 Found 39 hits of Enzyme Inhibition Constant Data
Found 39 hits of Enzyme Inhibition Constant Data Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50238854

(CHEBI:58987 | Revatio | Sildenafil Citrate | UK-92...)Show SMILES OC(=O)CC(O)(CC(O)=O)C(O)=O.CCCc1nn(C)c2c1nc([nH]c2=O)-c1cc(ccc1OCC)S(=O)(=O)N1CCN(C)CC1 Show InChI InChI=1S/C22H30N6O4S/c1-5-7-17-19-20(27(4)25-17)22(29)24-21(23-19)16-14-15(8-9-18(16)32-6-2)33(30,31)28-12-10-26(3)11-13-28/h8-9,14H,5-7,10-13H2,1-4H3,(H,23,24,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-Sen University
Curated by ChEMBL
| Assay Description
Inhibition of PDE5A1 catalytic domain (535 to 860 residues) (unknown origin) expressed in Escherichia coli BL21(DE3) using [3H]cGMP as substrate afte... |
J Med Chem 60: 6622-6637 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00523
BindingDB Entry DOI: 10.7270/Q23N25NF |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50238849
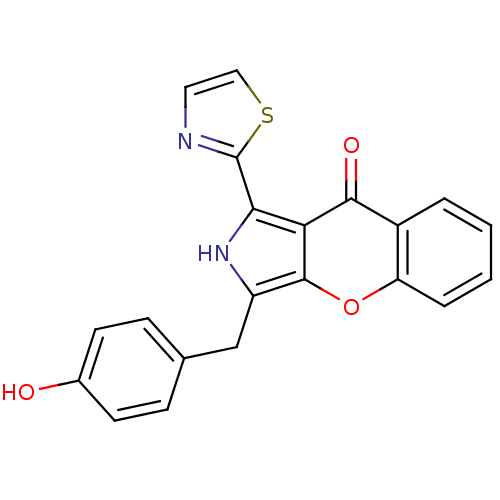
(CHEMBL4059555)Show SMILES Oc1ccc(Cc2[nH]c(-c3nccs3)c3c2oc2ccccc2c3=O)cc1 Show InChI InChI=1S/C21H14N2O3S/c24-13-7-5-12(6-8-13)11-15-20-17(18(23-15)21-22-9-10-27-21)19(25)14-3-1-2-4-16(14)26-20/h1-10,23-24H,11H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-Sen University
Curated by ChEMBL
| Assay Description
Inhibition of PDE5A1 catalytic domain (535 to 860 residues) (unknown origin) expressed in Escherichia coli BL21(DE3) using [3H]cGMP as substrate afte... |
J Med Chem 60: 6622-6637 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00523
BindingDB Entry DOI: 10.7270/Q23N25NF |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50238860

