

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Plasma kallikrein (Homo sapiens (Human)) | BDBM50210127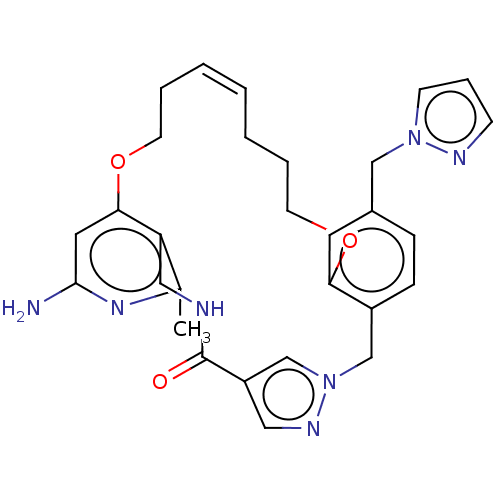 (CHEMBL3884851) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Global Blood Therapeutics Curated by ChEMBL | Assay Description Inhibition of human plasma kallikrein using D-Pro-Phe-Arg-pNA as substrate preincubated for 30 mins followed by substrate addition | ACS Med Chem Lett 8: 185-190 (2017) Article DOI: 10.1021/acsmedchemlett.6b00384 BindingDB Entry DOI: 10.7270/Q2DB83V8 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM50210072 (CHEMBL3884830) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Global Blood Therapeutics Curated by ChEMBL | Assay Description Inhibition of human plasma kallikrein using D-Pro-Phe-Arg-pNA as substrate preincubated for 30 mins followed by substrate addition | ACS Med Chem Lett 8: 185-190 (2017) Article DOI: 10.1021/acsmedchemlett.6b00384 BindingDB Entry DOI: 10.7270/Q2DB83V8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM50210075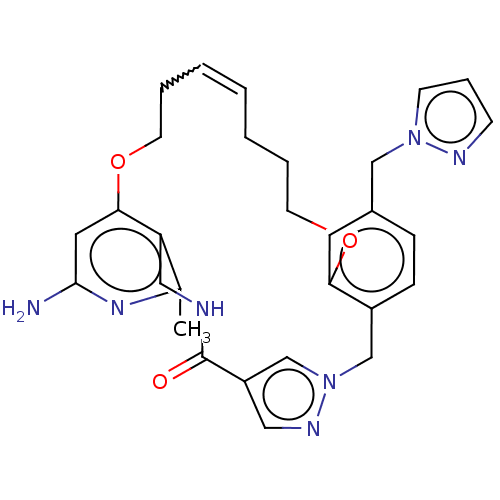 (CHEMBL3883788) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Global Blood Therapeutics Curated by ChEMBL | Assay Description Inhibition of human plasma kallikrein using D-Pro-Phe-Arg-pNA as substrate preincubated for 30 mins followed by substrate addition | ACS Med Chem Lett 8: 185-190 (2017) Article DOI: 10.1021/acsmedchemlett.6b00384 BindingDB Entry DOI: 10.7270/Q2DB83V8 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM50210073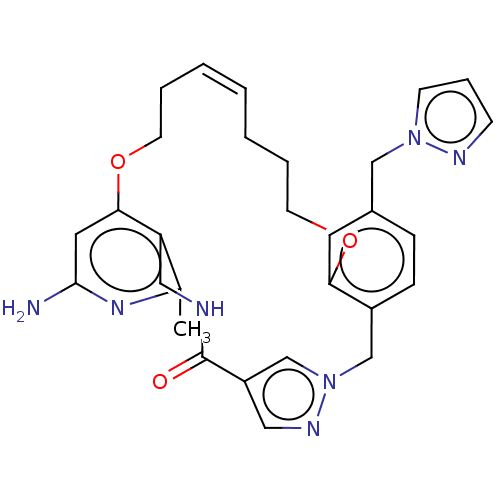 (CHEMBL3883461) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
Global Blood Therapeutics Curated by ChEMBL | Assay Description Inhibition of human plasma kallikrein using D-Pro-Phe-Arg-pNA as substrate preincubated for 30 mins followed by substrate addition | ACS Med Chem Lett 8: 185-190 (2017) Article DOI: 10.1021/acsmedchemlett.6b00384 BindingDB Entry DOI: 10.7270/Q2DB83V8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM50210074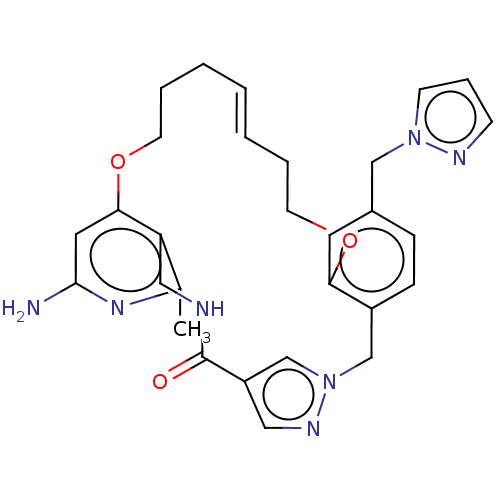 (CHEMBL3883423) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 153 | n/a | n/a | n/a | n/a | n/a | n/a |
Global Blood Therapeutics Curated by ChEMBL | Assay Description Inhibition of human plasma kallikrein using D-Pro-Phe-Arg-pNA as substrate preincubated for 30 mins followed by substrate addition | ACS Med Chem Lett 8: 185-190 (2017) Article DOI: 10.1021/acsmedchemlett.6b00384 BindingDB Entry DOI: 10.7270/Q2DB83V8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM50210129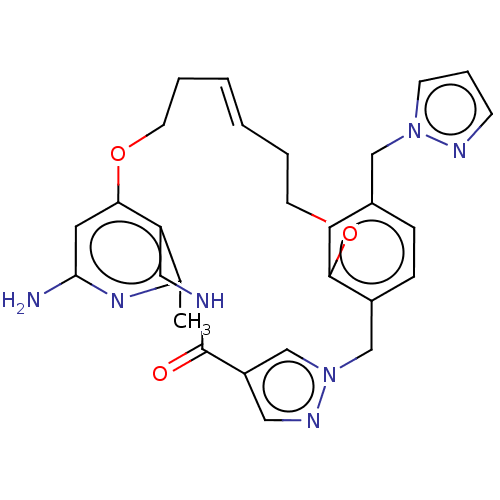 (CHEMBL3885334) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.21E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Global Blood Therapeutics Curated by ChEMBL | Assay Description Inhibition of human plasma kallikrein using D-Pro-Phe-Arg-pNA as substrate preincubated for 30 mins followed by substrate addition | ACS Med Chem Lett 8: 185-190 (2017) Article DOI: 10.1021/acsmedchemlett.6b00384 BindingDB Entry DOI: 10.7270/Q2DB83V8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM50210130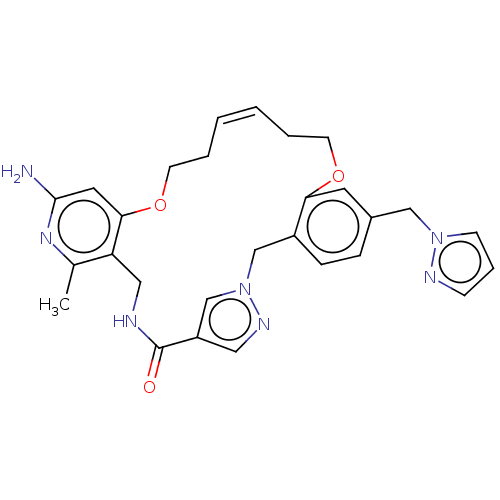 (CHEMBL3885037) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.57E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Global Blood Therapeutics Curated