

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Eukaryotic initiation factor 4A-III (Homo sapiens (Human)) | BDBM65493 (eIF4A3 inhibitor, 2) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
BC Cancer Agency | Assay Description Inhibitory activity was assessed under the following conditions: 350 uM ATP/1.5 ug mL−1 RNA indicated by black; 35 uM ATP/1.5 ug mL−1 RNA... | ACS Chem Biol 12: 1760-1768 (2017) Article DOI: 10.1021/acschembio.7b00041 BindingDB Entry DOI: 10.7270/Q2T151TG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Eukaryotic initiation factor 4A-III (Homo sapiens (Human)) | BDBM65492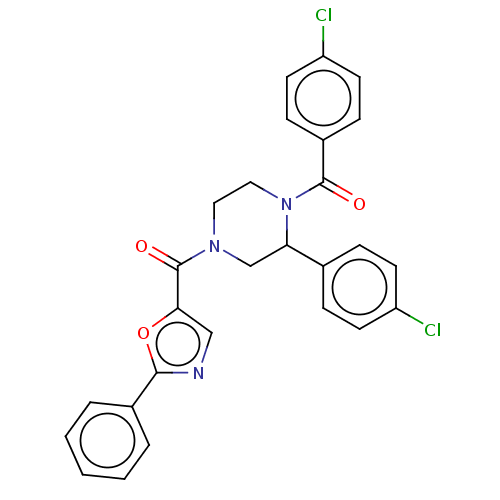 (eIF4A3 inhibitor, 1) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
BC Cancer Agency | Assay Description Inhibitory activity was assessed under the following conditions: 350 uM ATP/1.5 ug mL−1 RNA indicated by black; 35 uM ATP/1.5 ug mL−1 RNA... | ACS Chem Biol 12: 1760-1768 (2017) Article DOI: 10.1021/acschembio.7b00041 BindingDB Entry DOI: 10.7270/Q2T151TG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Eukaryotic initiation factor 4A-III (Homo sapiens (Human)) | BDBM65494 (eIF4A3 inhibitor, 3) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
BC Cancer Agency | Assay Description Inhibitory activity was assessed under the following conditions: 350 uM ATP/1.5 ug mL−1 RNA indicated by black; 35 uM ATP/1.5 ug mL−1 RNA... | ACS Chem Biol 12: 1760-1768 (2017) Article DOI: 10.1021/acschembio.7b00041 BindingDB Entry DOI: 10.7270/Q2T151TG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| U5 small nuclear ribonucleoprotein 200 kDa helicase (Homo sapiens (Human)) | BDBM65493 (eIF4A3 inhibitor, 2) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
BC Cancer Agency | Assay Description Inhibitory activity was assessed under the following conditions: 350 uM ATP/1.5 ug mL−1 RNA indicated by black; 35 uM ATP/1.5 ug mL−1 RNA... | ACS Chem Biol 12: 1760-1768 (2017) Article DOI: 10.1021/acschembio.7b00041 BindingDB Entry DOI: 10.7270/Q2T151TG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Eukaryotic initiation factor 4A-I (Homo sapiens (Human)) | BDBM65493 (eIF4A3 inhibitor, 2) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
BC Cancer Agency | Assay Description Inhibitory activity was assessed under the following conditions: 350 uM ATP/1.5 ug mL−1 RNA indicated by black; 35 uM ATP/1.5 ug mL−1 RNA... | ACS Chem Biol 12: 1760-1768 (2017) Article DOI: 10.1021/acschembio.7b00041 BindingDB Entry DOI: 10.7270/Q2T151TG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ATP-dependent RNA helicase DHX29 (Homo sapiens (Human)) | BDBM65493 (eIF4A3 inhibitor, 2) | MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
BC Cancer Agency | Assay Description Inhibitory activity was assessed under the following conditions: 350 uM ATP/1.5 ug mL−1 RNA indicated by black; 35 uM ATP/1.5 ug mL−1 RNA... | ACS Chem Biol 12: 1760-1768 (2017) Article DOI: 10.1021/acschembio.7b00041 BindingDB Entry DOI: 10.7270/Q2T151TG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Eukaryotic initiation factor 4A-II (Homo sapiens (Human)) | BDBM65493 (eIF4A3 inhibitor, 2) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
BC Cancer Agency | Assay Description Inhibitory activity was assessed under the following conditions: 350 uM ATP/1.5 ug mL−1 RNA indicated by black; 35 uM ATP/1.5 ug mL−1 RNA... | ACS Chem Biol 12: 1760-1768 (2017) Article DOI: 10.1021/acschembio.7b00041 BindingDB Entry DOI: 10.7270/Q2T151TG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||