 Found 86 hits of Enzyme Inhibition Constant Data
Found 86 hits of Enzyme Inhibition Constant Data Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Fibroblast growth factor receptor 2
(Homo sapiens (Human)) | BDBM286984

(8-(3-(4-acryloylpiperazin-1-yl)propyl)-6-(2,6-dich...)Show SMILES CNc1ncc2cc(-c3c(Cl)c(OC)cc(OC)c3Cl)c(=O)n(CCCN3CCN(CC3)C(=O)C=C)c2n1 |(-6.67,-.77,;-6.67,.77,;-5.33,1.54,;-5.33,3.08,;-4,3.85,;-2.67,3.08,;-1.33,3.85,;,3.08,;1.33,3.85,;1.33,5.39,;,6.16,;2.67,6.16,;2.67,7.7,;1.33,8.47,;4,5.39,;4,3.85,;5.33,3.08,;6.67,3.85,;2.67,3.08,;2.67,1.54,;,1.54,;1.33,.77,;-1.33,.77,;-1.33,-.77,;,-1.54,;,-3.08,;1.33,-3.85,;1.33,-5.39,;2.67,-6.16,;4,-5.39,;4,-3.85,;2.67,-3.08,;5.33,-6.16,;6.67,-5.39,;5.33,-7.7,;6.67,-8.47,;-2.67,1.54,;-4,.77,)| Show InChI InChI=1S/C26H30Cl2N6O4/c1-5-20(35)33-11-9-32(10-12-33)7-6-8-34-24-16(15-30-26(29-2)31-24)13-17(25(34)36)21-22(27)18(37-3)14-19(38-4)23(21)28/h5,13-15H,1,6-12H2,2-4H3,(H,29,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Principia Biopharma, Inc.
Curated by ChEMBL
| Assay Description
Irreversible inhibition of human FGFR2 preincubated with enzyme followed by peptide substrate addition by caliper capillary electrophoresis method |
J Med Chem 60: 6516-6527 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00360
BindingDB Entry DOI: 10.7270/Q2C53P3H |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor receptor 1
(Homo sapiens (Human)) | BDBM286984

(8-(3-(4-acryloylpiperazin-1-yl)propyl)-6-(2,6-dich...)Show SMILES CNc1ncc2cc(-c3c(Cl)c(OC)cc(OC)c3Cl)c(=O)n(CCCN3CCN(CC3)C(=O)C=C)c2n1 |(-6.67,-.77,;-6.67,.77,;-5.33,1.54,;-5.33,3.08,;-4,3.85,;-2.67,3.08,;-1.33,3.85,;,3.08,;1.33,3.85,;1.33,5.39,;,6.16,;2.67,6.16,;2.67,7.7,;1.33,8.47,;4,5.39,;4,3.85,;5.33,3.08,;6.67,3.85,;2.67,3.08,;2.67,1.54,;,1.54,;1.33,.77,;-1.33,.77,;-1.33,-.77,;,-1.54,;,-3.08,;1.33,-3.85,;1.33,-5.39,;2.67,-6.16,;4,-5.39,;4,-3.85,;2.67,-3.08,;5.33,-6.16,;6.67,-5.39,;5.33,-7.7,;6.67,-8.47,;-2.67,1.54,;-4,.77,)| Show InChI InChI=1S/C26H30Cl2N6O4/c1-5-20(35)33-11-9-32(10-12-33)7-6-8-34-24-16(15-30-26(29-2)31-24)13-17(25(34)36)21-22(27)18(37-3)14-19(38-4)23(21)28/h5,13-15H,1,6-12H2,2-4H3,(H,29,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Principia Biopharma, Inc.
Curated by ChEMBL
| Assay Description
Irreversible inhibition of human FGFR1 using 5-FAM-KKKKEEIYFFF-NH2 as substrate preincubated with enzyme followed by peptide substrate addition by ca... |
J Med Chem 60: 6516-6527 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00360
BindingDB Entry DOI: 10.7270/Q2C53P3H |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor receptor 3
(Homo sapiens (Human)) | BDBM286984

(8-(3-(4-acryloylpiperazin-1-yl)propyl)-6-(2,6-dich...)Show SMILES CNc1ncc2cc(-c3c(Cl)c(OC)cc(OC)c3Cl)c(=O)n(CCCN3CCN(CC3)C(=O)C=C)c2n1 |(-6.67,-.77,;-6.67,.77,;-5.33,1.54,;-5.33,3.08,;-4,3.85,;-2.67,3.08,;-1.33,3.85,;,3.08,;1.33,3.85,;1.33,5.39,;,6.16,;2.67,6.16,;2.67,7.7,;1.33,8.47,;4,5.39,;4,3.85,;5.33,3.08,;6.67,3.85,;2.67,3.08,;2.67,1.54,;,1.54,;1.33,.77,;-1.33,.77,;-1.33,-.77,;,-1.54,;,-3.08,;1.33,-3.85,;1.33,-5.39,;2.67,-6.16,;4,-5.39,;4,-3.85,;2.67,-3.08,;5.33,-6.16,;6.67,-5.39,;5.33,-7.7,;6.67,-8.47,;-2.67,1.54,;-4,.77,)| Show InChI InChI=1S/C26H30Cl2N6O4/c1-5-20(35)33-11-9-32(10-12-33)7-6-8-34-24-16(15-30-26(29-2)31-24)13-17(25(34)36)21-22(27)18(37-3)14-19(38-4)23(21)28/h5,13-15H,1,6-12H2,2-4H3,(H,29,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Principia Biopharma, Inc.
Curated by ChEMBL
| Assay Description
Irreversible inhibition of human FGFR3 preincubated with enzyme followed by peptide substrate addition by caliper capillary electrophoresis method |
J Med Chem 60: 6516-6527 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00360
BindingDB Entry DOI: 10.7270/Q2C53P3H |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor receptor 4
(Homo sapiens (Human)) | BDBM286984

(8-(3-(4-acryloylpiperazin-1-yl)propyl)-6-(2,6-dich...)Show SMILES CNc1ncc2cc(-c3c(Cl)c(OC)cc(OC)c3Cl)c(=O)n(CCCN3CCN(CC3)C(=O)C=C)c2n1 |(-6.67,-.77,;-6.67,.77,;-5.33,1.54,;-5.33,3.08,;-4,3.85,;-2.67,3.08,;-1.33,3.85,;,3.08,;1.33,3.85,;1.33,5.39,;,6.16,;2.67,6.16,;2.67,7.7,;1.33,8.47,;4,5.39,;4,3.85,;5.33,3.08,;6.67,3.85,;2.67,3.08,;2.67,1.54,;,1.54,;1.33,.77,;-1.33,.77,;-1.33,-.77,;,-1.54,;,-3.08,;1.33,-3.85,;1.33,-5.39,;2.67,-6.16,;4,-5.39,;4,-3.85,;2.67,-3.08,;5.33,-6.16,;6.67,-5.39,;5.33,-7.7,;6.67,-8.47,;-2.67,1.54,;-4,.77,)| Show InChI InChI=1S/C26H30Cl2N6O4/c1-5-20(35)33-11-9-32(10-12-33)7-6-8-34-24-16(15-30-26(29-2)31-24)13-17(25(34)36)21-22(27)18(37-3)14-19(38-4)23(21)28/h5,13-15H,1,6-12H2,2-4H3,(H,29,30,31) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 73 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Principia Biopharma, Inc.
Curated by ChEMBL
| Assay Description
Irreversible inhibition of human FGFR4 preincubated with enzyme followed by peptide substrate addition by caliper capillary electrophoresis method |
J Med Chem 60: 6516-6527 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00360
BindingDB Entry DOI: 10.7270/Q2C53P3H |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Fibroblast growth factor receptor 1
(Homo sapiens (Human)) | BDBM287008
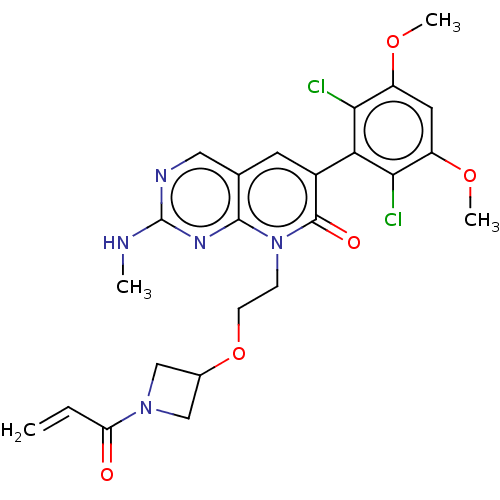
(8-(2-((1-acryloylazetidin-3-yl)oxy)ethyl)-6-(2,6-d...)Show SMILES CNc1ncc2cc(-c3c(Cl)c(OC)cc(OC)c3Cl)c(=O)n(CCOC3CN(C3)C(=O)C=C)c2n1 |(-6.94,-1.38,;-6.94,.16,;-5.61,.93,;-5.61,2.47,;-4.28,3.24,;-2.94,2.47,;-1.61,3.24,;-.28,2.47,;1.06,3.24,;1.06,4.78,;-.28,5.55,;2.39,5.55,;2.39,7.09,;1.06,7.86,;3.72,4.78,;3.72,3.24,;5.06,2.47,;6.39,3.24,;2.39,2.47,;2.39,.93,;-.28,.93,;1.06,.16,;-1.61,.16,;-1.61,-1.38,;-.28,-2.15,;-.28,-3.69,;1.06,-4.46,;1.46,-5.95,;2.94,-5.55,;2.55,-4.06,;4.28,-6.32,;4.28,-7.86,;5.61,-5.55,;6.94,-6.32,;-2.94,.93,;-4.28,.16,)| Show InChI InChI=1S/C24H25Cl2N5O5/c1-5-18(32)30-11-14(12-30)36-7-6-31-22-13(10-28-24(27-2)29-22)8-15(23(31)33)19-20(25)16(34-3)9-17(35-4)21(19)26/h5,8-10,14H,1,6-7,11-12H2,2-4H3,(H,27,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Principia Biopharma, Inc.
Curated by ChEMBL
| Assay Description
In vitro antagonist activity against rat prostatic androgen receptor (AR) |
J Med Chem 60: 6516-6527 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00360
BindingDB Entry DOI: 10.7270/Q2C53P3H |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor receptor 1
(Homo sapiens (Human)) | BDBM286984

(8-(3-(4-acryloylpiperazin-1-yl)propyl)-6-(2,6-dich...)Show SMILES CNc1ncc2cc(-c3c(Cl)c(OC)cc(OC)c3Cl)c(=O)n(CCCN3CCN(CC3)C(=O)C=C)c2n1 |(-6.67,-.77,;-6.67,.77,;-5.33,1.54,;-5.33,3.08,;-4,3.85,;-2.67,3.08,;-1.33,3.85,;,3.08,;1.33,3.85,;1.33,5.39,;,6.16,;2.67,6.16,;2.67,7.7,;1.33,8.47,;4,5.39,;4,3.85,;5.33,3.08,;6.67,3.85,;2.67,3.08,;2.67,1.54,;,1.54,;1.33,.77,;-1.33,.77,;-1.33,-.77,;,-1.54,;,-3.08,;1.33,-3.85,;1.33,-5.39,;2.67,-6.16,;4,-5.39,;4,-3.85,;2.67,-3.08,;5.33,-6.16,;6.67,-5.39,;5.33,-7.7,;6.67,-8.47,;-2.67,1.54,;-4,.77,)| Show InChI InChI=1S/C26H30Cl2N6O4/c1-5-20(35)33-11-9-32(10-12-33)7-6-8-34-24-16(15-30-26(29-2)31-24)13-17(25(34)36)21-22(27)18(37-3)14-19(38-4)23(21)28/h5,13-15H,1,6-12H2,2-4H3,(H,29,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Principia Biopharma, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of FGFR1 in HUVEC assessed as reduction in FGF2-induced ERK phosphorylation preincubated for 60 mins followed by FGF2 stimulation for 10 m... |
J Med Chem 60: 6516-6527 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00360
BindingDB Entry DOI: 10.7270/Q2C53P3H |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor receptor 1
(Homo sapiens (Human)) | BDBM286984