(CHEMBL4099219)Show SMILES O=c1c2c([nH]c(Cc3ccc4OCOc4c3)c2oc2ccccc12)-c1nccs1 Show InChI InChI=1S/C22H14N2O4S/c25-20-13-3-1-2-4-15(13)28-21-14(24-19(18(20)21)22-23-7-8-29-22)9-12-5-6-16-17(10-12)27-11-26-16/h1-8,10,24H,9,11H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-Sen University
Curated by ChEMBL
| Assay Description
Inhibition of PDE5A1 catalytic domain (535 to 860 residues) (unknown origin) expressed in Escherichia coli BL21(DE3) using [3H]cGMP as substrate afte... |
J Med Chem 60: 6622-6637 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00523
BindingDB Entry DOI: 10.7270/Q23N25NF |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50238851
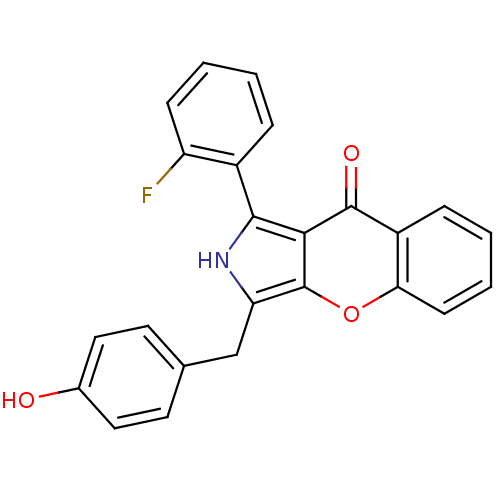
(CHEMBL4087872)Show SMILES Oc1ccc(Cc2[nH]c(-c3ccccc3F)c3c2oc2ccccc2c3=O)cc1 Show InChI InChI=1S/C24H16FNO3/c25-18-7-3-1-5-16(18)22-21-23(28)17-6-2-4-8-20(17)29-24(21)19(26-22)13-14-9-11-15(27)12-10-14/h1-12,26-27H,13H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-Sen University
Curated by ChEMBL
| Assay Description
Inhibition of PDE5A1 catalytic domain (535 to 860 residues) (unknown origin) expressed in Escherichia coli BL21(DE3) using [3H]cGMP as substrate afte... |
J Med Chem 60: 6622-6637 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00523
BindingDB Entry DOI: 10.7270/Q23N25NF |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50238864
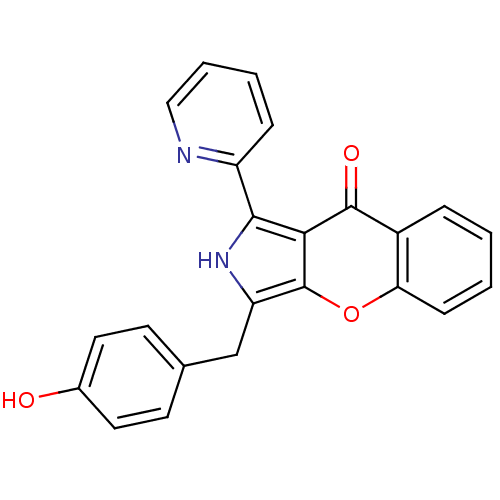
(CHEMBL4099081)Show SMILES Oc1ccc(Cc2[nH]c(-c3ccccn3)c3c2oc2ccccc2c3=O)cc1 Show InChI InChI=1S/C23H16N2O3/c26-15-10-8-14(9-11-15)13-18-23-20(21(25-18)17-6-3-4-12-24-17)22(27)16-5-1-2-7-19(16)28-23/h1-12,25-26H,13H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-Sen University
Curated by ChEMBL
| Assay Description
Inhibition of PDE5A1 catalytic domain (535 to 860 residues) (unknown origin) expressed in Escherichia coli BL21(DE3) using [3H]cGMP as substrate afte... |
J Med Chem 60: 6622-6637 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00523
BindingDB Entry DOI: 10.7270/Q23N25NF |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50238858
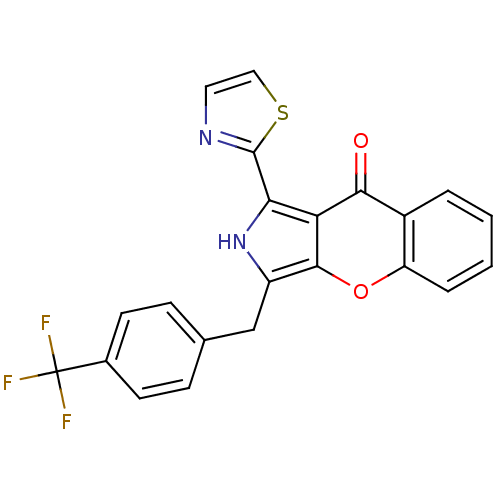
(CHEMBL4082746)Show SMILES FC(F)(F)c1ccc(Cc2[nH]c(-c3nccs3)c3c2oc2ccccc2c3=O)cc1 Show InChI InChI=1S/C22H13F3N2O2S/c23-22(24,25)13-7-5-12(6-8-13)11-15-20-17(18(27-15)21-26-9-10-30-21)19(28)14-3-1-2-4-16(14)29-20/h1-10,27H,11H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-Sen University
Curated by ChEMBL
| Assay Description
Inhibition of PDE5A1 catalytic domain (535 to 860 residues) (unknown origin) expressed in Escherichia coli BL21(DE3) using [3H]cGMP as substrate afte... |
J Med Chem 60: 6622-6637 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00523
BindingDB Entry DOI: 10.7270/Q23N25NF |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50238859
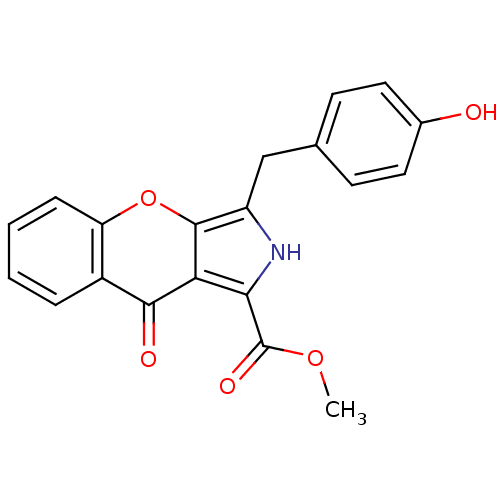
(CHEMBL4069398)Show SMILES COC(=O)c1[nH]c(Cc2ccc(O)cc2)c2oc3ccccc3c(=O)c12 Show InChI InChI=1S/C20H15NO5/c1-25-20(24)17-16-18(23)13-4-2-3-5-15(13)26-19(16)14(21-17)10-11-6-8-12(22)9-7-11/h2-9,21-22H,10H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-Sen University
Curated by ChEMBL
| Assay Description
Inhibition of PDE5A1 catalytic domain (535 to 860 residues) (unknown origin) expressed in Escherichia coli BL21(DE3) using [3H]cGMP as substrate afte... |
J Med Chem 60: 6622-6637 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00523
BindingDB Entry DOI: 10.7270/Q23N25NF |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50238862

(CHEMBL4079069)Show SMILES Oc1ccc(Cc2[nH]c(-c3ccc(Cl)cn3)c3c2oc2ccccc2c3=O)cc1 Show InChI InChI=1S/C23H15ClN2O3/c24-14-7-10-17(25-12-14)21-20-22(28)16-3-1-2-4-19(16)29-23(20)18(26-21)11-13-5-8-15(27)9-6-13/h1-10,12,26-27H,11H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-Sen University
Curated by ChEMBL
| Assay Description
Inhibition of PDE5A1 catalytic domain (535 to 860 residues) (unknown origin) expressed in Escherichia coli BL21(DE3) using [3H]cGMP as substrate afte... |
J Med Chem 60: 6622-6637 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00523
BindingDB Entry DOI: 10.7270/Q23N25NF |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50238852

(CHEMBL4084753)Show SMILES Oc1ccc(Cc2[nH]c(-c3ccncn3)c3c2oc2ccccc2c3=O)cc1 Show InChI InChI=1S/C22H15N3O3/c26-14-7-5-13(6-8-14)11-17-22-19(20(25-17)16-9-10-23-12-24-16)21(27)15-3-1-2-4-18(15)28-22/h1-10,12,25-26H,11H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 56 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-Sen University
Curated by ChEMBL
| Assay Description
Inhibition of PDE5A1 catalytic domain (535 to 860 residues) (unknown origin) expressed in Escherichia coli BL21(DE3) using [3H]cGMP as substrate afte... |
J Med Chem 60: 6622-6637 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00523
BindingDB Entry DOI: 10.7270/Q23N25NF |
More data for this
Ligand-Target Pair | |
Rod cGMP-specific 3',5'-cyclic phosphodiesterase subunit alpha
(Homo sapiens (Human)) | BDBM50238860

