

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pancreatic alpha-amylase (Homo sapiens (Human)) | BDBM223314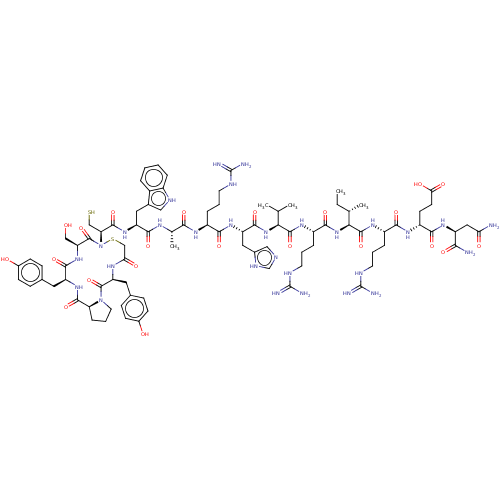 (YPYSCWARHVRIREN | piHA-D1) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | 7.0 | n/a |
The University of Tokyo | Assay Description The release of 2-chloro-4-nitrophenol resulting from the HPA-catalyzed hydrolysis of 2-chloro-4-nitrophenyl a-maltotrioside (CNPG3) was monitored at ... | Cell Chem Biol 24: 381-390 (2017) Article DOI: 10.1016/j.chembiol.2017.02.001 BindingDB Entry DOI: 10.7270/Q27D2T0M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pancreatic alpha-amylase (Homo sapiens (Human)) | BDBM223315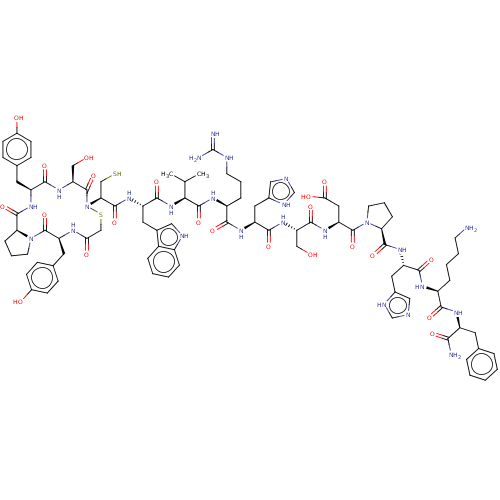 (YPYSCWVRHSDPHKF | piHA-D3) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | 7.0 | n/a |
The University of Tokyo | Assay Description The release of 2-chloro-4-nitrophenol resulting from the HPA-catalyzed hydrolysis of 2-chloro-4-nitrophenyl a-maltotrioside (CNPG3) was monitored at ... | Cell Chem Biol 24: 381-390 (2017) Article DOI: 10.1016/j.chembiol.2017.02.001 BindingDB Entry DOI: 10.7270/Q27D2T0M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pancreatic alpha-amylase (Homo sapiens (Human)) | BDBM223316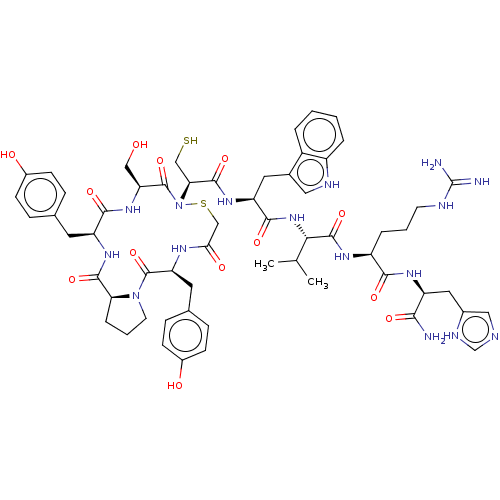 (YPYSCWVRH | piHA-Dm) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | 7.0 | n/a |
The University of Tokyo | Assay Description The release of 2-chloro-4-nitrophenol resulting from the HPA-catalyzed hydrolysis of 2-chloro-4-nitrophenyl a-maltotrioside (CNPG3) was monitored at ... | Cell Chem Biol 24: 381-390 (2017) Article DOI: 10.1016/j.chembiol.2017.02.001 BindingDB Entry DOI: 10.7270/Q27D2T0M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pancreatic alpha-amylase (Sus scrofa (Pig)) | BDBM223316 (YPYSCWVRH | piHA-Dm) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.5 | n/a | n/a | n/a | n/a | 7.0 | n/a |
The University of Tokyo | Assay Description After kinetic analysis with HPA, piHA-Dm was tested as an inhibitor of ten additional enzymes. The conditions are listed as follows: Agrobacterium fa... | Cell Chem Biol 24: 381-390 (2017) Article DOI: 10.1016/j.chembiol.2017.02.001 BindingDB Entry DOI: 10.7270/Q27D2T0M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-amylase 1A (Homo sapiens (Human)) | BDBM223316 (YPYSCWVRH | piHA-Dm) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 34 | n/a | n/a | n/a | n/a | 7.0 | n/a |
The University of Tokyo | Assay Description After kinetic analysis with HPA, piHA-Dm was tested as an inhibitor of ten additional enzymes. The conditions are listed as follows: Agrobacterium fa... | Cell Chem Biol 24: 381-390 (2017) Article DOI: 10.1016/j.chembiol.2017.02.001 BindingDB Entry DOI: 10.7270/Q27D2T0M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Maltase-glucoamylase (Homo sapiens (Human)) | BDBM223316 (YPYSCWVRH | piHA-Dm) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 520 | n/a | n/a | n/a | n/a | 7.0 | n/a |
The University of Tokyo | Assay Description After kinetic analysis with HPA, piHA-Dm was tested as an inhibitor of ten additional enzymes. The conditions are listed as follows: Agrobacterium fa... | Cell Chem Biol 24: 381-390 (2017) Article DOI: 10.1016/j.chembiol.2017.02.001 BindingDB Entry DOI: 10.7270/Q27D2T0M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Soluble maltase-glucoamylase (Mus musculus (Mouse)) | BDBM223316 (YPYSCWVRH | piHA-Dm) | UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 990 | n/a | n/a | n/a | n/a | 7.0 | n/a |
The University of Tokyo | Assay Description After kinetic analysis with HPA, piHA-Dm was tested as an inhibitor of ten additional enzymes. The conditions are listed as follows: Agrobacterium fa... | Cell Chem Biol 24: 381-390 (2017) Article DOI: 10.1016/j.chembiol.2017.02.001 BindingDB Entry DOI: 10.7270/Q27D2T0M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||