(8-(3-(4-acryloylpiperazin-1-yl)propyl)-6-(2,6-dich...)Show SMILES CNc1ncc2cc(-c3c(Cl)c(OC)cc(OC)c3Cl)c(=O)n(CCCN3CCN(CC3)C(=O)C=C)c2n1 |(-6.67,-.77,;-6.67,.77,;-5.33,1.54,;-5.33,3.08,;-4,3.85,;-2.67,3.08,;-1.33,3.85,;,3.08,;1.33,3.85,;1.33,5.39,;,6.16,;2.67,6.16,;2.67,7.7,;1.33,8.47,;4,5.39,;4,3.85,;5.33,3.08,;6.67,3.85,;2.67,3.08,;2.67,1.54,;,1.54,;1.33,.77,;-1.33,.77,;-1.33,-.77,;,-1.54,;,-3.08,;1.33,-3.85,;1.33,-5.39,;2.67,-6.16,;4,-5.39,;4,-3.85,;2.67,-3.08,;5.33,-6.16,;6.67,-5.39,;5.33,-7.7,;6.67,-8.47,;-2.67,1.54,;-4,.77,)| Show InChI InChI=1S/C26H30Cl2N6O4/c1-5-20(35)33-11-9-32(10-12-33)7-6-8-34-24-16(15-30-26(29-2)31-24)13-17(25(34)36)21-22(27)18(37-3)14-19(38-4)23(21)28/h5,13-15H,1,6-12H2,2-4H3,(H,29,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Principia Biopharma, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human FGFR1 using 5-FAM-KKKKEEIYFFF-NH2 as substrate preincubated for 15 mins followed by peptide substrate addition measured after 3 h... |
J Med Chem 60: 6516-6527 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00360
BindingDB Entry DOI: 10.7270/Q2C53P3H |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor receptor 1
(Homo sapiens (Human)) | BDBM286381
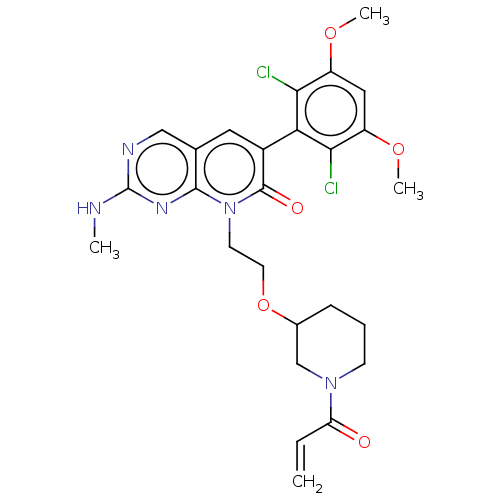
(8-(2-((1-acryloylpiperidin-3-yl)oxy)ethyl)-6-(2,6-...)Show SMILES CNc1ncc2cc(-c3c(Cl)c(OC)cc(OC)c3Cl)c(=O)n(CCOC3CCCN(C3)C(=O)C=C)c2n1 |(-6.67,,;-6.67,1.54,;-5.33,2.31,;-5.33,3.85,;-4,4.62,;-2.67,3.85,;-1.33,4.62,;,3.85,;1.33,4.62,;1.33,6.16,;,6.93,;2.67,6.93,;2.67,8.47,;4,9.24,;4,6.16,;4,4.62,;5.33,3.85,;6.67,4.62,;2.67,3.85,;2.67,2.31,;,2.31,;1.33,1.54,;-1.33,1.54,;-1.33,,;,-.77,;,-2.31,;1.33,-3.08,;2.67,-2.31,;4,-3.08,;4,-4.62,;2.67,-5.39,;1.33,-4.62,;2.67,-6.93,;1.33,-7.7,;4,-7.7,;4,-9.24,;-2.67,2.31,;-4,1.54,)| Show InChI InChI=1S/C26H29Cl2N5O5/c1-5-20(34)32-8-6-7-16(14-32)38-10-9-33-24-15(13-30-26(29-2)31-24)11-17(25(33)35)21-22(27)18(36-3)12-19(37-4)23(21)28/h5,11-13,16H,1,6-10,14H2,2-4H3,(H,29,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Principia Biopharma, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of FGFR1 in HUVEC assessed as reduction in FGF2-induced ERK phosphorylation preincubated for 60 mins followed by FGF2 stimulation for 10 m... |
J Med Chem 60: 6516-6527 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00360
BindingDB Entry DOI: 10.7270/Q2C53P3H |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor receptor 1
(Homo sapiens (Human)) | BDBM50238813
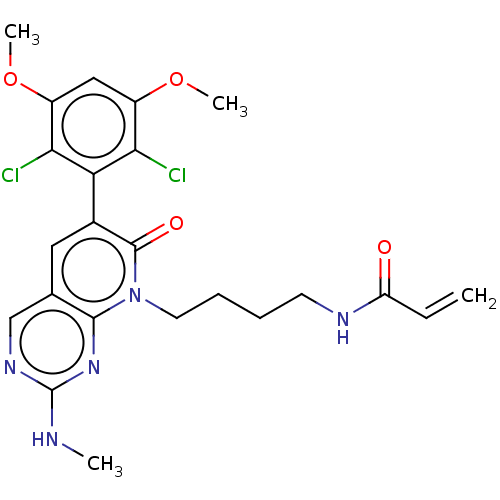
(CHEMBL4083740)Show SMILES CNc1ncc2cc(-c3c(Cl)c(OC)cc(OC)c3Cl)c(=O)n(CCCCNC(=O)C=C)c2n1 |(28.89,-34.94,;28.89,-33.4,;30.23,-32.63,;30.23,-31.09,;31.56,-30.32,;32.89,-31.08,;34.23,-30.3,;35.57,-31.08,;36.9,-30.32,;36.9,-28.79,;35.56,-28.02,;38.23,-28.02,;38.23,-26.48,;39.56,-25.71,;39.57,-28.79,;39.56,-30.33,;40.9,-31.11,;42.23,-30.34,;38.23,-31.1,;38.22,-32.64,;35.56,-32.63,;36.89,-33.4,;34.22,-33.4,;34.22,-34.94,;35.55,-35.71,;35.54,-37.25,;36.88,-38.03,;36.87,-39.57,;38.2,-40.34,;39.54,-39.57,;38.2,-41.88,;39.53,-42.65,;32.89,-32.63,;31.56,-33.4,)| Show InChI InChI=1S/C23H25Cl2N5O4/c1-5-17(31)27-8-6-7-9-30-21-13(12-28-23(26-2)29-21)10-14(22(30)32)18-19(24)15(33-3)11-16(34-4)20(18)25/h5,10-12H,1,6-9H2,2-4H3,(H,27,31)(H,26,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Principia Biopharma, Inc.
Curated by ChEMBL
| Assay Description
In vitro antagonist activity against rat prostatic androgen receptor (AR) |
J Med Chem 60: 6516-6527 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00360
BindingDB Entry DOI: 10.7270/Q2C53P3H |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor receptor 1
(Homo sapiens (Human)) | BDBM50238811
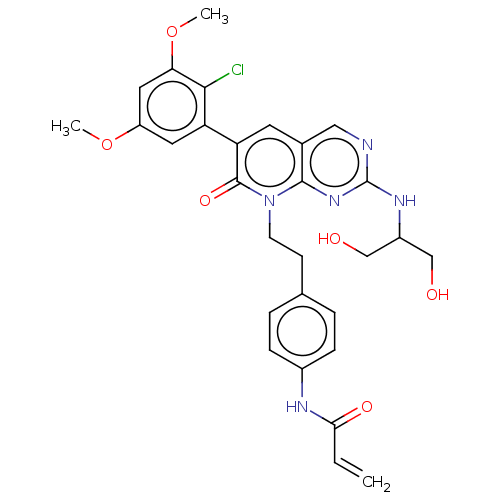
(CHEMBL4073347)Show SMILES COc1cc(OC)c(Cl)c(c1)-c1cc2cnc(NC(CO)CO)nc2n(CCc2ccc(NC(=O)C=C)cc2)c1=O Show InChI InChI=1S/C29H30ClN5O6/c1-4-25(38)32-19-7-5-17(6-8-19)9-10-35-27-18(14-31-29(34-27)33-20(15-36)16-37)11-23(28(35)39)22-12-21(40-2)13-24(41-3)26(22)30/h4-8,11-14,20,36-37H,1,9-10,15-16H2,2-3H3,(H,32,38)(H,31,33,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Principia Biopharma, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of FGFR1 in HUVEC assessed as reduction in FGF2-induced ERK phosphorylation preincubated for 60 mins followed by FGF2 stimulation for 10 m... |
J Med Chem 60: 6516-6527 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00360
BindingDB Entry DOI: 10.7270/Q2C53P3H |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor receptor 1
(Homo sapiens (Human)) | BDBM50238823
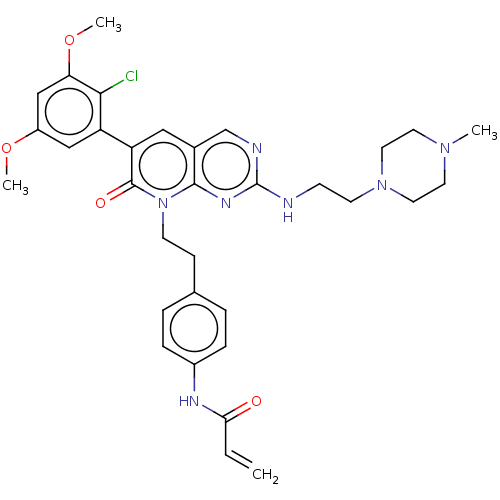
(CHEMBL4086567)Show SMILES COc1cc(OC)c(Cl)c(c1)-c1cc2cnc(NCCN3CCN(C)CC3)nc2n(CCc2ccc(NC(=O)C=C)cc2)c1=O Show InChI InChI=1S/C33H38ClN7O4/c1-5-29(42)37-24-8-6-22(7-9-24)10-12-41-31-23(21-36-33(38-31)35-11-13-40-16-14-39(2)15-17-40)18-27(32(41)43)26-19-25(44-3)20-28(45-4)30(26)34/h5-9,18-21H,1,10-17H2,2-4H3,(H,37,42)(H,35,36,38) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Principia Biopharma, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human FGFR1 using 5-FAM-KKKKEEIYFFF-NH2 as substrate preincubated for 15 mins followed by peptide substrate addition measured after 3 h... |
J Med Chem 60: 6516-6527 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00360
BindingDB Entry DOI: 10.7270/Q2C53P3H |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor receptor 1
(Homo sapiens (Human)) | BDBM287067

(8-(2-((1-acryloylazetidin-3-yl)(methyl)amino)ethyl...)Show SMILES CNc1ncc2cc(-c3c(Cl)c(OC)cc(OC)c3Cl)c(=O)n(CCN(C)C3CN(C3)C(=O)C=C)c2n1 |(-6.94,-1.38,;-6.94,.16,;-5.61,.93,;-5.61,2.47,;-4.28,3.24,;-2.94,2.47,;-1.61,3.24,;-.28,2.47,;1.06,3.24,;1.06,4.78,;-.28,5.55,;2.39,5.55,;2.39,7.09,;1.06,7.86,;3.72,4.78,;3.72,3.24,;5.06,2.47,;6.39,3.24,;2.39,2.47,;2.39,.93,;-.28,.93,;1.06,.16,;-1.61,.16,;-1.61,-1.38,;-.28,-2.15,;-.28,-3.69,;-1.61,-4.46,;1.06,-4.46,;1.46,-5.95,;2.94,-5.55,;2.55,-4.06,;4.28,-6.32,;4.28,-7.86,;5.61,-5.55,;6.94,-6.32,;-2.94,.93,;-4.28,.16,)| Show InChI InChI=1S/C25H28Cl2N6O4/c1-6-19(34)32-12-15(13-32)31(3)7-8-33-23-14(11-29-25(28-2)30-23)9-16(24(33)35)20-21(26)17(36-4)10-18(37-5)22(20)27/h6,9-11,15H,1,7-8,12-13H2,2-5H3,(H,28,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Principia Biopharma, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of FGFR1 in HUVEC assessed as reduction in FGF2-induced ERK phosphorylation preincubated for 60 mins followed by FGF2 stimulation for 10 m... |
J Med Chem 60: 6516-6527 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00360
BindingDB Entry DOI: 10.7270/Q2C53P3H |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor receptor 1
(Homo sapiens (Human)) | BDBM50238809
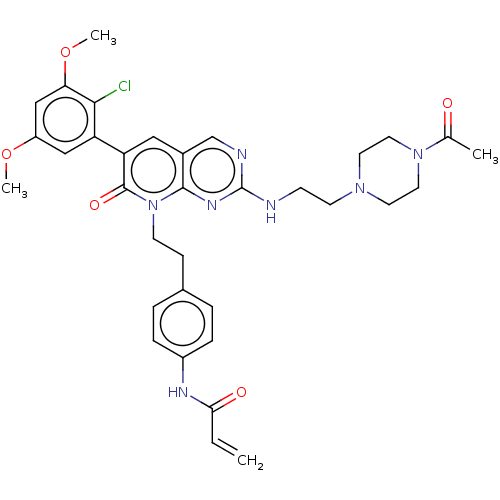
(CHEMBL4084708)Show SMILES COc1cc(OC)c(Cl)c(c1)-c1cc2cnc(NCCN3CCN(CC3)C(C)=O)nc2n(CCc2ccc(NC(=O)C=C)cc2)c1=O Show InChI InChI=1S/C34H38ClN7O5/c1-5-30(44)38-25-8-6-23(7-9-25)10-12-42-32-24(18-28(33(42)45)27-19-26(46-3)20-29(47-4)31(27)35)21-37-34(39-32)36-11-13-40-14-16-41(17-15-40)22(2)43/h5-9,18-21H,1,10-17H2,2-4H3,(H,38,44)(H,36,37,39) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Principia Biopharma, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human FGFR1 using 5-FAM-KKKKEEIYFFF-NH2 as substrate preincubated for 15 mins followed by peptide substrate addition measured after 3 h... |
J Med Chem 60: 6516-6527 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00360
BindingDB Entry DOI: 10.7270/Q2C53P3H |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor receptor 1
(Homo sapiens (Human)) | BDBM287008

(8-(2-((1-acryloylazetidin-3-yl)oxy)ethyl)-6-(2,6-d...)Show SMILES CNc1ncc2cc(-c3c(Cl)c(OC)cc(OC)c3Cl)c(=O)n(CCOC3CN(C3)C(=O)C=C)c2n1 |(-6.94,-1.38,;-6.94,.16,;-5.61,.93,;-5.61,2.47,;-4.28,3.24,;-2.94,2.47,;-1.61,3.24,;-.28,2.47,;1.06,3.24,;1.06,4.78,;-.28,5.55,;2.39,5.55,;2.39,7.09,;1.06,7.86,;3.72,4.78,;3.72,3.24,;5.06,2.47,;6.39,3.24,;2.39,2.47,;2.39,.93,;-.28,.93,;1.06,.16,;-1.61,.16,;-1.61,-1.38,;-.28,-2.15,;-.28,-3.69,;1.06,-4.46,;1.46,-5.95,;2.94,-5.55,;2.55,-4.06,;4.28,-6.32,;4.28,-7.86,;5.61,-5.55,;6.94,-6.32,;-2.94,.93,;-4.28,.16,)| Show InChI InChI=1S/C24H25Cl2N5O5/c1-5-18(32)30-11-14(12-30)36-7-6-31-22-13(10-28-24(27-2)29-22)8-15(23(31)33)19-20(25)16(34-3)9-17(35-4)21(19)26/h5,8-10,14H,1,6-7,11-12H2,2-4H3,(H,27,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Principia Biopharma, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of FGFR1 in HUVEC assessed as reduction in FGF2-induced ERK phosphorylation preincubated for 60 mins followed by FGF2 stimulation for 10 m... |
J Med Chem 60: 6516-6527 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00360
BindingDB Entry DOI: 10.7270/Q2C53P3H |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor receptor 1
(Homo sapiens (Human)) | BDBM287067

(8-(2-((1-acryloylazetidin-3-yl)(methyl)amino)ethyl...)Show SMILES CNc1ncc2cc(-c3c(Cl)c(OC)cc(OC)c3Cl)c(=O)n(CCN(C)C3CN(C3)C(=O)C=C)c2n1 |(-6.94,-1.38,;-6.94,.16,;-5.61,.93,;-5.61,2.47,;-4.28,3.24,;-2.94,2.47,;-1.61,3.24,;-.28,2.47,;1.06,3.24,;1.06,4.78,;-.28,5.55,;2.39,5.55,;2.39,7.09,;1.06,7.86,;3.72,4.78,;3.72,3.24,;5.06,2.47,;6.39,3.24,;2.39,2.47,;2.39,.93,;-.28,.93,;1.06,.16,;-1.61,.16,;-1.61,-1.38,;-.28,-2.15,;-.28,-3.69,;-1.61,-4.46,;1.06,-4.46,;1.46,-5.95,;2.94,-5.55,;2.55,-4.06,;4.28,-6.32,;4.28,-7.86,;5.61,-5.55,;6.94,-6.32,;-2.94,.93,;-4.28,.16,)| Show InChI InChI=1S/C25H28Cl2N6O4/c1-6-19(34)32-12-15(13-32)31(3)7-8-33-23-14(11-29-25(28-2)30-23)9-16(24(33)35)20-21(26)17(36-4)10-18(37-5)22(20)27/h6,9-11,15H,1,7-8,12-13H2,2-5H3,(H,28,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Principia Biopharma, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of FGFR1 in HUVEC assessed as reduction in FGF2-induced ERK phosphorylation preincubated for 60 mins followed by FGF2 stimulation for 10 m... |
J Med Chem 60: 6516-6527 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00360
BindingDB Entry DOI: 10.7270/Q2C53P3H |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor receptor 1
(Homo sapiens (Human)) | BDBM50238806

(CHEMBL4083151)Show SMILES CNc1ncc2cc(-c3cc(OC)cc(OC)c3Cl)c(=O)n(CCc3ccc(NC(=O)C=C)cn3)c2n1 Show InChI InChI=1S/C26H25ClN6O4/c1-5-22(34)31-17-7-6-16(29-14-17)8-9-33-24-15(13-30-26(28-2)32-24)10-20(25(33)35)19-11-18(36-3)12-21(37-4)23(19)27/h5-7,10-14H,1,8-9H2,2-4H3,(H,31,34)(H,28,30,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Principia Biopharma, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human FGFR1 using 5-FAM-KKKKEEIYFFF-NH2 as substrate preincubated for 15 mins followed by peptide substrate addition measured after 3 h... |
J Med Chem 60: 6516-6527 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00360
BindingDB Entry DOI: 10.7270/Q2C53P3H |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor receptor 1
(Homo sapiens (Human)) | BDBM50238804