(CHEMBL4099219)Show SMILES O=c1c2c([nH]c(Cc3ccc4OCOc4c3)c2oc2ccccc12)-c1nccs1 Show InChI InChI=1S/C22H14N2O4S/c25-20-13-3-1-2-4-15(13)28-21-14(24-19(18(20)21)22-23-7-8-29-22)9-12-5-6-16-17(10-12)27-11-26-16/h1-8,10,24H,9,11H2 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 58 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-Sen University
Curated by ChEMBL
| Assay Description
Inhibition of PDE6A (484 to 817 residues) (unknown origin) expressed in Escherichia coli BL21(DE3) using [3H]cGMP or [3H]cAMP as substrate after 15 m... |
J Med Chem 60: 6622-6637 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00523
BindingDB Entry DOI: 10.7270/Q23N25NF |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50238850
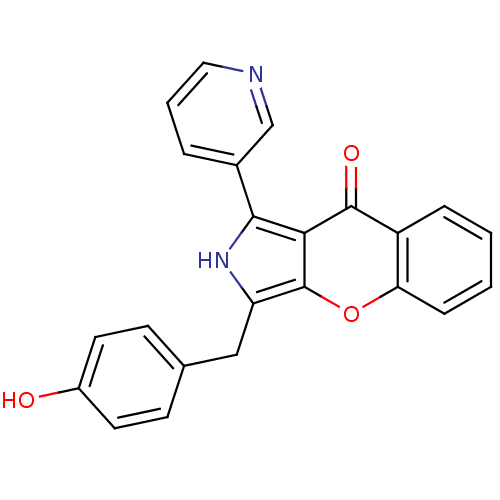
(CHEMBL4065489)Show SMILES Oc1ccc(Cc2[nH]c(-c3cccnc3)c3c2oc2ccccc2c3=O)cc1 Show InChI InChI=1S/C23H16N2O3/c26-16-9-7-14(8-10-16)12-18-23-20(21(25-18)15-4-3-11-24-13-15)22(27)17-5-1-2-6-19(17)28-23/h1-11,13,25-26H,12H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 77 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-Sen University
Curated by ChEMBL
| Assay Description
Inhibitory concentration (isomer B) against Angiotensin I converting enzyme |
J Med Chem 60: 6622-6637 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00523
BindingDB Entry DOI: 10.7270/Q23N25NF |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50238844
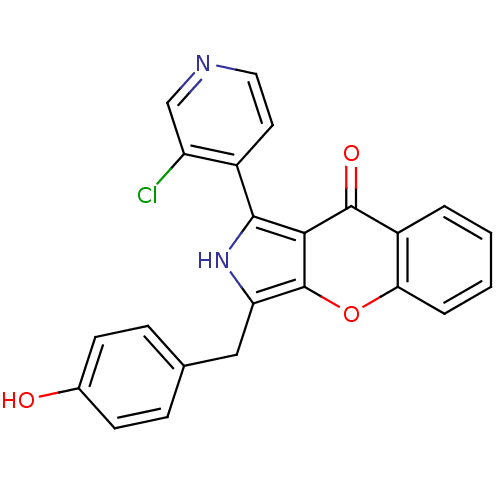
(CHEMBL4068199)Show SMILES Oc1ccc(Cc2[nH]c(-c3ccncc3Cl)c3c2oc2ccccc2c3=O)cc1 Show InChI InChI=1S/C23H15ClN2O3/c24-17-12-25-10-9-15(17)21-20-22(28)16-3-1-2-4-19(16)29-23(20)18(26-21)11-13-5-7-14(27)8-6-13/h1-10,12,26-27H,11H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >100 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-Sen University
Curated by ChEMBL
| Assay Description
Inhibition of PDE5A1 catalytic domain (535 to 860 residues) (unknown origin) expressed in Escherichia coli BL21(DE3) using [3H]cGMP as substrate afte... |
J Med Chem 60: 6622-6637 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00523
BindingDB Entry DOI: 10.7270/Q23N25NF |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50238845

