253
BindingDB Entry DOI: 10.7270/Q2FJ2K2V |
More data for this
Ligand-Target Pair | |
Thioredoxin reductase 1, cytoplasmic
(Rattus norvegicus) | BDBM50236288

(CHEMBL4098945)Show SMILES Cl.CC1(C)N=C(N)N=C(N)N1OCCCCOc1ccc(cc1)C(=O)\C=C\c1ccccc1 |t:3,6| Show InChI InChI=1S/C24H29N5O3/c1-24(2)28-22(25)27-23(26)29(24)32-17-7-6-16-31-20-13-11-19(12-14-20)21(30)15-10-18-8-4-3-5-9-18/h3-5,8-15H,6-7,16-17H2,1-2H3,(H4,25,26,27,28)/b15-10+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.45E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National University of Singapore
Curated by ChEMBL
| Assay Description
Inhibition of recombinant rat liver thioredoxin reductase after 30 mins by DTNB reduction assay |
J Med Chem 60: 1734-1745 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01253
BindingDB Entry DOI: 10.7270/Q2FJ2K2V |
More data for this
Ligand-Target Pair | |
Thioredoxin reductase 1, cytoplasmic
(Rattus norvegicus) | BDBM29143

(CHEMBL7976 | Chalcone 1 | Chalcone, 13 | cid_63776...)Show InChI InChI=1S/C15H12O/c16-15(14-9-5-2-6-10-14)12-11-13-7-3-1-4-8-13/h1-12H/b12-11+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.64E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National University of Singapore
Curated by ChEMBL
| Assay Description
Inhibition of recombinant rat liver thioredoxin reductase after 30 mins by DTNB reduction assay |
J Med Chem 60: 1734-1745 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01253
BindingDB Entry DOI: 10.7270/Q2FJ2K2V |
More data for this
Ligand-Target Pair | |
Thioredoxin reductase 1, cytoplasmic
(Rattus norvegicus) | BDBM50236284

(CHEMBL4069255)Show SMILES Cl.CC1(C)N=C(N)N=C(N)N1OCCCOc1ccc(cc1)C(=O)\C=C\c1ccccc1 |t:3,6| Show InChI InChI=1S/C23H27N5O3/c1-23(2)27-21(24)26-22(25)28(23)31-16-6-15-30-19-12-10-18(11-13-19)20(29)14-9-17-7-4-3-5-8-17/h3-5,7-14H,6,15-16H2,1-2H3,(H4,24,25,26,27)/b14-9+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 4.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National University of Singapore
Curated by ChEMBL
| Assay Description
Inhibition of recombinant rat liver thioredoxin reductase after 30 mins by DTNB reduction assay |
J Med Chem 60: 1734-1745 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01253
BindingDB Entry DOI: 10.7270/Q2FJ2K2V |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data