(CHEMBL4099578)Show SMILES CNc1ncc2cc(-c3cc(OC)cc(OC)c3Cl)c(=O)n(CCc3ccc(NC(=O)C=C)cc3)c2n1 Show InChI InChI=1S/C27H26ClN5O4/c1-5-23(34)31-18-8-6-16(7-9-18)10-11-33-25-17(15-30-27(29-2)32-25)12-21(26(33)35)20-13-19(36-3)14-22(37-4)24(20)28/h5-9,12-15H,1,10-11H2,2-4H3,(H,31,34)(H,29,30,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Principia Biopharma, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of FGFR1 in HUVEC assessed as reduction in FGF2-induced ERK phosphorylation preincubated for 60 mins followed by FGF2 stimulation for 10 m... |
J Med Chem 60: 6516-6527 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00360
BindingDB Entry DOI: 10.7270/Q2C53P3H |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor receptor 2
(Homo sapiens (Human)) | BDBM286984

(8-(3-(4-acryloylpiperazin-1-yl)propyl)-6-(2,6-dich...)Show SMILES CNc1ncc2cc(-c3c(Cl)c(OC)cc(OC)c3Cl)c(=O)n(CCCN3CCN(CC3)C(=O)C=C)c2n1 |(-6.67,-.77,;-6.67,.77,;-5.33,1.54,;-5.33,3.08,;-4,3.85,;-2.67,3.08,;-1.33,3.85,;,3.08,;1.33,3.85,;1.33,5.39,;,6.16,;2.67,6.16,;2.67,7.7,;1.33,8.47,;4,5.39,;4,3.85,;5.33,3.08,;6.67,3.85,;2.67,3.08,;2.67,1.54,;,1.54,;1.33,.77,;-1.33,.77,;-1.33,-.77,;,-1.54,;,-3.08,;1.33,-3.85,;1.33,-5.39,;2.67,-6.16,;4,-5.39,;4,-3.85,;2.67,-3.08,;5.33,-6.16,;6.67,-5.39,;5.33,-7.7,;6.67,-8.47,;-2.67,1.54,;-4,.77,)| Show InChI InChI=1S/C26H30Cl2N6O4/c1-5-20(35)33-11-9-32(10-12-33)7-6-8-34-24-16(15-30-26(29-2)31-24)13-17(25(34)36)21-22(27)18(37-3)14-19(38-4)23(21)28/h5,13-15H,1,6-12H2,2-4H3,(H,29,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Principia Biopharma, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human FGFR2 preincubated for 15 mins followed by peptide substrate addition measured after 3 hrs by caliper capillary electrophoresis m... |
J Med Chem 60: 6516-6527 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00360
BindingDB Entry DOI: 10.7270/Q2C53P3H |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor receptor 1
(Homo sapiens (Human)) | BDBM50238805

(CHEMBL4061286)Show SMILES CNc1ncc2cc(-c3cc(OC)cc(OC)c3Cl)c(=O)n(CCc3ccc(NC(=O)C=C)nc3)c2n1 Show InChI InChI=1S/C26H25ClN6O4/c1-5-22(34)31-21-7-6-15(13-29-21)8-9-33-24-16(14-30-26(28-2)32-24)10-19(25(33)35)18-11-17(36-3)12-20(37-4)23(18)27/h5-7,10-14H,1,8-9H2,2-4H3,(H,28,30,32)(H,29,31,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Principia Biopharma, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of FGFR1 in HUVEC assessed as reduction in FGF2-induced ERK phosphorylation preincubated for 60 mins followed by FGF2 stimulation for 10 m... |
J Med Chem 60: 6516-6527 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00360
BindingDB Entry DOI: 10.7270/Q2C53P3H |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor receptor 1
(Homo sapiens (Human)) | BDBM50238805

(CHEMBL4061286)Show SMILES CNc1ncc2cc(-c3cc(OC)cc(OC)c3Cl)c(=O)n(CCc3ccc(NC(=O)C=C)nc3)c2n1 Show InChI InChI=1S/C26H25ClN6O4/c1-5-22(34)31-21-7-6-15(13-29-21)8-9-33-24-16(14-30-26(28-2)32-24)10-19(25(33)35)18-11-17(36-3)12-20(37-4)23(18)27/h5-7,10-14H,1,8-9H2,2-4H3,(H,28,30,32)(H,29,31,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Principia Biopharma, Inc.
Curated by ChEMBL
| Assay Description
In vitro antagonist activity against rat prostatic androgen receptor (AR) |
J Med Chem 60: 6516-6527 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00360
BindingDB Entry DOI: 10.7270/Q2C53P3H |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor receptor 1
(Homo sapiens (Human)) | BDBM50238818
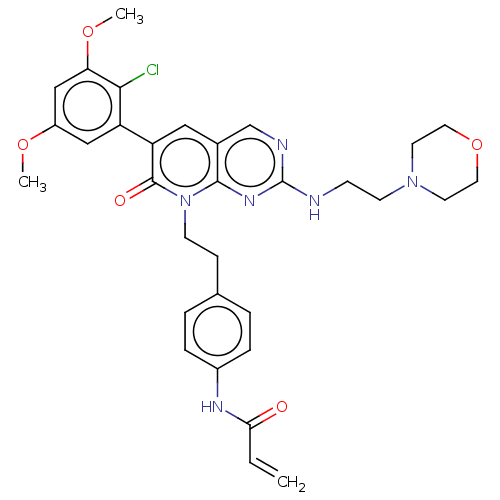
(CHEMBL4064081)Show SMILES COc1cc(OC)c(Cl)c(c1)-c1cc2cnc(NCCN3CCOCC3)nc2n(CCc2ccc(NC(=O)C=C)cc2)c1=O Show InChI InChI=1S/C32H35ClN6O5/c1-4-28(40)36-23-7-5-21(6-8-23)9-11-39-30-22(20-35-32(37-30)34-10-12-38-13-15-44-16-14-38)17-26(31(39)41)25-18-24(42-2)19-27(43-3)29(25)33/h4-8,17-20H,1,9-16H2,2-3H3,(H,36,40)(H,34,35,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Principia Biopharma, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of FGFR1 in HUVEC assessed as reduction in FGF2-induced ERK phosphorylation preincubated for 60 mins followed by FGF2 stimulation for 10 m... |
J Med Chem 60: 6516-6527 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00360
BindingDB Entry DOI: 10.7270/Q2C53P3H |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor receptor 1
(Homo sapiens (Human)) | BDBM50238818

(CHEMBL4064081)Show SMILES COc1cc(OC)c(Cl)c(c1)-c1cc2cnc(NCCN3CCOCC3)nc2n(CCc2ccc(NC(=O)C=C)cc2)c1=O Show InChI InChI=1S/C32H35ClN6O5/c1-4-28(40)36-23-7-5-21(6-8-23)9-11-39-30-22(20-35-32(37-30)34-10-12-38-13-15-44-16-14-38)17-26(31(39)41)25-18-24(42-2)19-27(43-3)29(25)33/h4-8,17-20H,1,9-16H2,2-3H3,(H,36,40)(H,34,35,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Principia Biopharma, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human FGFR1 using 5-FAM-KKKKEEIYFFF-NH2 as substrate preincubated for 15 mins followed by peptide substrate addition measured after 3 h... |
J Med Chem 60: 6516-6527 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00360
BindingDB Entry DOI: 10.7270/Q2C53P3H |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor receptor 1
(Homo sapiens (Human)) | BDBM50238824

(CHEMBL4064756)Show SMILES COc1cc(OC)c(Cl)c(c1)-c1cc2cnc(NCCCN3CCOCC3)nc2n(CCc2ccc(NC(=O)C=C)cc2)c1=O Show InChI InChI=1S/C33H37ClN6O5/c1-4-29(41)37-24-8-6-22(7-9-24)10-13-40-31-23(21-36-33(38-31)35-11-5-12-39-14-16-45-17-15-39)18-27(32(40)42)26-19-25(43-2)20-28(44-3)30(26)34/h4,6-9,18-21H,1,5,10-17H2,2-3H3,(H,37,41)(H,35,36,38) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Principia Biopharma, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human FGFR1 using 5-FAM-KKKKEEIYFFF-NH2 as substrate preincubated for 15 mins followed by peptide substrate addition measured after 3 h... |
J Med Chem 60: 6516-6527 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00360
BindingDB Entry DOI: 10.7270/Q2C53P3H |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor receptor 1
(Homo sapiens (Human)) | BDBM50238803

(CHEMBL4066763)Show SMILES CNc1ncc2cc(-c3cc(OC)cc(OC)c3Cl)c(=O)n(CCc3cccc(NC(=O)C=C)n3)c2n1 Show InChI InChI=1S/C26H25ClN6O4/c1-5-22(34)31-21-8-6-7-16(30-21)9-10-33-24-15(14-29-26(28-2)32-24)11-19(25(33)35)18-12-17(36-3)13-20(37-4)23(18)27/h5-8,11-14H,1,9-10H2,2-4H3,(H,28,29,32)(H,30,31,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Principia Biopharma, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of FGFR1 in HUVEC assessed as reduction in FGF2-induced ERK phosphorylation preincubated for 60 mins followed by FGF2 stimulation for 10 m... |
J Med Chem 60: 6516-6527 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00360
BindingDB Entry DOI: 10.7270/Q2C53P3H |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor receptor 1
(Homo sapiens (Human)) | BDBM50238803

(CHEMBL4066763)Show SMILES CNc1ncc2cc(-c3cc(OC)cc(OC)c3Cl)c(=O)n(CCc3cccc(NC(=O)C=C)n3)c2n1 Show InChI InChI=1S/C26H25ClN6O4/c1-5-22(34)31-21-8-6-7-16(30-21)9-10-33-24-15(14-29-26(28-2)32-24)11-19(25(33)35)18-12-17(36-3)13-20(37-4)23(18)27/h5-8,11-14H,1,9-10H2,2-4H3,(H,28,29,32)(H,30,31,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Principia Biopharma, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of FGFR1 in HUVEC assessed as reduction in FGF2-induced ERK phosphorylation preincubated for 60 mins followed by FGF2 stimulation for 10 m... |
J Med Chem 60: 6516-6527 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00360
BindingDB Entry DOI: 10.7270/Q2C53P3H |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor receptor 1
(Homo sapiens (Human)) | BDBM286523

(8-(2-((1-acryloylpyrrolidin-3-yl)oxy)ethyl)-6-(2,6...)Show SMILES CNc1ncc2cc(-c3c(Cl)c(OC)cc(OC)c3Cl)c(=O)n(CCOC3CCN(C3)C(=O)C=C)c2n1 |(-6.67,-.74,;-6.67,.8,;-5.33,1.57,;-5.33,3.11,;-4,3.88,;-2.67,3.11,;-1.33,3.88,;,3.11,;1.33,3.88,;1.33,5.42,;,6.19,;2.67,6.19,;2.67,7.73,;1.33,8.5,;4,5.42,;4,3.88,;5.33,3.11,;6.67,3.88,;2.67,3.11,;2.67,1.57,;,1.57,;1.33,.8,;-1.33,.8,;-1.33,-.74,;,-1.51,;,-3.05,;1.33,-3.82,;2.8,-3.34,;3.7,-4.59,;2.8,-5.83,;1.33,-5.36,;3.57,-7.17,;2.8,-8.5,;5.11,-7.17,;5.88,-8.5,;-2.67,1.57,;-4,.8,)| Show InChI InChI=1S/C25H27Cl2N5O5/c1-5-19(33)31-7-6-15(13-31)37-9-8-32-23-14(12-29-25(28-2)30-23)10-16(24(32)34)20-21(26)17(35-3)11-18(36-4)22(20)27/h5,10-12,15H,1,6-9,13H2,2-4H3,(H,28,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Principia Biopharma, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of FGFR1 in HUVEC assessed as reduction in FGF2-induced ERK phosphorylation preincubated for 60 mins followed by FGF2 stimulation for 10 m... |
J Med Chem 60: 6516-6527 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00360
BindingDB Entry DOI: 10.7270/Q2C53P3H |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor receptor 1
(Homo sapiens (Human)) | BDBM50238821
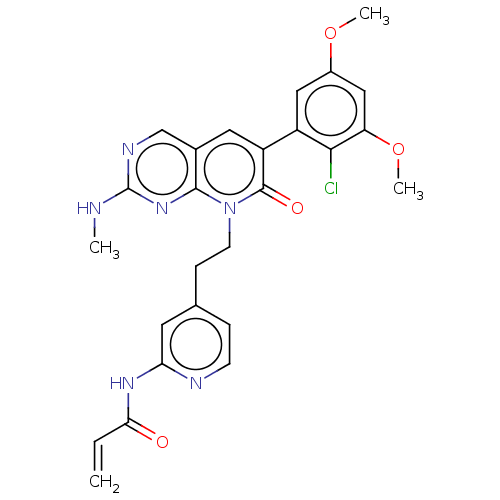
(CHEMBL4088057)Show SMILES CNc1ncc2cc(-c3cc(OC)cc(OC)c3Cl)c(=O)n(CCc3ccnc(NC(=O)C=C)c3)c2n1 Show InChI InChI=1S/C26H25ClN6O4/c1-5-22(34)31-21-10-15(6-8-29-21)7-9-33-24-16(14-30-26(28-2)32-24)11-19(25(33)35)18-12-17(36-3)13-20(37-4)23(18)27/h5-6,8,10-14H,1,7,9H2,2-4H3,(H,28,30,32)(H,29,31,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Principia Biopharma, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human FGFR1 using 5-FAM-KKKKEEIYFFF-NH2 as substrate preincubated for 15 mins followed by peptide substrate addition measured after 3 h... |
J Med Chem 60: 6516-6527 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00360
BindingDB Entry DOI: 10.7270/Q2C53P3H |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor receptor 1
(Homo sapiens (Human)) | BDBM50238816

(CHEMBL4084079)Show SMILES CNc1ncc2cc(-c3cc(OC)cc(OC)c3Cl)c(=O)n(CCc3cnc(NC(=O)C=C)cn3)c2n1 Show InChI InChI=1S/C25H24ClN7O4/c1-5-21(34)31-20-13-28-15(12-29-20)6-7-33-23-14(11-30-25(27-2)32-23)8-18(24(33)35)17-9-16(36-3)10-19(37-4)22(17)26/h5,8-13H,1,6-7H2,2-4H3,(H,27,30,32)(H,29,31,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Principia Biopharma, Inc.
Curated by ChEMBL
| Assay Description
In vitro antagonist activity against rat prostatic androgen receptor (AR) |
J Med Chem 60: 6516-6527 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00360
BindingDB Entry DOI: 10.7270/Q2C53P3H |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor receptor 1
(Homo sapiens (Human)) | BDBM50238821