(CHEMBL4073050)Show SMILES COc1ncccc1-c1[nH]c(Cc2ccc(O)cc2)c2oc3ccccc3c(=O)c12 Show InChI InChI=1S/C24H18N2O4/c1-29-24-17(6-4-12-25-24)21-20-22(28)16-5-2-3-7-19(16)30-23(20)18(26-21)13-14-8-10-15(27)11-9-14/h2-12,26-27H,13H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >100 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-Sen University
Curated by ChEMBL
| Assay Description
Inhibition of PDE5A1 catalytic domain (535 to 860 residues) (unknown origin) expressed in Escherichia coli BL21(DE3) using [3H]cGMP as substrate afte... |
J Med Chem 60: 6622-6637 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00523
BindingDB Entry DOI: 10.7270/Q23N25NF |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50238855
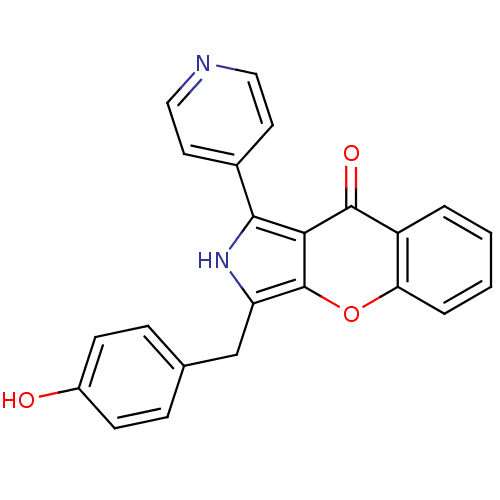
(CHEMBL4086923)Show SMILES Oc1ccc(Cc2[nH]c(-c3ccncc3)c3c2oc2ccccc2c3=O)cc1 Show InChI InChI=1S/C23H16N2O3/c26-16-7-5-14(6-8-16)13-18-23-20(21(25-18)15-9-11-24-12-10-15)22(27)17-3-1-2-4-19(17)28-23/h1-12,25-26H,13H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >100 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-Sen University
Curated by ChEMBL
| Assay Description
Inhibition of PDE5A1 catalytic domain (535 to 860 residues) (unknown origin) expressed in Escherichia coli BL21(DE3) using [3H]cGMP as substrate afte... |
J Med Chem 60: 6622-6637 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00523
BindingDB Entry DOI: 10.7270/Q23N25NF |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50238857
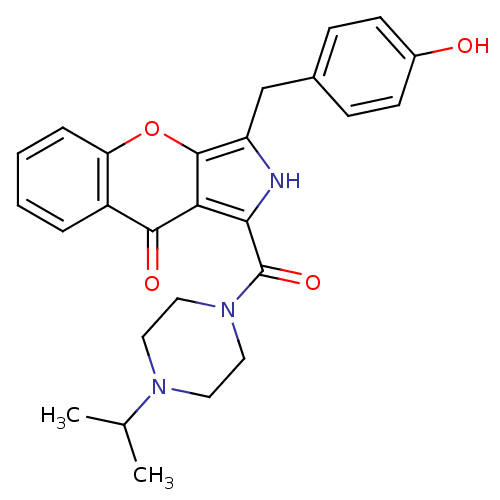
(CHEMBL4060717)Show SMILES CC(C)N1CCN(CC1)C(=O)c1[nH]c(Cc2ccc(O)cc2)c2oc3ccccc3c(=O)c12 Show InChI InChI=1S/C26H27N3O4/c1-16(2)28-11-13-29(14-12-28)26(32)23-22-24(31)19-5-3-4-6-21(19)33-25(22)20(27-23)15-17-7-9-18(30)10-8-17/h3-10,16,27,30H,11-15H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >100 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-Sen University
Curated by ChEMBL
| Assay Description
Inhibition of PDE5A1 catalytic domain (535 to 860 residues) (unknown origin) expressed in Escherichia coli BL21(DE3) using [3H]cGMP as substrate afte... |
J Med Chem 60: 6622-6637 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00523
BindingDB Entry DOI: 10.7270/Q23N25NF |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50238846
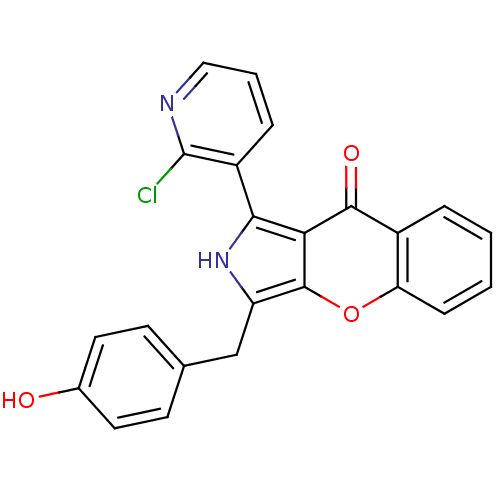
(CHEMBL4095575)Show SMILES Oc1ccc(Cc2[nH]c(-c3cccnc3Cl)c3c2oc2ccccc2c3=O)cc1 Show InChI InChI=1S/C23H15ClN2O3/c24-23-16(5-3-11-25-23)20-19-21(28)15-4-1-2-6-18(15)29-22(19)17(26-20)12-13-7-9-14(27)10-8-13/h1-11,26-27H,12H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >100 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-Sen University
Curated by ChEMBL
| Assay Description
Inhibition of PDE5A1 catalytic domain (535 to 860 residues) (unknown origin) expressed in Escherichia coli BL21(DE3) using [3H]cGMP as substrate afte... |
J Med Chem 60: 6622-6637 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00523
BindingDB Entry DOI: 10.7270/Q23N25NF |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50238847