by ChEMBL | Assay Description Inhibition of human plasma kallikrein using D-Pro-Phe-Arg-pNA as substrate preincubated for 30 mins followed by substrate addition | ACS Med Chem Lett 8: 185-190 (2017) Article DOI: 10.1021/acsmedchemlett.6b00384 BindingDB Entry DOI: 10.7270/Q2DB83V8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50210127 (CHEMBL3884851) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 3.61E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Global Blood Therapeutics Curated by ChEMBL | Assay Description Inhibition of factor 11a (unknown origin) using S-2366 as substrate incubated for 30 mins followed by substrate addition | ACS Med Chem Lett 8: 185-190 (2017) Article DOI: 10.1021/acsmedchemlett.6b00384 BindingDB Entry DOI: 10.7270/Q2DB83V8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM50210076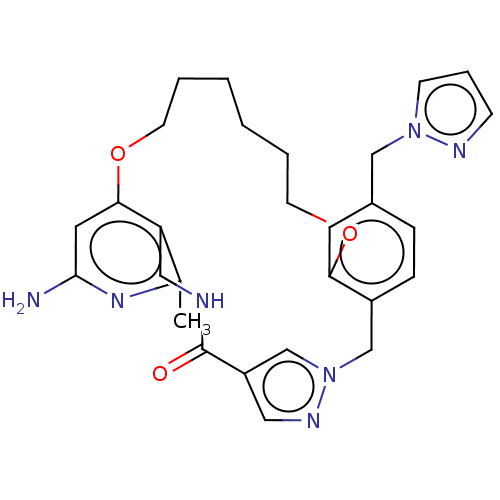 (CHEMBL3884030) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.31E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Global Blood Therapeutics Curated by ChEMBL | Assay Description Inhibition of human plasma kallikrein using D-Pro-Phe-Arg-pNA as substrate preincubated for 30 mins followed by substrate addition | ACS Med Chem Lett 8: 185-190 (2017) Article DOI: 10.1021/acsmedchemlett.6b00384 BindingDB Entry DOI: 10.7270/Q2DB83V8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Homo sapiens (Human)) | BDBM50210074 (CHEMBL3883423) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.97E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Global Blood Therapeutics Curated by ChEMBL | Assay Description Inhibition of Lys-plasmin (unknown origin) using Tosyl-Gly-Pro-Lys-4-nitranilide as substrate preincubated for 30 mins followed by substrate addition | ACS Med Chem Lett 8: 185-190 (2017) Article DOI: 10.1021/acsmedchemlett.6b00384 BindingDB Entry DOI: 10.7270/Q2DB83V8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50210075 (CHEMBL3883788) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 1.17E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Global Blood Therapeutics Curated by ChEMBL | Assay Description Inhibition of factor 11a (unknown origin) using S-2366 as substrate incubated for 30 mins followed by substrate addition | ACS Med Chem Lett 8: 185-190 (2017) Article DOI: 10.1021/acsmedchemlett.6b00384 BindingDB Entry DOI: 10.7270/Q2DB83V8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50210074 (CHEMBL3883423) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.27E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Global Blood Therapeutics Curated by ChEMBL | Assay Description Inhibition of factor 11a (unknown origin) using S-2366 as substrate incubated for 30 mins followed by substrate addition | ACS Med Chem Lett 8: 185-190 (2017) Article DOI: 10.1021/acsmedchemlett.6b00384 BindingDB Entry DOI: 10.7270/Q2DB83V8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Homo sapiens (Human)) | BDBM50210075 (CHEMBL3883788) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 2.14E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Global Blood Therapeutics Curated by ChEMBL | Assay Description Inhibition of Lys-plasmin (unknown origin) using Tosyl-Gly-Pro-Lys-4-nitranilide as substrate preincubated for 30 mins followed by substrate addition | ACS