(CHEMBL4088057)Show SMILES CNc1ncc2cc(-c3cc(OC)cc(OC)c3Cl)c(=O)n(CCc3ccnc(NC(=O)C=C)c3)c2n1 Show InChI InChI=1S/C26H25ClN6O4/c1-5-22(34)31-21-10-15(6-8-29-21)7-9-33-24-16(14-30-26(28-2)32-24)11-19(25(33)35)18-12-17(36-3)13-20(37-4)23(18)27/h5-6,8,10-14H,1,7,9H2,2-4H3,(H,28,30,32)(H,29,31,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Principia Biopharma, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of FGFR1 in HUVEC assessed as reduction in FGF2-induced ERK phosphorylation preincubated for 60 mins followed by FGF2 stimulation for 10 m... |
J Med Chem 60: 6516-6527 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00360
BindingDB Entry DOI: 10.7270/Q2C53P3H |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor receptor 1
(Homo sapiens (Human)) | BDBM286388

(8-(2-(4-acryloylpiperazin-1-yl)ethyl)-6-(2,6-dichl...)Show SMILES CNc1ncc2cc(-c3c(Cl)c(OC)cc(OC)c3Cl)c(=O)n(CCN3CCN(CC3)C(=O)C=C)c2n1 |(-6.67,1.54,;-5.33,.77,;-4,1.54,;-4,3.08,;-2.67,3.85,;-1.33,3.08,;,3.85,;1.33,3.08,;2.67,3.85,;2.67,5.39,;1.33,6.16,;4,6.16,;4,7.7,;2.67,8.47,;5.33,5.39,;5.33,3.85,;6.67,3.08,;6.67,1.54,;4,3.08,;4,1.54,;1.33,1.54,;2.67,.77,;,.77,;,-.77,;1.33,-1.54,;1.33,-3.08,;,-3.85,;,-5.39,;1.33,-6.16,;2.67,-5.39,;2.67,-3.85,;1.33,-7.7,;,-8.47,;2.67,-8.47,;4,-7.7,;-1.33,1.54,;-2.67,.77,)| Show InChI InChI=1S/C25H28Cl2N6O4/c1-5-19(34)32-9-6-31(7-10-32)8-11-33-23-15(14-29-25(28-2)30-23)12-16(24(33)35)20-21(26)17(36-3)13-18(37-4)22(20)27/h5,12-14H,1,6-11H2,2-4H3,(H,28,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Principia Biopharma, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of FGFR1 in HUVEC assessed as reduction in FGF2-induced ERK phosphorylation preincubated for 60 mins followed by FGF2 stimulation for 10 m... |
J Med Chem 60: 6516-6527 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00360
BindingDB Entry DOI: 10.7270/Q2C53P3H |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor receptor 1
(Homo sapiens (Human)) | BDBM286388

(8-(2-(4-acryloylpiperazin-1-yl)ethyl)-6-(2,6-dichl...)Show SMILES CNc1ncc2cc(-c3c(Cl)c(OC)cc(OC)c3Cl)c(=O)n(CCN3CCN(CC3)C(=O)C=C)c2n1 |(-6.67,1.54,;-5.33,.77,;-4,1.54,;-4,3.08,;-2.67,3.85,;-1.33,3.08,;,3.85,;1.33,3.08,;2.67,3.85,;2.67,5.39,;1.33,6.16,;4,6.16,;4,7.7,;2.67,8.47,;5.33,5.39,;5.33,3.85,;6.67,3.08,;6.67,1.54,;4,3.08,;4,1.54,;1.33,1.54,;2.67,.77,;,.77,;,-.77,;1.33,-1.54,;1.33,-3.08,;,-3.85,;,-5.39,;1.33,-6.16,;2.67,-5.39,;2.67,-3.85,;1.33,-7.7,;,-8.47,;2.67,-8.47,;4,-7.7,;-1.33,1.54,;-2.67,.77,)| Show InChI InChI=1S/C25H28Cl2N6O4/c1-5-19(34)32-9-6-31(7-10-32)8-11-33-23-15(14-29-25(28-2)30-23)12-16(24(33)35)20-21(26)17(36-3)13-18(37-4)22(20)27/h5,12-14H,1,6-11H2,2-4H3,(H,28,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Principia Biopharma, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human FGFR1 using 5-FAM-KKKKEEIYFFF-NH2 as substrate preincubated for 15 mins followed by peptide substrate addition measured after 3 h... |
J Med Chem 60: 6516-6527 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00360
BindingDB Entry DOI: 10.7270/Q2C53P3H |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor receptor 1
(Homo sapiens (Human)) | BDBM50238810

(CHEMBL4092456)Show SMILES COc1cc(OC)c(Cl)c(c1)-c1cc2cnc(NCCO)nc2n(CCc2ccc(NC(=O)C=C)cc2)c1=O Show InChI InChI=1S/C28H28ClN5O5/c1-4-24(36)32-19-7-5-17(6-8-19)9-11-34-26-18(16-31-28(33-26)30-10-12-35)13-22(27(34)37)21-14-20(38-2)15-23(39-3)25(21)29/h4-8,13-16,35H,1,9-12H2,2-3H3,(H,32,36)(H,30,31,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Principia Biopharma, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of FGFR1 in HUVEC assessed as reduction in FGF2-induced ERK phosphorylation preincubated for 60 mins followed by FGF2 stimulation for 10 m... |
J Med Chem 60: 6516-6527 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00360
BindingDB Entry DOI: 10.7270/Q2C53P3H |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor receptor 1
(Homo sapiens (Human)) | BDBM50238810

(CHEMBL4092456)Show SMILES COc1cc(OC)c(Cl)c(c1)-c1cc2cnc(NCCO)nc2n(CCc2ccc(NC(=O)C=C)cc2)c1=O Show InChI InChI=1S/C28H28ClN5O5/c1-4-24(36)32-19-7-5-17(6-8-19)9-11-34-26-18(16-31-28(33-26)30-10-12-35)13-22(27(34)37)21-14-20(38-2)15-23(39-3)25(21)29/h4-8,13-16,35H,1,9-12H2,2-3H3,(H,32,36)(H,30,31,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Principia Biopharma, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of FGFR1 in HUVEC assessed as reduction in FGF2-induced ERK phosphorylation preincubated for 60 mins followed by FGF2 stimulation for 10 m... |
J Med Chem 60: 6516-6527 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00360
BindingDB Entry DOI: 10.7270/Q2C53P3H |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor receptor 1
(Homo sapiens (Human)) | BDBM50238802

(CHEMBL4095783)Show SMILES CNc1ncc2cc(-c3cc(OC)cc(OC)c3Cl)c(=O)n(CCc3cccc(NC(=O)C=C)c3)c2n1 Show InChI InChI=1S/C27H26ClN5O4/c1-5-23(34)31-18-8-6-7-16(11-18)9-10-33-25-17(15-30-27(29-2)32-25)12-21(26(33)35)20-13-19(36-3)14-22(37-4)24(20)28/h5-8,11-15H,1,9-10H2,2-4H3,(H,31,34)(H,29,30,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Principia Biopharma, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of FGFR1 in HUVEC assessed as reduction in FGF2-induced ERK phosphorylation preincubated for 60 mins followed by FGF2 stimulation for 10 m... |
J Med Chem 60: 6516-6527 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00360
BindingDB Entry DOI: 10.7270/Q2C53P3H |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor receptor 1
(Homo sapiens (Human)) | BDBM50238804

(CHEMBL4099578)Show SMILES CNc1ncc2cc(-c3cc(OC)cc(OC)c3Cl)c(=O)n(CCc3ccc(NC(=O)C=C)cc3)c2n1 Show InChI InChI=1S/C27H26ClN5O4/c1-5-23(34)31-18-8-6-16(7-9-18)10-11-33-25-17(15-30-27(29-2)32-25)12-21(26(33)35)20-13-19(36-3)14-22(37-4)24(20)28/h5-9,12-15H,1,10-11H2,2-4H3,(H,31,34)(H,29,30,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Principia Biopharma, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of FGFR1 in HUVEC assessed as reduction in FGF2-induced ERK phosphorylation preincubated for 60 mins followed by FGF2 stimulation for 10 m... |
J Med Chem 60: 6516-6527 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00360
BindingDB Entry DOI: 10.7270/Q2C53P3H |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor receptor 1
(Homo sapiens (Human)) | BDBM50238812
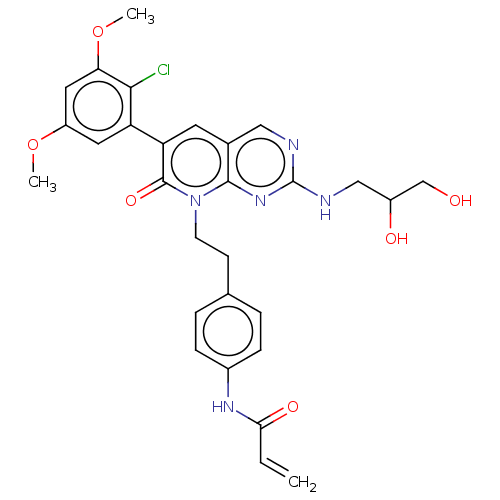
(CHEMBL4100201)Show SMILES COc1cc(OC)c(Cl)c(c1)-c1cc2cnc(NCC(O)CO)nc2n(CCc2ccc(NC(=O)C=C)cc2)c1=O Show InChI InChI=1S/C29H30ClN5O6/c1-4-25(38)33-19-7-5-17(6-8-19)9-10-35-27-18(14-31-29(34-27)32-15-20(37)16-36)11-23(28(35)39)22-12-21(40-2)13-24(41-3)26(22)30/h4-8,11-14,20,36-37H,1,9-10,15-16H2,2-3H3,(H,33,38)(H,31,32,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Principia Biopharma, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of FGFR1 in HUVEC assessed as reduction in FGF2-induced ERK phosphorylation preincubated for 60 mins followed by FGF2 stimulation for 10 m... |
J Med Chem 60: 6516-6527 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00360
BindingDB Entry DOI: 10.7270/Q2C53P3H |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor receptor 1
(Homo sapiens (Human)) | BDBM287559

(8-(2-((1-acryloylpiperidin-4-yl)(methyl)amino)ethy...)Show SMILES CNc1ncc2cc(-c3c(Cl)c(OC)cc(OC)c3Cl)c(=O)n(CCN(C)C3CCN(CC3)C(=O)C=C)c2n1 |(-7.34,-1.16,;-7.34,.38,;-6,1.15,;-6,2.69,;-4.67,3.46,;-3.33,2.69,;-2,3.46,;-.67,2.69,;.67,3.46,;.67,5,;-.67,5.77,;2,5.78,;2,7.32,;3.33,8.09,;3.33,5,;3.33,3.46,;4.67,2.69,;6,3.46,;2,2.69,;2,1.15,;-.67,1.15,;.67,.38,;-2,.38,;-2,-1.16,;-.67,-1.93,;-.67,-3.47,;-2,-4.24,;.67,-4.24,;.67,-5.78,;2,-6.55,;3.33,-5.78,;3.33,-4.24,;2,-3.47,;4.67,-6.55,;4.67,-8.09,;6,-5.78,;7.34,-6.55,;-3.33,1.15,;-4.67,.38,)| Show InChI InChI=1S/C27H32Cl2N6O4/c1-6-21(36)34-9-7-17(8-10-34)33(3)11-12-35-25-16(15-31-27(30-2)32-25)13-18(26(35)37)22-23(28)19(38-4)14-20(39-5)24(22)29/h6,13-15,17H,1,7-12H2,2-5H3,(H,30,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Principia Biopharma, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of FGFR1 in HUVEC assessed as reduction in FGF2-induced ERK phosphorylation preincubated for 60 mins followed by FGF2 stimulation for 10 m... |
J Med Chem 60: 6516-6527 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00360
BindingDB Entry DOI: 10.7270/Q2C53P3H |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor receptor 3
(Homo sapiens (Human)) | BDBM286984

(8-(3-(4-acryloylpiperazin-1-yl)propyl)-6-(2,6-dich...)Show SMILES CNc1ncc2cc(-c3c(Cl)c(OC)cc(OC)c3Cl)c(=O)n(CCCN3CCN(CC3)C(=O)C=C)c2n1 |(-6.67,-.77,;-6.67,.77,;-5.33,1.54,;-5.33,3.08,;-4,3.85,;-2.67,3.08,;-1.33,3.85,;,3.08,;1.33,3.85,;1.33,5.39,;,6.16,;2.67,6.16,;2.67,7.7,;1.33,8.47,;4,5.39,;4,3.85,;5.33,3.08,;6.67,3.85,;2.67,3.08,;2.67,1.54,;,1.54,;1.33,.77,;-1.33,.77,;-1.33,-.77,;,-1.54,;,-3.08,;1.33,-3.85,;1.33,-5.39,;2.67,-6.16,;4,-5.39,;4,-3.85,;2.67,-3.08,;5.33,-6.16,;6.67,-5.39,;5.33,-7.7,;6.67,-8.47,;-2.67,1.54,;-4,.77,)| Show InChI InChI=1S/C26H30Cl2N6O4/c1-5-20(35)33-11-9-32(10-12-33)7-6-8-34-24-16(15-30-26(29-2)31-24)13-17(25(34)36)21-22(27)18(37-3)14-19(38-4)23(21)28/h5,13-15H,1,6-12H2,2-4H3,(H,29,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Principia Biopharma, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human FGFR3 preincubated for 15 mins followed by peptide substrate addition measured after 3 hrs by caliper capillary electrophoresis m... |
J Med Chem 60: 6516-6527 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00360
BindingDB Entry DOI: 10.7270/Q2C53P3H |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor receptor 1
(Homo sapiens (Human)) | BDBM287559

(8-(2-((1-acryloylpiperidin-4-yl)(methyl)amino)ethy...)Show SMILES CNc1ncc2cc(-c3c(Cl)c(OC)cc(OC)c3Cl)c(=O)n(CCN(C)C3CCN(CC3)C(=O)C=C)c2n1 |(-7.34,-1.16,;-7.34,.38,;-6,1.15,;-6,2.69,;-4.67,3.46,;-3.33,2.69,;-2,3.46,;-.67,2.69,;.67,3.46,;.67,5,;-.67,5.77,;2,5.78,;2,7.32,;3.33,8.09,;3.33,5,;3.33,3.46,;4.67,2.69,;6,3.46,;2,2.69,;2,1.15,;-.67,1.15,;.67,.38,;-2,.38,;-2,-1.16,;-.67,-1.93,;-.67,-3.47,;-2,-4.24,;.67,-4.24,;.67,-5.78,;2,-6.55,;3.33,-5.78,;3.33,-4.24,;2,-3.47,;4.67,-6.55,;4.67,-8.09,;6,-5.78,;7.34,-6.55,;-3.33,1.15,;-4.67,.38,)| Show InChI InChI=1S/C27H32Cl2N6O4/c1-6-21(36)34-9-7-17(8-10-34)33(3)11-12-35-25-16(15-31-27(30-2)32-25)13-18(26(35)37)22-23(28)19(38-4)14-20(39-5)24(22)29/h6,13-15,17H,1,7-12H2,2-5H3,(H,30,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Principia Biopharma, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of FGFR1 in HUVEC assessed as reduction in FGF2-induced ERK phosphorylation preincubated for 60 mins followed by FGF2 stimulation for 10 m... |
J Med Chem 60: 6516-6527 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00360
BindingDB Entry DOI: 10.7270/Q2C53P3H |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor receptor 1
(Homo sapiens (Human)) | BDBM50238820