(CHEMBL4099915)Show SMILES Cc1ccc(cc1)-c1[nH]c(Cc2ccc(O)cc2)c2oc3ccccc3c(=O)c12 Show InChI InChI=1S/C25H19NO3/c1-15-6-10-17(11-7-15)23-22-24(28)19-4-2-3-5-21(19)29-25(22)20(26-23)14-16-8-12-18(27)13-9-16/h2-13,26-27H,14H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >100 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-Sen University
Curated by ChEMBL
| Assay Description
Inhibition of PDE5A1 catalytic domain (535 to 860 residues) (unknown origin) expressed in Escherichia coli BL21(DE3) using [3H]cGMP as substrate afte... |
J Med Chem 60: 6622-6637 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00523
BindingDB Entry DOI: 10.7270/Q23N25NF |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50238865
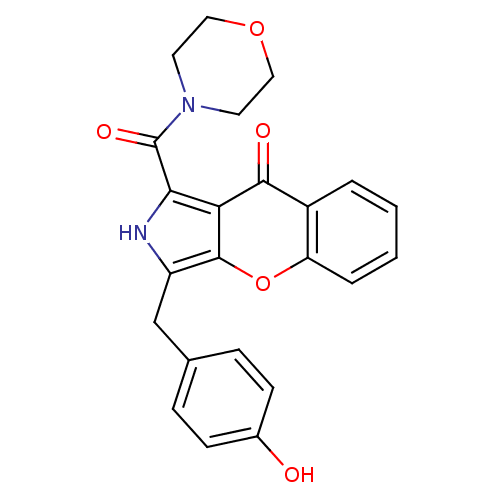
(CHEMBL4096629)Show SMILES Oc1ccc(Cc2[nH]c(C(=O)N3CCOCC3)c3c2oc2ccccc2c3=O)cc1 Show InChI InChI=1S/C23H20N2O5/c26-15-7-5-14(6-8-15)13-17-22-19(21(27)16-3-1-2-4-18(16)30-22)20(24-17)23(28)25-9-11-29-12-10-25/h1-8,24,26H,9-13H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >100 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-Sen University
Curated by ChEMBL
| Assay Description
Inhibition of PDE5A1 catalytic domain (535 to 860 residues) (unknown origin) expressed in Escherichia coli BL21(DE3) using [3H]cGMP as substrate afte... |
J Med Chem 60: 6622-6637 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00523
BindingDB Entry DOI: 10.7270/Q23N25NF |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50238853
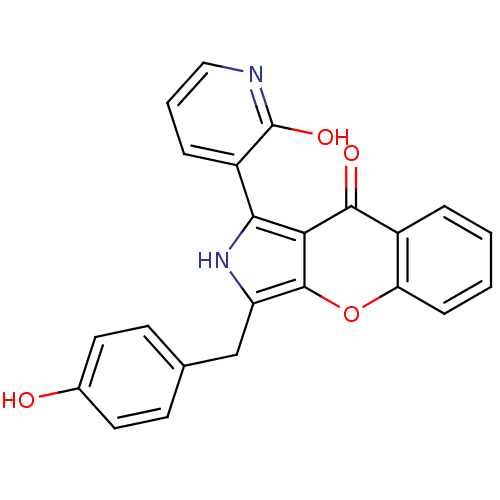
(CHEMBL4063667)Show SMILES Oc1ccc(Cc2[nH]c(-c3cccnc3O)c3c2oc2ccccc2c3=O)cc1 Show InChI InChI=1S/C23H16N2O4/c26-14-9-7-13(8-10-14)12-17-22-19(20(25-17)16-5-3-11-24-23(16)28)21(27)15-4-1-2-6-18(15)29-22/h1-11,25-26H,12H2,(H,24,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >100 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-Sen University
Curated by ChEMBL
| Assay Description
Inhibition of PDE5A1 catalytic domain (535 to 860 residues) (unknown origin) expressed in Escherichia coli BL21(DE3) using [3H]cGMP as substrate afte... |
J Med Chem 60: 6622-6637 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00523
BindingDB Entry DOI: 10.7270/Q23N25NF |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50238856
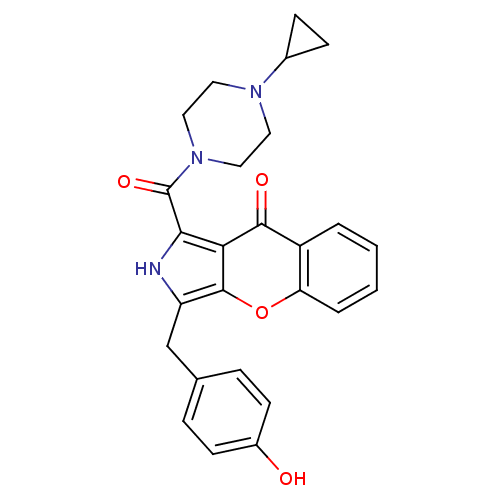
(CHEMBL4088934)Show SMILES Oc1ccc(Cc2[nH]c(C(=O)N3CCN(CC3)C3CC3)c3c2oc2ccccc2c3=O)cc1 Show InChI InChI=1S/C26H25N3O4/c30-18-9-5-16(6-10-18)15-20-25-22(24(31)19-3-1-2-4-21(19)33-25)23(27-20)26(32)29-13-11-28(12-14-29)17-7-8-17/h1-6,9-10,17,27,30H,7-8,11-15H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >100 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-Sen University
Curated by ChEMBL
| Assay Description
Inhibition of PDE5A1 catalytic domain (535 to 860 residues) (unknown origin) expressed in Escherichia coli BL21(DE3) using [3H]cGMP as substrate afte... |
J Med Chem 60: 6622-6637 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00523
BindingDB Entry DOI: 10.7270/Q23N25NF |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50238863
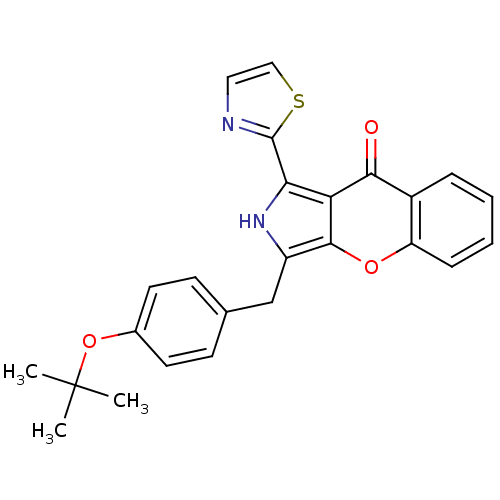
(CHEMBL4072265)Show SMILES CC(C)(C)Oc1ccc(Cc2[nH]c(-c3nccs3)c3c2oc2ccccc2c3=O)cc1 Show InChI InChI=1S/C25H22N2O3S/c1-25(2,3)30-16-10-8-15(9-11-16)14-18-23-20(21(27-18)24-26-12-13-31-24)22(28)17-6-4-5-7-19(17)29-23/h4-13,27H,14H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >100 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-Sen University
Curated by ChEMBL
| Assay Description
Inhibition of PDE5A1 catalytic domain (535 to 860 residues) (unknown origin) expressed in Escherichia coli BL21(DE3) using [3H]cGMP as substrate afte... |
J Med Chem 60: 6622-6637 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00523
BindingDB Entry DOI: 10.7270/Q23N25NF |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50238848
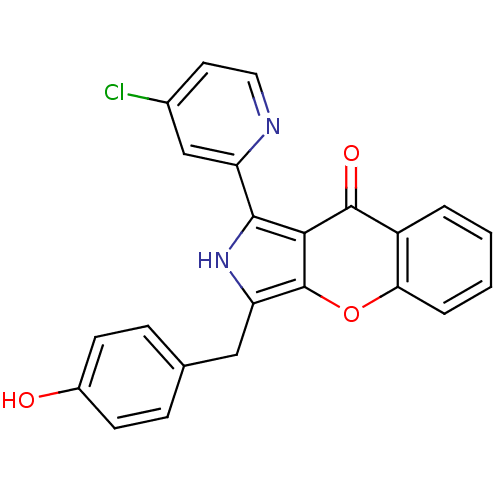
(CHEMBL4103327)Show SMILES Oc1ccc(Cc2[nH]c(-c3cc(Cl)ccn3)c3c2oc2ccccc2c3=O)cc1 Show InChI InChI=1S/C23H15ClN2O3/c24-14-9-10-25-17(12-14)21-20-22(28)16-3-1-2-4-19(16)29-23(20)18(26-21)11-13-5-7-15(27)8-6-13/h1-10,12,26-27H,11H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >100 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-Sen University
Curated by ChEMBL
| Assay Description
Inhibition of PDE5A1 catalytic domain (535 to 860 residues) (unknown origin) expressed in Escherichia coli BL21(DE3) using [3H]cGMP as substrate afte... |
J Med Chem 60: 6622-6637 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00523
BindingDB Entry DOI: 10.7270/Q23N25NF |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50238861
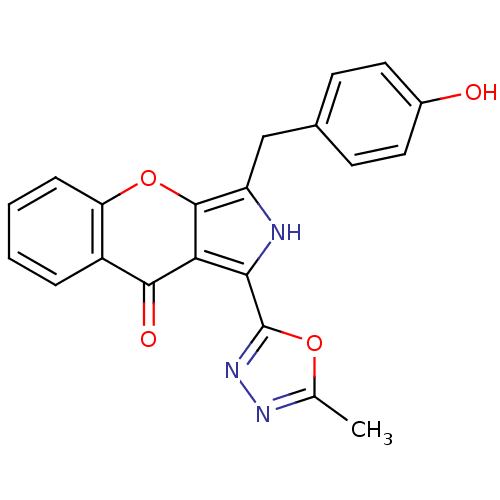
(CHEMBL4091487)Show SMILES Cc1nnc(o1)-c1[nH]c(Cc2ccc(O)cc2)c2oc3ccccc3c(=O)c12 Show InChI InChI=1S/C21H15N3O4/c1-11-23-24-21(27-11)18-17-19(26)14-4-2-3-5-16(14)28-20(17)15(22-18)10-12-6-8-13(25)9-7-12/h2-9,22,25H,10H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >100 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-Sen University
Curated by ChEMBL
| Assay Description
Inhibition of PDE5A1 catalytic domain (535 to 860 residues) (unknown origin) expressed in Escherichia coli BL21(DE3) using [3H]cGMP as substrate afte... |
J Med Chem 60: 6622-6637 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00523
BindingDB Entry DOI: 10.7270/Q23N25NF |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50238860