Med Chem Lett 8: 185-190 (2017) Article DOI: 10.1021/acsmedchemlett.6b00384 BindingDB Entry DOI: 10.7270/Q2DB83V8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50210073 (CHEMBL3883461) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.09E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Global Blood Therapeutics Curated by ChEMBL | Assay Description Inhibition of factor 11a (unknown origin) using S-2366 as substrate incubated for 30 mins followed by substrate addition | ACS Med Chem Lett 8: 185-190 (2017) Article DOI: 10.1021/acsmedchemlett.6b00384 BindingDB Entry DOI: 10.7270/Q2DB83V8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM50210077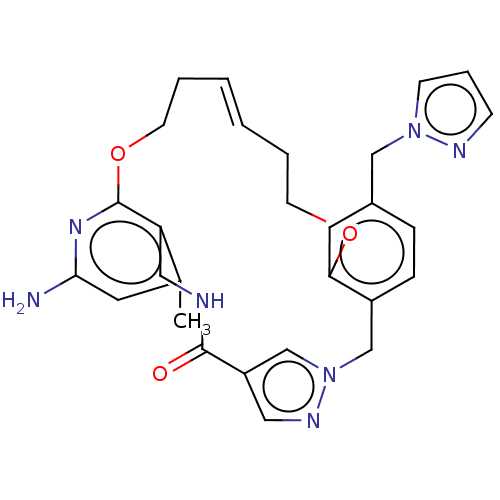 (CHEMBL3883550) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5.32E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Global Blood Therapeutics Curated by ChEMBL | Assay Description Inhibition of human plasma kallikrein using D-Pro-Phe-Arg-pNA as substrate preincubated for 30 mins followed by substrate addition | ACS Med Chem Lett 8: 185-190 (2017) Article DOI: 10.1021/acsmedchemlett.6b00384 BindingDB Entry DOI: 10.7270/Q2DB83V8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50210072 (CHEMBL3884830) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.06E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Global Blood Therapeutics Curated by ChEMBL | Assay Description Inhibition of factor 11a (unknown origin) using S-2366 as substrate incubated for 30 mins followed by substrate addition | ACS Med Chem Lett 8: 185-190 (2017) Article DOI: 10.1021/acsmedchemlett.6b00384 BindingDB Entry DOI: 10.7270/Q2DB83V8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tissue-type plasminogen activator (Homo sapiens (Human)) | BDBM50210073 (CHEMBL3883461) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Global Blood Therapeutics Curated by ChEMBL | Assay Description Inhibition of tPA (unknown origin) using Methylsulfonyl-D-Phe-Gly-Arg-pNA as substrate preincubated for 30 mins followed by substrate addition | ACS Med Chem Lett 8: 185-190 (2017) Article DOI: 10.1021/acsmedchemlett.6b00384 BindingDB Entry DOI: 10.7270/Q2DB83V8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50210073 (CHEMBL3883461) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Global Blood Therapeutics Curated by ChEMBL | Assay Description Inhibition of factor 10a (unknown origin) using N-Z-D-Arg-Gly-Arg-pNA as substrate preincubated for 30 mins followed by substrate addition | ACS Med Chem Lett 8: 185-190 (2017) Article DOI: 10.1021/acsmedchemlett.6b00384 BindingDB Entry DOI: 10.7270/Q2DB83V8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50210074 (CHEMBL3883423) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Global Blood Therapeutics Curated by ChEMBL | Assay Description Inhibition of factor 10a (unknown origin) using N-Z-D-Arg-Gly-Arg-pNA as substrate preincubated for 30 mins followed by substrate addition | ACS Med Chem Lett 8: 185-190 (2017) Article DOI: 10.1021/acsmedchemlett.6b00384 BindingDB Entry DOI: 10.7270/Q2DB83V8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50210127 (CHEMBL3884851) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Global Blood Therapeutics Curated by ChEMBL | Assay Description Inhibition of factor 10a (unknown origin) using N-Z-D-Arg-Gly-Arg-pNA as substrate preincubated for 30 mins followed by substrate addition | ACS Med Chem Lett 8: 185-190 (2017) Article DOI: 10.1021/acsmedchemlett.6b00384 BindingDB Entry DOI: 10.7270/Q2DB83V8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tissue-type plasminogen activator (Homo sapiens (Human)) | BDBM50210075 (CHEMBL3883788) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Global Blood Therapeutics Curated by ChEMBL | Assay Description Inhibition of tPA (unknown origin) using Methylsulfonyl-D-Phe-Gly-Arg-pNA as substrate preincubated for 30 mins followed by substrate addition | ACS Med Chem Lett 8: 185-190 (2017) Article DOI: 10.1021/acsmedchemlett.6b00384 BindingDB Entry DOI: 10.7270/Q2DB83V8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Homo sapiens (Human)) | BDBM50210127 (CHEMBL3884851) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Global Blood Therapeutics Curated by ChEMBL | Assay Description Inhibition of Lys-plasmin (unknown origin) using Tosyl-Gly-Pro-Lys-4-nitranilide as substrate preincubated for 30 mins followed by substrate addition | ACS Med Chem Lett 8: 185-190 (2017) Article DOI: 10.1021/acsmedchemlett.6b00384 BindingDB Entry DOI: 10.7270/Q2DB83V8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XII (Homo sapiens (Human)) | BDBM50210075 (CHEMBL3883788) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Global Blood Therapeutics Curated by ChEMBL | Assay Description Inhibition of factor 12a (unknown origin) using D-Pro-Phe-Arg-pNA as substrate preincubated for 30 mins followed by substrate addition | ACS Med Chem Lett 8: 185-190 (2017) Article DOI: 10.1021/acsmedchemlett.6b00384 BindingDB Entry DOI: 10.7270/Q2DB83V8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Homo sapiens (Human)) | BDBM50210073 (CHEMBL3883461) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Global Blood Therapeutics Curated by ChEMBL | Assay Description Inhibition of Lys-plasmin (unknown origin) using Tosyl-Gly-Pro-Lys-4-nitranilide as substrate preincubated for 30 mins followed by substrate addition | ACS Med Chem Lett 8: 185-190 (2017) Article DOI: 10.1021/acsmedchemlett.6b00384 BindingDB Entry DOI: 10.7270/Q2DB83V8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50210074 (CHEMBL3883423) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Global Blood Therapeutics Curated by ChEMBL | Assay Description Inhibition of alpha-thrombin (unknown origin) using D-Phe-Pip-Arg-pNA as substrate preincubated for 30 mins followed by substrate addition | ACS Med Chem Lett 8: 185-190 (2017) Article DOI: 10.1021/acsmedchemlett.6b00384 BindingDB Entry DOI: 10.7270/Q2DB83V8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XII (Homo sapiens (Human)) | BDBM50210074 (CHEMBL3883423) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Global Blood Therapeutics Curated by ChEMBL | Assay Description Inhibition of factor 12a (unknown origin) using D-Pro-Phe-Arg-pNA as substrate preincubated for 30 mins followed by substrate addition | ACS Med Chem Lett 8: 185-190 (2017) Article DOI: 10.1021/acsmedchemlett.6b00384 BindingDB Entry DOI: 10.7270/Q2DB83V8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tissue-type plasminogen activator (Homo sapiens (Human)) | BDBM50210074 (CHEMBL3883423) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Global Blood Therapeutics Curated by ChEMBL | Assay Description Inhibition of tPA (unknown origin) using Methylsulfonyl-D-Phe-Gly-Arg-pNA as substrate preincubated for 30 mins followed by substrate addition | ACS Med Chem Lett 8: 185-190 (2017) Article DOI: 10.1021/acsmedchemlett.6b00384 BindingDB Entry DOI: 10.7270/Q2DB83V8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50210075 (CHEMBL3883788) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Global Blood Therapeutics Curated by ChEMBL | Assay Description Inhibition of alpha-thrombin (unknown origin) using D-Phe-Pip-Arg-pNA as substrate preincubated for 30 mins followed by substrate addition | ACS Med Chem Lett 8: 185-190 (2017) Article DOI: 10.1021/acsmedchemlett.6b00384 BindingDB Entry DOI: 10.7270/Q2DB83V8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50210075 (CHEMBL3883788) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Global Blood Therapeutics Curated by ChEMBL | Assay Description Inhibition of factor 10a (unknown origin) using N-Z-D-Arg-Gly-Arg-pNA as substrate preincubated for 30 mins followed by substrate addition | ACS Med Chem Lett 8: 185-190 (2017) Article DOI: 10.1021/acsmedchemlett.6b00384 BindingDB Entry DOI: 10.7270/Q2DB83V8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XII (Homo sapiens (Human)) | BDBM50210073 (CHEMBL3883461) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Global Blood Therapeutics Curated by ChEMBL | Assay Description Inhibition of factor 12a (unknown origin) using D-Pro-Phe-Arg-pNA as substrate preincubated for 30 mins followed by substrate addition | ACS Med Chem Lett 8: 185-190 (2017) Article DOI: 10.1021/acsmedchemlett.6b00384 BindingDB Entry DOI: 10.7270/Q2DB83V8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50210073 (CHEMBL3883461) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Global Blood Therapeutics Curated by ChEMBL | Assay Description Inhibition of alpha-thrombin (unknown origin) using D-Phe-Pip-Arg-pNA as substrate preincubated for 30 mins followed by substrate addition | ACS Med Chem Lett 8: 185-190 (2017) Article DOI: 10.1021/acsmedchemlett.6b00384 BindingDB Entry DOI: 10.7270/Q2DB83V8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tissue-type plasminogen activator (Homo sapiens (Human)) | BDBM50210127 (CHEMBL3884851) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Global Blood Therapeutics Curated by ChEMBL | Assay Description Inhibition of tPA (unknown origin) using Methylsulfonyl-D-Phe-Gly-Arg-pNA as substrate preincubated for 30 mins followed by substrate addition | ACS Med Chem Lett 8: 185-190 (2017) Article DOI: 10.1021/acsmedchemlett.6b00384 BindingDB Entry DOI: 10.7270/Q2DB83V8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XII (Homo sapiens (Human)) | BDBM50210127 (CHEMBL3884851) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Global Blood Therapeutics Curated by ChEMBL | Assay Description Inhibition of factor 12a (unknown origin) using D-Pro-Phe-Arg-pNA as substrate preincubated for 30 mins followed by substrate addition | ACS Med Chem Lett 8: 185-190 (2017) Article DOI: 10.1021/acsmedchemlett.6b00384 BindingDB Entry DOI: 10.7270/Q2DB83V8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50210127 (CHEMBL3884851) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Global Blood Therapeutics Curated by ChEMBL | Assay Description Inhibition of alpha-thrombin (unknown origin) using D-Phe-Pip-Arg-pNA as substrate preincubated for 30 mins followed by substrate addition | ACS Med Chem Lett 8: 185-190 (2017) Article DOI: 10.1021/acsmedchemlett.6b00384 BindingDB Entry DOI: 10.7270/Q2DB83V8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50210072 (CHEMBL3884830) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Global Blood Therapeutics Curated by ChEMBL | Assay Description Inhibition of alpha-thrombin (unknown origin) using D-Phe-Pip-Arg-pNA as substrate preincubated for 30 mins followed by substrate addition | ACS Med Chem Lett 8: 185-190 (2017) Article DOI: 10.1021/acsmedchemlett.6b00384 BindingDB Entry DOI: 10.7270/Q2DB83V8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XII (Homo sapiens (Human)) | BDBM50210072 (CHEMBL3884830) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Global Blood Therapeutics Curated by ChEMBL | Assay Description Inhibition of factor 12a (unknown origin) using D-Pro-Phe-Arg-pNA as substrate preincubated for 30 mins followed by substrate addition | ACS Med Chem Lett 8: 185-190 (2017) Article DOI: 10.1021/acsmedchemlett.6b00384 BindingDB Entry DOI: 10.7270/Q2DB83V8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50210072 (CHEMBL3884830) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Global Blood Therapeutics Curated by ChEMBL | Assay Description Inhibition of factor 10a (unknown origin) using N-Z-D-Arg-Gly-Arg-pNA as substrate preincubated for 30 mins followed by substrate addition | ACS Med Chem Lett 8: 185-190 (2017) Article DOI: 10.1021/acsmedchemlett.6b00384 BindingDB Entry DOI: 10.7270/Q2DB83V8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Homo sapiens (Human)) | BDBM50210072 (CHEMBL3884830) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Global Blood Therapeutics Curated by ChEMBL | Assay Description Inhibition of Lys-plasmin (unknown origin) using Tosyl-Gly-Pro-Lys-4-nitranilide as substrate preincubated for 30 mins followed by substrate addition | ACS Med Chem Lett 8: 185-190 (2017) Article DOI: 10.1021/acsmedchemlett.6b00384 BindingDB Entry DOI: 10.7270/Q2DB83V8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tissue-type plasminogen activator (Homo sapiens (Human)) | BDBM50210072 (CHEMBL3884830) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Global Blood Therapeutics Curated by ChEMBL | Assay Description Inhibition of tPA (unknown origin) using Methylsulfonyl-D-Phe-Gly-Arg-pNA as substrate preincubated for 30 mins followed by substrate addition | ACS Med Chem Lett 8: 185-190 (2017) Article DOI: 10.1021/acsmedchemlett.6b00384 BindingDB Entry DOI: 10.7270/Q2DB83V8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM50210128 (CHEMBL3884218) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Global Blood Therapeutics Curated by ChEMBL | Assay Description Inhibition of human plasma kallikrein using D-Pro-Phe-Arg-pNA as substrate preincubated for 30 mins followed by substrate addition | ACS Med Chem Lett 8: 185-190 (2017) Article DOI: 10.1021/acsmedchemlett.6b00384 BindingDB Entry DOI: 10.7270/Q2DB83V8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||