(CHEMBL4072676)Show SMILES CNc1ncc2cc(-c3cc(OC)cc(OC)c3Cl)c(=O)n(CCc3ccc(cc3)N(C)C(=O)C=C)c2n1 Show InChI InChI=1S/C28H28ClN5O4/c1-6-24(35)33(3)19-9-7-17(8-10-19)11-12-34-26-18(16-31-28(30-2)32-26)13-22(27(34)36)21-14-20(37-4)15-23(38-5)25(21)29/h6-10,13-16H,1,11-12H2,2-5H3,(H,30,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Principia Biopharma, Inc.
Curated by ChEMBL
| Assay Description
In vitro antagonist activity against rat prostatic androgen receptor (AR) |
J Med Chem 60: 6516-6527 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00360
BindingDB Entry DOI: 10.7270/Q2C53P3H |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor receptor 1
(Homo sapiens (Human)) | BDBM50238814

(CHEMBL4085605)Show SMILES CNc1ncc2cc(-c3cc(OC)cc(OC)c3Cl)c(=O)n(Cc3cccc(NC(=O)C=C)c3)c2n1 Show InChI InChI=1S/C26H24ClN5O4/c1-5-22(33)30-17-8-6-7-15(9-17)14-32-24-16(13-29-26(28-2)31-24)10-20(25(32)34)19-11-18(35-3)12-21(36-4)23(19)27/h5-13H,1,14H2,2-4H3,(H,30,33)(H,28,29,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Principia Biopharma, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of FGFR1 in HUVEC assessed as reduction in FGF2-induced ERK phosphorylation preincubated for 60 mins followed by FGF2 stimulation for 10 m... |
J Med Chem 60: 6516-6527 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00360
BindingDB Entry DOI: 10.7270/Q2C53P3H |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor receptor 1
(Homo sapiens (Human)) | BDBM286381

(8-(2-((1-acryloylpiperidin-3-yl)oxy)ethyl)-6-(2,6-...)Show SMILES CNc1ncc2cc(-c3c(Cl)c(OC)cc(OC)c3Cl)c(=O)n(CCOC3CCCN(C3)C(=O)C=C)c2n1 |(-6.67,,;-6.67,1.54,;-5.33,2.31,;-5.33,3.85,;-4,4.62,;-2.67,3.85,;-1.33,4.62,;,3.85,;1.33,4.62,;1.33,6.16,;,6.93,;2.67,6.93,;2.67,8.47,;4,9.24,;4,6.16,;4,4.62,;5.33,3.85,;6.67,4.62,;2.67,3.85,;2.67,2.31,;,2.31,;1.33,1.54,;-1.33,1.54,;-1.33,,;,-.77,;,-2.31,;1.33,-3.08,;2.67,-2.31,;4,-3.08,;4,-4.62,;2.67,-5.39,;1.33,-4.62,;2.67,-6.93,;1.33,-7.7,;4,-7.7,;4,-9.24,;-2.67,2.31,;-4,1.54,)| Show InChI InChI=1S/C26H29Cl2N5O5/c1-5-20(34)32-8-6-7-16(14-32)38-10-9-33-24-15(13-30-26(29-2)31-24)11-17(25(33)35)21-22(27)18(36-3)12-19(37-4)23(21)28/h5,11-13,16H,1,6-10,14H2,2-4H3,(H,29,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Principia Biopharma, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of FGFR1 in HUVEC assessed as reduction in FGF2-induced ERK phosphorylation preincubated for 60 mins followed by FGF2 stimulation for 10 m... |
J Med Chem 60: 6516-6527 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00360
BindingDB Entry DOI: 10.7270/Q2C53P3H |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor receptor 1
(Homo sapiens (Human)) | BDBM50238802

(CHEMBL4095783)Show SMILES CNc1ncc2cc(-c3cc(OC)cc(OC)c3Cl)c(=O)n(CCc3cccc(NC(=O)C=C)c3)c2n1 Show InChI InChI=1S/C27H26ClN5O4/c1-5-23(34)31-18-8-6-7-16(11-18)9-10-33-25-17(15-30-27(29-2)32-25)12-21(26(33)35)20-13-19(36-3)14-22(37-4)24(20)28/h5-8,11-15H,1,9-10H2,2-4H3,(H,31,34)(H,29,30,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Principia Biopharma, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of FGFR1 in HUVEC assessed as reduction in FGF2-induced ERK phosphorylation preincubated for 60 mins followed by FGF2 stimulation for 10 m... |
J Med Chem 60: 6516-6527 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00360
BindingDB Entry DOI: 10.7270/Q2C53P3H |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor receptor 1
(Homo sapiens (Human)) | BDBM286523

(8-(2-((1-acryloylpyrrolidin-3-yl)oxy)ethyl)-6-(2,6...)Show SMILES CNc1ncc2cc(-c3c(Cl)c(OC)cc(OC)c3Cl)c(=O)n(CCOC3CCN(C3)C(=O)C=C)c2n1 |(-6.67,-.74,;-6.67,.8,;-5.33,1.57,;-5.33,3.11,;-4,3.88,;-2.67,3.11,;-1.33,3.88,;,3.11,;1.33,3.88,;1.33,5.42,;,6.19,;2.67,6.19,;2.67,7.73,;1.33,8.5,;4,5.42,;4,3.88,;5.33,3.11,;6.67,3.88,;2.67,3.11,;2.67,1.57,;,1.57,;1.33,.8,;-1.33,.8,;-1.33,-.74,;,-1.51,;,-3.05,;1.33,-3.82,;2.8,-3.34,;3.7,-4.59,;2.8,-5.83,;1.33,-5.36,;3.57,-7.17,;2.8,-8.5,;5.11,-7.17,;5.88,-8.5,;-2.67,1.57,;-4,.8,)| Show InChI InChI=1S/C25H27Cl2N5O5/c1-5-19(33)31-7-6-15(13-31)37-9-8-32-23-14(12-29-25(28-2)30-23)10-16(24(32)34)20-21(26)17(35-3)11-18(36-4)22(20)27/h5,10-12,15H,1,6-9,13H2,2-4H3,(H,28,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Principia Biopharma, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of FGFR1 in HUVEC assessed as reduction in FGF2-induced ERK phosphorylation preincubated for 60 mins followed by FGF2 stimulation for 10 m... |
J Med Chem 60: 6516-6527 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00360
BindingDB Entry DOI: 10.7270/Q2C53P3H |
More data for this
Ligand-Target Pair | |
Macrophage colony-stimulating factor 1 receptor
(Homo sapiens (Human)) | BDBM286984

(8-(3-(4-acryloylpiperazin-1-yl)propyl)-6-(2,6-dich...)Show SMILES CNc1ncc2cc(-c3c(Cl)c(OC)cc(OC)c3Cl)c(=O)n(CCCN3CCN(CC3)C(=O)C=C)c2n1 |(-6.67,-.77,;-6.67,.77,;-5.33,1.54,;-5.33,3.08,;-4,3.85,;-2.67,3.08,;-1.33,3.85,;,3.08,;1.33,3.85,;1.33,5.39,;,6.16,;2.67,6.16,;2.67,7.7,;1.33,8.47,;4,5.39,;4,3.85,;5.33,3.08,;6.67,3.85,;2.67,3.08,;2.67,1.54,;,1.54,;1.33,.77,;-1.33,.77,;-1.33,-.77,;,-1.54,;,-3.08,;1.33,-3.85,;1.33,-5.39,;2.67,-6.16,;4,-5.39,;4,-3.85,;2.67,-3.08,;5.33,-6.16,;6.67,-5.39,;5.33,-7.7,;6.67,-8.47,;-2.67,1.54,;-4,.77,)| Show InChI InChI=1S/C26H30Cl2N6O4/c1-5-20(35)33-11-9-32(10-12-33)7-6-8-34-24-16(15-30-26(29-2)31-24)13-17(25(34)36)21-22(27)18(37-3)14-19(38-4)23(21)28/h5,13-15H,1,6-12H2,2-4H3,(H,29,30,31) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 8.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Principia Biopharma, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human CSF1R preincubated for 15 mins followed by peptide substrate addition measured after 3 hrs by caliper capillary electrophoresis m... |
J Med Chem 60: 6516-6527 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00360
BindingDB Entry DOI: 10.7270/Q2C53P3H |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor receptor 1
(Homo sapiens (Human)) | BDBM50238808

(CHEMBL4102642)Show SMILES CCN1CCN(CCNc2ncc3cc(-c4cc(OC)cc(OC)c4Cl)c(=O)n(CCc4ccc(NC(=O)C=C)cc4)c3n2)CC1 Show InChI InChI=1S/C34H40ClN7O4/c1-5-30(43)38-25-9-7-23(8-10-25)11-13-42-32-24(19-28(33(42)44)27-20-26(45-3)21-29(46-4)31(27)35)22-37-34(39-32)36-12-14-41-17-15-40(6-2)16-18-41/h5,7-10,19-22H,1,6,11-18H2,2-4H3,(H,38,43)(H,36,37,39) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Principia Biopharma, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of FGFR1 in HUVEC assessed as reduction in FGF2-induced ERK phosphorylation preincubated for 60 mins followed by FGF2 stimulation for 10 m... |
J Med Chem 60: 6516-6527 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00360
BindingDB Entry DOI: 10.7270/Q2C53P3H |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor receptor 1
(Homo sapiens (Human)) | BDBM50238808

(CHEMBL4102642)Show SMILES CCN1CCN(CCNc2ncc3cc(-c4cc(OC)cc(OC)c4Cl)c(=O)n(CCc4ccc(NC(=O)C=C)cc4)c3n2)CC1 Show InChI InChI=1S/C34H40ClN7O4/c1-5-30(43)38-25-9-7-23(8-10-25)11-13-42-32-24(19-28(33(42)44)27-20-26(45-3)21-29(46-4)31(27)35)22-37-34(39-32)36-12-14-41-17-15-40(6-2)16-18-41/h5,7-10,19-22H,1,6,11-18H2,2-4H3,(H,38,43)(H,36,37,39) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Principia Biopharma, Inc.
Curated by ChEMBL
| Assay Description
In vitro antagonist activity against rat prostatic androgen receptor (AR) |
J Med Chem 60: 6516-6527 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00360
BindingDB Entry DOI: 10.7270/Q2C53P3H |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor receptor 1
(Homo sapiens (Human)) | BDBM50238815
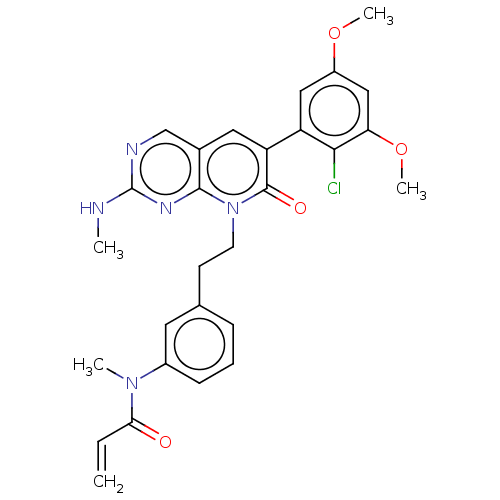
(CHEMBL4068712)Show SMILES CNc1ncc2cc(-c3cc(OC)cc(OC)c3Cl)c(=O)n(CCc3cccc(c3)N(C)C(=O)C=C)c2n1 Show InChI InChI=1S/C28H28ClN5O4/c1-6-24(35)33(3)19-9-7-8-17(12-19)10-11-34-26-18(16-31-28(30-2)32-26)13-22(27(34)36)21-14-20(37-4)15-23(38-5)25(21)29/h6-9,12-16H,1,10-11H2,2-5H3,(H,30,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 9.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Principia Biopharma, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of FGFR1 in HUVEC assessed as reduction in FGF2-induced ERK phosphorylation preincubated for 60 mins followed by FGF2 stimulation for 10 m... |
J Med Chem 60: 6516-6527 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00360
BindingDB Entry DOI: 10.7270/Q2C53P3H |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor receptor 1
(Homo sapiens (Human)) | BDBM50238806

(CHEMBL4083151)Show SMILES CNc1ncc2cc(-c3cc(OC)cc(OC)c3Cl)c(=O)n(CCc3ccc(NC(=O)C=C)cn3)c2n1 Show InChI InChI=1S/C26H25ClN6O4/c1-5-22(34)31-17-7-6-16(29-14-17)8-9-33-24-15(13-30-26(28-2)32-24)10-20(25(33)35)19-11-18(36-3)12-21(37-4)23(19)27/h5-7,10-14H,1,8-9H2,2-4H3,(H,31,34)(H,28,30,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 9.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Principia Biopharma, Inc.
Curated by ChEMBL
| Assay Description
In vitro antagonist activity against rat prostatic androgen receptor (AR) |
J Med Chem 60: 6516-6527 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00360
BindingDB Entry DOI: 10.7270/Q2C53P3H |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor receptor 1
(Homo sapiens (Human)) | BDBM50238809

(CHEMBL4084708)Show SMILES COc1cc(OC)c(Cl)c(c1)-c1cc2cnc(NCCN3CCN(CC3)C(C)=O)nc2n(CCc2ccc(NC(=O)C=C)cc2)c1=O Show InChI InChI=1S/C34H38ClN7O5/c1-5-30(44)38-25-8-6-23(7-9-25)10-12-42-32-24(18-28(33(42)45)27-19-26(46-3)20-29(47-4)31(27)35)21-37-34(39-32)36-11-13-40-14-16-41(17-15-40)22(2)43/h5-9,18-21H,1,10-17H2,2-4H3,(H,38,44)(H,36,37,39) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Principia Biopharma, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of FGFR1 in HUVEC assessed as reduction in FGF2-induced ERK phosphorylation preincubated for 60 mins followed by FGF2 stimulation for 10 m... |
J Med Chem 60: 6516-6527 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00360
BindingDB Entry DOI: 10.7270/Q2C53P3H |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor receptor 1
(Homo sapiens (Human)) | BDBM50238824

(CHEMBL4064756)Show SMILES COc1cc(OC)c(Cl)c(c1)-c1cc2cnc(NCCCN3CCOCC3)nc2n(CCc2ccc(NC(=O)C=C)cc2)c1=O Show InChI InChI=1S/C33H37ClN6O5/c1-4-29(41)37-24-8-6-22(7-9-24)10-13-40-31-23(21-36-33(38-31)35-11-5-12-39-14-16-45-17-15-39)18-27(32(40)42)26-19-25(43-2)20-28(44-3)30(26)34/h4,6-9,18-21H,1,5,10-17H2,2-3H3,(H,37,41)(H,35,36,38) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Principia Biopharma, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of FGFR1 in HUVEC assessed as reduction in FGF2-induced ERK phosphorylation preincubated for 60 mins followed by FGF2 stimulation for 10 m... |
J Med Chem 60: 6516-6527 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00360
BindingDB Entry DOI: 10.7270/Q2C53P3H |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor receptor 1
(Homo sapiens (Human)) | BDBM50238819

