

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acyl-protein thioesterase 2 (Homo sapiens (Human)) | BDBM207992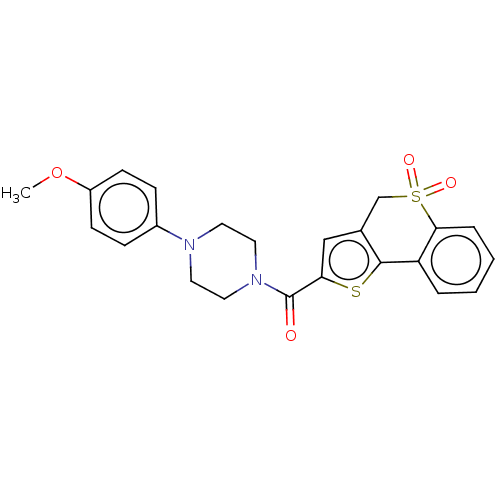 (ML349) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Competitive inhibition of HEK293T cells-derived His-tagged APT2 expressed in Escherichia coli BL21(DE3) preincubated for 30 mins followed by substrat... | ACS Med Chem Lett 8: 215-220 (2017) Article DOI: 10.1021/acsmedchemlett.6b00441 BindingDB Entry DOI: 10.7270/Q2V98B7S | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acyl-protein thioesterase 2 (Homo sapiens (Human)) | BDBM50212216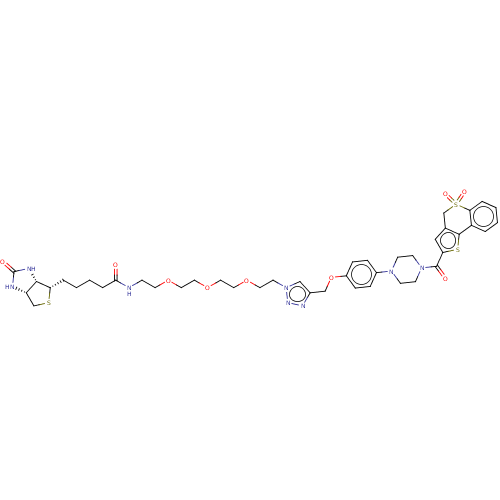 (CHEMBL3944678) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Competitive inhibition of HEK293T cells-derived His-tagged APT2 expressed in Escherichia coli BL21(DE3) preincubated for 30 mins followed by substrat... | ACS Med Chem Lett 8: 215-220 (2017) Article DOI: 10.1021/acsmedchemlett.6b00441 BindingDB Entry DOI: 10.7270/Q2V98B7S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acyl-protein thioesterase 2 (Homo sapiens (Human)) | BDBM50212217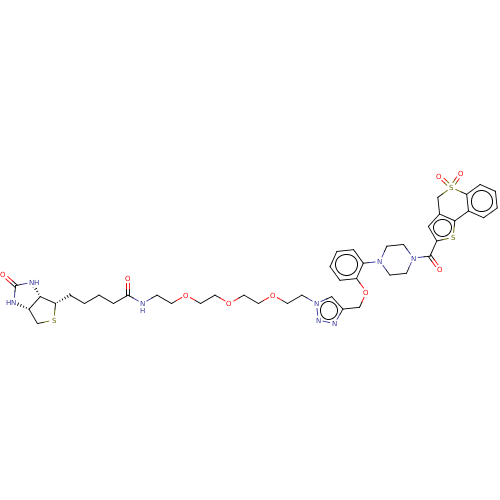 (CHEMBL3917640) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Competitive inhibition of HEK293T cells-derived His-tagged APT2 expressed in Escherichia coli BL21(DE3) preincubated for 30 mins followed by substrat... | ACS Med Chem Lett 8: 215-220 (2017) Article DOI: 10.1021/acsmedchemlett.6b00441 BindingDB Entry DOI: 10.7270/Q2V98B7S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acyl-protein thioesterase 2 (Homo sapiens (Human)) | BDBM50212218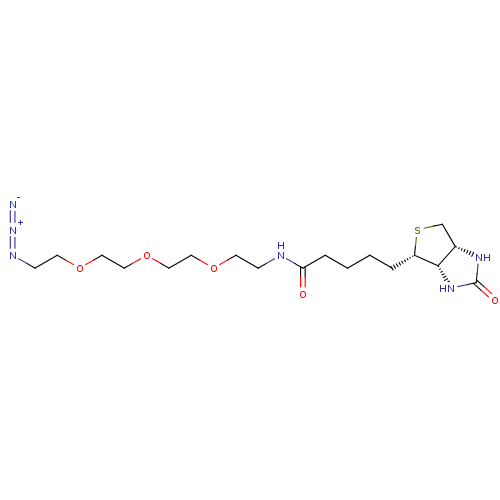 (CHEMBL3974081) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Competitive inhibition of HEK293T cells-derived His-tagged APT2 expressed in Escherichia coli BL21(DE3) preincubated for 30 mins followed by substrat... | ACS Med Chem Lett 8: 215-220 (2017) Article DOI: 10.1021/acsmedchemlett.6b00441 BindingDB Entry DOI: 10.7270/Q2V98B7S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ribosyldihydronicotinamide dehydrogenase [quinone] (Homo sapiens (Human)) | BDBM13530 (4-[(4-methylpiperazin-1-yl)methyl]-N-[4-methyl-3-[...