(CHEMBL4099219)Show SMILES O=c1c2c([nH]c(Cc3ccc4OCOc4c3)c2oc2ccccc12)-c1nccs1 Show InChI InChI=1S/C22H14N2O4S/c25-20-13-3-1-2-4-15(13)28-21-14(24-19(18(20)21)22-23-7-8-29-22)9-12-5-6-16-17(10-12)27-11-26-16/h1-8,10,24H,9,11H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 153 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-Sen University
Curated by ChEMBL
| Assay Description
Inhibition of PDE10A (449 to 770 residues) (unknown origin) expressed in Escherichia coli BL21(DE3) using [3H]cGMP or [3H]cAMP as substrate after 15 ... |
J Med Chem 60: 6622-6637 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00523
BindingDB Entry DOI: 10.7270/Q23N25NF |
More data for this
Ligand-Target Pair | |
cGMP-dependent 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50238860

(CHEMBL4099219)Show SMILES O=c1c2c([nH]c(Cc3ccc4OCOc4c3)c2oc2ccccc12)-c1nccs1 Show InChI InChI=1S/C22H14N2O4S/c25-20-13-3-1-2-4-15(13)28-21-14(24-19(18(20)21)22-23-7-8-29-22)9-12-5-6-16-17(10-12)27-11-26-16/h1-8,10,24H,9,11H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 362 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-Sen University
Curated by ChEMBL
| Assay Description
Inhibition of PDE2A (580 to 919 residues) (unknown origin) expressed in Escherichia coli BL21(DE3) using [3H]cGMP or [3H]cAMP as substrate after 15 m... |
J Med Chem 60: 6622-6637 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00523
BindingDB Entry DOI: 10.7270/Q23N25NF |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4D
(Homo sapiens (Human)) | BDBM50238860