(CHEMBL4065128)Show SMILES COc1cc(OC)c(Cl)c(c1)-c1cc2cnc(NCCN3CCCCC3)nc2n(CCc2ccc(NC(=O)C=C)cc2)c1=O Show InChI InChI=1S/C33H37ClN6O4/c1-4-29(41)37-24-10-8-22(9-11-24)12-16-40-31-23(21-36-33(38-31)35-13-17-39-14-6-5-7-15-39)18-27(32(40)42)26-19-25(43-2)20-28(44-3)30(26)34/h4,8-11,18-21H,1,5-7,12-17H2,2-3H3,(H,37,41)(H,35,36,38) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Principia Biopharma, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of FGFR1 in HUVEC assessed as reduction in FGF2-induced ERK phosphorylation preincubated for 60 mins followed by FGF2 stimulation for 10 m... |
J Med Chem 60: 6516-6527 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00360
BindingDB Entry DOI: 10.7270/Q2C53P3H |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor receptor 1
(Homo sapiens (Human)) | BDBM50238811

(CHEMBL4073347)Show SMILES COc1cc(OC)c(Cl)c(c1)-c1cc2cnc(NC(CO)CO)nc2n(CCc2ccc(NC(=O)C=C)cc2)c1=O Show InChI InChI=1S/C29H30ClN5O6/c1-4-25(38)32-19-7-5-17(6-8-19)9-10-35-27-18(14-31-29(34-27)33-20(15-36)16-37)11-23(28(35)39)22-12-21(40-2)13-24(41-3)26(22)30/h4-8,11-14,20,36-37H,1,9-10,15-16H2,2-3H3,(H,32,38)(H,31,33,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Principia Biopharma, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of FGFR1 in HUVEC assessed as reduction in FGF2-induced ERK phosphorylation preincubated for 60 mins followed by FGF2 stimulation for 10 m... |
J Med Chem 60: 6516-6527 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00360
BindingDB Entry DOI: 10.7270/Q2C53P3H |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor receptor 4
(Homo sapiens (Human)) | BDBM286984

(8-(3-(4-acryloylpiperazin-1-yl)propyl)-6-(2,6-dich...)Show SMILES CNc1ncc2cc(-c3c(Cl)c(OC)cc(OC)c3Cl)c(=O)n(CCCN3CCN(CC3)C(=O)C=C)c2n1 |(-6.67,-.77,;-6.67,.77,;-5.33,1.54,;-5.33,3.08,;-4,3.85,;-2.67,3.08,;-1.33,3.85,;,3.08,;1.33,3.85,;1.33,5.39,;,6.16,;2.67,6.16,;2.67,7.7,;1.33,8.47,;4,5.39,;4,3.85,;5.33,3.08,;6.67,3.85,;2.67,3.08,;2.67,1.54,;,1.54,;1.33,.77,;-1.33,.77,;-1.33,-.77,;,-1.54,;,-3.08,;1.33,-3.85,;1.33,-5.39,;2.67,-6.16,;4,-5.39,;4,-3.85,;2.67,-3.08,;5.33,-6.16,;6.67,-5.39,;5.33,-7.7,;6.67,-8.47,;-2.67,1.54,;-4,.77,)| Show InChI InChI=1S/C26H30Cl2N6O4/c1-5-20(35)33-11-9-32(10-12-33)7-6-8-34-24-16(15-30-26(29-2)31-24)13-17(25(34)36)21-22(27)18(37-3)14-19(38-4)23(21)28/h5,13-15H,1,6-12H2,2-4H3,(H,29,30,31) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Principia Biopharma, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human FGFR4 preincubated for 15 mins followed by peptide substrate addition measured after 3 hrs by caliper capillary electrophoresis m... |
J Med Chem 60: 6516-6527 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00360
BindingDB Entry DOI: 10.7270/Q2C53P3H |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Fibroblast growth factor receptor 1
(Homo sapiens (Human)) | BDBM50238817
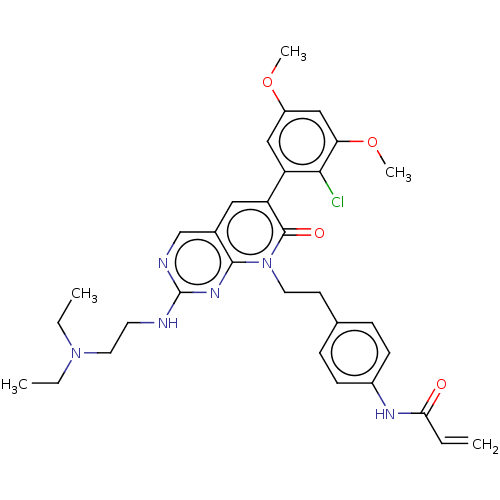
(CHEMBL4091839)Show SMILES CCN(CC)CCNc1ncc2cc(-c3cc(OC)cc(OC)c3Cl)c(=O)n(CCc3ccc(NC(=O)C=C)cc3)c2n1 Show InChI InChI=1S/C32H37ClN6O4/c1-6-28(40)36-23-11-9-21(10-12-23)13-15-39-30-22(20-35-32(37-30)34-14-16-38(7-2)8-3)17-26(31(39)41)25-18-24(42-4)19-27(43-5)29(25)33/h6,9-12,17-20H,1,7-8,13-16H2,2-5H3,(H,36,40)(H,34,35,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Principia Biopharma, Inc.
Curated by ChEMBL
| Assay Description
In vitro antagonist activity against rat prostatic androgen receptor (AR) |
J Med Chem 60: 6516-6527 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00360
BindingDB Entry DOI: 10.7270/Q2C53P3H |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor receptor 1
(Homo sapiens (Human)) | BDBM50238807

(CHEMBL4102058)Show SMILES COc1cc(OC)c(Cl)c(c1)-c1cc2cnc(NC3CCN(C)CC3)nc2n(CCc2ccc(NC(=O)C=C)cc2)c1=O Show InChI InChI=1S/C32H35ClN6O4/c1-5-28(40)35-22-8-6-20(7-9-22)10-15-39-30-21(19-34-32(37-30)36-23-11-13-38(2)14-12-23)16-26(31(39)41)25-17-24(42-3)18-27(43-4)29(25)33/h5-9,16-19,23H,1,10-15H2,2-4H3,(H,35,40)(H,34,36,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Principia Biopharma, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of FGFR1 in HUVEC assessed as reduction in FGF2-induced ERK phosphorylation preincubated for 60 mins followed by FGF2 stimulation for 10 m... |
J Med Chem 60: 6516-6527 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00360
BindingDB Entry DOI: 10.7270/Q2C53P3H |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor receptor 1
(Homo sapiens (Human)) | BDBM50238807

(CHEMBL4102058)Show SMILES COc1cc(OC)c(Cl)c(c1)-c1cc2cnc(NC3CCN(C)CC3)nc2n(CCc2ccc(NC(=O)C=C)cc2)c1=O Show InChI InChI=1S/C32H35ClN6O4/c1-5-28(40)35-22-8-6-20(7-9-22)10-15-39-30-21(19-34-32(37-30)36-23-11-13-38(2)14-12-23)16-26(31(39)41)25-17-24(42-3)18-27(43-4)29(25)33/h5-9,16-19,23H,1,10-15H2,2-4H3,(H,35,40)(H,34,36,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Principia Biopharma, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of FGFR1 in HUVEC assessed as reduction in FGF2-induced ERK phosphorylation preincubated for 60 mins followed by FGF2 stimulation for 10 m... |
J Med Chem 60: 6516-6527 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00360
BindingDB Entry DOI: 10.7270/Q2C53P3H |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor receptor 2
(Homo sapiens (Human)) | BDBM50238799
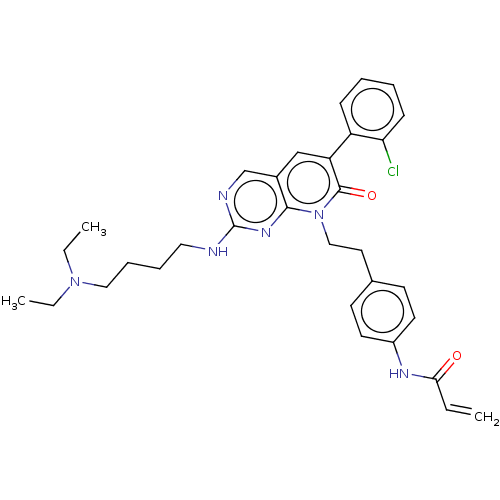
(CHEMBL4103536)Show SMILES CCN(CC)CCCCNc1ncc2cc(-c3ccccc3Cl)c(=O)n(CCc3ccc(NC(=O)C=C)cc3)c2n1 Show InChI InChI=1S/C32H37ClN6O2/c1-4-29(40)36-25-15-13-23(14-16-25)17-20-39-30-24(21-27(31(39)41)26-11-7-8-12-28(26)33)22-35-32(37-30)34-18-9-10-19-38(5-2)6-3/h4,7-8,11-16,21-22H,1,5-6,9-10,17-20H2,2-3H3,(H,36,40)(H,34,35,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
Principia Biopharma, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human FGFR2 preincubated for 15 mins followed by peptide substrate addition measured after 3 hrs by caliper capillary electrophoresis m... |
J Med Chem 60: 6516-6527 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00360
BindingDB Entry DOI: 10.7270/Q2C53P3H |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor receptor 1
(Homo sapiens (Human)) | BDBM50238812

(CHEMBL4100201)Show SMILES COc1cc(OC)c(Cl)c(c1)-c1cc2cnc(NCC(O)CO)nc2n(CCc2ccc(NC(=O)C=C)cc2)c1=O Show InChI InChI=1S/C29H30ClN5O6/c1-4-25(38)33-19-7-5-17(6-8-19)9-10-35-27-18(14-31-29(34-27)32-15-20(37)16-36)11-23(28(35)39)22-12-21(40-2)13-24(41-3)26(22)30/h4-8,11-14,20,36-37H,1,9-10,15-16H2,2-3H3,(H,33,38)(H,31,32,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
Principia Biopharma, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of FGFR1 in HUVEC assessed as reduction in FGF2-induced ERK phosphorylation preincubated for 60 mins followed by FGF2 stimulation for 10 m... |
J Med Chem 60: 6516-6527 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00360
BindingDB Entry DOI: 10.7270/Q2C53P3H |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor receptor 1
(Homo sapiens (Human)) | BDBM50238799

(CHEMBL4103536)Show SMILES CCN(CC)CCCCNc1ncc2cc(-c3ccccc3Cl)c(=O)n(CCc3ccc(NC(=O)C=C)cc3)c2n1 Show InChI InChI=1S/C32H37ClN6O2/c1-4-29(40)36-25-15-13-23(14-16-25)17-20-39-30-24(21-27(31(39)41)26-11-7-8-12-28(26)33)22-35-32(37-30)34-18-9-10-19-38(5-2)6-3/h4,7-8,11-16,21-22H,1,5-6,9-10,17-20H2,2-3H3,(H,36,40)(H,34,35,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
Principia Biopharma, Inc.
Curated by ChEMBL
| Assay Description
In vitro antagonist activity against rat prostatic androgen receptor (AR) |
J Med Chem 60: 6516-6527 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00360
BindingDB Entry DOI: 10.7270/Q2C53P3H |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor receptor 1
(Homo sapiens (Human)) | BDBM50238798

(CHEMBL4093349)Show SMILES CCN(CC)CCCCNc1ncc2cc(-c3ccccc3Cl)c(=O)n(CCc3cccc(NC(=O)C=C)c3)c2n1 Show InChI InChI=1S/C32H37ClN6O2/c1-4-29(40)36-25-13-11-12-23(20-25)16-19-39-30-24(21-27(31(39)41)26-14-7-8-15-28(26)33)22-35-32(37-30)34-17-9-10-18-38(5-2)6-3/h4,7-8,11-15,20-22H,1,5-6,9-10,16-19H2,2-3H3,(H,36,40)(H,34,35,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
Principia Biopharma, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of FGFR1 in HUVEC assessed as reduction in FGF2-induced ERK phosphorylation preincubated for 60 mins followed by FGF2 stimulation for 10 m... |
J Med Chem 60: 6516-6527 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00360
BindingDB Entry DOI: 10.7270/Q2C53P3H |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor receptor 3
(Homo sapiens (Human)) | BDBM50238799

(CHEMBL4103536)Show SMILES CCN(CC)CCCCNc1ncc2cc(-c3ccccc3Cl)c(=O)n(CCc3ccc(NC(=O)C=C)cc3)c2n1 Show InChI InChI=1S/C32H37ClN6O2/c1-4-29(40)36-25-15-13-23(14-16-25)17-20-39-30-24(21-27(31(39)41)26-11-7-8-12-28(26)33)22-35-32(37-30)34-18-9-10-19-38(5-2)6-3/h4,7-8,11-16,21-22H,1,5-6,9-10,17-20H2,2-3H3,(H,36,40)(H,34,35,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 269 | n/a | n/a | n/a | n/a | n/a | n/a |
Principia Biopharma, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human FGFR3 preincubated for 15 mins followed by peptide substrate addition measured after 3 hrs by caliper capillary electrophoresis m... |
J Med Chem 60: 6516-6527 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00360
BindingDB Entry DOI: 10.7270/Q2C53P3H |
More data for this
Ligand-Target Pair | |
Cytoplasmic tyrosine-protein kinase BMX
(Homo sapiens (Human)) | BDBM286984

(8-(3-(4-acryloylpiperazin-1-yl)propyl)-6-(2,6-dich...)Show SMILES CNc1ncc2cc(-c3c(Cl)c(OC)cc(OC)c3Cl)c(=O)n(CCCN3CCN(CC3)C(=O)C=C)c2n1 |(-6.67,-.77,;-6.67,.77,;-5.33,1.54,;-5.33,3.08,;-4,3.85,;-2.67,3.08,;-1.33,3.85,;,3.08,;1.33,3.85,;1.33,5.39,;,6.16,;2.67,6.16,;2.67,7.7,;1.33,8.47,;4,5.39,;4,3.85,;5.33,3.08,;6.67,3.85,;2.67,3.08,;2.67,1.54,;,1.54,;1.33,.77,;-1.33,.77,;-1.33,-.77,;,-1.54,;,-3.08,;1.33,-3.85,;1.33,-5.39,;2.67,-6.16,;4,-5.39,;4,-3.85,;2.67,-3.08,;5.33,-6.16,;6.67,-5.39,;5.33,-7.7,;6.67,-8.47,;-2.67,1.54,;-4,.77,)| Show InChI InChI=1S/C26H30Cl2N6O4/c1-5-20(35)33-11-9-32(10-12-33)7-6-8-34-24-16(15-30-26(29-2)31-24)13-17(25(34)36)21-22(27)18(37-3)14-19(38-4)23(21)28/h5,13-15H,1,6-12H2,2-4H3,(H,29,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 272 | n/a | n/a | n/a | n/a | n/a | n/a |
Principia Biopharma, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human BMX preincubated for 15 mins followed by peptide substrate addition measured after 3 hrs by caliper capillary electrophoresis met... |
J Med Chem 60: 6516-6527 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00360
BindingDB Entry DOI: 10.7270/Q2C53P3H |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor receptor 2
(Homo sapiens (Human)) | BDBM50238798