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 82 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Inhibition of full length human His6/GST-tagged NQO2 expressed in Escherichia coli Tuner(DE3)pLysS using menadione as substrate and CCHP as co-substr... | ACS Med Chem Lett 8: 215-220 (2017) Article DOI: 10.1021/acsmedchemlett.6b00441 BindingDB Entry DOI: 10.7270/Q2V98B7S | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Ribosyldihydronicotinamide dehydrogenase [quinone] (Homo sapiens (Human)) | BDBM50237710 (4-methyl-N-[3-(4-methyl-1H-imidazol-1-yl)-5-(trifl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 381 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Inhibition of full length human His6/GST-tagged NQO2 expressed in Escherichia coli Tuner(DE3)pLysS using menadione as substrate and CCHP as co-substr... | ACS Med Chem Lett 8: 215-220 (2017) Article DOI: 10.1021/acsmedchemlett.6b00441 BindingDB Entry DOI: 10.7270/Q2V98B7S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acyl-protein thioesterase 2 (Homo sapiens (Human)) | BDBM207992 (ML349) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Inhibition of fluorescein-labeled ML349 binding to HEK293T cells-derived His-tagged APT2 expressed in Escherichia coli BL21(DE3) after 30 mins by flu... | ACS Med Chem Lett 8: 215-220 (2017) Article DOI: 10.1021/acsmedchemlett.6b00441 BindingDB Entry DOI: 10.7270/Q2V98B7S | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Pyridoxal kinase (Homo sapiens (Human)) | BDBM207992 (ML349) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Inhibition of fluorescein-labeled ML349 binding to HEK293T cells-derived His-tagged PDXK expressed in Escherichia coli BL21(DE3) after 30 mins by flu... | ACS Med Chem Lett 8: 215-220 (2017) Article DOI: 10.1021/acsmedchemlett.6b00441 BindingDB Entry DOI: 10.7270/Q2V98B7S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pyridoxal kinase (Homo sapiens (Human)) | BDBM50212216 (CHEMBL3944678) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Inhibition of fluorescein-labeled ML349 binding to HEK293T cells-derived His-tagged PDXK expressed in Escherichia coli BL21(DE3) after 30 mins by flu... | ACS Med Chem Lett 8: 215-220 (2017) Article DOI: 10.1021/acsmedchemlett.6b00441 BindingDB Entry DOI: 10.7270/Q2V98B7S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acyl-protein thioesterase 2 (Homo sapiens (Human)) | BDBM50212216 (CHEMBL3944678) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Inhibition of fluorescein-labeled ML349 binding to HEK293T cells-derived His-tagged APT2 expressed in Escherichia coli BL21(DE3) after 30 mins by flu... | ACS Med Chem Lett 8: 215-220 (2017) Article DOI: 10.1021/acsmedchemlett.6b00441 BindingDB Entry DOI: 10.7270/Q2V98B7S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||