(CHEMBL4099219)Show SMILES O=c1c2c([nH]c(Cc3ccc4OCOc4c3)c2oc2ccccc12)-c1nccs1 Show InChI InChI=1S/C22H14N2O4S/c25-20-13-3-1-2-4-15(13)28-21-14(24-19(18(20)21)22-23-7-8-29-22)9-12-5-6-16-17(10-12)27-11-26-16/h1-8,10,24H,9,11H2 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.77E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-Sen University
Curated by ChEMBL
| Assay Description
Inhibition of PDE4D2 (86 to 413 residues) (unknown origin) expressed in Escherichia coli BL21(DE3) using [3H]cAMP or [3H]cGMP as substrate after 15 m... |
J Med Chem 60: 6622-6637 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00523
BindingDB Entry DOI: 10.7270/Q23N25NF |
More data for this
Ligand-Target Pair | |
High affinity cAMP-specific and IBMX-insensitive 3',5'-cyclic phosphodiesterase 8A
(Homo sapiens (Human)) | BDBM50238860

(CHEMBL4099219)Show SMILES O=c1c2c([nH]c(Cc3ccc4OCOc4c3)c2oc2ccccc12)-c1nccs1 Show InChI InChI=1S/C22H14N2O4S/c25-20-13-3-1-2-4-15(13)28-21-14(24-19(18(20)21)22-23-7-8-29-22)9-12-5-6-16-17(10-12)27-11-26-16/h1-8,10,24H,9,11H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.22E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-Sen University
Curated by ChEMBL
| Assay Description
Inhibition of PDE8A1 (480 to 820 residues) (unknown origin) using [3H]cGMP or [3H]cAMP as substrate after 15 mins by liquid scintillation counting me... |
J Med Chem 60: 6622-6637 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00523
BindingDB Entry DOI: 10.7270/Q23N25NF |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50238860

(CHEMBL4099219)Show SMILES O=c1c2c([nH]c(Cc3ccc4OCOc4c3)c2oc2ccccc12)-c1nccs1 Show InChI InChI=1S/C22H14N2O4S/c25-20-13-3-1-2-4-15(13)28-21-14(24-19(18(20)21)22-23-7-8-29-22)9-12-5-6-16-17(10-12)27-11-26-16/h1-8,10,24H,9,11H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-Sen University
Curated by ChEMBL
| Assay Description
Inhibition of CYP1A2 in human microsomes in presence of NADPH |
J Med Chem 60: 6622-6637 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00523
BindingDB Entry DOI: 10.7270/Q23N25NF |
More data for this
Ligand-Target Pair | |
High affinity cAMP-specific 3',5'-cyclic phosphodiesterase 7A
(Homo sapiens (Human)) | BDBM50238860

(CHEMBL4099219)Show SMILES O=c1c2c([nH]c(Cc3ccc4OCOc4c3)c2oc2ccccc12)-c1nccs1 Show InChI InChI=1S/C22H14N2O4S/c25-20-13-3-1-2-4-15(13)28-21-14(24-19(18(20)21)22-23-7-8-29-22)9-12-5-6-16-17(10-12)27-11-26-16/h1-8,10,24H,9,11H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-Sen University
Curated by ChEMBL
| Assay Description
Inhibition of PDE7A1 (130 to 482 residues) (unknown origin) expressed in Escherichia coli BL21(DE3) using [3H]cGMP or [3H]cAMP as substrate after 15 ... |
J Med Chem 60: 6622-6637 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00523
BindingDB Entry DOI: 10.7270/Q23N25NF |
More data for this
Ligand-Target Pair | |
Dual specificity calcium/calmodulin-dependent 3',5'-cyclic nucleotide phosphodiesterase 1B
(Homo sapiens (Human)) | BDBM50238860

(CHEMBL4099219)Show SMILES O=c1c2c([nH]c(Cc3ccc4OCOc4c3)c2oc2ccccc12)-c1nccs1 Show InChI InChI=1S/C22H14N2O4S/c25-20-13-3-1-2-4-15(13)28-21-14(24-19(18(20)21)22-23-7-8-29-22)9-12-5-6-16-17(10-12)27-11-26-16/h1-8,10,24H,9,11H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-Sen University
Curated by ChEMBL
| Assay Description
Inhibition of PDE1B (10 to 487 residues) (unknown origin) expressed in Escherichia coli BL21(DE3) using [3H]cAMP or [3H]cGMP as substrate after 15 mi... |
J Med Chem 60: 6622-6637 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00523
BindingDB Entry DOI: 10.7270/Q23N25NF |
More data for this
Ligand-Target Pair | |
cGMP-inhibited 3',5'-cyclic phosphodiesterase 3A
(Homo sapiens (Human)) | BDBM50238860

(CHEMBL4099219)Show SMILES O=c1c2c([nH]c(Cc3ccc4OCOc4c3)c2oc2ccccc12)-c1nccs1 Show InChI InChI=1S/C22H14N2O4S/c25-20-13-3-1-2-4-15(13)28-21-14(24-19(18(20)21)22-23-7-8-29-22)9-12-5-6-16-17(10-12)27-11-26-16/h1-8,10,24H,9,11H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-Sen University
Curated by ChEMBL
| Assay Description
Inhibition of PDE3A (679 to 1087 residues) (unknown origin) expressed in Escherichia coli BL21(DE3) using [3H]cAMP or [3H]cGMP as substrate after 15 ... |
J Med Chem 60: 6622-6637 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00523
BindingDB Entry DOI: 10.7270/Q23N25NF |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50238860