(CHEMBL4093349)Show SMILES CCN(CC)CCCCNc1ncc2cc(-c3ccccc3Cl)c(=O)n(CCc3cccc(NC(=O)C=C)c3)c2n1 Show InChI InChI=1S/C32H37ClN6O2/c1-4-29(40)36-25-13-11-12-23(20-25)16-19-39-30-24(21-27(31(39)41)26-14-7-8-15-28(26)33)22-35-32(37-30)34-17-9-10-18-38(5-2)6-3/h4,7-8,11-15,20-22H,1,5-6,9-10,16-19H2,2-3H3,(H,36,40)(H,34,35,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 369 | n/a | n/a | n/a | n/a | n/a | n/a |
Principia Biopharma, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human FGFR2 preincubated for 15 mins followed by peptide substrate addition measured after 3 hrs by caliper capillary electrophoresis m... |
J Med Chem 60: 6516-6527 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00360
BindingDB Entry DOI: 10.7270/Q2C53P3H |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Blk
(Homo sapiens (Human)) | BDBM286984

(8-(3-(4-acryloylpiperazin-1-yl)propyl)-6-(2,6-dich...)Show SMILES CNc1ncc2cc(-c3c(Cl)c(OC)cc(OC)c3Cl)c(=O)n(CCCN3CCN(CC3)C(=O)C=C)c2n1 |(-6.67,-.77,;-6.67,.77,;-5.33,1.54,;-5.33,3.08,;-4,3.85,;-2.67,3.08,;-1.33,3.85,;,3.08,;1.33,3.85,;1.33,5.39,;,6.16,;2.67,6.16,;2.67,7.7,;1.33,8.47,;4,5.39,;4,3.85,;5.33,3.08,;6.67,3.85,;2.67,3.08,;2.67,1.54,;,1.54,;1.33,.77,;-1.33,.77,;-1.33,-.77,;,-1.54,;,-3.08,;1.33,-3.85,;1.33,-5.39,;2.67,-6.16,;4,-5.39,;4,-3.85,;2.67,-3.08,;5.33,-6.16,;6.67,-5.39,;5.33,-7.7,;6.67,-8.47,;-2.67,1.54,;-4,.77,)| Show InChI InChI=1S/C26H30Cl2N6O4/c1-5-20(35)33-11-9-32(10-12-33)7-6-8-34-24-16(15-30-26(29-2)31-24)13-17(25(34)36)21-22(27)18(37-3)14-19(38-4)23(21)28/h5,13-15H,1,6-12H2,2-4H3,(H,29,30,31) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 371 | n/a | n/a | n/a | n/a | n/a | n/a |
Principia Biopharma, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human BLK preincubated for 15 mins followed by peptide substrate addition measured after 3 hrs by caliper capillary electrophoresis met... |
J Med Chem 60: 6516-6527 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00360
BindingDB Entry DOI: 10.7270/Q2C53P3H |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 3
(Homo sapiens (Human)) | BDBM286984

(8-(3-(4-acryloylpiperazin-1-yl)propyl)-6-(2,6-dich...)Show SMILES CNc1ncc2cc(-c3c(Cl)c(OC)cc(OC)c3Cl)c(=O)n(CCCN3CCN(CC3)C(=O)C=C)c2n1 |(-6.67,-.77,;-6.67,.77,;-5.33,1.54,;-5.33,3.08,;-4,3.85,;-2.67,3.08,;-1.33,3.85,;,3.08,;1.33,3.85,;1.33,5.39,;,6.16,;2.67,6.16,;2.67,7.7,;1.33,8.47,;4,5.39,;4,3.85,;5.33,3.08,;6.67,3.85,;2.67,3.08,;2.67,1.54,;,1.54,;1.33,.77,;-1.33,.77,;-1.33,-.77,;,-1.54,;,-3.08,;1.33,-3.85,;1.33,-5.39,;2.67,-6.16,;4,-5.39,;4,-3.85,;2.67,-3.08,;5.33,-6.16,;6.67,-5.39,;5.33,-7.7,;6.67,-8.47,;-2.67,1.54,;-4,.77,)| Show InChI InChI=1S/C26H30Cl2N6O4/c1-5-20(35)33-11-9-32(10-12-33)7-6-8-34-24-16(15-30-26(29-2)31-24)13-17(25(34)36)21-22(27)18(37-3)14-19(38-4)23(21)28/h5,13-15H,1,6-12H2,2-4H3,(H,29,30,31) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 410 | n/a | n/a | n/a | n/a | n/a | n/a |
Principia Biopharma, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human FLT4 preincubated for 15 mins followed by peptide substrate addition measured after 3 hrs by caliper capillary electrophoresis me... |
J Med Chem 60: 6516-6527 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00360
BindingDB Entry DOI: 10.7270/Q2C53P3H |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor receptor 4
(Homo sapiens (Human)) | BDBM50238799

(CHEMBL4103536)Show SMILES CCN(CC)CCCCNc1ncc2cc(-c3ccccc3Cl)c(=O)n(CCc3ccc(NC(=O)C=C)cc3)c2n1 Show InChI InChI=1S/C32H37ClN6O2/c1-4-29(40)36-25-15-13-23(14-16-25)17-20-39-30-24(21-27(31(39)41)26-11-7-8-12-28(26)33)22-35-32(37-30)34-18-9-10-19-38(5-2)6-3/h4,7-8,11-16,21-22H,1,5-6,9-10,17-20H2,2-3H3,(H,36,40)(H,34,35,37) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 526 | n/a | n/a | n/a | n/a | n/a | n/a |
Principia Biopharma, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human FGFR4 preincubated for 15 mins followed by peptide substrate addition measured after 3 hrs by caliper capillary electrophoresis m... |
J Med Chem 60: 6516-6527 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00360
BindingDB Entry DOI: 10.7270/Q2C53P3H |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK3
(Homo sapiens (Human)) | BDBM286984

(8-(3-(4-acryloylpiperazin-1-yl)propyl)-6-(2,6-dich...)Show SMILES CNc1ncc2cc(-c3c(Cl)c(OC)cc(OC)c3Cl)c(=O)n(CCCN3CCN(CC3)C(=O)C=C)c2n1 |(-6.67,-.77,;-6.67,.77,;-5.33,1.54,;-5.33,3.08,;-4,3.85,;-2.67,3.08,;-1.33,3.85,;,3.08,;1.33,3.85,;1.33,5.39,;,6.16,;2.67,6.16,;2.67,7.7,;1.33,8.47,;4,5.39,;4,3.85,;5.33,3.08,;6.67,3.85,;2.67,3.08,;2.67,1.54,;,1.54,;1.33,.77,;-1.33,.77,;-1.33,-.77,;,-1.54,;,-3.08,;1.33,-3.85,;1.33,-5.39,;2.67,-6.16,;4,-5.39,;4,-3.85,;2.67,-3.08,;5.33,-6.16,;6.67,-5.39,;5.33,-7.7,;6.67,-8.47,;-2.67,1.54,;-4,.77,)| Show InChI InChI=1S/C26H30Cl2N6O4/c1-5-20(35)33-11-9-32(10-12-33)7-6-8-34-24-16(15-30-26(29-2)31-24)13-17(25(34)36)21-22(27)18(37-3)14-19(38-4)23(21)28/h5,13-15H,1,6-12H2,2-4H3,(H,29,30,31) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 556 | n/a | n/a | n/a | n/a | n/a | n/a |
Principia Biopharma, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human JAK3 preincubated for 15 mins followed by peptide substrate addition measured after 3 hrs by caliper capillary electrophoresis me... |
J Med Chem 60: 6516-6527 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00360
BindingDB Entry DOI: 10.7270/Q2C53P3H |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lyn
(Homo sapiens (Human)) | BDBM286984

(8-(3-(4-acryloylpiperazin-1-yl)propyl)-6-(2,6-dich...)Show SMILES CNc1ncc2cc(-c3c(Cl)c(OC)cc(OC)c3Cl)c(=O)n(CCCN3CCN(CC3)C(=O)C=C)c2n1 |(-6.67,-.77,;-6.67,.77,;-5.33,1.54,;-5.33,3.08,;-4,3.85,;-2.67,3.08,;-1.33,3.85,;,3.08,;1.33,3.85,;1.33,5.39,;,6.16,;2.67,6.16,;2.67,7.7,;1.33,8.47,;4,5.39,;4,3.85,;5.33,3.08,;6.67,3.85,;2.67,3.08,;2.67,1.54,;,1.54,;1.33,.77,;-1.33,.77,;-1.33,-.77,;,-1.54,;,-3.08,;1.33,-3.85,;1.33,-5.39,;2.67,-6.16,;4,-5.39,;4,-3.85,;2.67,-3.08,;5.33,-6.16,;6.67,-5.39,;5.33,-7.7,;6.67,-8.47,;-2.67,1.54,;-4,.77,)| Show InChI InChI=1S/C26H30Cl2N6O4/c1-5-20(35)33-11-9-32(10-12-33)7-6-8-34-24-16(15-30-26(29-2)31-24)13-17(25(34)36)21-22(27)18(37-3)14-19(38-4)23(21)28/h5,13-15H,1,6-12H2,2-4H3,(H,29,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 631 | n/a | n/a | n/a | n/a | n/a | n/a |
Principia Biopharma, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human LYN-B preincubated for 15 mins followed by peptide substrate addition measured after 3 hrs by caliper capillary electrophoresis m... |
J Med Chem 60: 6516-6527 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00360
BindingDB Entry DOI: 10.7270/Q2C53P3H |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lyn
(Homo sapiens (Human)) | BDBM286984

(8-(3-(4-acryloylpiperazin-1-yl)propyl)-6-(2,6-dich...)Show SMILES CNc1ncc2cc(-c3c(Cl)c(OC)cc(OC)c3Cl)c(=O)n(CCCN3CCN(CC3)C(=O)C=C)c2n1 |(-6.67,-.77,;-6.67,.77,;-5.33,1.54,;-5.33,3.08,;-4,3.85,;-2.67,3.08,;-1.33,3.85,;,3.08,;1.33,3.85,;1.33,5.39,;,6.16,;2.67,6.16,;2.67,7.7,;1.33,8.47,;4,5.39,;4,3.85,;5.33,3.08,;6.67,3.85,;2.67,3.08,;2.67,1.54,;,1.54,;1.33,.77,;-1.33,.77,;-1.33,-.77,;,-1.54,;,-3.08,;1.33,-3.85,;1.33,-5.39,;2.67,-6.16,;4,-5.39,;4,-3.85,;2.67,-3.08,;5.33,-6.16,;6.67,-5.39,;5.33,-7.7,;6.67,-8.47,;-2.67,1.54,;-4,.77,)| Show InChI InChI=1S/C26H30Cl2N6O4/c1-5-20(35)33-11-9-32(10-12-33)7-6-8-34-24-16(15-30-26(29-2)31-24)13-17(25(34)36)21-22(27)18(37-3)14-19(38-4)23(21)28/h5,13-15H,1,6-12H2,2-4H3,(H,29,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 639 | n/a | n/a | n/a | n/a | n/a | n/a |
Principia Biopharma, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human LYN-A preincubated for 15 mins followed by peptide substrate addition measured after 3 hrs by caliper capillary electrophoresis m... |
J Med Chem 60: 6516-6527 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00360
BindingDB Entry DOI: 10.7270/Q2C53P3H |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM286984

(8-(3-(4-acryloylpiperazin-1-yl)propyl)-6-(2,6-dich...)Show SMILES CNc1ncc2cc(-c3c(Cl)c(OC)cc(OC)c3Cl)c(=O)n(CCCN3CCN(CC3)C(=O)C=C)c2n1 |(-6.67,-.77,;-6.67,.77,;-5.33,1.54,;-5.33,3.08,;-4,3.85,;-2.67,3.08,;-1.33,3.85,;,3.08,;1.33,3.85,;1.33,5.39,;,6.16,;2.67,6.16,;2.67,7.7,;1.33,8.47,;4,5.39,;4,3.85,;5.33,3.08,;6.67,3.85,;2.67,3.08,;2.67,1.54,;,1.54,;1.33,.77,;-1.33,.77,;-1.33,-.77,;,-1.54,;,-3.08,;1.33,-3.85,;1.33,-5.39,;2.67,-6.16,;4,-5.39,;4,-3.85,;2.67,-3.08,;5.33,-6.16,;6.67,-5.39,;5.33,-7.7,;6.67,-8.47,;-2.67,1.54,;-4,.77,)| Show InChI InChI=1S/C26H30Cl2N6O4/c1-5-20(35)33-11-9-32(10-12-33)7-6-8-34-24-16(15-30-26(29-2)31-24)13-17(25(34)36)21-22(27)18(37-3)14-19(38-4)23(21)28/h5,13-15H,1,6-12H2,2-4H3,(H,29,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 705 | n/a | n/a | n/a | n/a | n/a | n/a |
Principia Biopharma, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human VEGFR2 preincubated for 15 mins followed by peptide substrate addition measured after 3 hrs by caliper capillary electrophoresis ... |
J Med Chem 60: 6516-6527 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00360
BindingDB Entry DOI: 10.7270/Q2C53P3H |
More data for this
Ligand-Target Pair | |
Eukaryotic translation initiation factor 2-alpha kinase 3
(Homo sapiens (Human)) | BDBM286984

(8-(3-(4-acryloylpiperazin-1-yl)propyl)-6-(2,6-dich...)Show SMILES CNc1ncc2cc(-c3c(Cl)c(OC)cc(OC)c3Cl)c(=O)n(CCCN3CCN(CC3)C(=O)C=C)c2n1 |(-6.67,-.77,;-6.67,.77,;-5.33,1.54,;-5.33,3.08,;-4,3.85,;-2.67,3.08,;-1.33,3.85,;,3.08,;1.33,3.85,;1.33,5.39,;,6.16,;2.67,6.16,;2.67,7.7,;1.33,8.47,;4,5.39,;4,3.85,;5.33,3.08,;6.67,3.85,;2.67,3.08,;2.67,1.54,;,1.54,;1.33,.77,;-1.33,.77,;-1.33,-.77,;,-1.54,;,-3.08,;1.33,-3.85,;1.33,-5.39,;2.67,-6.16,;4,-5.39,;4,-3.85,;2.67,-3.08,;5.33,-6.16,;6.67,-5.39,;5.33,-7.7,;6.67,-8.47,;-2.67,1.54,;-4,.77,)| Show InChI InChI=1S/C26H30Cl2N6O4/c1-5-20(35)33-11-9-32(10-12-33)7-6-8-34-24-16(15-30-26(29-2)31-24)13-17(25(34)36)21-22(27)18(37-3)14-19(38-4)23(21)28/h5,13-15H,1,6-12H2,2-4H3,(H,29,30,31) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 792 | n/a | n/a | n/a | n/a | n/a | n/a |
Principia Biopharma, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human PERK preincubated for 15 mins followed by peptide substrate addition measured after 3 hrs by caliper capillary electrophoresis me... |
J Med Chem 60: 6516-6527 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00360
BindingDB Entry DOI: 10.7270/Q2C53P3H |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor receptor 3
(Homo sapiens (Human)) | BDBM50238798

(CHEMBL4093349)Show SMILES CCN(CC)CCCCNc1ncc2cc(-c3ccccc3Cl)c(=O)n(CCc3cccc(NC(=O)C=C)c3)c2n1 Show InChI InChI=1S/C32H37ClN6O2/c1-4-29(40)36-25-13-11-12-23(20-25)16-19-39-30-24(21-27(31(39)41)26-14-7-8-15-28(26)33)22-35-32(37-30)34-17-9-10-18-38(5-2)6-3/h4,7-8,11-15,20-22H,1,5-6,9-10,16-19H2,2-3H3,(H,36,40)(H,34,35,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 820 | n/a | n/a | n/a | n/a | n/a | n/a |
Principia Biopharma, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human FGFR3 preincubated for 15 mins followed by peptide substrate addition measured after 3 hrs by caliper capillary electrophoresis m... |
J Med Chem 60: 6516-6527 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00360
BindingDB Entry DOI: 10.7270/Q2C53P3H |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50238798

(CHEMBL4093349)Show SMILES CCN(CC)CCCCNc1ncc2cc(-c3ccccc3Cl)c(=O)n(CCc3cccc(NC(=O)C=C)c3)c2n1 Show InChI InChI=1S/C32H37ClN6O2/c1-4-29(40)36-25-13-11-12-23(20-25)16-19-39-30-24(21-27(31(39)41)26-14-7-8-15-28(26)33)22-35-32(37-30)34-17-9-10-18-38(5-2)6-3/h4,7-8,11-15,20-22H,1,5-6,9-10,16-19H2,2-3H3,(H,36,40)(H,34,35,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 997 | n/a | n/a | n/a | n/a | n/a | n/a |
Principia Biopharma, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human VEGFR2 preincubated for 15 mins followed by peptide substrate addition measured after 3 hrs by caliper capillary electrophoresis ... |
J Med Chem 60: 6516-6527 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00360
BindingDB Entry DOI: 10.7270/Q2C53P3H |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor receptor 1
(Homo sapiens (Human)) | BDBM50238800
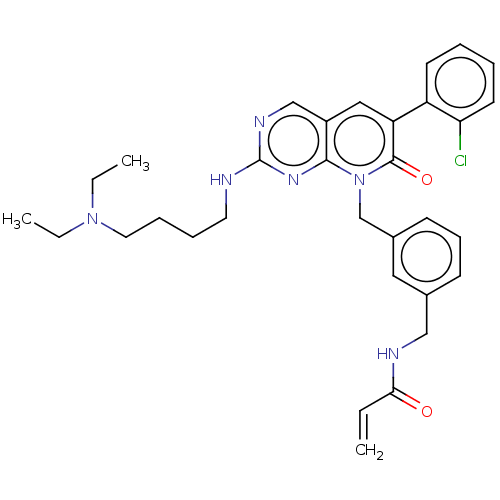
(CHEMBL4101091)Show SMILES CCN(CC)CCCCNc1ncc2cc(-c3ccccc3Cl)c(=O)n(Cc3cccc(CNC(=O)C=C)c3)c2n1 Show InChI InChI=1S/C32H37ClN6O2/c1-4-29(40)35-20-23-12-11-13-24(18-23)22-39-30-25(19-27(31(39)41)26-14-7-8-15-28(26)33)21-36-32(37-30)34-16-9-10-17-38(5-2)6-3/h4,7-8,11-15,18-19,21H,1,5-6,9-10,16-17,20,22H2,2-3H3,(H,35,40)(H,34,36,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Principia Biopharma, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of FGFR1 in HUVEC assessed as reduction in FGF2-induced ERK phosphorylation preincubated for 60 mins followed by FGF2 stimulation for 10 m... |
J Med Chem 60: 6516-6527 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00360
BindingDB Entry DOI: 10.7270/Q2C53P3H |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor receptor 3
(Homo sapiens (Human)) | BDBM50238801

(CHEMBL4065736)Show SMILES CCN(CC)CCCCNc1ncc2cc(-c3ccccc3Cl)c(=O)n(Cc3ccc(NC(=O)C=C)cc3)c2n1 Show InChI InChI=1S/C31H35ClN6O2/c1-4-28(39)35-24-15-13-22(14-16-24)21-38-29-23(19-26(30(38)40)25-11-7-8-12-27(25)32)20-34-31(36-29)33-17-9-10-18-37(5-2)6-3/h4,7-8,11-16,19-20H,1,5-6,9-10,17-18,21H2,2-3H3,(H,35,39)(H,33,34,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Principia Biopharma, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human FGFR3 preincubated for 15 mins followed by peptide substrate addition measured after 3 hrs by caliper capillary electrophoresis m... |
J Med Chem 60: 6516-6527 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00360
BindingDB Entry DOI: 10.7270/Q2C53P3H |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor receptor 1
(Homo sapiens (Human)) | BDBM50238801

(CHEMBL4065736)Show SMILES CCN(CC)CCCCNc1ncc2cc(-c3ccccc3Cl)c(=O)n(Cc3ccc(NC(=O)C=C)cc3)c2n1 Show InChI InChI=1S/C31H35ClN6O2/c1-4-28(39)35-24-15-13-22(14-16-24)21-38-29-23(19-26(30(38)40)25-11-7-8-12-27(25)32)20-34-31(36-29)33-17-9-10-18-37(5-2)6-3/h4,7-8,11-16,19-20H,1,5-6,9-10,17-18,21H2,2-3H3,(H,35,39)(H,33,34,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Principia Biopharma, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of FGFR1 in HUVEC assessed as reduction in FGF2-induced ERK phosphorylation preincubated for 60 mins followed by FGF2 stimulation for 10 m... |
J Med Chem 60: 6516-6527 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00360
BindingDB Entry DOI: 10.7270/Q2C53P3H |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor receptor 3
(Homo sapiens (Human)) | BDBM50238800

(CHEMBL4101091)Show SMILES CCN(CC)CCCCNc1ncc2cc(-c3ccccc3Cl)c(=O)n(Cc3cccc(CNC(=O)C=C)c3)c2n1 Show InChI InChI=1S/C32H37ClN6O2/c1-4-29(40)35-20-23-12-11-13-24(18-23)22-39-30-25(19-27(31(39)41)26-14-7-8-15-28(26)33)21-36-32(37-30)34-16-9-10-17-38(5-2)6-3/h4,7-8,11-15,18-19,21H,1,5-6,9-10,16-17,20,22H2,2-3H3,(H,35,40)(H,34,36,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Principia Biopharma, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human FGFR3 preincubated for 15 mins followed by peptide substrate addition measured after 3 hrs by caliper capillary electrophoresis m... |
J Med Chem 60: 6516-6527 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00360
BindingDB Entry DOI: 10.7270/Q2C53P3H |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor receptor 2
(Homo sapiens (Human)) | BDBM50238801

(CHEMBL4065736)Show SMILES CCN(CC)CCCCNc1ncc2cc(-c3ccccc3Cl)c(=O)n(Cc3ccc(NC(=O)C=C)cc3)c2n1 Show InChI InChI=1S/C31H35ClN6O2/c1-4-28(39)35-24-15-13-22(14-16-24)21-38-29-23(19-26(30(38)40)25-11-7-8-12-27(25)32)20-34-31(36-29)33-17-9-10-18-37(5-2)6-3/h4,7-8,11-16,19-20H,1,5-6,9-10,17-18,21H2,2-3H3,(H,35,39)(H,33,34,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Principia Biopharma, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human FGFR2 preincubated for 15 mins followed by peptide substrate addition measured after 3 hrs by caliper capillary electrophoresis m... |
J Med Chem 60: 6516-6527 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00360
BindingDB Entry DOI: 10.7270/Q2C53P3H |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor receptor 2
(Homo sapiens (Human)) | BDBM50238800

(CHEMBL4101091)Show SMILES CCN(CC)CCCCNc1ncc2cc(-c3ccccc3Cl)c(=O)n(Cc3cccc(CNC(=O)C=C)c3)c2n1 Show InChI InChI=1S/C32H37ClN6O2/c1-4-29(40)35-20-23-12-11-13-24(18-23)22-39-30-25(19-27(31(39)41)26-14-7-8-15-28(26)33)21-36-32(37-30)34-16-9-10-17-38(5-2)6-3/h4,7-8,11-15,18-19,21H,1,5-6,9-10,16-17,20,22H2,2-3H3,(H,35,40)(H,34,36,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Principia Biopharma, Inc.
Curated by ChEMBL
| Assay Description
In vitro antagonist activity against rat prostatic androgen receptor (AR) |
J Med Chem 60: 6516-6527 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00360
BindingDB Entry DOI: 10.7270/Q2C53P3H |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor receptor 4
(Homo sapiens (Human)) | BDBM50238801

(CHEMBL4065736)Show SMILES CCN(CC)CCCCNc1ncc2cc(-c3ccccc3Cl)c(=O)n(Cc3ccc(NC(=O)C=C)cc3)c2n1 Show InChI InChI=1S/C31H35ClN6O2/c1-4-28(39)35-24-15-13-22(14-16-24)21-38-29-23(19-26(30(38)40)25-11-7-8-12-27(25)32)20-34-31(36-29)33-17-9-10-18-37(5-2)6-3/h4,7-8,11-16,19-20H,1,5-6,9-10,17-18,21H2,2-3H3,(H,35,39)(H,33,34,36) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Principia Biopharma, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human FGFR4 preincubated for 15 mins followed by peptide substrate addition measured after 3 hrs by caliper capillary electrophoresis m... |
J Med Chem 60: 6516-6527 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00360
BindingDB Entry DOI: 10.7270/Q2C53P3H |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor receptor 4
(Homo sapiens (Human)) | BDBM50238800

(CHEMBL4101091)Show SMILES CCN(CC)CCCCNc1ncc2cc(-c3ccccc3Cl)c(=O)n(Cc3cccc(CNC(=O)C=C)c3)c2n1 Show InChI InChI=1S/C32H37ClN6O2/c1-4-29(40)35-20-23-12-11-13-24(18-23)22-39-30-25(19-27(31(39)41)26-14-7-8-15-28(26)33)21-36-32(37-30)34-16-9-10-17-38(5-2)6-3/h4,7-8,11-15,18-19,21H,1,5-6,9-10,16-17,20,22H2,2-3H3,(H,35,40)(H,34,36,37) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Principia Biopharma, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human FGFR4 preincubated for 15 mins followed by peptide substrate addition measured after 3 hrs by caliper capillary electrophoresis m... |
J Med Chem 60: 6516-6527 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00360
BindingDB Entry DOI: 10.7270/Q2C53P3H |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50238799

(CHEMBL4103536)Show SMILES CCN(CC)CCCCNc1ncc2cc(-c3ccccc3Cl)c(=O)n(CCc3ccc(NC(=O)C=C)cc3)c2n1 Show InChI InChI=1S/C32H37ClN6O2/c1-4-29(40)36-25-15-13-23(14-16-25)17-20-39-30-24(21-27(31(39)41)26-11-7-8-12-28(26)33)22-35-32(37-30)34-18-9-10-19-38(5-2)6-3/h4,7-8,11-16,21-22H,1,5-6,9-10,17-20H2,2-3H3,(H,36,40)(H,34,35,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 1.04E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Principia Biopharma, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human VEGFR2 preincubated for 15 mins followed by peptide substrate addition measured after 3 hrs by caliper capillary electrophoresis ... |
J Med Chem 60: 6516-6527 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00360
BindingDB Entry DOI: 10.7270/Q2C53P3H |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase TAO2
(Homo sapiens (Human)) | BDBM286984

(8-(3-(4-acryloylpiperazin-1-yl)propyl)-6-(2,6-dich...)Show SMILES CNc1ncc2cc(-c3c(Cl)c(OC)cc(OC)c3Cl)c(=O)n(CCCN3CCN(CC3)C(=O)C=C)c2n1 |(-6.67,-.77,;-6.67,.77,;-5.33,1.54,;-5.33,3.08,;-4,3.85,;-2.67,3.08,;-1.33,3.85,;,3.08,;1.33,3.85,;1.33,5.39,;,6.16,;2.67,6.16,;2.67,7.7,;1.33,8.47,;4,5.39,;4,3.85,;5.33,3.08,;6.67,3.85,;2.67,3.08,;2.67,1.54,;,1.54,;1.33,.77,;-1.33,.77,;-1.33,-.77,;,-1.54,;,-3.08,;1.33,-3.85,;1.33,-5.39,;2.67,-6.16,;4,-5.39,;4,-3.85,;2.67,-3.08,;5.33,-6.16,;6.67,-5.39,;5.33,-7.7,;6.67,-8.47,;-2.67,1.54,;-4,.77,)| Show InChI InChI=1S/C26H30Cl2N6O4/c1-5-20(35)33-11-9-32(10-12-33)7-6-8-34-24-16(15-30-26(29-2)31-24)13-17(25(34)36)21-22(27)18(37-3)14-19(38-4)23(21)28/h5,13-15H,1,6-12H2,2-4H3,(H,29,30,31) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 1.18E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Principia Biopharma, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human TAOK2 preincubated for 15 mins followed by peptide substrate addition measured after 3 hrs by caliper capillary electrophoresis m... |
J Med Chem 60: 6516-6527 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00360
BindingDB Entry DOI: 10.7270/Q2C53P3H |
More data for this
Ligand-Target Pair | |
Macrophage colony-stimulating factor 1 receptor
(Homo sapiens (Human)) | BDBM286984

(8-(3-(4-acryloylpiperazin-1-yl)propyl)-6-(2,6-dich...)Show SMILES CNc1ncc2cc(-c3c(Cl)c(OC)cc(OC)c3Cl)c(=O)n(CCCN3CCN(CC3)C(=O)C=C)c2n1 |(-6.67,-.77,;-6.67,.77,;-5.33,1.54,;-5.33,3.08,;-4,3.85,;-2.67,3.08,;-1.33,3.85,;,3.08,;1.33,3.85,;1.33,5.39,;,6.16,;2.67,6.16,;2.67,7.7,;1.33,8.47,;4,5.39,;4,3.85,;5.33,3.08,;6.67,3.85,;2.67,3.08,;2.67,1.54,;,1.54,;1.33,.77,;-1.33,.77,;-1.33,-.77,;,-1.54,;,-3.08,;1.33,-3.85,;1.33,-5.39,;2.67,-6.16,;4,-5.39,;4,-3.85,;2.67,-3.08,;5.33,-6.16,;6.67,-5.39,;5.33,-7.7,;6.67,-8.47,;-2.67,1.54,;-4,.77,)| Show InChI InChI=1S/C26H30Cl2N6O4/c1-5-20(35)33-11-9-32(10-12-33)7-6-8-34-24-16(15-30-26(29-2)31-24)13-17(25(34)36)21-22(27)18(37-3)14-19(38-4)23(21)28/h5,13-15H,1,6-12H2,2-4H3,(H,29,30,31) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | >1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Principia Biopharma, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human CSF1R by cell based assay |
J Med Chem 60: 6516-6527 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00360
BindingDB Entry DOI: 10.7270/Q2C53P3H |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor receptor 4
(Homo sapiens (Human)) | BDBM50238798

(CHEMBL4093349)Show SMILES CCN(CC)CCCCNc1ncc2cc(-c3ccccc3Cl)c(=O)n(CCc3cccc(NC(=O)C=C)c3)c2n1 Show InChI InChI=1S/C32H37ClN6O2/c1-4-29(40)36-25-13-11-12-23(20-25)16-19-39-30-24(21-27(31(39)41)26-14-7-8-15-28(26)33)22-35-32(37-30)34-17-9-10-18-38(5-2)6-3/h4,7-8,11-15,20-22H,1,5-6,9-10,16-19H2,2-3H3,(H,36,40)(H,34,35,37) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.77E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Principia Biopharma, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human FGFR4 preincubated for 15 mins followed by peptide substrate addition measured after 3 hrs by caliper capillary electrophoresis m... |
J Med Chem 60: 6516-6527 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00360
BindingDB Entry DOI: 10.7270/Q2C53P3H |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data