(CHEMBL4099219)Show SMILES O=c1c2c([nH]c(Cc3ccc4OCOc4c3)c2oc2ccccc12)-c1nccs1 Show InChI InChI=1S/C22H14N2O4S/c25-20-13-3-1-2-4-15(13)28-21-14(24-19(18(20)21)22-23-7-8-29-22)9-12-5-6-16-17(10-12)27-11-26-16/h1-8,10,24H,9,11H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-Sen University
Curated by ChEMBL
| Assay Description
Inhibition of human ERG expressed in CHO cells by automated patch clamp assay |
J Med Chem 60: 6622-6637 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00523
BindingDB Entry DOI: 10.7270/Q23N25NF |
More data for this
Ligand-Target Pair | |
High affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A
(Homo sapiens (Human)) | BDBM50238860

(CHEMBL4099219)Show SMILES O=c1c2c([nH]c(Cc3ccc4OCOc4c3)c2oc2ccccc12)-c1nccs1 Show InChI InChI=1S/C22H14N2O4S/c25-20-13-3-1-2-4-15(13)28-21-14(24-19(18(20)21)22-23-7-8-29-22)9-12-5-6-16-17(10-12)27-11-26-16/h1-8,10,24H,9,11H2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-Sen University
Curated by ChEMBL
| Assay Description
Inhibition of PDE9A2 (181 to 506 residues) (unknown origin) expressed in Escherichia coli BL21(DE3) using [3H]cGMP or [3H]cAMP as substrate after 15 ... |
J Med Chem 60: 6622-6637 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00523
BindingDB Entry DOI: 10.7270/Q23N25NF |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50238860

(CHEMBL4099219)Show SMILES O=c1c2c([nH]c(Cc3ccc4OCOc4c3)c2oc2ccccc12)-c1nccs1 Show InChI InChI=1S/C22H14N2O4S/c25-20-13-3-1-2-4-15(13)28-21-14(24-19(18(20)21)22-23-7-8-29-22)9-12-5-6-16-17(10-12)27-11-26-16/h1-8,10,24H,9,11H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-Sen University
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C19 in human microsomes in presence of NADPH |
J Med Chem 60: 6622-6637 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00523
BindingDB Entry DOI: 10.7270/Q23N25NF |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C8
(Homo sapiens (Human)) | BDBM50238860

(CHEMBL4099219)Show SMILES O=c1c2c([nH]c(Cc3ccc4OCOc4c3)c2oc2ccccc12)-c1nccs1 Show InChI InChI=1S/C22H14N2O4S/c25-20-13-3-1-2-4-15(13)28-21-14(24-19(18(20)21)22-23-7-8-29-22)9-12-5-6-16-17(10-12)27-11-26-16/h1-8,10,24H,9,11H2 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-Sen University
Curated by ChEMBL
| Assay Description
Inhibitory concentration (isomer A) against Angiotensin I converting enzyme |
J Med Chem 60: 6622-6637 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00523
BindingDB Entry DOI: 10.7270/Q23N25NF |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2B6
(Homo sapiens (Human)) | BDBM50238860

(CHEMBL4099219)Show SMILES O=c1c2c([nH]c(Cc3ccc4OCOc4c3)c2oc2ccccc12)-c1nccs1 Show InChI InChI=1S/C22H14N2O4S/c25-20-13-3-1-2-4-15(13)28-21-14(24-19(18(20)21)22-23-7-8-29-22)9-12-5-6-16-17(10-12)27-11-26-16/h1-8,10,24H,9,11H2 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-Sen University
Curated by ChEMBL
| Assay Description
Inhibition of CYP2B6 in human microsomes in presence of NADPH |
J Med Chem 60: 6622-6637 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00523
BindingDB Entry DOI: 10.7270/Q23N25NF |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50238860

(CHEMBL4099219)Show SMILES O=c1c2c([nH]c(Cc3ccc4OCOc4c3)c2oc2ccccc12)-c1nccs1 Show InChI InChI=1S/C22H14N2O4S/c25-20-13-3-1-2-4-15(13)28-21-14(24-19(18(20)21)22-23-7-8-29-22)9-12-5-6-16-17(10-12)27-11-26-16/h1-8,10,24H,9,11H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-Sen University
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 in human microsomes in presence of NADPH |
J Med Chem 60: 6622-6637 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00523
BindingDB Entry DOI: 10.7270/Q23N25NF |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50238860

(CHEMBL4099219)Show SMILES O=c1c2c([nH]c(Cc3ccc4OCOc4c3)c2oc2ccccc12)-c1nccs1 Show InChI InChI=1S/C22H14N2O4S/c25-20-13-3-1-2-4-15(13)28-21-14(24-19(18(20)21)22-23-7-8-29-22)9-12-5-6-16-17(10-12)27-11-26-16/h1-8,10,24H,9,11H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-Sen University
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 in human microsomes in presence of NADPH |
J Med Chem 60: 6622-6637 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00523
BindingDB Entry DOI: 10.7270/Q23N25NF |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50238860

(CHEMBL4099219)Show SMILES O=c1c2c([nH]c(Cc3ccc4OCOc4c3)c2oc2ccccc12)-c1nccs1 Show InChI InChI=1S/C22H14N2O4S/c25-20-13-3-1-2-4-15(13)28-21-14(24-19(18(20)21)22-23-7-8-29-22)9-12-5-6-16-17(10-12)27-11-26-16/h1-8,10,24H,9,11H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-Sen University
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 in human microsomes in presence of NADPH |
J Med Chem 60: 6622-6637 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00523
BindingDB Entry DOI: 10.7270/Q23N25NF |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data